Abstract
Foot pad dermatitis (FPD) is a serious problem of the modern poultry industry, negatively affecting birds' welfare and health status, walking and feeding activity, growth performance, carcass quality, and economic performance of meat production. The gut microbiome in poultry with FPD has not been previously investigated. Therefore, we compared the cecal microbiomes of 8 breeding ducks with FPD to 8 control ducks (breeders with apparently healthy feet) by pyrosequencing the bacterial 16S ribosomal RNA gene. The results showed a significant β-diversity (P < 0.05) of cecal microbiota presented between healthy and FPD-affected breeder ducks. The plasma endotoxins, interleukin 1β (IL-1β), IL-17, IL-6, IL-10, and tumor necrosis factor-α concentration, and the abundance of class Clostridia in FPD-affected ducks was markedly higher (P < 0.05), however, the abundance of genus Prevotella, Lactobacillus, Lachnospiraceae UCG-008, and the Firmicutes to Bacteroidetes ratio in FPD-affected ducks was significantly lower (P < 0.05) when compared to healthy ducks. These findings suggest when duck breeders are affected with FPD, ducks show an increased inflammatory response and a difference of structure and composition of the cecal microbiome.
key words: cecal microbiome, 16SrRNA, foot pad dermatitis, duck breeders, inflammatory response
INTRODUCTION
Foot pad dermatitis (FPD), a condition of mild to severe inflammation and sometimes necrotic lesions on the plantar surface of the footpads, is commonly observed in fast-growing broiler chickens and turkeys (Shepherd and Fairchild, 2010). Macroscopically FPD appears as brown-black coloration, inflammation, ulcers on the foot skin, hyperkeratosis and in more severe cases, necrosis of the epidermis as found in histopathological examination (Greene et al., 1985). Foot pad dermatitis is a serious problem of the modern poultry industry, negatively affecting birds' welfare and health status, walking and feeding activity, growth performance, carcass quality, and economic performance of meat production. According to Haslam et al. (2007), the mean flock percentage of moderate to severe FPD lesions was 11.0%, ranging from 0 to 71.5% for broiler production. In fact, the occurrence of FPD is now used as an audit criterion in welfare assessments of poultry production systems in Europe and the United States (Martrenchar et al., 2002; Berg and Algers, 2004). Recently, FPD has an increasing incidence in breeder ducks, which results in a sharp decrease of reproductive performance, as well has a high mortality (about 20 to 30%) in China.
The etiology of FPD is complex with many risk factors, including stocking density, flock management, bedding type and quality, high litter moisture, and nutrition (Swiatkiewicz et al., 2016). Weber Wyneken et al. (2015) observed a linear relationship between FPD and litter moisture in broilers. However, Eija et al. (2016) observed that maintaining good litter quality alone is not enough to ensure healthy foot pads in broiler breeders, and the occurrence of foot pad lesions increased and became more severe as broiler breeders aged, with severe lesions reaching a maximum of 64% at the end of breeding cycle. However, little investigation has been done into the pathophysiology in birds with FDP, especially duck breeders.
In recent years, there has been increasing interest in gut microbiome-host interaction as accumulating evidence suggests that microbial populations of different makeup within the gut play an important role in the initiation and progression of many diseases in human, including metabolic disorders and rheumatoid arthritis (RA) (Wu et al., 2016). In fact, the gut microbiome is thought to be one of the important environmental factors affecting the development of RA (Maeda and Takeda, 2017). Moreover, animal models suggest a role for intestinal bacteria in supporting the systemic immune response required for joint inflammation (Scher et al., 2013). With the considerable progress made in next-generation sequencing techniques, and the vital role of gut microbiota in regulating immunity and inflammatory disease (Sun et al., 2015), the identified gut microbiota difference between FPD-affected and healthy breeder ducks will provide a new insight of the association between gut microbiota and FPD in poultry. Therefore, the objective of this study was to compare the composition and structure of the cecal microbiome, as well as clinical phenotypes in healthy breeder ducks and those with FDP.
MATERIALS AND METHODS
All experimental procedures were approved by Animal Care and Use Committee of Sichuan Agricultural University.
The Selection of Duck Breeders
This case-control study compared apparently healthy breeder ducks to those with classic signs of FPD. Healthy and FPD-affected breeder ducks were matched based on sex, age, body weight (BW), nutrition, environmental, and management criteria. Eight FDP-affected duck breeders (BW = 3.09 kg, 400 D of age) and 8 control healthy duck breeders (BW = 3.05 kg, 400 D of age) were included in the final analysis.
Sample Collection
Blood from the jugular vein of 16 birds was harvested for analysis of plasma endotoxin and cytokine measurement. Following this, all birds were euthanized by cervical dislocation, and cecal contents were collected and kept in liquid nitrogen for microbial community and short-chain fatty acid (SCFA) analysis. Then, samples from the same areas of the dermis of the feet of healthy and FPD-affected ducks were collected for histopathological evaluation.
