Abstract
Electrochemical and photoelectrochemical water splitting offers a scalable approach to producing hydrogen from renewable sources for sustainable energy storage. Depending on the applications, oxygen evolution catalysts (OECs) may perform water splitting under a variety of conditions. However, low stability and/or activity present challenges to the design of OECs, prompting the design of self-healing OECs composed of earth-abundant first-row transition metal oxides. The concept of self-healing catalysis offers a new tool to be employed in the design of stable and functionally active OECs under operating conditions ranging from acidic to basic solutions and from a variety of water sources.
Subject terms: Energy, Electrocatalysis, Catalytic mechanisms
Large scale sustainable energy storage by water splitting benefits from performing the oxygen evolution reaction under a variety of conditions. Here, the authors discuss self-healing catalysis as a new tool in the design of stable and functionally active catalysts in acidic to basic solutions, and a variety of water sources
Introduction
Large-scale implementation of sustainable energy is needed to address the rising energy demands of our society while avoiding the detrimental impacts of fossil fuels. Whereas sustainable energy supplies (e.g., solar energy) are abundant, their implementation is bottlenecked by the challenge of storing this energy at the scale of societal demand1–3. One potentially scalable approach to energy storage is the production of hydrogen gas (H2) through renewably-driven electrochemical water splitting1,2,4,5. Hydrogen is a reliable energy carrier that can be used directly as a green fuel2, or be employed to furnish increasingly energy-dense products, including liquid fuels6–9 ammonia10,11. Water splitting (Eq. (1)) is an endergonic process that demands an external energy input greater than the thermodynamic minimum of 1.23 V to proceed:
| 1 |
| 2 |
| 3 |
Thus, renewable energy, whether it be from solar or wind, may be stored in the rearranged bonds of water as H2 and O21. Of the two half-reactions of water splitting (Eqs. (2), (3)), the 4e–/4H+ oxygen evolution reaction (OER) is particularly demanding12. The most active and stable oxide catalysts for OER comprise critical metals (i.e., Ru, Ir)13. However, with regard to commercially relevant applications, they are not stable enough14,15 and they are costly owing to their scarcity. These challenges provide an imperative for the development of oxygen evolution catalysts (OECs) from earth-abundant elements that are both highly active and stable in various water sources under a range of operating conditions16–18.
Earth-abundant first-row transition metal oxides, however, have been relegated to operation in concentrated base13,19. The presence of base is required because metal oxides are themselves basic according to the Lux classification20 and will readily react with protons produced through OER (Eq. (2)), leading to damage (i.e., dissolution, corrosion, protonation). In concentrated base, hydroxide (OH−) is the strongest base and will neutralize these protons to protect the oxide; however, in less basic solutions, the concentration of OH− is not sufficient making the primary base the oxide itself, leading to catalyst damage and inactivation. The ability to operate in nonbasic conditions has advantages of using natural water sources21, facilitating the interfacing of catalysts with materials such as Si22–26, which is unstable in corrosive basic conditions, reducing liability associated with technology advancement especially for distributed systems, and enabling the interfacing of water splitting catalysis to bio-organisms in hybrid inorganic–biological devices8,9,27,28. A unique strategy for operating earth-abundant first-row transition metal oxide OECs outside of strongly basic conditions is through the implementation of self-healing.
Within the catalysis community, multiple definitions of self-healing and self-repairing catalysts have been proposed29,30. The concept of self-healing has historically been used to describe any material with the ability to repair itself. Materials ranging from bio-inspired systems31,32 to synthetic organic polymers33–35, inorganic–organic hybrid materials, and metallic systems36–38 have all been considered self-healing. This can be through autonomous or stimuli-triggered processes that occur without or with external input (e.g., energy, pressure, chemical healing agents), respectively34–38. Many materials described as self-healing, including bio-inspired synthetic materials and metallic systems, however, induce repair after significant functional damage has already occurred, involve processes outside of normal operation, or rely upon the presence of chemical healing agents that are continually depleted34–38. We restrict the term “self-healing” herein to describe systems that continually regenerate themselves through a dynamic equilibrium during catalysis and under a given set of operating conditions. For example, self-healing notably differs from the repair mechanism of the Oxygen-Evolving Complex in Photosystem II, where the damaged reaction center is continuously replaced by a newly synthesized copy39,40. Furthermore, based on our criteria, catalyst regeneration mechanisms that rely on chemical oxidants (e.g., Ce4+)41, require activation by light to release oxygen and regenerate the precatalytic resting state42, or involve regeneration at conditions outside of that required for catalysis43 are considered self-repairing but not self-healing. Self-repairing metal oxide-based OECs have been comprehensively reviewed44.
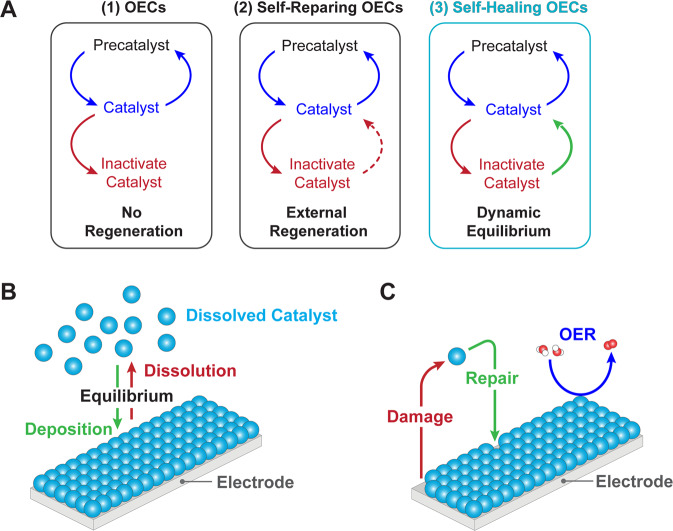
Figure 1 schematically contrasts OECs that degrade with ones that self-repair and self-heal. Electrochemical and photoelectrochemical OER relies upon applying an external bias (potential) to the OEC to promote reactivity (Eq. (2)). Self-healing OECs are realized when this external bias is sufficient to drive regeneration such that the rate of repair is greater than or equal to the rate of damage. The required potential for this process is therefore less than or equal to the potential required to drive OER (Fig. 1C). This requirement is not fulfilled by self-repairing OECs, thus distinguishing self-healing from self-repairing catalysts. This is illustrated schematically in Fig. 1B where metal ions at equilibrium in solution can (re)deposit onto the still active and functional catalyst, assuming adequate mass transport from the solution to the electrode surface30. At equilibrium, it may be considered that there is no net degradation of catalyst, to reconcile a previous definition of self-healing29. We emphasize that self-healing as defined here is not established universally by structure/composition but rather is defined operationally. For oxide catalysts, structural and compositional damage occurs most commonly by protonation of surface oxo-species and the subsequent amorphization or dissolution of surface metal species; degradation mechanisms of OECs may also involve other structural rearrangement, mechanical, and poisoning effects45. The ability of the catalyst to regenerate itself will depend on the types and amounts of electrolytes and buffers, bulk pH values, applied currents and potentials, temperature, mass transport conditions, etc. Consequently, a homogeneous or heterogeneous OER catalyst of a given composition, crystallinity, or polymorphism may degrade under one set of conditions and not another. Thus, self-healing is determined by the set of conditions in which the catalyst operates.
Fig. 1. Schematic depiction of self-healing OECs.
