Abstract
The aim of this study was to determine if body weight or range use has a significant impact on bone health in commercial free-range laying hens, and to correlate tibia bone quality parameters with individual range usage and body weight. A total of 30 Lohmann Brown hens at 74 wk of age were selected from a commercial free-range farm and were either classified as heavy (mean ± SEM body weight 2.11 ± 0.034 kg, n = 14) or light (1.68 ± 0.022 kg, n = 16) body weight, and also classified as rangers (accessed the range for 86.7% of available days, n = 16) or stayers (accessed the range for 5.00% of available days, n = 14). The left tibiae of all individuals were analyzed for morphological parameters using computed tomography, evaluated for bone breaking strength, and ashed to determine mineral composition. Keel bone scoring was performed based on observation. Data were analyzed using a 2 × 2 factorial ANOVA, and regression analysis was performed. There was no measurable effect of range usage on any of the tibia parameters investigated. The body weight was significantly correlated with tibia breaking strength (r = 0.59), tibia weight (r = 0.56), tibia length (r = 0.64), diaphyseal diameter (r = 0.61), and total tibia volume (r = 0.67). In conclusion, range access had no beneficial effect on bone health. The impact of internal hen house furnishing and movement on bone health needs further investigation.
Key words: breaking strength, calcium metabolism, keel bone, quantitative computed tomography (CT), welfare
INTRODUCTION
Bone health and subsequently bone metabolism of laying hens has been a longstanding concern for egg producers, veterinarians, nutritionists, and geneticists. Common targets of pullet and layer production are meeting recommended body weight, producing adequate egg mass with a sufficient shell quality, maintaining long term laying persistency, and preventing skeletal disorders (Jahja et al., 2013). Bone health has a fundamental impact on skeletal support and eggshell quality and is important in meeting these industry targets (Fleming et al., 1996; Leyendecker et al., 2005; Jahja et al., 2013; Kerschnitzki et al., 2014).
Bones are dynamic tissues, and their quality is influenced by nutritional, hormonal, and physiological factors including mechanical stress and physical activity (Rath et al., 1999). Bone structure is able to adapt its mass, shape, and internal architecture according to the mechanical loading experienced within an environment. The most important factors influencing these features are locally acting stresses and strains created by intrinsic muscle forces as well as the external loads (Shipov et al., 2010). An increase in bone load due to body mass or physical activity stimulates bone formation and increases bone mass, whereas hypoactivity or decreased load due to disuse or rest induces bone loss or reduced mass in chickens due to modeling and remodeling mediated by the activity of osteoblasts and osteoclasts (Knowles and Broom, 1990; Norgaard-Nielsen, 1990; Fleming et al., 2006; Shipov et al., 2010; Jahja et al., 2013; Aguado et al., 2015; Rodriguez-Navarro et al., 2018). The physical loading of bones directs the deposition of bone materials towards the sites of highest physical stress (Jahja et al., 2013).
Severe osteoporosis is commonly observed in caged laying hens and highlights the importance of physical exercise (Regmi et al., 2016; Casey-Trott et al., 2017). Modern commercial housing systems such as barn or free-range facilities provide a variety of internal furniture such as perches, aviaries, dust bathing, and scratching areas (Miao et al., 2005). This increases load-bearing exercise due to expression of a large variety of behaviors including general locomotion as well as dust bathing, sun bathing, preening, stretching, nesting, running, wing flapping, or flying (Leyendecker et al., 2005; Miao et al., 2005). The choice to exercise can affect the overall bone biology significantly (Whitehead, 2002, 2004; Leyendecker et al., 2005). The increased physical activity of hens housed in the free-range system has shown to increase the cortical area and bone stiffness, and thus might influence the incidence and severity of osteoporosis in laying hens (Shipov et al., 2010; Rodriguez-Navarro et al., 2018).
When a range is provided, some hens prefer to spend most of their time ranging (hereafter referred to as rangers) while others prefer to stay inside the shed and rarely access the outdoors (hereafter referred to as stayers) (Gebhardt-Henrich et al., 2014). The development of these subpopulations has been associated with different hen performances. Hens that range frequently come earlier into lay and maintain their laying rate for longer (Ruhnke and Sibanda, 2018). However, hens with access to pasture have also been reported to be heavier compared to hens that prefer to stay in the shed which are less exposed to pasture (Singh et al., 2016; Iqbal et al., 2017). Given that physical movement and body weight can significantly impact bone health, we hypothesize that free-range hens that spent more time on the range or hens that are heavier may have superior bone health compared to hens that stay in the shed or hens that are lighter. Therefore, the aim of this study was 1) to determine if body weight or range use has a significant impact on bone health in commercial free-range laying hens and 2) to determine whether there is a correlation between tibia bone quality parameters and individual range usage or body weight.
MATERIALS AND METHODS
Ethical Statement
The study was approved by the Animal Ethics Committee (AEC17-120), University of New England, NSW, Australia, prior to the start of the data collection.
Housing and Management
A total of 40,000 Lohmann Brown hens were housed on a commercial free-range farm equipped with a multi-tier aviary system. All the hens were reared in a 3-tier aviary system to match the later housing system. A randomly selected subpopulation of 3,125 hens were partitioned and monitored for range usage using a custom-made RFID system (Science and Engineering workshop at the University of New England, Armidale, NSW, Australia) as previously described by Sibanda et al. (2019) (unpublished data). Briefly, radio frequency antennae were placed along the entire length of the partitioned inner and outer pop-holes to determine the direction of hen movement. All hens were equipped with individual numbered RFID leg bands (Monza R6 UHF RFID leg band, Impinj, Seattle, WA) at the age of 16 wk and monitored for range access daily until 74 wk of age. All hens were subjected to the same management and environmental conditions. At the time of depopulation (74 wk of age), a cohort of 30 hens was selected based on their individual range use and body weight. Selected hens were either classified as heavy (average 2.11 ± 0.034 kg, n = 14) or light (average 1.68 ± 0.02 kg, n = 16), and also classified as rangers (accessed the range for 86.7% of available days, n = 16) or stayers (accessed the range for 5.00% of available days, n = 14; Table 1).