Measurements
Histopathology
Segments of the healthy and FPD-affected (about 1.0 cm2) feet were removed, flushed gently with ice-cold physiological saline solution, and then fixed in in 4% (w/v) paraformaldehyde, embedded in paraffin, sectioned to a 5-μm thickness and stained with hematoxylin and eosin (HE) for histo-pathological examination by a veterinary pathologist.
Plasma Endotoxin and Cytokines Assay
Plasma concentration of endotoxin was determined using a commercially available ELISA kit according to the manufacturer's protocol (MM-1626O1, mmbio industrial Co., Ltd, Jiangsu, China). Endotoxin was expressed as pg/mL. This kit was sensitive to 5 pg endotoxin per mL. Plasma tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-17, IL-6 and IL-10 concentrations were measured using ELISA commercial kits (mmbio industrial Co., Ltd, Jiangsu, China). Cytokines were performed as described by the manufacturer's instructions. All assays were done in duplicate.
Cecal Short-Chain Fatty Acid Analysis
Approximately 0.5 g of cecal contents were thoroughly mixed with 2 mL ultrapure water and centrifuged (3,000 × g, 15 min) after sitting at room temperature for 30 min. Supernatants (1 mL) were mixed with 0.2 mL ice-cold 25% (w/v) metaphosphoric acid solution, mixing under 4°C incubation for 30 min, followed by 11,000 × g centrifugation for 10 min. The SCFA concentrations including acetate, propionate and butyrate were separated and measured by gas chromatographic system (Varian CP-3800, USA) as described by Qin et al. (2019).
DNA Extraction, Sequencing of 16S rRNA, Sequence Processing, and Data Analysis
DNA extraction and high-throughput sequencing and analysis of 16S rRNA gene amplicons were performed using the Illumina Hiseq platform Novo gene (Novo gene Bioinformation Technology, Beijing, China). All the procedures of DNA extraction, sequencing of 16S rRNA, sequence processing, and data analysis were referenced according to our previous study of Dai et al. (2018). Briefly, DNA was diluted to 10 ng/µL using sterile water. 16S rRNA genes of distinct regions (16S V4) were amplified using specific primer (515F GTGCCAGCMGCCGCGGTAA; 806R GGACTACHVGGGTWTCTAAT) with unique barcodes. All PCR reactions were carried out with Phusion High-Fidelity PCR Master Mix (New England Biolabs, USA). PCR products were mixed in equal density ratios. Then, mixed PCR products were purified with Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer's recommendations, and index codes were added.
The raw sequencing data produced were processed by removing the sequence reads of too low quality (only “passing filter” reads were selected) and discarding reads containing adaptor sequences or failing PhiX Control with an in-house filtering protocol. Reads were filtered by QIIME quality filters. Sequences with ≥97% similarity were assigned to the same optimal taxonomic units (OTUs). Then, a representative sequence was chosen for each OTU to annotate the taxonomic information of that unit. All OTUs were subsequently analyzed for abundance and diversity. For α-diversity analysis, rarefaction curves and rank abundance curves were generated by R project (Version 2.15.3). Sequences (Clean Data) were analyzed using the Quantitative Insights into Microbial Ecology (QIIME, Version 1.7.0) software package. A jackknifed β-diversity analysis was conducted to assess the statistical variation of sample location in principal coordinate analysis (PCoA) plots based on unweighted UniFrac distances and perMANOVA (Lozupone et al., 2011).
Statistical Analysis
The data were analyzed by using the mixed model procedure of SAS 9.4 software (SAS Institute, Inc., Cary, NC). When significant, post hoc comparisons of treatment means were made using Tukey's test.P < 0.05 was considered to be statistically significant.