A Self-repairing OECs (center) are a specific type of OECs (left) that may operate for a prolonged time as they are regenerated once they become inactive, usually with the aid of an external input (e.g., energy, pressure, chemical healing agents). Self-healing OECs (right) are a specific type of OECs that continually regenerate themselves through an equilibrium process that occurs under the operating conditions of OER. B Graphical representation of the competing effects of catalyst deposition and dissolution that give rise to the equilibrium implicit for self-healing OECs. C Based on the equilibrium shown in (B), a damaged site is continuously repaired during OER for self-healing OECs, and as the rate of repair is greater than or equal to the rate of damage, no loss of catalytic species is observed. Blue spheres represent a catalytically competent metal capable of self-healing (e.g., Mn, Co, Ni, Cu).
We now survey the current state of OECs based on first-row transition metal oxides that exhibit self-healing behavior. Dimensional reduction of first-row metal oxides of Mn, Co, and Ni gives rise to metallate oxygen evolution catalysts (M-OECs) that exhibit high activity for OER46–49. We focus on the kinetics of OER and self-assembly, which form the foundation for the inherent self-healing properties of M-OECs. We highlight how the distinct kinetics of these processes determine the stability and activity of catalysts under different operating conditions, how the concept of self-healing is extended to multimetallic systems and discuss future approaches to developing increasingly active self-healing OECs.
Self-healing Co-OECs
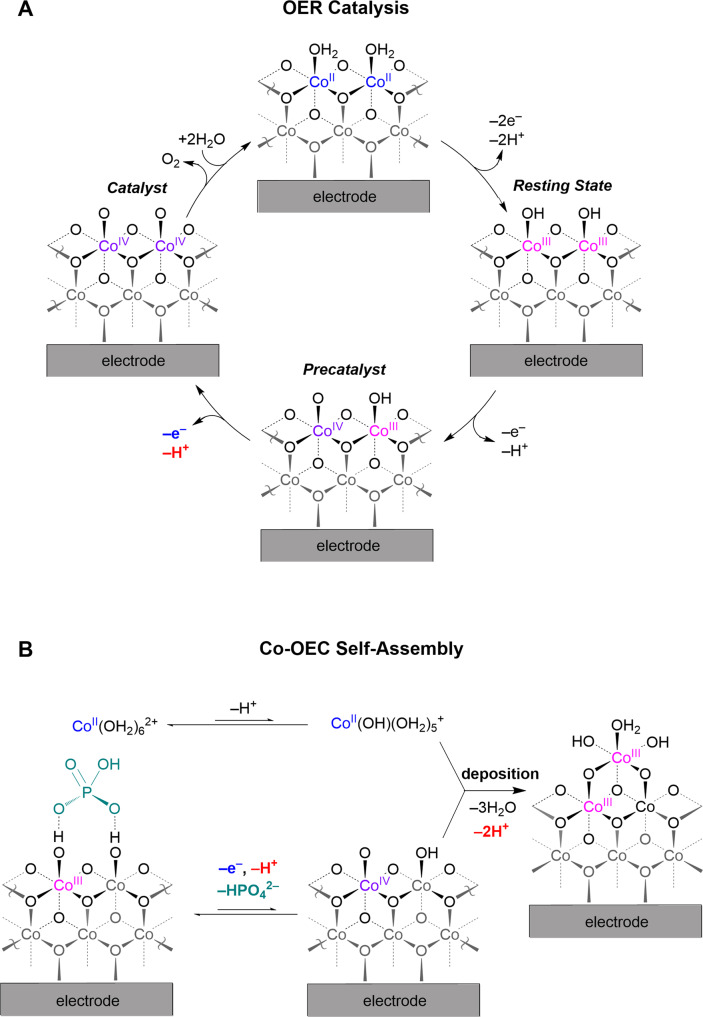
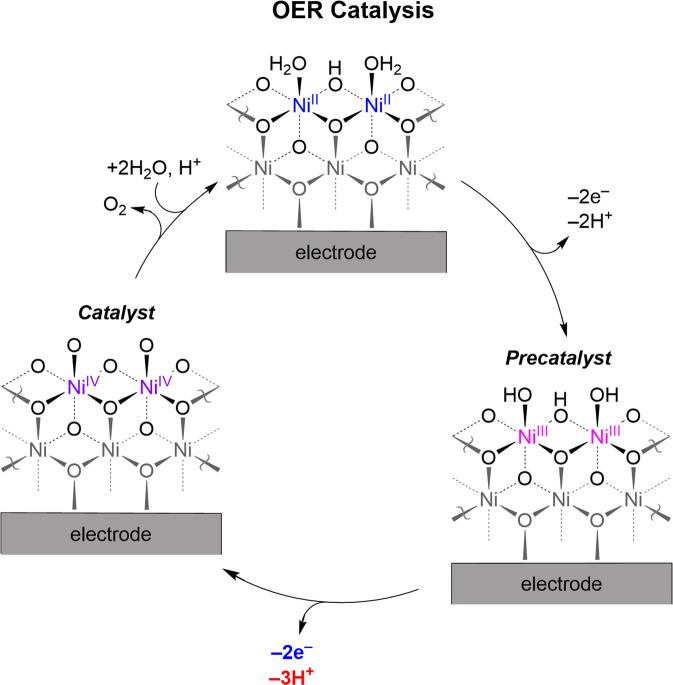
Cobalt-based M-OECs (Co-OECs) in the presence of phosphate (Pi) and borate (Bi) are exemplar self-healing OECs (Co-OECs generated in the presence of Pi or Bi are referred to hereafter as CoPi and CoBi, respectively). These catalysts are generated as amorphous thin films on various conductive substrates (e.g., indium tin oxide, fluorine-doped tin oxide) during anodic electrodeposition from dilute aqueous Co2+ solutions in the presence of Pi50, methylphosphonate (MePi)51, or Bi21,52 electrolytes in neutral to mildly basic conditions. These oxoanions facilitate the dimensional reduction of extended metal oxides by capping cluster growth to give rise to metallate active sites, which range from 10 to 60 metal centers, as deduced from X-ray pair distribution function (PDF) analysis of heterogeneous films52–55. The molecular nature of the M-OECs has allowed the mechanism of OER catalysis to be defined at a molecular and atomistic level. Isotopic measurements using differential electrochemical mass spectrometry56 together with electrokinetics51, spectroscopic57, and computational58,59 studies establish that O–O bond formation occurs by proton-coupled electron transfer (PCET) at cobaltate cluster edge sites.
Figure 2A shows the mechanism for OER catalyzed by Co-OECs. The catalyst resides in a CoIIICoIII resting state (vide infra), as highlighted in Fig. 2B, from which the CoIIICoIV precatalytic state is generated. Here, the terminal CoIV–oxo (i.e., CoIV = O) is better formulated as a CoIII–oxyl (i.e., CoIII–O•) radical based on the electronic considerations embodied by the “oxo wall”60. Tafel analysis at pH 7 reveals a slope of 59 mV dec−1, indicating that the active CoIVCoIV catalyst is generated by a 1e–/1H+ pre-equilibrium step followed by a turnover rate-limiting chemical step involving O–O bond formation and O2 release61. Accordingly, the rate of OER possesses an inverse first-order dependence of log(jOER) on proton activity (i.e., first-order dependence on pH), as summarized in the following electrochemical rate law obtained from electrokinetics studies:
| 4 |
where jOER is the current density for OER and k0OER is a potential-independent constant that is proportional to the exchange current density for OER.
Fig. 2. Co-OEC catalysis and self-assembly.