Table 1.
Descriptive statistics of the study population.
| Rangers |
Stayers |
Light |
Heavy |
||
|---|---|---|---|---|---|
| (n = 16) | (n = 14) | (n = 16) | (n = 14) | ||
| Range use (% of the available days) | Mean | 86.7 | 5.0 | 45.9 | 51.6 |
| SEM | 3.38 | 2.68 | 11.5 | 10.9 | |
| Minimum | 37.2 | 0 | 0 | 0.4 | |
| Maximum | 94.7 | 30.8 | 92.1 | 94.7 | |
| Absolute number of days hens accessed the range | Mean | 230.6 | 12.5 | 122 | 136.6 |
| SEM | 8.98 | 6.69 | 29.2 | 30.7 | |
| Minimum | 99 | 0 | 0 | 1 | |
| Maximum | 252 | 77 | 245 | 252 | |
| Body weight (kg) | Mean | 1.88 | 1.89 | 1.69 | 2.11 |
| SEM | 0.07 | 0.05 | 0.02 | 0.03 | |
| Minimum | 1.51 | 1.65 | 1.51 | 1.89 | |
| Maximum | 2.30 | 2.23 | 1.82 | 2.24 |
Sample Collection
Individual body weight of the selected hens was measured using poultry weighing scales (Veit BAT 1, Moravany, Czech Republic) with a precision of 0.001 kg. The hens were then humanely sacrificed by cervical dislocation and their left tibiae were collected, the associated muscle mass was manually removed, and the bones were stored at –20°C.
Keel Bone Damage
Keel bone damage on sacrificed and skinned hens was physically evaluated by the same observer using a scoring system modified from Scholz et al. (2008). The keel bone integrity was determined by comparison with an ordinal scale where “0” indicates no fracture, “1” a minor fracture, and “2” severe or multiple fractures.
Tibia Weight, Length, and Diameter
Individual tibia weight was measured using a commercial scale (Shinko Denshi, RoHS Compliant, Japan), tibia length was determined from the intercondylar eminence to the most proximal point of the lateral malleolus, and the diameter was determined at the mid shaft diaphyseal region using digital Vernier calipers (Model, Kincrome, Melbourne, Australia) with an accuracy of ± 0.01 mm. Relative bone weight was calculated by referring to individual body weight. The values were calculated using the following equation:
Quantitative Computed Tomography Scan for Cortical Bone Mineral Density
The tibiae were imaged using a GE-Phoenix V|tome|xs 240 micro-computed tomography (CT) scanner (GE Sensing and Inspection Technologies GmbH, Wunstorf, Germany) to determine the cortical bone mineral density, the relative proportion of blood vessels to total bone volume, the proportion of tibia bone marrow, and the proportion of cortical bone relative to total bone volume. Briefly, the bones were thawed, immediately cleaned at room temperature of remaining debris, and mounted onto a rotating stage along with 2 calibration calcium hydroxyapatite phantom equivalents (0.25 and 0.75 g/cm3 density, Bruker-MicroCT, Melbourne, Australia). Images were taken with the x-ray settings maintained at 160 kV, 120 mA, 200 ms integration time per projection with a focal spot of 4 µm diameter. Scans were captured using a 1,000 × 2,000 pixel “virtual” detector array (DXR-250) with 3,600 projection angles per revolution using a constant rotation CT method. The isotropic voxel side length was 124.91 µm after reconstruction. Volumes were imported into FIJI, ImageJ version 2.0.0.0-rc-15/1.49k, Java 1.6.0_65, and the “threshold” tool was used to isolate pixels representing different phases (cortex, marrow, and air) for a sample from each scan. These pixels were then used to create a mask of classified pixels, and any misclassification was manually removed. These masks were then used to train a classifier in the “trainable Weka segmentation” machine learning toolkit (v3.2.29) available in ImageJ (Frank et al., 2016). This classification algorithm determined for the training sample was assessed for accuracy and when acceptable was applied to the remainder of the dataset. Voxel counting methods were used for volumetric analysis.
Three-Point Bone Breaking Strength
After CT scanning, the left tibiae underwent a 3-point bending to failure procedure following the method described by Toscano et al. (2013) using an Instron Lx 600 machine (Instron Lv 600, Instron, UK). Bones were mounted on a metal pin support of 50 mm distance from the mid-diaphysis testing site of the tibia, and a perpendicular load cell was used to apply loading to the midpoint of anterior surface until fracture. The result was recorded as the force (N) required to reach the structural failure of tibia.
Tibia Ash Percentage and Mineral Analysis
Following quantitative CT scanning and bone breaking strength measures, the whole tibia was weighted (wet weight) using a commercial scale (Shinko Denshi, RoHS Compliant, Japan) and dried in an oven at 100°C for 24 h until constant weight (dry weight). The bones were then ashed at 600°C overnight in a muffle furnace (Carbolite Gero Limited, Hope Valley, UK), cooled in a desiccator, and weighted again. The moisture percentage and tibia ash percentage were calculated using the following equations:
The concentrations of calcium, magnesium, phosphorus, and sulfur in the ash were further analyzed using the ultrawave microwave digestion system (Milestone Srl, Sorisole, Italy), and the mineral percentages were determined. Briefly, approx. 0.2 g of homogenized ashed sample were used for the digestion in 4 mL concentrated nitric acid using the single reactor chamber of the microwave. The temperature control ranged from 110 to 240°C, and the pressure was applied up to 110 bar. After digestion, the samples were quantitatively transferred to a 25 mL container and adjusted for volume with high purity deionized water. Analysis for calcium (422.673 nm), magnesium (285.213 nm), phosphorus (213.618 nm), and sulfur (181.972 nm) was carried out using an inductively coupled plasma optical emission spectrometer (ICP-OES; Agilent Australia, Victoria, Australia).