RESULTS
Histopathology
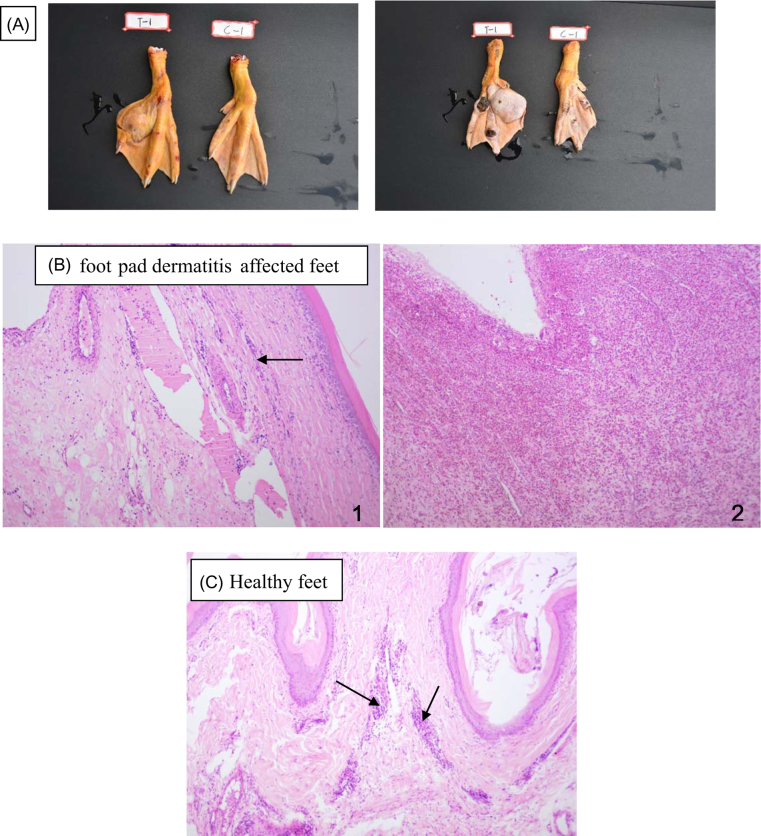
The gross appearance and histopathology of the feet in representative healthy and FPD-affected duck breeders are presented in Figure 1. Figure 1 A shows representative pictures of feet categorized as normal as well as those considered to have FPD. The FPD-affected feet have dark, irregular, rough, raised nodules, and at least one foot has developed a large cystic structure as well. Histopathologically, there is obvious infiltration of lymphocytes and neutrophils throughout the solid nodules and around the cyst (Figure 1 B). To the contrary, the histology of the healthy feet, the section showed normal tissue with only small numbers of lymphocytes (Figure 1 C).
Figure 1.
The appearance and histopathology of the feet in healthy or foot pad dermatitis-affected duck breeders. A: the appearance of healthy or foot pad dermatitis (FPD)-affected feet, T-1 represents the FPD feet; C-1 represents the healthy feet. B: the histopathology of the FPD feet, 1 shows a large area of inflammatory cells infiltration (black arrow); 2 shows an area of cyst. C: the histology of the healthy feet, the section shows normal histology with few lymphocytes(2 black arrows).
Plasma Endotoxin and Cytokine Concentration
Plasma endotoxin, IL-17, IL-1β, IL-6, IL-10, and TNF-α concentration in FDP-affected duck breeders were significantly higher (P < 0.05, Table 1) than those in healthy duck breeders.
Table 1.
The plasma endotoxin and cytokine concentration in foot pad dermatitis-affected and healthy duck breeders (n = 8).
| Items (ng/L) | Disease1 | Health1 | SEM | P-value |
|---|---|---|---|---|
| Endotoxin | 52.82 | 20.81 | 4.53 | <0.05 |
| IL-17 | 28.14 | 20.14 | 0.91 | <0.05 |
| TNF-α | 339.47 | 191.64 | 16.20 | <0.05 |
| IL-1β | 28.95 | 15.48 | 1.48 | <0.05 |
| IL-6 | 220.70 | 111.14 | 4.86 | <0.05 |
| IL-10 | 63.23 | 50.43 | 2.71 | <0.05 |
Disease means foot pad dermatitis (FPD)-affected duck breeders; Health means control healthy duck breeders. IL-7: interleukin 17; TNF-α: tumor necrosis factor-α.
Cecal Short-Chain Fatty Acid Content
No difference (P > 0.05) of cecal SCFA content was detected in healthy and FPD-affected duck breeders (Table 2).
Table 2.
The cecal short-chain fatty acid content in foot pad dermatitis-affected and healthy duck breeders (n = 8).
| Items (μmol/g) | Disease1 | Health1 | SEM | P-value |
|---|---|---|---|---|
| Acetate | 69.03 | 65.35 | 5.43 | 0.64 |
| Propionate | 37.61 | 29.23 | 3.29 | 0.09 |
| Butyrate | 14.29 | 16.00 | 1.93 | 0.54 |
Disease means foot pad dermatitis (FPD)-affected duck breeders; Health means control healthy duck breeders.