A Proposed OER catalytic cycle (top cycle in Fig. 1A (3)) for Co-OECs (i.e., CoPi, CoMePi, CoBi), as determined from spectroscopic analysis and electrochemical kinetics studies for CoPi. B Proposed mechanism for the generation of Co-OEC films (bottom cycle in Fig. 1A (3), and green arrows in Fig. 1B, C). The protons (H+) that appear in the electrochemical rate laws for OER and catalyst deposition/ regeneration are highlighted in red.
Self-healing is established between the interplay of the potential required for OER and that required for self-assembly (i.e., deposition and regeneration) of the Co-OECs. Electrochemical kinetics have revealed the mechanism for catalyst deposition/regeneration to be as shown in Fig. 2B. At the deposition potential of 1.0 V vs. the normal hydrogen electrode (NHE) there is a minor Nernstian population of Co4+, as verified by EPR spectra of CoPi films62, and consistent with the redox Co3+/Co4+ midpotential63. The Co4+ on the electrode oxidizes Co2+ in solution, which is in the form of Co(OH)(OH2)5+ at the 1.0 V deposition potential, by inner-sphere electron transfer upon dissociation of Pi, giving rise to substitutionally inert Co3+ that adds to the exposed edge sites for metallate cluster growth. The pH dependence of CoPi film deposition reveals an inverse third-order dependence on proton activity (i.e., third-order dependence of log(jOER) on pH), as well as an inverse first-order dependence on [Pi], and first-order dependence on [Co2+], owing to the need to dissociate Pi for the inner-sphere electron transfer between Co4+ on the surface and Co(OH)(OH2)5+ in solution64. Thus, the overall rate law for deposition of CoPi is65:
| 5 |
where jdep is the current density for catalyst deposition/regeneration and k0dep is a potential-independent constant that is proportional to the exchange current density for the deposition/regeneration process.
Self-healing is achieved because the potential needed to drive CoPi film self-assembly (i.e., produce Co4+ for deposition) occurs at 0.2 V below the potential required for sustaining OER. Accordingly, potentials sufficient to sustain OER will oxidize any Co2+ in solution that may have leached from the film during operation and, thus, induce instantaneous redeposition. The transport of Co4+ in CoPi films (i.e., the oxidizing hole equivalent) is predicted to be fast based on the Co3+/4+ self-exchange electron transfer rate constant of kET(Co3+/4+) = 3 × 105 M–1 s–1 as measured in cobalt cubane model complexes66. Because such hole hopping through the film is fast relative to Co2+ deposition62,67, very little Co2+ is produced in solution. Nonetheless, as confirmed by Co-57 radiolabeling of CoPi films, any Co2+ released in solution is redeposited via the mechanism shown in Fig. 2B68. Consequently, no film dissolution is observed during OER when Pi is present at intermediate-to-high concentration (>~1 mM). Furthermore, this self-healing mechanism also applies for Co-OECs with MePi and Bi oxoanions, and similar self-healing Co-OECs are posited to form from the decomposition of molecular cobaloxime precursors in Bi buffer solutions (pH 9.2) upon application of high positive potentials69.
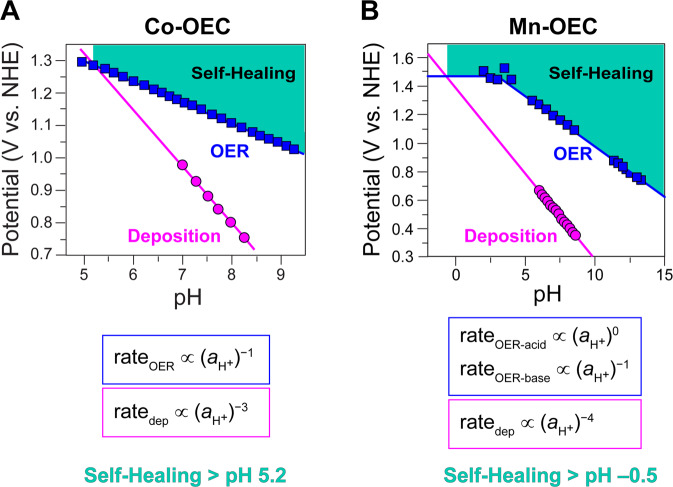
The disparate pH profiles for OER and catalyst generation for Co-OECs give rise to a “Pourbaix” diagram for self-healing. Because of the steeper inverse third-order dependence on proton activity for deposition, the potential necessary for catalyst self-assembly rises much more rapidly with decreasing pH as compared to that for OER. As highlighted by the teal zone in Fig. 3A, the potentials necessary to sustain catalyst film formation are well below those required for OER. Thus, as long as Co-OECs are operated in the teal zone in Fig. 3A (pH > 5.2) the catalysts are indefinitely stable in aqueous solutions under the operational conditions that promote self-healing. This functional stability range for CoPi is further supported by the observation of catalyst damage at pH 5 and below47,65. By introducing 0.1–1 mM Co2+ to the buffered electrolyte, however, Co-OECs may remain functionally stable down to pH of ~3 as the increased concentration of Co2+ drives a dynamic equilibrium, as shown in Fig. 2B, toward catalyst deposition and thus regeneration70,71. Below pH ~3, however, these low concentrations of Co2+ are insufficient to offset metal oxide dissolution and catalyst damage cannot be reversed. Notably, operational stability of Co-OECs at pH 1.6 can be achieved at higher Co2+ concentrations of 0.6 M in phosphate or sulfate electrolytes under application of potentials above 2.05 V vs. NHE72.
Fig. 3. Pourbaix diagrams for Co- and Mn-OECs.
Potential–pH diagrams for (A) Co-OECs and (B) Mn-OECs at fixed current densities of j = 30 and 1.3 μA cm−2 (based on geometric electrode surface area), respectively. The dependence of the rate of OER and the rate of catalyst deposition/regeneration on proton activity (aH+) are highlighted in blue and magenta, respectively, for each catalyst type. The different pH dependence for the two processes forms the foundation for the self-healing properties of Co-OECs and Mn-OECs above pH 5.2 and −0.5, respectively, in solutions devoid of component metals as indicated with the teal zones in the graphs. All potentials are referenced to the NHE scale.
The CoPi OER catalyst is exemplary of the concept of self-healing from an operational as opposed to a structural or compositional viewpoint. As emphasized for CoPi, self-healing is not achieved when the buffer concentration is too low29,71, as the rate of catalyst redeposition is smaller than the rate of dissolution. Hence under one set of conditions (e.g., low Pi concentration or a given current, potential, etc.) CoPi is self-repairing, while under a different set of conditions that establishes Fig. 1C, CoPi is self-healing. As a metric, a self-healing catalyst under operating conditions has a turnover number that is extremely large and in the limit approaches infinity.