Statistical Analysis
Statistical analysis was performed using SPSS statistics v.24 (IBM Corp., Armonk, NY). A 2 × 2 factorial ANOVA was conducted to compare the main effects and interaction of range use and body weight on different bone parameters. Both range use and body weight included 2 levels of analysis: “rangers” and “stayers”, as well as “heavy” and “light” hens, respectively. The data that were not normally distributed were log transformed before the analysis. For the ordinal data of the keel bone damage, a chi-square probability test was performed to determine the relationship between the keel bone damage and range usage and body weight using JMP 14 (SAS Institute Inc., Cary, NC). Spearman correlation tests were performed between the body weight and range usage and the parameters of bones to evaluate the potential contribution of body weight or range. An overall correlation matrix of body weight and range usage with different bone parameters and box and whisker diagram was created using JMP 14 (SAS Institute Inc.). The level of significance (α) was set at P < 0.05.
RESULTS
The Impact of Range Usage and Body Weight on Tibia Bone Architecture and Keel Bone Damage
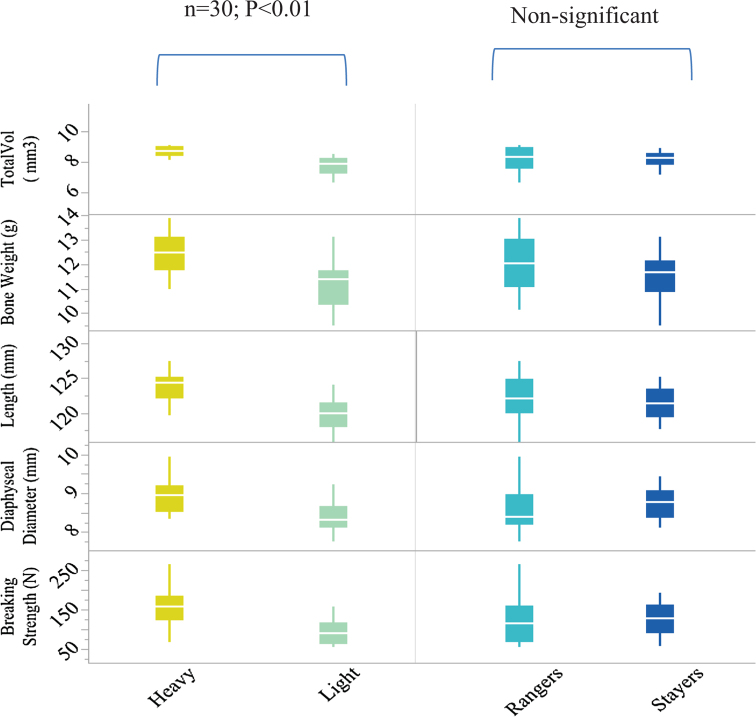
Body weight but not range usage was a significant factor for individual tibial length, weight, diaphyseal diameter, total tibia volume, or tibia breaking strength (Table 2, Figure 1). Similarly, there were no interactions between the body weight and range usage for any of the obtained bone parameters. Heavy hens showed 35.6% higher tibia breaking strength (P < 0.001), 3.31% larger diaphyseal diameter (P = 0.001), 7.14% larger total bone volume (P < 0.001), 5.08% higher bone weight (P = 0.003), and 1.98% greater bone length (P = 0.001) compared to the lighter hens (Figure 1). In contrast, lighter hens had 3.34% higher relative bone weight (P = 0.001) than heavy hens. There was also a significant effect of range usage on the relative bone weight where rangers had heavier relative bone weight compared to the stayers (P = 0.044). However, there was no effect of body weight or range use on composition of the bone minerals (Table 2).
Table 2.
The impact of body weight and range usage on tibia mineral concentration and the relative proportion of different bone parameters obtained from hens at the age of 74 wk housed in commercial free-range system.