Cecal Microbiome
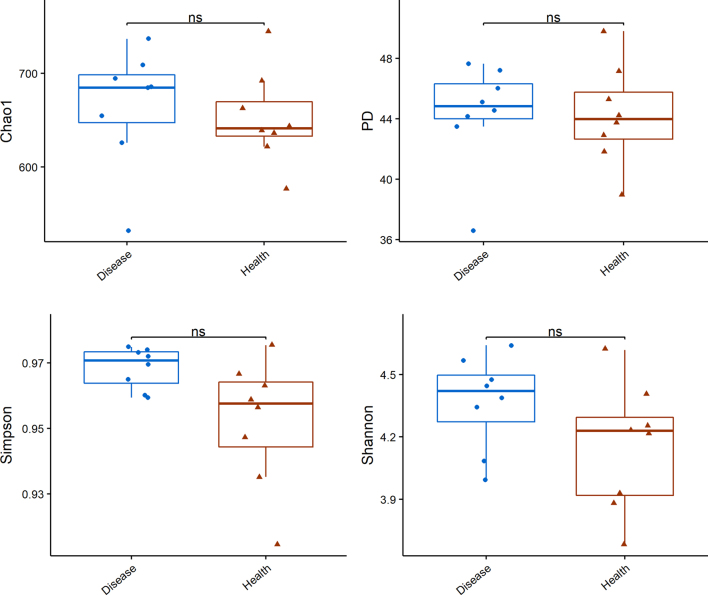
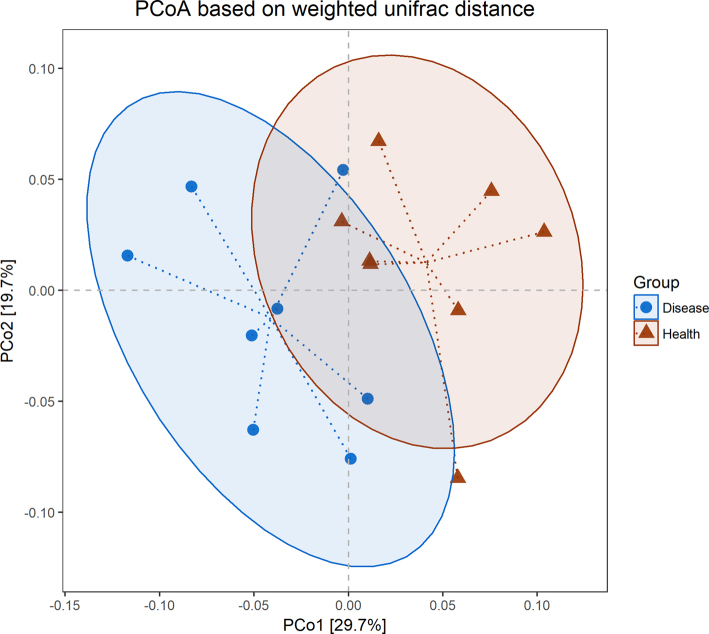
The full dataset included bacteria from 278 genera, 126 families, 73 orders, 37 classes, and 23 phyla. Although no statistically significant differences were found with respect to commonly used α- diversity indices (Chao1, PD, Simpson, and Shannon, Figure 2), comparisons of the clustering of patient and control samples in dendrograms based on β-diversity metrics (PCoA, Figure 3) showed a significant difference between groups (unweighted UniFrac P < 0.05, brayP < 0.05, and jaccard P < 0.05, Table 3).
Figure 2.
α-diversity analysis of cecal microbiota in healthy and foot pad dermatitis affected duck breeders. Values are shown as min to max with the mean value calculated for each groups. Statistical tests were performed using post hoc ANOVA and ns indicated no significant difference. Disease (blue) means foot pad dermatitis (FPD)-affected duck breeders; Health (red) means control healthy duck breeders.
Figure 3.
Principal coordinate analysis (PCoA) of weighted UniFrac distances. Each point represents a sample. In principal coordinates analysis (PCoA), points that are closer together represent microbial communities that are more similar in sequence composition. Axes are scaled by the percent of variation explained by each principal coordinate. Disease(blue) means foot pad dermatitis (FPD)-affected duck breeders; Health (red) means control healthy duck breeders.
Table 3.
The results of perMANOVA for β-diversity analysis.
| Groups | Measure | Permutations | R2 | P-value |
|---|---|---|---|---|
| Disease1 vs. Health1 | wei_unifrac | 999 | 0.1838 | 0.002 |
| Disease vs. Health | unwei_unifrac | 999 | 0.0842 | 0.113 |
| Disease vs. Health | bray | 999 | 0.1868 | 0.002 |
| Disease vs. Health | jaccard | 999 | 0.1504 | 0.001 |
Disease means foot pad dermatitis (FPD)-affected duck breeders; Health means control healthy duck breeders.