Self-healing Mn-OECs
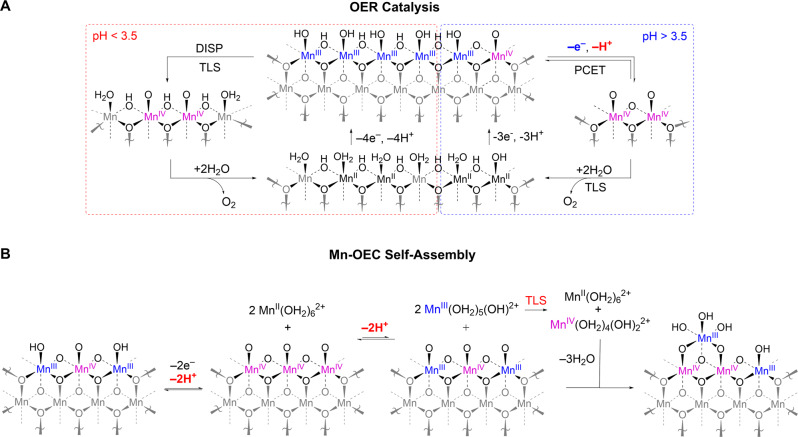
Modulating the relationship between potential and pH for OER and self-assembly can lead to extraordinary catalytic properties. Self-healing in a Mn-OEC produced by electrodeposition from dilute Mn2+ solutions in a weakly basic electrolyte furnishes a stable OER catalyst at pHs as low as –0.573–75. The rate of OER by Mn-OECs in Pi and MePi electrolytes is zeroth order in proton activity at pH < 3.5 and inverse first order in proton activity at pH > 3.5. Based on Tafel analysis, two parallel OER mechanisms shown in Fig. 4A have been proposed in these different pH regions: (1) a Mn3+ disproportionation process with zeroth-order dependence on proton activity that predominates at pH < 3.5 and (2) a 1e–/1H+ pathway that is dominant at pH > 3.5. This modest pH dependence for OER is juxtaposed to a significant pH dependence for deposition. An inverse fourth-order dependence on proton activity arises from a turnover-limiting disproportionation reaction, which also gives rise to a second-order dependence on Mn2+ concentration73. This steep pH dependence for catalyst deposition results in a potential–pH diagram with a crossing for OER and catalyst deposition/regeneration at pH –0.5 (Fig. 3B), allowing the Mn-OEC to operate in concentrated acid. The functional stability of Mn-OECs is supported by 31P NMR line width analysis when using MePi as an electrolyte74. The stability of Mn-OECs, however, is complicated by the formation of MnO4− ions at high potentials, preventing operation at high current densities (and attendant higher potentials) for OER76. As such, the stability of Mn-OECs in acidic electrolytes is maintained only at low OER current densities (< 1 mA cm–2). Activation of Mn-OECs by potential cycling affords a substantial improvement in OER activity, resulting in two orders of magnitude increase in current density at pH 2.573. This activity enhancement for Mn-OECs upon varying the synthesis protocol73,77 suggests that the generation of acid-stable Mn-OECs that show higher OER activity is possible.
Fig. 4. Mn-OEC catalysis and self-assembly.
A Proposed OER catalytic cycle for Mn-OECs, as determined from spectroscopic analysis and electrochemical kinetics studies at different pHs. B Proposed mechanism for the generation of Mn-OEC films. The protons (H+) that appear in the electrochemical rate laws for OER and catalyst deposition/regeneration are highlighted in red. Owing to the disproportionation reaction involving two manganese complexes, an inverse fourth-order proton dependence is observed, enabling self-healing to be preserved at pH > –0.5. DISP and TLS denote disproportionation and turnover-limiting step, respectively.
Self-healing Ni-OECs
Self-healing Ni-OEC may be established with Bi as the electrolyte, as Pi is unable to support nickelate cluster formation78–80. The NiBi catalyst is prepared by anodic electrodeposition from dilute aqueous Ni2+ solutions in the presence of Bi at pH 9.2. Subsequent activation by anodization affords enhanced OER activity owing to an average increase in the Ni oxidation state from +3.2 to +3.6, indicating substantial generation of formally Ni4+ species, whereas nonactivated films are predominately Ni3+79,80. The mechanism for NiBi self-healing resembles that of CoPi with the caveat that Tafel analysis of NiBi furnishes an OER rate law that is inverse first order in [Bi] (i.e., for 20–300 mM Bi) and inverse third order in proton activity (i.e., for pH 8.5–12). The 2e–/3H+ pre-equilibrium followed by a rate-limiting O–O bond formation and O2 release is shown in Fig. 5. Despite the ostensible “inhibitory” effect of Bi on OER kinetics, the activity of NiBi is high owing to the differences of Bi vs. Pi substitution. Whereas Pi must exchange at dicobalt edge sites by a dissociative substitution mechanism, Bi can exchange with more facility at edge sites via Lewis acid–base mechanism56:
Fig. 5. Ni-OEC catalytic cycle.
Proposed OER catalytic cycle for Ni-OEC with Bi as an electrolyte, as determined from spectroscopic analysis and electrochemical kinetics studies for NiBi.
The differences in the role of electrolyte between Co-OECs and Ni-OECs highlight the pivotal role that electrolytes play in OER and self-healing by facilitating proton transfer at intermediate pHs and by establishing the equilibrium for catalyst deposition/regeneration. The different characteristics of the PCET pre-equilibrium (and thus Tafel slope) for CoPi and NiBi and associated disparate pH dependences for these catalysts render NiBi increasingly more active than CoPi as the pH is elevated. However, CoPi outperforms NiBi at neutral and slightly acidic conditions79. On that note, Ni-OECs are not stable in acidic electrolytes at moderate overpotentials owing to dissolution of the oxides, as judged by the Pourbaix diagram of Ni in water76. In contrast, Ni-OECs are stable at alkaline pHs and display self-healing in concentrated base (e.g., 0.1–1.0 M KOH) devoid of buffer electrolytes79. Accordingly, Ni-OECs exhibit self-healing properties at pH ~9–14, however, the pH dependence of the Ni-OEC film assembly process remains to be defined to enable the construction of potential-pH diagrams such as those shown for Co-OECs and Mn-OECs in Fig. 3.
As with Co-OECs, catalytically active Ni-OEC films may form from molecular complexes that provide the requisite metal ions81. The use of a proton-accepting Bi electrolyte has been shown to be necessary to achieve and maintain high catalytic activity for these Ni-OECs at pH 9.2. The facility with which M-OEC films form from molecular complexes highlights the difficulties associated with deploying molecular systems as OER catalysts. Specifically, determining whether catalytic activity is derived from the initial molecular species or from the generation of small amounts of M-OECs, where the molecular complex serves as a metal source, remains a challenging task.
Self-healing in Cu-OECs
Self-healing has been imparted to Cu-OECs where sustained OER is achieved in a carbonate buffer (pH ~ 10.8) containing sufficient dissolved Cu2+ to drive catalyst self-assembly82. Here, formation of a compact film of CuO on a Cu surface prevents anodic corrosion and enables sustained OER catalysis.
Self-healing mixed-metal OECs
Mixed-metal oxide OECs with catalytic and structural metals
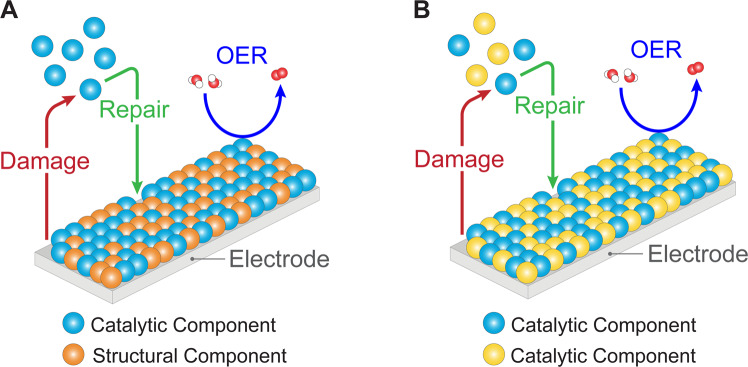
Self-healing may be augmented by alloying catalytically active metal elements with structural metal elements to further enhance stability. In a common class of mixed-metal oxide OECs (M′M–OECs), some components may themselves be formally self-healing while other components function predominantly as structural elements (Fig. 6A) such that OER activity and stability are decoupled and therefore may be optimized independently83. The incorporation of various catalytically active metals into a shared inert oxide framework can fortify the metal–oxygen bonds that are generally weakened during OER, particularly at active sites, leading to enhanced stability while preserving high OER activity. Among the most common types of M′M–OECs are systems where a catalytically competent metal (e.g., Mn, Fe, Co, Ni) is doped into a conductive and thermodynamically stable matrix (e.g., PbOx) to achieve materials with simultaneous high activity and stability under acidic conditions83,84. For example, the self-healing and structurally fortified CoFePbOx is functionally stable under optimized reaction conditions (i.e., 5 mM Co2+, 1 mM Fe3+, 0.5 mM Pb2+) at both ambient and elevated temperatures in solutions of pH 0–1 with no loss of activity after even one week of continuous operation85. Since catalyst repair and damage, as well as OER, are all kinetically controlled processes, tuning the concentrations of the component metals, along with temperature and pH, appears to be a promising means of imparting self-healing to M′M–OECs.