| Main effect | Bone dry matter | Total ash (%) | Ca (%) | Mg (%) | P (%) | S (%) | Relative proportion of cortical bone to total bone volume (%) | Relative proportion of tibia bone marrow (%) | Relative proportion of blood vessels to total bone volume (%) | Bone mineral density (mg/cm3) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range use (RU) | Rangers | 9.80 ± 0.95 | 45.4 ± 2.06 | 41.6 ± 0.15 | 0.49 ± 0.01 | 17.62 ± 0.07 | 0.08 ± 0.01 | 0.46 ± 0.02 | 0.49 ± 0.03 | 0.03 ± 0.001 | 0.53 ± 0.02 | |
| Stayers | 8.49 ± 0.23 | 48.3 ± 2.97 | 41.7 ± 0.09 | 0.48 ± 0.01 | 17.61 ± 0.06 | 0.07 ± 0.01 | 0.48 ± 0.02 | 0.49 ± 0.03 | 0.002 ± 0.002 | 0.51 ± 0.01 | ||
| Body weight (BW) | Light | 8.99 ± 0.58 | 48.7 ± 3.21 | 41.7 ± 0.01 | 0.49 ± 0.01 | 17.61 ± 0.07 | 0.08 ± 0.007 | 0.47 ± 0.03 | 0.488 ± 0.02 | 0.025 ± 0.001 | 0.50 ± 0.01 | |
| Heavy | 9.72 ± 0.89 | 44.4 ± 0.70 | 41.6 ± 0.01 | 0.48 ± 0.01 | 17.61 ± 0.05 | 0.07 ± 0.004 | 0.46 ± 0.02 | 0.49 ± 0.03 | 0.026 ± 0.002 | 0.54 ± 0.02 | ||
| Two-way interaction | ||||||||||||
| RU*BW | Rangers | Light | 9.70 ± 1.11 | 45.7 ± 4.17 | 41.6 ± 0.28 | 0.48 ± 0.01 | 17.63 ± 0.12 | 0.08 ± 0.01 | 0.48 ± 0.03 | 0.48 ± 0.03 | 0.03 ± 0.002 | 0.50 ± 0.02 |
| Rangers | Heavy | 10.6 ± 1.62 | 45.1 ± 0.84 | 41.5 ± 0.15 | 0.49 ± 0.01 | 17.61 ± 0.06 | 0.08 ± 0.01 | 0.44 ± 0.04 | 0.50 ± 0.05 | 0.03 ± 0.001 | 0.57 ± 0.03 | |
| Stayers | Light | 8.28 ± 0.28 | 51.7 ± 4.90 | 41.8 ± 0.10 | 0.49 ± 0.01 | 17.60 ± 0.09 | 0.08 ± 0.01 | 0.47 ± 0.04 | 0.49 ± 0.04 | 0.028 ± 0.003 | 0.51 ± 0.02 | |
| Stayers | Heavy | 8.51 ± 0.43 | 43.8 ± 1.21 | 41.7 ± 0.17 | 0.48 ± 0.01 | 17.62 ± 0.08 | 0.06 ± 0.01 | 0.49 ± 0.03 | 0.48 ± 0.03 | 0.02 ± 0.003 | 0.50 ± 0.03 | |
| P-values | ||||||||||||
| Range use | 0.487 | 0.515 | 0.403 | 0.512 | 0.912 | 0.304 | 0.582 | 0.975 | 0.910 | 0.323 | ||
| Body weight | 0.326 | 0.239 | 0.479 | 0.913 | 0.982 | 0.154 | 0.813 | 0.992 | 0.601 | 0.259 | ||
| RU*BW | 0.261 | 0.307 | 0.897 | 0.384 | 0.877 | 0.449 | 0.328 | 0.764 | 0.458 | 0.197 |
The following abbreviations are used: Ca: calcium, Mg: magnesium, P: phosphorus, S: sulfur, RU: range usage, BW: body weight.
Figure 1.
Box and whisker diagrams illustrating the impact of body weight and range usage on several tibia characteristics in commercial Lohman Brown hens at the age of 74 wk. Boxes are bounded by the 25th and 75th percentiles with the median shown by the line bisecting the box. Whiskers extend to the full range of the data. The P values shown above the box relate to the main effect of the factors (body weight and ranging use) on each bone parameters obtained from 2 × 2 factorial ANOVA. The following abbreviations are used: BS: breaking strength (N), DD: diaphyseal diameter (mm), TotalVol: total bone volume (mm3).
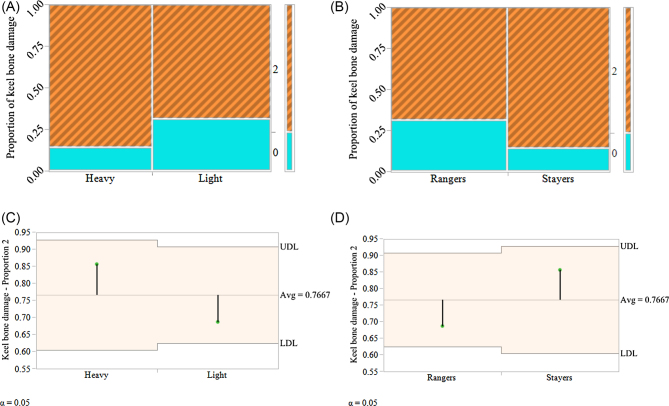
No significant effect of body weight or range usage on the occurrence of keel bone fractures could be observed (Table 3). There was no difference in the incidence of occurrence of keel bone damage in rangers and stayers group. Although not significant, the stayers had 3.33% (P = 0.27) higher keel bone damage compared to the rangers (Figure 4). Similarly, the proportion of keel bone damage in heavy and light hens was almost similar (40.0% and 36.7%, respectively, P = 0.27; Figure 4).
Table 3.
The relationship between the keel bone damage and the range usage and body weight of the commercial free-range laying hens of 74 wk of age.
| Test | Chi-Square | DF | P-value |
|---|---|---|---|
| a. Range usage | |||
| Pearson | 1.201 | 1 | 0.273 |
| Likelihood ratio | 1.238 | 1 | 0.265 |
| b. Body weight | |||
| Pearson | 1.201 | 1 | 0.273 |
| Likelihood ratio | 1.238 | 2 | 0.265 |
Figure 4.
The mosaic plot (A and B) and analysis of mean plot (C and D) illustrating the proportion of keel bone damage in rangers and stayers and heavy and light group of hens of 74 wk of age in a commercial free-range flock. The “score 0” represents no damage and “score 2” represents severely damaged. There was no hens with “score 1” that represented mild damage. There was no significant difference between any of the parameters. Hens of all groups (rangers, stayers, heavy, and light) were severely affected by keel bone damage with incidence typical for commercial flocks housed in non-cage system.