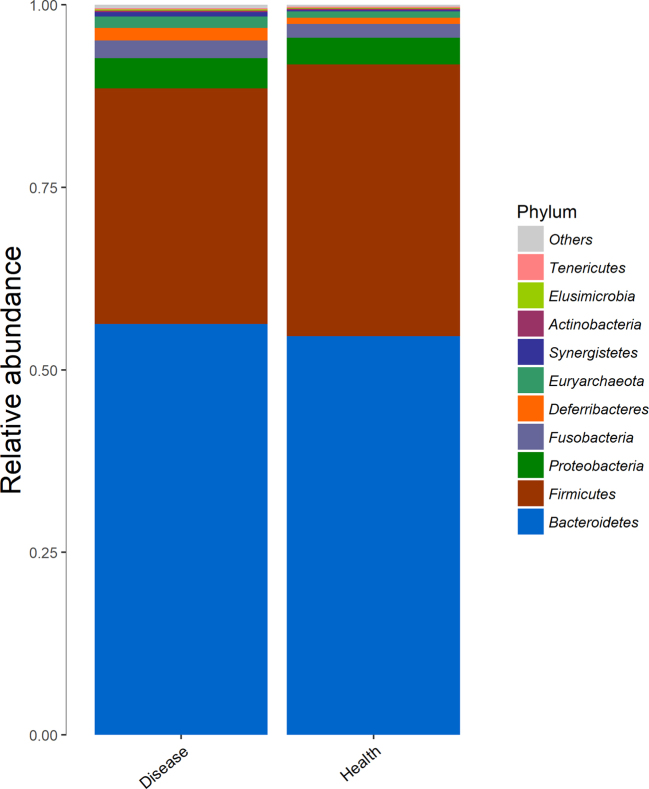
The relative taxa abundance of bacteria was analyzed from the phylum level (Figure 4). Bacteroidetes, Firmicutes, and Proteobacteria were 3 most dominant phyla in the duck's cecal digesta (92.70% FPD-affected ducks vs. 95.49% control healthy ducks). The Firmicutes to Bacteroidetes ratio is 0.57 in FPD-affected ducks, which is lower (P < 0.05) than this (0.68) in control healthy ducks. The abundance of Proteobacteria is higher in FPD-affected ducks (0.042%) than that in control healthy ducks (0.037%).
Figure 4.
Depicts phylum level classifications for observed operational taxonomic units (OTUs). OTUs representing higher proportions of the population grouped according to diseased and healthy. Disease means foot pad dermatitis (FPD)-affected duck breeders; Health means control healthy duck breeders.
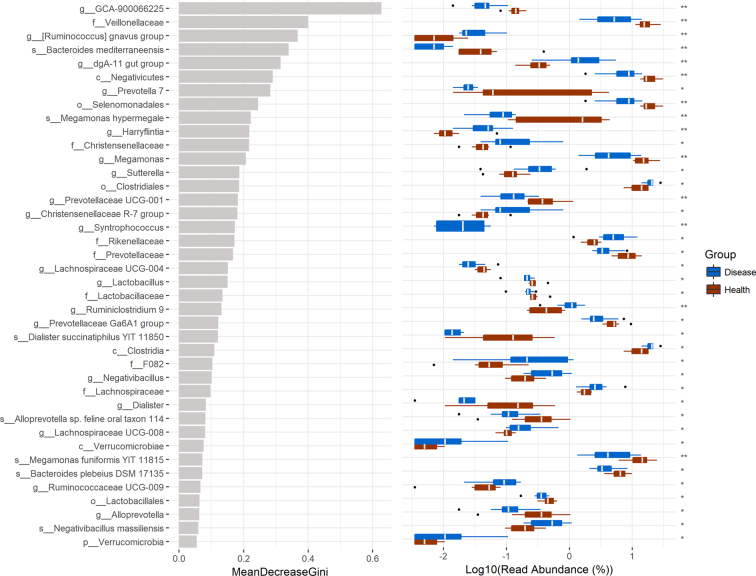
Based on random forest analyses (Figure 5), the abundance of genus Prevotella 7, Prevotellaceae UCG-001, Prevotellaceae Ga6A1 group, Alloprevotella, Prevotella 9, family Prevotellaceac, and species Alloprevotella, as well as the abundance of order Lactobacillales, genus Lactobacillus, family Lactobacillaceae, and species Lactobacillus reuteri, and genus Lachnospiraceae UCG-008, family Lachnospiraceae in ceca of FDP-affected breeder ducks were markedly lower (P < 0.05) than those in ceca of healthy breeder ducks. However, breeder ducks with FPD showed a higher (P < 0.05) abundance of order Clostridiales and class Clostridia compared to the healthy ducks.
Figure 5.
Random Forest for reads abundance. **means P < 0.01; *means P < 0.05. Disease (blue) means foot pad dermatitis (FPD)-affected duck breeders; Health (red) means control healthy duck breeders.
DISCUSSION
Initially, the control and experimental groups were selected based on phenotype; those with grossly normal feet were in the control group and those with classic FPD-type lesions were placed in the experimental (FPD-affected) group. The histopathologic examinations verified that the ducks chosen were placed into the appropriate group, and could be used with confidence for further analysis.