Fig. 6. Schematic depiction of two common types of mixed-metal oxide OECs (M′M–OECs) that may possess self-healing properties.
A Self-healing M′M–OEC system composed of separate catalytic (e.g., Co, Ni; blue spheres) and structural components (e.g., PbOx; orange spheres). B Self-healing M′M–OEC system comprising two types of catalytic components (blue, yellow spheres). Self-healing in both types of systems involves the catalytic component(s) undergoing damage and repair while performing OER. The colored spheres above the electrode surface denote dissolved catalyst capable of repair.
Mixed-metal oxide OECs with different catalytic metals
Another common type of M′M–OECs is generated by alloying metals to increase the activity of a unary metal oxide that already displays OER activity (Fig. 6B). The introduction of Fe3+ into NiBi and CoPi has been shown to be especially effective at increasing OER activity86–91, though the reason for this enhancement continues to be explored92. As Fe-based OECs are not self-healing93, NiFe-OECs and CoFe-OECs incur a significant loss in stability owing to oxide dissolution under varying conditions83,84. Notwithstanding, a dynamically stable CoFe-OEC may be prepared using a nanorod array of CoMoO4 as a host matrix for the redeposition of FeOOH and CoOOH in alkaline media94. The resulting CoFe-OEC exhibits high OER activity, sustaining an OER current density of 100 mA cm−2 (based on geometric electrode surface area) with an overpotential of 298 mV in 1 M KOH. Furthermore, recent work has demonstrated that self-healing may also be achieved by operating in alkaline electrolytes containing a small amount of Fe95,96. For example, addition of 0.1 ppm Fe to the operating KOH electrolyte solution was shown to promote self-healing in a range of first-row transition metal oxyhydroxides by facilitating dynamic Fe exchange95. Isotopic labeling experiments with 56Fe and 57Fe revealed rapid exchange of isotopes while preserving overall Fe content, indicating a fast rate of Fe regeneration as compared to the rate of dissolution. Consequently, the electrolyte must contain a sufficiently high concentration of Fe so as to raise the rate of Fe regeneration to engender a high number of dynamic active sites, and thus preserve high OER activity and functional stability. In a related study, introduction of Co into NiFe-OECs was shown to promote redeposition of Fe in situ and, thus, engender catalyst self-healing when operating in strongly alkaline (pH 14) electrolytes containing both ferrous and borate ions96. Here, the Fe ions could only be redeposited on sites adjacent to Co sites, preventing the deposition of thick Fe oxyhydroxide overlayers. This unique self-limiting thickness was demonstrated to be ideal for integration with photoelectrodes as a high light transmittance through the catalyst layer could be maintained during OER operation. A similar approach has been taken with NiFe-OECs in carbonate buffer, where long-term stability and self-healing was induced by adding Ni2+ and Fe3+ to the operating electrolyte in weakly to strongly basic solutions97. Together these examples highlight that the competing rates of metal oxide repair and damage may be influenced by tuning the concentrations of dissolved component metals in solution.
Other mixed-metal oxide OECs
Self-healing M′M-OECs beyond typical electrodeposited metal oxide films have also been reported. A NiFe-OEC generated in situ on a Mo-doped BiVO4/Ni/Sn photoelectrode is self-healing in a site-specific manner when operated in a Bi electrolyte by using a passivated Ni contact layer as the source of Ni2+98. Similarly, dynamic cycling of dissolution, diffusion, and deposition of Ni and Fe in a Si-based photoanode coated with a dual layer electrocatalyst engenders extended electrode stability99. Furthermore, a nanoparticle-based system composed of NiFe-layered double hydroxide nanoparticles deposited onto a Ni electrode has been shown to possess self-healing properties in an alkaline electrolyser100. This catalyst is generated in situ during electrolysis driven by electrostatic forces; self-healing is induced by operating in an electrolyte containing the component nanoparticles that continually deposit onto the underlying Ni electrode during operation. Along these lines, a ligand-induced self-healing mechanism was recently reported for a Fe-based electrocatalyst operating in strongly alkaline media101. These self-healing approaches are analogous to those described above but rely on a component material or a stabilizing ligand, as opposed to a dissolved metal ion, to direct the damage vs. repair equilibrium toward repair.
Finally, self-healing may directly involve oxide lattice sites. For perovskite systems such as SrCoO3102, self-healing is derived from surface adsorbed H2O molecules, which dissociate to form reactive oxygen atoms that fill oxygen vacancies on the surface produced during OER. As the formation of such oxide vacancies define catalyst degradation, their depletion accommodates catalyst repair. This system is self-healing as the onset potential required to drive the filling of the oxygen vacancy is lower than the potential at which OER proceeds. A similar oxygen vacancy-based self-healing property has been proposed for TiO2103 and α-MnO2104 OECs.
Summary and outlook
The need to implement sustainable energy sources on a global scale has promoted H2 as a potential energy carrier. Electrochemical water splitting provides a scalable route to H2 production from renewable sources and has prompted the development of OECs to drive the more challenging oxygen evolution half-reaction. In this effort, self-healing OECs based on earth-abundant first-row transition metal oxides have garnered significant attention owing to their ability to drive OER at relatively low overpotentials while remaining stable under various operating conditions. Self-healing unary metal oxide OECs such as MnOx, CoPi, and NiBi provide activity and stability in acidic, neutral, and basic conditions, whereas emerging mixed-metal oxide OECs such as CoFePbOx and NiFeOx offer improved activity and/or stability across a similar range of conditions. Under a wide range of operating conditions, OER catalysts may be more easily interfaced with (1) materials for direct conversion of water to oxygen and hydrogen at high efficiency24–26,105 and (2) biological organisms to perform artificial photosynthesis8,9. A better fundamental understanding of damage and repair mechanisms in existing and future systems may expand the self-healing capabilities of OECs to a wider range of conditions and promote self-healing as a general design principle in catalysis development, opening paths to a broad set of future applications. Overall, this review has highlighted the prospects for self-healing first-row transition metal oxides as OECs in water splitting systems. The increasing need to transition away from fossil fuels necessitates further research into self-healing OECs based on earth-abundant elements, expanding the practicality and utility of these versatile chemical platforms.
Acknowledgements
Material is based upon work supported under the Solar Photochemistry Program of the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences of the U.S. Department of Energy DE-SC0017619. A.E.T. acknowledges the Harvard University Center for the Environment (HUCE) for a postdoctoral fellowship and S.V.V. acknowledges support from the Herchel Smith Graduate Fellowship in the Sciences.