Correlation of Body Weight and Ranging Activity on Tibia Characteristics
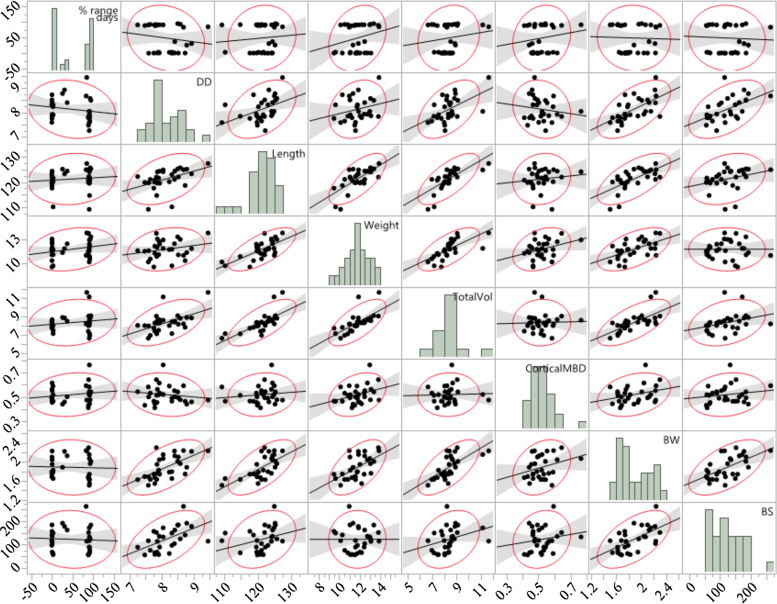
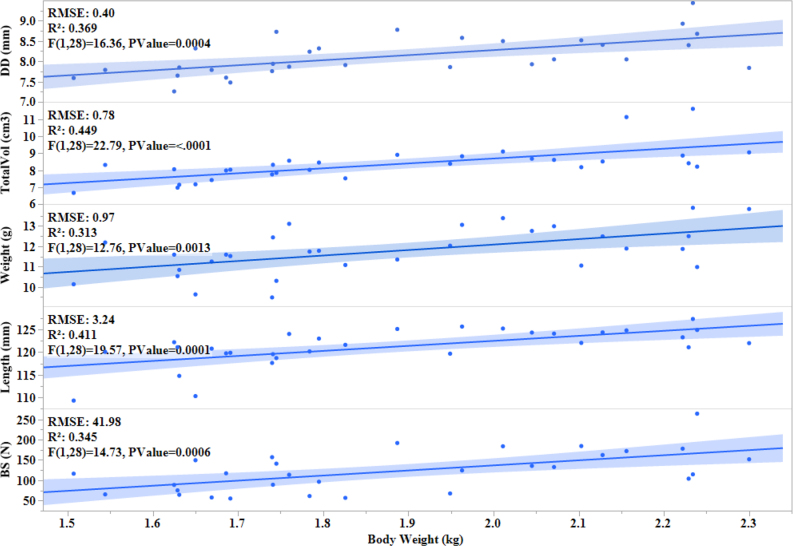
An overall scatterplot correlation matrix of body weight, range usage, and different bone parameters with histogram is shown in Figure 2. Correlation analysis indicated a positive relationship between the hen weight and tibia breaking strength (r = 0.33,P < 0.001), diaphyseal diameter (r = 0.61, P < 0.001), tibia length (r = 0.74, P < 0.001), weight (r = 0.56,P < 0.001), and total volume (r = 0.67, P < 0.001) (Table 3). Significant positive linear relationships between the body weight and different bone parameters were observed with r2 = 0.345 (P < 0.001), 0.411 (P < 0.001), 0.313 (P < 0.001), 0.449 (P < 0.001), and 0.369 (P < 0.001) for breaking strength, tibia length, tibia weight, total volume, and diaphyseal diameter, respectively (Figure 3).
Figure 2.
Overall scatterplot correlation matrix of body weight, range usage, and different bone parameters of Lohmann Brown hens at the age of 74 wk. The histogram on the diagonal axis shows the distribution of different parameters. The lower and upper triangles at the off-diagonal section illustrate the same relationships between the variables but the axis has been switched. The density eclipses show the magnitude of the linear association between the variables (the tighter the eclipses, the stronger is the correlation). The following abbreviations are used: DD: diaphyseal diameter, TotalVol: total volume, BMD: bone mineral density, BW: body weight, BS: breaking strength.
Figure 3.
Linear regression fit plot for the body weight and different bone parameters of commercial laying hens of 74 wk of age. Each dot represents an individual hen and the blue line represents the linear fit with R2 values and P -values displayed. The following abbreviations are used: BS: breaking strength, TotalVol: total volume, DD: diaphyseal diameter.
DISCUSSION
The Impact of Body Weight and Tibia Morphology/Compositional Parameters
To our knowledge, there is very limited information available about tibia characteristics from individual hens/flock subpopulations housed in the same free-range or barn system and subject to the same rearing conditions. Therefore, in the present study, we evaluated the tibia from these subpopulation, stayers, and rangers to determine the impact of body weight and range usage on bone quality. The tibiae of heavy hens were found to have greater breaking strength, diameter, length, bone weight, and total bone volume compared to the tibiae of lighter hens. Such differences can be attributed to the overall body size knowing that bone geometry responds to the changes in body weight and additional body mass increases the loading strain applied to the skeletal system, and therefore its composition and strength (Harner and Wilson, 1985; Cooper et al., 1995). This is in agreement with previous research performed on 16-wk-old pullets where body weight was associated with greater total, cortical, and trabecular area as well as bone mineral content (Casey-Trott et al., 2017). Therefore, it is important to maintain the breeder standard body weight in a flock to maintain the bone health and strength. Further research on determination of lower threshold for body weight to maintain the bone health is warranted.