The microbiota within the hindgut plays a distinct role in defense against pathogens and maintenance of intestinal health (Pourabedin and Zhao, 2015). In the present study, FPD-affected in breeder ducks did not affect the predominant phylum in the cecum, which is consistent with previous studies in ducks that Bacteroidetes, Firmcutes, and Proteobacteria were the 3 dominant phyla in the cecum, with Bacteroidetes possessing a higher proportion (Vasaï et al., 2014; Best et al., 2017). However, in our study, we found FPD-affected ducks had a lower Firmicutes to Bacteroidetes ratio, and a higher abundance of Proteobacteria in ceca. These results are in line with several studies on prebiotics, which have shown expansion of Firmicutes (Yacoubi et al., 2018) or higher Firmicutes to Bacteroidetes ratio (Molist et al., 2011). It was demonstrated that the abundance of Firmicutes was strongly negative correlated with pathogenic bacterial populations in the intestine (Mulder et al., 2009). Meanwhile, mediterranean diet (a balanced intake of fruits, grains, monounsaturated fat, vegetables, and polyunsaturated fat) had lower numbers of Proteobacteria and acute phase C-reactive proteins (Marlow et al., 2013; De Filippis et al., 2016), suggesting the decrease of the relative abundance of the phylum Proteobacteria represents a healthier condition. These results indicated that FPD-affected ducks had shown an unhealthier cecal microbial community.
The main findings of our study are the reduced abundance of Prevotella and the increased plasma IL-17, IL-1β, IL-6, IL-10, and TNF-α concentration in FPD-affected ducks. These results agree with the study by Luo et al. (2019), which found that the reduced abundance of Prevotella, and the increased mRNA expressions of TNF-α, IL-1β, IL-6, IL-17A in ceca of ducks infected with duck origin parvovirus. In addition, these results are also consistent with findings in other species. For example, Liu et al. (2016) has reported that mice inoculated with Prevotellaceae histicola have a significantly reduced incidence of arthritis as a result of the suppression of the serum levels of several pro-inflammatory cytokines, such as IL-2, IL-17, and TNF-α. IL-17 is considered a key driver of joint, cartilage, and bone damage (Joosten et al., 2008). In vivo and in vitro experiments have consistently shown that IL-17 induces the receptor activator of NF-κB ligand (RANKL) expression in human synovial fibroblasts, leading to the loss of the RANKL/osteoprotegerin balance and the subsequently enhanced osteoclastogenesis and bone erosion in autoimmune arthritis (Lubeerts et al., 2003; Kim et al., 2012). In addition, IL-17 increases the production of vascular endothelial growth factor in rheumatoid fibroblast like synoviocytes, contributing to the angiogenesis in rheumatoid synovium (Ryu et al., 2006). Moreover, IL-17 stimulates the expressions of various pro-inflammatory cytokines (e.g., IL-1β, TNF-α, and IL-6) in whole synovial tissue, synovial fibroblasts, and cartilage, thus promoting inflammation, matrix turnover, and cartilage destruction during RA development (Jovanovic et al., 1998; Moran et al., 2009).
Especially important, Prevotella is a commensal microbe in the colon that can not only degrade a broad spectrum of plant polysaccharides and mucin glycoproteins in the mucosal layer of the gut, but also may interact with the immune system (Arumugam et al.,2011; Scher et al., 2013; Wu et al., 2011). Prevotella was recently suggested as a main contributor to the gut microbiome enterotypes (Arumugam et al., 2011). This enterotype is related to higher levels of health-promoting neuroactive SCFA and a high capacity for biosynthesis of thiamine and folate (Cryan et al., 2012; Ou et al., 2013). Recently, many studies have found that some people who have autism (Kang et al., 2013), type I diabetes (Brown et al., 2011), and constipation (Zhu et al., 2014) also have decreased Prevotella. Decreased Prevotella abundance also fits well with observations of increased gut permeability in Parkinson's disease (Brown et al., 2011; Forsyth et al., 2011). Increased mucosal permeability could lead to local and systemic exposure to bacterial endotoxin, which has been suggested as an environmental trigger of Parkinson's disease and can lead to increased alpha-synuclein expression in the colon (Kelly et al., 2013). Similarity, we also found that the plasma endotoxin concentration was higher in FPD-affected ducks, indicating an increased gut permeability in FPD-affected ducks.
In the current study, we also found that the abundance of Clostridia was higher, and the abundance of Lachnospiraceae and Lactobacillus was lower in the cecum of FPD-affected duck breeders. These results are consistent with findings in human being with RA. Zhang et al. (2015) found that the abundance of Clostridia and Coliforms increased, and the abundance of Lactobacteria decreased in the stool of RA patients compared to healthy persons. Lactobacillus, a well-known lactate-producing bacterium, is important in mediating innate and adaptive immune defenses against microbial pathogens (Martin et al., 2003). In addition, Biddle et al. (2013) reported that Lachnospiraceae is linked with gut health for its butyrate-producing properties. However, in our current study, we did not observe a significantly lower butyrate concentration in cecal contents of FPD-affected duck breeders. Therefore, the mechanisms of the difference in cecal microbiome structure and composition of FDP-affected breeder ducks needs further study.