Author contributions
D.G.N. developed the idea and supervised the work. A.E.T. and S.S.V. organized content and designed figures. All authors contributed to the writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Agnes E. Thorarinsdottir, Samuel S. Veroneau.
References
- 1.Lewis NS, Nocera DG. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook TR, et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010;110:6474–6502. doi: 10.1021/cr100246c. [DOI] [PubMed] [Google Scholar]
- 3.Abbott D. Keeping the energy debate clean: how do we supply the world’s energy needs? Proc. IEEE. 2010;98:42–66. [Google Scholar]
- 4.Nocera DG. Chemistry of personalized solar energy. Inorg. Chem. 2009;48:10001–10017. doi: 10.1021/ic901328v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitler MT, et al. Practical challenges in the development of photoelectrochemical solar fuels production. Sustain. Energy Fuels. 2020;4:985–995. [Google Scholar]
- 6.Li CW, Ciston J, Kanan MW. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature. 2014;508:504–507. doi: 10.1038/nature13249. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz JA, Kanan MW. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 2020;11:5231. doi: 10.1038/s41467-020-19135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Colón BC, Ziesack M, Silver PA, Nocera DG. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science. 2016;352:1210–1213. doi: 10.1126/science.aaf5039. [DOI] [PubMed] [Google Scholar]
- 9.Dogutan DK, Nocera DG. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis. Acc. Chem. Res. 2019;52:3143–3148. doi: 10.1021/acs.accounts.9b00380. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J, Grönkvist S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy. 2019;44:11901–11919. [Google Scholar]
- 11.Klerke A, Christensen CH, Nørskov JK, Vegge T. Ammonia for hydrogen storage: challenges and opportunities. J. Mater. Chem. 2008;18:2304–2310. [Google Scholar]
- 12.Eisenberg R, Gray HB. Preface on making oxygen. Inorg. Chem. 2008;47:1697–1699. doi: 10.1021/ic800155g. [DOI] [PubMed] [Google Scholar]
- 13.Surendranath Y, Nocera DG. Oxygen evolution reaction chemistry of oxide-based electrodes. Prog. Inorg. Chem. 2011;57:505–560. [Google Scholar]
- 14.Kötz R, Stucki S, Scherson D, Kolb DM. In-situ identification of RuO4 as the corrosion product during oxygen evolution on ruthenium in acid media. J. Electroanal. Chem. Interfacial Electrochem. 1984;172:211–219. [Google Scholar]
- 15.Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2012;2:1765–1772. [Google Scholar]
- 16.Mavrokefalos CK, Patzke GR. Water oxidation catalysts: the quest for new oxide-based materials. Inorganics. 2019;7:29. [Google Scholar]
- 17.Thorarinsdottir AE, Nocera DG. Energy catalysis needs ligands with high oxidative stability. Chem. Catal. 2021;1:32–43. [Google Scholar]
- 18.Li J, et al. Molecular and heterogeneous water oxidation catalysts: recent progress and joint perspectives. Chem. Soc. Rev. 2021;50:2444–2485. doi: 10.1039/d0cs00978d. [DOI] [PubMed] [Google Scholar]
- 19.Sapountzi FM, Gracia JM, Westrate CJ, Fredriksson HOA, Niemantsverdriet JW. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017;58:1–35. [Google Scholar]
- 20.Lux H. “Säuren” und “basen” im schmelzfluss: die bestimmung der sauerstoffionen-konzentration. Ztschr. Elektrochem. 1939;45:303–309. [Google Scholar]
- 21.Esswein AJ, Surendranath Y, Reece SY, Nocera DG. Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters. Energy Environ. Sci. 2011;4:499–504. [Google Scholar]
- 22.Steinmiller EMP, Choi K-S. Photochemical deposition of cobalt-based oxygen evolving catalyst on a semiconductor photoanode for solar oxygen production. Proc. Natl. Acad. Sci. USA. 2009;106:20633–20636. doi: 10.1073/pnas.0910203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong DK, Choi S, Gamelin DR. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J. Am. Chem. Soc. 2011;133:18370–18377. doi: 10.1021/ja207348x. [DOI] [PubMed] [Google Scholar]
- 24.Pijpers JJH, Winkler MT, Surendranath Y, Buonassisi T, Nocera DG. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc. Natl. Acad. Sci. USA. 2011;108:10056–10061. doi: 10.1073/pnas.1106545108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reece SY, et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science. 2011;334:645–648. doi: 10.1126/science.1209816. [DOI] [PubMed] [Google Scholar]
- 26.Nocera DG. The artificial leaf. Acc. Chem. Res. 2012;45:767–776. doi: 10.1021/ar2003013. [DOI] [PubMed] [Google Scholar]
- 27.Kornienko N, Zhang JZ, Sakimoto KK, Yang P, Reisner E. Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat. Nanotechnol. 2018;13:890–899. doi: 10.1038/s41565-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 28.Sherbo, R. S., Loh, D. M. & Nocera, D. G. Hybrid biological-inorganic systems for CO2 conversion to fuels. In Carbon Dioxide Electrochemistry: Homogeneous and Heterogeneous Catalysis (eds Robert, M., Costentin, C. & Daasbjerg, K.) Ch. 8 (Royal Society of Chemistry, 2021).
- 29.Mohammadi, M. R. et al. Exploring the limits of self-repair in cobalt oxide films for electrocatalytic water oxidation. ACS Catal. 10, 7990–7999 (2020). Definition of self-healing from a structure/composition viewpoint as opposed to operational viewpoint.
- 30.Costentin C, Nocera DG. Self-healing catalysis in water. Proc. Natl. Acad. Sci. USA. 2017;114:13380–13384. doi: 10.1073/pnas.1711836114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremaldi JC, Bhushan B. Bioinspired self-healing materials: lessons from nature. Beilstein J. Nanotechnol. 2018;9:907–935. doi: 10.3762/bjnano.9.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speck O, Speck T. An overview of bioinspired and biomimetic self-repairing materials. Biomimetics. 2019;4:26. doi: 10.3390/biomimetics4010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, G. Self-Healing Composites: Shape Memory Polymer Based Structures, 21–34 (John Wiley & Sons, 2014).
- 34.Blaiszik BJ, et al. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010;40:179–211. [Google Scholar]
- 35.Yang Y, Urban MW. Self-healing polymeric materials. Chem. Soc. Rev. 2013;42:7446–7467. doi: 10.1039/c3cs60109a. [DOI] [PubMed] [Google Scholar]
- 36.Self Healing Materials—An Alternative Approach to 20 Centuries of Materials Science (ed. van der Zwaag, S.) (Springer, 2007).
- 37.Amendola V, Meneghetti M. Self-healing at the nanoscale. Nanoscale. 2009;1:74–88. doi: 10.1039/b9nr00146h. [DOI] [PubMed] [Google Scholar]
- 38.Self-Healing Materials: Fundamentals, Design Strategies, and Applications (ed. Ghosh, S. K.) (Wiley-VCH, 2009).