The Impact of Range Use and Tibia Morphology/Compositional Parameters
Multiple studies have demonstrated an impact of the housing system on various bone parameters including bending, modulus of elasticity, weight capacity, and stiffness (Newman and Leeson, 1998; Shipov et al., 2010). Specifically, Shipov et al. (2010) demonstrated that the quality of tibiae and humeri of free-range hens were mechanically superior including the ultimate load, load to fracture, yield load, and stiffness compared to tibiae obtained from caged hens. In the present study, the rangers had heavier relative tibia compared to stayers and there was no interaction between the body weight and range usage. On the contrary, the results from Knowles and Broom (1990) showed a positive relationship between the movement of laying hens, body weight, and bone strength, where body weight impacted bone loading only in perched hens where movement was possible compared to the hens housed in cages. While our results clearly demonstrated that bone characteristics are not affected by range use and activity in the range as such (Figure 1, Table 2), other possible factors that might have affected relative tibiae weight such as the impact of movement within the hen house should be investigated.
The fact that the range use did not influence any other bone parameters in this study which was unexpected since in other studies, bone density of the cortical and trabecular regions in adult hens has been reported to differ depending on the housing system and the level of physical restriction they experience (Jendral et al., 2008; Shipov et al., 2010; Regmi et al., 2015). Moreover, bone breaking strength has been found to be consistently higher in hens kept in aviary systems compared to hens kept in conventional and furnished cages (Leyendecker et al., 2005). Furthermore, bone density can be positively related to the exercise level of the individual (Rutten et al., 2002; Jahja et al., 2013). One possible explanation for the results observed in this study might be that although the stayers did not access the range as frequently as the rangers, their activity in the shed could have been comparable to the activity of rangers outdoors. Stayers and rangers were hens from the same flock and subject to the same rearing conditions which included a 3-tier aviary system. While bone is a dynamic tissue and constantly subject to osteoclastic and osteoblastic activity, the fundamental of the quality of the skeletal system is significantly influenced during early development, e.g., the pullet growing phase that represents a critical period for structural bone growth (Enneking et al., 2012; Hester et al., 2013; Regmi et al., 2015; Casey-Trott et al., 2017) and will only be maintained during the laying cycle if the opportunities for movement are continued (Regmi et al., 2016). This weight loading exercise during rearing and the ongoing hen exercise later in life regardless of the individual hen location (in the shed or on the range) might have contributed more significantly to physical bone quality parameters than range use in this study. Similarly, Donaldson et al. (2012) have shown that the availability of aerial perches on tibial bone strength was not beneficial when compared with the hens housed in alternative system but with no perches, concluding that hens might have similar opportunities to exercise and improve leg health by using the shed environment. Moreover, vigorous exercise such as flying or running rather than walking or hopping and the extent of movement allowed in the husbandry system are more important factors than just the provision of perches alone to markedly improve bone quality in laying hens (Knowles and Broom, 1990; Whitehead and Fleming 2000; Leyendecker et al., 2005). Therefore, future research on the behavioral study tracking individual hen movement within the shed but also on the range would be required to determine factors that impact bone quality in hens housed in loose husbandry systems in more detail.
In the present study, we were not able to observe differences in the cortical bone density, bone ash, or any of the mineral content (calcium, phosphorus, sulfur, and magnesium) between the stayers and rangers which is in line with several other research studies investigating the cortical and trabecular structure in laying hens (Jendral et al., 2008; Shipov et al., 2010; Regmi et al., 2016) suggesting that exercise improves bone quality chiefly by altering its structural properties rather than mineral composition.
The Impact of Body Weight and Range Use on Keel Bone Damage
Keel bone fractures are common in hens housed in aviary and free-range systems due to more frequent collisions (Gregory et al., 1990; Bosh and Van Nieker, 1994; Leyendecker et al., 2005; Jahja et al., 2013). The prevalence of keel bone damage is estimated to increase up to 90% amongst hen's housed in non-cage system (Rodenburg et al., 2008; Kappeli et al., 2011; Gilani et al., 2013; Gebhardt-Henrich and Fröhlich, 2015). Heavier hens are also more likely to suffer from keel bone damages due to the higher impact force when experiencing a collision incident (Toscano et al., 2013; Gebhardt-Henrich et al., 2017). Incongruously, Donaldson et al. (2012) showed no effects of using horizontal structures such as perches on the prevalence and severity of keel bone damage observed on commercial free-range layer farms. Moreover, they also did not find an association between body mass and keel bone fracture which can be confirmed by the results obtained from our research. The prevalence of keel bone fractures depends not only on the housing condition but also on the rearing system that was used during pullet age (Casey-Trott et al., 2017). For example, if hens were raised in an aviary system rather than a caged system, the percentage of fractures is significantly lower (e.g 41.6% compared to 60.3%, respectively; Casey-Trott et al., 2017). The hens in the current study were reared in an aviary system, and early life experience may have prevented them from exhibiting significant differences in keel bone damage later in life. Environmental components such as the housing design, perches, or factors contributing to accidents seem to be a major driver for the incidence and severity of keel bone fractures (Gebhardt-Henrich et al., 2017) making both the rangers and stayers equally susceptible to the keel bone fractures.