CONCLUSIONS
Taken together, our results demonstrate that differences of cecal microbiome diversity and compositions exist between in healthy and FPD-affected duck breeders. These findings also indicate that FPD-affected can significantly affect the abundance of some beneficial bacteria, such as Prevotella, Lachnospiraceae, Lactobacillus, and Clostridia, and result in severe inflammatory response in duck breeders. Further studies are necessary to explore the mechanisms underlying the protective or predisposing role of the gut microbiota in FPD of poultry and leverage these to develop novel preventative and therapeutic approaches.
ACKNOWLEDGMENTS
The research was supported by China Agriculture Research System (CARS-42-10), and the 111 project of Foreign Experts Affairs, and Sichuan Agricultural University 211 Foundation of China. The authors wish to thank Dr. Susan M. Fraley for critical comments in the preparation of this manuscript.
REFERENCES
- Arumugam M., Raes J., Pelletier E., Dusko Ehrlich S., Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C., Algers B. EAAP 55th Annual Meeting L4.101. Bled; Slovenia: 2004. The effect of floor heating and feed protein level on the incidence of foot pad dermatitis in turkeys poults; p. 359. [Google Scholar]
- Best A.A., Porter A.L., Fraley S.M., Fraley G.S. Characterization gut microbiome dynamics in developing Pekin ducks and impact of management system. Front. Microbiol. 2017;7 doi: 10.3389/fmicb.2016.02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Brown C.T., Davis-Richardson A.G., Giongo A., Gano K.A., Crabb D.B. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Dai S.J., Zhang K.Y., Ding X.M., Bai S.P., Luo Y.H., Wang J.P., Zeng Q.F. Effect of dietary non-phytate phosphorus levels on the diversity and structure of cecal microbiota in meat duck from 1 to 21d of age. Poult. Sci. 2018;97:2441–2450. doi: 10.3382/ps/pey090. [DOI] [PubMed] [Google Scholar]
- De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- Eija K., Marianna N., Anna V. Effect of litter quality on foot pad dermatitis, hock burns and breast blisters in broiler breeders during the production period. Avian Pathol. 2016;45:667–673. doi: 10.1080/03079457.2016.1197377. [DOI] [PubMed] [Google Scholar]
- Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J.A., McCracken R.M., Evans R.T. A contact dermatitis of broilers-clinical and pathological findings. Avian Pathol. 1985;14:23–38. doi: 10.1080/03079458508436205. [DOI] [PubMed] [Google Scholar]
- Haslam S.M., Knowles T.G., Brown S.N., Wilkins L.J., Kestin S.C., Warriss P.D., Nicol C.J. Factors affecting the prevalence of foot pad dermatitis, hock burn and breast burn in broiler chicken. Br. Poult. Sci. 2007;48:264–275. doi: 10.1080/00071660701371341. [DOI] [PubMed] [Google Scholar]
- Joosten L.A., Abdollahi-Roodsaz S., Heuvelmass-Jacobs M., Helsen M.M.A., van den Bersselaar L.A.M., Oppers-Walgreen B., Koenders M.I., van den Berg W.B. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: crucial role of both interleukin-1beta and interleukin-17. Arthritis Rheum. 2008;58:98–108. doi: 10.1002/art.23152. [DOI] [PubMed] [Google Scholar]
- Jovanovic D.V., DiBattista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Kang D.W., Park J.G., Ilhan Z.E., Wallstrom G., Labaer J.S., Adams J.B., Krajmalnik-Brown R. Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L.P., Carves P.M., Keshavarzian A., Shannon K.M., Shaikh M., Bakay R.A.E., Kordower J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov. Disord. 2013;29:999–1000. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.W., Kim H.R., Park J.Y., Park J.S., Oh H.J., Woo Y.J., Park M.K., Cho M.L., Lee S.H. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- Liu X.F., Zeng B.H., Zhang J., Li W.X., Mou F.X., Wang H., Zou Q.H., Zhong B., Wu L.K., Wei H., Fang Y.F. Role of the gut microbiome in modulating arthritis progression in mice. Sci. Rep. 2016;6 doi: 10.1038/srep30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Xu J., Huang C., Lei X., Cheng D., Liu W., Cheng A., Tang L., Fang J., Ou Y., Geng Y., Chen Z. Impacts of duck-origin parvovirus infection on Cherry Valley ducklings from the perspective of gut microbiota. Front. Microbiol. 2019;10:624. doi: 10.3389/fmicb.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Lladser M.E., Knights D., Stombaugh J., Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E., van den Bersselaar L., Oppers-Walgreen B., Schwarzenberger P., Coenen-de Roo C.J., Kolls J.K., Joosten L.A., van den Berg W.B. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κB ligand/osteoprotegerin balance. J. Immunol. 