- 39.Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 40.Theis J, Schroda M. Revisiting the photosystem II repair cycle. Plant Signal. Behav. 2016;11:e1218587. doi: 10.1080/15592324.2016.1218587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafpour MM, Khoshkam M, Sedigh DJ, Zahraei A, Kompany-Zareh M. Self-healing for nanolayered manganese oxides in the presence of cerium(IV) ammonium nitrate: new findings. New J. Chem. 2015;39:2547–2550. [Google Scholar]
- 42.Hocking RK, et al. Water-oxidation catalysis by manganese in a geochemical-like cycle. Nat. Chem. 2011;3:461–466. doi: 10.1038/nchem.1049. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Wang J, Meng F, Zhang X. In situ activating ubiquitous rust towards low‐cost, efficient, free‐standing, and recoverable oxygen evolution electrodes. Angew. Chem. Int. Ed. 2016;55:9937–9941. doi: 10.1002/anie.201604040. [DOI] [PubMed] [Google Scholar]
- 44.Najafpour MM, et al. Damage management in water-oxidizing catalysts: from photosystem II to nanosized metal oxides. ACS Catal. 2015;5:1499–1512. [Google Scholar]
- 45.Spöri C, Kwan JTH, Bonakdarpour A, Wilkinson DP, Strasser P. The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 2017;56:5994–6021. doi: 10.1002/anie.201608601. [DOI] [PubMed] [Google Scholar]
- 46.Kanan MW, Surendranath Y, Nocera DG. Cobalt-phosphate oxygen-evolving compound. Chem. Soc. Rev. 2009;38:109–114. doi: 10.1039/b802885k. [DOI] [PubMed] [Google Scholar]
- 47.Bediako DK, Ullman AM, Nocera DG. Catalytic oxygen evolution by cobalt oxido thin films. Top. Curr. Chem. 2016;371:173–213. doi: 10.1007/128_2015_649. [DOI] [PubMed] [Google Scholar]
- 48.Suen N-T, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 2017;46:337–365. doi: 10.1039/c6cs00328a. [DOI] [PubMed] [Google Scholar]
- 49.Roger I, Shipman MA, Symes MD. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017;1:0003. [Google Scholar]
- 50.Kanan MW, Nocera DG. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ Science. 2008;321:1072–1075. doi: 10.1126/science.1162018. [DOI] [PubMed] [Google Scholar]
- 51.Surendranath Y, Dincǎ M, Nocera DG. Electrolyte-dependent electrosynthesis and activity of cobalt-based water oxidation catalysts. J. Am. Chem. Soc. 2009;131:2615–2620. doi: 10.1021/ja807769r. [DOI] [PubMed] [Google Scholar]
- 52.Farrow CL, Bediako DK, Surendranath Y, Nocera DG, Billinge SJL. Intermediate-range structure of self-assembled cobalt-based oxygen-evolving catalyst. J. Am. Chem. Soc. 2013;135:6403–6406. doi: 10.1021/ja401276f. [DOI] [PubMed] [Google Scholar]
- 53.Du P, Kokhan O, Chapman KW, Chupas PJ, Tiede DM. Elucidating the domain structure of the cobalt oxide water splitting catalyst by x-ray pair distribution function analysis. J. Am. Chem. Soc. 2012;134:11096–11099. doi: 10.1021/ja303826a. [DOI] [PubMed] [Google Scholar]
- 54.Kanan MW, et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ x-ray spectroscopy. J. Am. Chem. Soc. 2010;132:13692–13701. doi: 10.1021/ja1023767. [DOI] [PubMed] [Google Scholar]
- 55.Kwon G, et al. Resolution of electronic and structural factors underlying oxygen-evolving performance in amorphous cobalt oxide catalysts. J. Am. Chem. Soc. 2018;140:10710–10720. doi: 10.1021/jacs.8b02719. [DOI] [PubMed] [Google Scholar]
- 56.Ullman AM, Brodsky CN, Li N, Zheng S-L, Nocera DG. Probing edge site reactivity of oxidic cobalt water oxidation catalysts. J. Am. Chem. Soc. 2016;138:4229–4236. doi: 10.1021/jacs.6b00762. [DOI] [PubMed] [Google Scholar]
- 57.Bergmann A, et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 2018;1:711–719. [Google Scholar]
- 58.Mattioli G, Giannozzi P, Amore Bonapasta A, Guidoni L. Reaction pathways for oxygen evolution promoted by cobalt catalyst. J. Am. Chem. Soc. 2013;135:15353–15363. doi: 10.1021/ja401797v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Selloni A. First principles study of cobalt (hydr)oxides under electrochemical conditions. J. Phys. Chem. C. 2013;117:20002–20006. [Google Scholar]
- 60.Winkler, J. R. & Gray, H. B. Electronic structures of oxo-metal ions. In Molecular Electronic Structures of Transition Metal Complexes I. Structure and Bonding, Vol. 142 (eds Mingos, D. M. P., Day, P. & Dahl, J. P.) 17–28 (Springer, 2012).
- 61.Surendranath Y, Kanan MW, Nocera DG. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 2010;132:16501–16509. doi: 10.1021/ja106102b. [DOI] [PubMed] [Google Scholar]
- 62.McAlpin JG, et al. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 2010;132:6882–6883. doi: 10.1021/ja1013344. [DOI] [PubMed] [Google Scholar]
- 63.Risch M, et al. Water oxidation by amorphous cobalt-based oxides: in situ tracking of redox transitions and mode of catalysis. Energy Environ. Sci. 2015;8:661–674. [Google Scholar]
- 64.Sisley MJ, Jordan RB. First hydrolysis constants of hexaaquacobalt(III) and -manganese(III): longstanding issues resolved. Inorg. Chem. 2006;45:10758–10763. doi: 10.1021/ic0612956. [DOI] [PubMed] [Google Scholar]
- 65.Surendranath Y, Lutterman DA, Liu Y, Nocera DG. Nucleation, growth, and repair of a cobalt-based oxygen evolving catalyst. J. Am. Chem. Soc. 2012;134:6326–6336. doi: 10.1021/ja3000084. [DOI] [PubMed] [Google Scholar]
- 66.Symes MD, Surendranath Y, Lutterman DA, Nocera DG. Bidirectional and unidirectional PCET in a molecular model of a cobalt-based oxygen-evolving catalyst. J. Am. Chem. Soc. 2011;133:5174–5177. doi: 10.1021/ja110908v. [DOI] [PubMed] [Google Scholar]
- 67.Costentin C, Nocera DG. Dual-phase molecular-like charge transport in nanoporous transition metal oxides. J. Phys. Chem. C. 2019;123:1966–1973. [Google Scholar]
- 68.Lutterman DA, Surendranath Y, Nocera DG. A self-healing oxygen-evolving catalyst. J. Am. Chem. Soc. 2009;131:3838–3839. doi: 10.1021/ja900023k. [DOI] [PubMed] [Google Scholar]
- 69.Han A, Wu H, Sun Z, Jia H, Du P. Facile deposition of nanostructured cobalt oxide catalysts from molecular cobaloximes for efficient water oxidation. Phys. Chem. Chem. Phys. 2013;15:12534–12538. doi: 10.1039/c3cp52275j. [DOI] [PubMed] [Google Scholar]
- 70.Gerken JB, Landis EC, Hamers RJ, Stahl SS. Fluoride-modulated cobalt catalysts for electrochemical oxidation of water under non-alkaline conditions. ChemSusChem. 2010;3:1176–1179. doi: 10.1002/cssc.201000161. [DOI] [PubMed] [Google Scholar]
- 71.Gerken JB, et al. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14; the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 2011;133:14431–14442. doi: 10.1021/ja205647m. [DOI] [PubMed] [Google Scholar]
- 72.Bloor LG, Molina PI, Symes MD, Cronin L. Low pH electrolytic water splitting using earth-abundant metastable catalysts that self-assemble in situ. J. Am. Chem. Soc. 2014;136:3304–3311. doi: 10.1021/ja5003197. [DOI] [PubMed] [Google Scholar]
- 73.Huynh M, Bediako DK, Liu Y, Nocera DG. Nucleation and growth mechanisms of an electrodeposited manganese oxide oxygen evolution catalyst. J. Phys. Chem. C. 2014;118:17142–17152. [Google Scholar]
- 74.Huynh M, Bediako DK, Nocera DG. A functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 2014;136:6002–6010. doi: 10.1021/ja413147e. [DOI] [PubMed] [Google Scholar]
- 75.Huynh M, Shi C, Billinge SJL, Nocera DG. Nature of activated manganese oxide for oxygen evolution. J. Am. Chem. Soc. 2015;137:14887–14904. doi: 10.1021/jacs.5b06382. [DOI] [PubMed] [Google Scholar]
- 76.Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions (National Association of Corrosion Engineers, 1974).