Correlation of Bone Parameters With Body Weight and Range Usage
There was a strong correlation of bone breaking strength with other bone parameters such as bone length, diaphyseal diameter, and total bone volume. A strong positive linear association of the breaking strength and body weight observed in this study indicates that heavier hens experience bones that are harder to break. The range usage was not able to predict any of the bone parameters which again strengthen our conclusion that the range usage was not beneficial to improve any of the bone health parameters. Lighter hens, therefore, might have a greater risk of tibia breakage which compromises their health and welfare. These findings are in agreement with Harner and Wilson (1985) and Nogaard-Nielsen (1990). Casey-Trott et al. (2017) also found body weight to have a positive linear effect on the majority of the quantitative computed tomographic parameters such as total, cortical, and trabecular cross-sectional areas as well as total bone mineral content of tibia. The known effects of weight loading and shear force on bone remodeling suggest that the relationship is causal rather than coincidental (Knowles and Broom, 1990). Since range usage was not correlated to the tibia characteristics in the present study, we might suggest that the opportunity for hens to use horizontal structures such as perches in the hen house was equivalent irrespective of the time hens spent on the range. In fact, in order to prevent floor eggs, it is crucial to ensure hens are roosting at night. Training of hens to use nest boxes and sleep on horizontal structures would have been the same for stayers and rangers, given that all hens were subject to the same environmental and management conditions. It is therefore likely that there is an equivalency in the stressors placed on hens with indoor activities and that range usage is not a dominant factor in improving bone strength or resilience parameters.
CONCLUSION
Range access appears to be of minor importance for tibia health, suggesting that other husbandry strategies such as the rearing environment, horizontal structures in the hen house, or hen management practices may have a stronger impact on skeletal quality. Tibia quality was predominantly correlated with body weight, highlighting the importance of flock uniformity and managing for a target body weight to ensure nutrient requirements are met for all individuals. Further research including the characterization of detailed hen movement patterns within the hen house is warranted to determine the ideal indoor hen environment regarding bone health.
Footnotes
Supplementary data are available at Poultry Science online.
Supplementary Material
REFERENCES
- Aguado E., Pascaretti-Grizon F., Goyenvalle E., Audran M., Chappard D. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosh J.G.M.J., Van Niekkerk Th.G.G.C.M. The aviary system for laying hens. Health (N. Y) 1994;75:106. [Google Scholar]
- Casey-Trott T.M., Korver D.R., Guerin M.T., Sandilands V., Torrey S., Widowski T.M. Opportunities for exercise during pullet rearing, Part I: effect on the musculoskeletal characteristics of pullets. Poult. Sci. 2017;96:2509–2517. doi: 10.3382/ps/pex059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Cawley M., Bhalla A., Egger P., Ring F., Morton L., Barker D. Childhood growth, physical activity, and peak bone mass in women. J. Bone Miner. Res. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- Donaldson C.J., Ball M.E.E., O'Connell N.E. Aerial perches and free-range laying hens: the effect of access to aerial perches and of individual bird parameters on keel bone injuries in commercial free-range laying hens. Poult. Sci. 2012;91:304–315. doi: 10.3382/ps.2011-01774. [DOI] [PubMed] [Google Scholar]
- Enneking S.A., Cheng H.W., Jefferson-Moore K.Y., Einstein M.E., Rubin D.A., Hester P.Y. Early access to perches in caged white leghorn pullets. Poult. Sci. 2012;91:2114–2120. doi: 10.3382/ps.2012-02328. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. The influence of medullary bone on humeral breaking strength. Br. Poult. Sci. 1996;37:30–32. [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Relationships between genetic, environmental and nutritional factors influencing osteoporosis in laying hens. Br. Poult. Sci. 2006;47:742–755. doi: 10.1080/00071660601077949. [DOI] [PubMed] [Google Scholar]
- Frank E., Hall M.A., Witten I.H. 4th ed. Morgan Kaufmann; Burlington, MA, USA: 2016. The WEKA Workbench. Online Appendix for “Data Mining: Practical Machine Learning Tools and Techniques”. [Google Scholar]
- Gebhardt-Henrich S.G., Frohlich E.K.F. Early onset of laying and bumblefoot favor keel bone fractures. Animals. 2015;5:1192–1206. doi: 10.3390/ani5040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt-Henrich S.G., Pfulg A., Frohlich E.K.F., Kappeli S., Guggisberg D., Liesegang A., Stoffel M.H. Limited associations between keel bone damage and bone properties measured with computer tomography, three-point bending test and analysis of minerals in Swiss laying hens. Frontiers Vet. Sci. 2017;4:1–9. doi: 10.3389/fvets.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt-Henrich S.G., Toscano M.J., Frohlich E.K.F. Use of outdoor ranges by laying hens in different sized flocks. Appl. Anim. Behav. Sci. 2014;155:74–81. [Google Scholar]
- Gilani A.M., Knowles T.G., Nicol C.J. The effect of rearing environment on feather pecking in young and adult laying hens. Appl. Anim. Behav. Sci. 2013;148:54–63. [Google Scholar]
- Gregory N.G., Wilkins L.J., Ballantyne E.S.D., Overfield N.D. Broken bones in domestic fowls, effects of husbandry system and stunning method in end-of-lay hens. Br. Poult. Sci. 1990;31:59–69. [Google Scholar]