2003;17:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Takeda K. Role of gut microbiota in rheumatoid arthritis. J. Clin. Med. 2017;6:60. doi: 10.3390/jcm6060060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow G., Ellett S., Ferguson I.R., Zhu S., Karunasinghe N., Jesuthasan A.C. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum. Genomics. 2013;7:24. doi: 10.1186/1479-7364-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Langa S., Reviriego C., Jiminez E., Marin M.L., Xaus J., Fernandez L., Rodriguez J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003;143:754–758. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Martrenchar A., Boilletot E., Huonnic D., Pol F. Risk factors for foot pad dermatitis in chicken and turkey broilers in France. Prev. Vet. Med. 2002;52:213–226. doi: 10.1016/s0167-5877(01)00259-8. [DOI] [PubMed] [Google Scholar]
- Molist F., Manzanilla E.G., Pérez J.F., Nyachoti C.M. Coarse, but not finely ground, dietary fibre increases intestinal Firmicutes: Bacteroidetes ratio and reduces diarrhoea induced by experimental infection in piglets. Br. J. Nutr. 2011;108:9–15. doi: 10.1017/S0007114511005216. [DOI] [PubMed] [Google Scholar]
- Moran E.M., Mullan R., McCormick J., Connolly M., Sullivan O., Fitzgerald O., Bresnihan B., Veale D.J., Fearon U. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-α, Oncostatin M and response to biologic therapies. Arthritis Res. Ther. 2009;11:R113. doi: 10.1186/ar2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder I.E., Schmidt B., Stokes R.C., Lewis M., Bailey M., Aminov R.I. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Carbonero F., Zoetendal E.G., DeLany J.P., Wang M., Newton K., Gaskins H.R., OKeefe S.J.D. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin Nutr. 2013;98:111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Qin S.M., Zhang K.Y., Ding X.M., Bai S.P., Wang J.P., Zeng Q.F. Effect of dietary graded resistant potato starch levels on growth performance, plasma cytokines concentration, and intestinal health in meat ducks. Poult. Sci. 2019 doi: 10.3382/ps/pez186. [DOI] [PubMed] [Google Scholar]
- Ryu S., Lee J.H., Kim S.I. IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin. Rheumatol. 2006;25:16–20. doi: 10.1007/s10067-005-1081-1. [DOI] [PubMed] [Google Scholar]
- Scher U.J., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., Huttenhower C., Littman D.R. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2 doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd E.M., Fairchild B.D. Footpad dermatitis in poultry. Poult. Sci. 2010;89:2043–2051. doi: 10.3382/ps.2010-00770. [DOI] [PubMed] [Google Scholar]
- Sun M., He C., Cong Y., Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8:969–978. doi: 10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatkiewicz S., Arczewska-Wlosek A., Jozefiak D. The nutrition of poultry as a factor affecting litter quality and foot pad dermatitis - an updated review. J. Anim. Physiol. Anim. Nutr. 2016;101:e14–e20. doi: 10.1111/jpn.12630. [DOI] [PubMed] [Google Scholar]
- Vasaï F., Brugirard Ricaud K., Bernadet M.D., Cauquil L., Bouchez O., Combes S. Overfeeding and genetics affect the composition of intestinal microbiota in Anasplatyrhynchos (Pekin) and Cairinamoschata (Muscovy) ducks. FEMS Microbiol. Ecol. 2014;87:204–216. doi: 10.1111/1574-6941.12217. [DOI] [PubMed] [Google Scholar]
- Weber Wyneken C., Sinclair A., Veldkamp T., Vinco L.J., Hocking P.M. Footpad dermatitis and pain assessment in turkey poults using analgesia and objective gait analysis. Br. Poult. Sci. 2015;56:522–530. doi: 10.1080/00071668.2015.1077203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keibaugh S.A., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.H., He B., Liu J., Feng H., Ma Y.H., Li D.F., Guo B. S, Liang C., Dang L., Wang L.Y., Tian J., Zhu H.L., Xiao L.B., Lu C., Lu A.P., Zhang G. Molecular insight into gut microbiota and rheumatoid arthritis. Int. J. Mol. Sci. 2016;17:431. doi: 10.3390/ijms17030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi N., Saulnier L., Bonnin E., Devillard E., Eeckhaut V., Rhayat L. Short-chain arabinoxylans prepared from enzymatically treated wheat grain exert prebiotic effects during the broiler starter period. Poult. Sci. 2018;97:412–424. doi: 10.3382/ps/pex297. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang D., Jia H., Feng Q., Wang D., Liang D. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- Zhu L., Liu W., Alkhouri R., Baker R.D., Bard J.B., Quigley E.M., Baker S.S. Structural changes in the gut microbiome of constipated patients. Physiol. Genomics. 2014;46:679–686. doi: 10.1152/physiolgenomics.00082.2014. [DOI] [PubMed] [Google Scholar]