- 77.Zaharieva I, et al. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 2012;5:7081–7089. [Google Scholar]
- 78.Dincă M, Surendranath Y, Nocera DG. A nickel-borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl. Acad. Sci. USA. 2010;107:10337–10341. doi: 10.1073/pnas.1001859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bediako DK, Surendranath Y, Nocera DG. Mechanistic studies of the oxygen evolution reaction mediated by a nickel–borate thin film electrocatalyst. J. Am. Chem. Soc. 2013;135:3662–3674. doi: 10.1021/ja3126432. [DOI] [PubMed] [Google Scholar]
- 80.Bediako DK, et al. Structure–activity correlations in a nickel–borate oxygen evolution catalyst. J. Am. Chem. Soc. 2012;134:6801–6809. doi: 10.1021/ja301018q. [DOI] [PubMed] [Google Scholar]
- 81.Singh A, Chang SLY, Hocking RK, Bach U, Spiccia L. Highly active nickel oxide water oxidation catalysts deposited from molecular complexes. Energy Environ. Sci. 2013;6:579–586. [Google Scholar]
- 82.Du J, Chen Z, Ye S, Wiley BJ, Meyer TJ. Copper as a robust and transparent electrocatalyst for water oxidation. Angew. Chem. Int. Ed. 2015;54:2073–2078. doi: 10.1002/anie.201408854. [DOI] [PubMed] [Google Scholar]
- 83.Huynh M, Ozel T, Liu C, Lau EC, Nocera DG. Design of template-stabilized active and earth-abundant oxygen evolution catalysts in acid. Chem. Sci. 2017;8:4779–4794. doi: 10.1039/c7sc01239j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N, et al. Template-stabilized oxidic nickel oxygen evolution catalysts. Proc. Natl. Acad. Sci. USA. 2020;117:16187–16192. doi: 10.1073/pnas.2001529117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chatti M, et al. Intrinsically stable in situ generated electrocatalyst for long-term oxidation of acidic water at up to 80 °C. Nat. Catal. 2019;2:457–465. [Google Scholar]
- 86.Trotochaud L, Ranney JK, Williams KN, Boettcher SW. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012;134:17253–17261. doi: 10.1021/ja307507a. [DOI] [PubMed] [Google Scholar]
- 87.Trotochaud L, Young SL, Ranney JK, Boettcher SW. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014;136:6744–6753. doi: 10.1021/ja502379c. [DOI] [PubMed] [Google Scholar]
- 88.Swierk JR, Klaus S, Trotochaud L, Bell AT, Tilley TD. Electrochemical study of the energetics of the oxygen evolution reaction at nickel iron (oxy)hydroxide catalysts. J. Chem. Phys. C. 2015;119:19022–19029. [Google Scholar]
- 89.Li N, Hadt RG, Hayes D, Chen LX, Nocera DG. Detection of high-valent iron species in alloyed oxidic cobaltates for catalysing the oxygen evolution reaction. Nat. Commun. 2021;12:4218. doi: 10.1038/s41467-021-24453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Görlin M, et al. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni−Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 2016;138:5603–5614. doi: 10.1021/jacs.6b00332. [DOI] [PubMed] [Google Scholar]
- 91.Shin H, Xiao H, Goddard WA., III In silico discovery of new dopants for Fe-doped Ni oxyhydroxide (Ni1–xFexOOH) catalysts for oxygen evolution reaction. J. Am. Chem. Soc. 2018;140:6745–6748. doi: 10.1021/jacs.8b02225. [DOI] [PubMed] [Google Scholar]
- 92.Li N, et al. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl. Acad. Sci. USA. 2017;114:1486–1491. doi: 10.1073/pnas.1620787114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou S, et al. Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution. Chem. Mater. 2015;27:8011–8020. [Google Scholar]
- 94.Yang M, et al. Fe(Co)OOH dynamically stable interface based on self-sacrificial reconstruction for long-term electrochemical water oxidation. ACS Appl. Mater. Interfaces. 2021;13:17450–17458. doi: 10.1021/acsami.0c22620. [DOI] [PubMed] [Google Scholar]
- 95.Chung DY, et al. Dynamic stability of active sites in hydr(oxy) oxides for the oxygen evolution reaction. Nat. Energy. 2020;5:222–230. [Google Scholar]
- 96.Feng C, et al. A self-healing catalyst for electrocatalytic and photoelectrochemical oxygen evolution in highly alkaline conditions. Nat. Commun. 2021;12:5980. doi: 10.1038/s41467-021-26281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Ji L, Chen Z. In situ rapid formation of a nickel–iron-based electrocatalyst for water oxidation. ACS Catal. 2016;6:6987–6992. [Google Scholar]
- 98.Kuang Y, et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy. 2016;2:16191. [Google Scholar]
- 99.Boddula R, et al. Synergetic effects of dual electrocatalysts for high-performance solar-driven water oxidation. ACS Appl. Energy Mater. 2019;2:7256–7262. [Google Scholar]
- 100.Barwe S, et al. Overcoming the instability of nanoparticle-based catalyst films in alkaline electrolyzers by using self-assembling and self-healing films. Angew. Chem. Int. Ed. 2017;56:8573–8577. doi: 10.1002/anie.201703963. [DOI] [PubMed] [Google Scholar]
- 101.Xiao M, et al. Regenerable catalyst for highly alkaline water oxidation. ACS Energy Lett. 2021;6:1677–1683. [Google Scholar]
- 102.Tahini HA, Tan X, Schwingenschlögl U, Smith SC. In operando self-healing of perovskite electrocatalysts: a case study of SrCoO3 for the oxygen evolution reaction. Part. Part. Syst. Charact. 2017;34:1600280. [Google Scholar]
- 103.Zhang Y, et al. Direct observation of oxygen vacancy self-healing on TiO2 photocatalysts for solar water splitting. Angew. Chem. Int. Ed. 2019;58:14229–14233. doi: 10.1002/anie.201907954. [DOI] [PubMed] [Google Scholar]
- 104.Ganesan K, Murugan P. First principles calculations on oxygen vacant hydrated α-MnO2 for activating water oxidation and its self-healing mechanism. Phys. Chem. Chem. Phys. 2016;18:22196–22202. doi: 10.1039/c6cp02032a. [DOI] [PubMed] [Google Scholar]
- 105.Cox CR, Lee JZ, Nocera DG, Buonassisi T. Ten-percent solar-to-fuel conversion with nonprecious materials. Proc. Natl. Acad. Sci. USA. 2014;111:14057–14061. doi: 10.1073/pnas.1414290111. [DOI] [PMC free article] [PubMed] [Google Scholar]