- Harner J.P., Wilson J.H. Effect of body size and cage profile on the shear strength of bones of caged layers. Br. Poult. Sci. 1985;26:543–548. doi: 10.1080/00071668508416846. [DOI] [PubMed] [Google Scholar]
- Hester P.Y., Enneking S.A., Haley B.K., Cheng H.W., Einstein M.E., Rubin D.A. The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 2013;92:1972–1980. doi: 10.3382/ps.2013-03008. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Roberts J., Perez-Maldonado R.A., Goodarzi Boroojeni F., Swick R.A., Ruhnke I. Pasture, multi-enzymes, benzoic acid and essential oils positively influence performance, intestinal organ weight and egg quality in free-range laying hens. Br. Poult. Sci. 2017;59:180–189. doi: 10.1080/00071668.2017.1403566. [DOI] [PubMed] [Google Scholar]
- Jahja A., Bessei W., Grashorn M.A., Stuhec I. Effect of physical activity of laying hens on bone condition. Arch. Geflügelkunde. 2013;77:171–178. [Google Scholar]
- Jendral M.J., Krover D.R., Church J.S., Feddes J.R. Bone mineral density and breaking strength of White Leghorns housed in conventional, modified and commercially available colony battery cages. Poult. Sci. 2008;87:828–837. doi: 10.3382/ps.2007-00192. [DOI] [PubMed] [Google Scholar]
- Kappeli S., Gebhardt-Henrich S., Frohlich E., Pfulg A., Stoffel M.H. Prevalence of keel bone deformities in Swiss laying hens. Br. Poult. Sci. 2011;52:531–536. doi: 10.1080/00071668.2011.615059. [DOI] [PubMed] [Google Scholar]
- Kerschnitzki M., Zander T., Zaslansky P., Fratzl P. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone. 2014;69:109–117. doi: 10.1016/j.bone.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Knowles T.G., Broom D.M. Limb bone strength and movement in laying hens from different housing systems. Vet. Rec. 1990;126:354–356. doi: 10.1136/vr.126.15.354. [DOI] [PubMed] [Google Scholar]
- Leyendecker M., Hamann H., Hartung J., Kamphues J., Neumann U., Surie C., Distl P.O. Keeping laying hens in furnished cages and an aviary housing system enhances their bone stability. Br. Poult. Sci. 2005;46:536–544. doi: 10.1080/00071660500273094. [DOI] [PubMed] [Google Scholar]
- Miao Z.H., Glatz P.C., Ru Y.J. Free-range poultry production—a review. Asian-Australas. J. Anim. Sci. 2005;18:113–132. [Google Scholar]
- Newman S., Leeson S. Effect of housing birds in cages or an aviary system on bone characteristics. Poult. Sci. 1998;77:1492–1496. doi: 10.1093/ps/77.10.1492. [DOI] [PubMed] [Google Scholar]
- Nogaard-Nielsen G.L. Bone strength of laying hens kept in an alternative system, compared with hens in cages and on deep-litter. Br. Poult. Sci. 1990;31:81–89. doi: 10.1080/00071669008417233. [DOI] [PubMed] [Google Scholar]
- Rath N.C., Balog J.M., Huff W.E., Huff G.R., Kulkarni G.B., Tierce J.F. Comparative differences in the composition and biomechanical properties of tibiae of seven- and seventy-two-week-old male and female broiler breeder chicken. Poult. Sci. 1999;78:1232–1239. doi: 10.1093/ps/78.8.1232. [DOI] [PubMed] [Google Scholar]
- Rodenburg T.B., Tuyttens F.A.M., De Reu K., Herman L., Zoons J., Sonck B. Welfare assessment of laying hens in furnished cages and non-cage systems: an on-farm comparison. Anim. Welf. 2008;17:363–373. [Google Scholar]
- Rodriguez-Navarro A.B., McCormack H.M., Fleming R.H., Alvarez-Lloret P., Romero-Pastor J., Casey-Trott T., Krover D.R., Guerin M.T., Sandilands V., Torrey S., Widowski T.M. Opportunities for exercise during pullet rearing, Part I: effect on the musculoskeletal characteristics of pullets. Poult. Sci. 2018;96:2509–2517. doi: 10.3382/ps/pex059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi P., Deland T.S., Steibel J.P., Robison C.I., Haut R.C., Orth M.W., Karcher D.M. Effect of rearing environment on bone growth of pullets. Poult. Sci. 2015;94:502–511. doi: 10.3382/ps/peu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi P., Nelson N., Steibel J.P., Anderson K.E., Karcher D.M. Comparisons of bone properties and keel deformities between strains and housing systems in end-of-lay hens. Poult. Sci. 2016;95:2225–2234. doi: 10.3382/ps/pew199. [DOI] [PubMed] [Google Scholar]
- Ruhnke I., Sibanda T.Z. Proceedings of the Poultry Information Exchange. Gold Coast, Australia. 2018. Nutritional management of free-range laying hens; pp. 147–148. [Google Scholar]
- Rutten M., Letterrier C., Constantin P., Retter K., Bessei W. Bone development and activity in chickens in response to reduced weight- load on legs. Anim. Res. 2002;51:327–336. [Google Scholar]
- Scholz B., Ronchen S., Hamann H., Christian S., Neumann U., Josef K., Ottmar D. Evaluation of bone strength, keel bone deformity and egg quality of laying hens housed in small group housing systems and furnished cages in comparison to an aviary housing system. Arch. Tierz. Dummerstorf. 2008;51:179–186. [Google Scholar]
- Shipov A., Sharir A., Zelzer E., Milgram J., Monsonego-Ornan E., Shahar R. The influence of severe prolonged exercise restriction on the mechanical and structural properties of bone in an Avian model. Vet. J. 2010;183:153–160. doi: 10.1016/j.tvjl.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Singh M., Hernandez C.E., Hinch G., Cowieson A.J. Wanderers versus stay at home: Who has the better guts? Aust. Poult. Sci. Symp. 2016;27:78–81. [Google Scholar]
- Toscano M.J., Wilkins L.J., Millburn G., Thorpe K., Tarlton J.F. Development of an ex vivo protocol to model bone fracture in laying hens resulting from collisions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C. Proc. Aust. Poult. Sci. Symp. Poultry research foundation; Camden, NSW: 2002. Bone breakage and osteoporosis in laying hens: causes and solutions; pp. 61–68. [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.