Abstract
This study was aimed to assess the protective effects of γ-irradiated Astragalus polysaccharides (IAPS) on the development of small intestine and intestinal mucosal immunity of immunosuppressed broilers induced by cyclophosphamide (CPM). A total of 384 one-day-old broiler chicks with similar initial weight were randomly assigned into 6 groups: non-treated group (control), and CPM-treated groups fed either a basal diet or the diets containing 900 mg/kg APS, or 900, 600, 300 mg/kg IAPS, respectively. On days 16, 18, and 20, all broilers except for control group were intramuscularly injected with 0.5 mL CPM (40 mg/kg of BW). Broilers in the control group were intramuscularly injected with 0.5 mL sterilized saline (0.75%, wt/vol). This trial was lasted for 21 d. The results revealed that both APS and IAPS treatment elevated the duodenal IgA-producing cells number and the jejunal mRNA expression of interleukin-2 (IL-2), interleukin-10 (IL-10), and interferon γ of CPM-injected broilers (P < 0.05). The decreased jejunal villus height (VH), the ratio of VH to crypt depth (V/C), as well as the intestinal intraepithelial lymphocytes (IELs) and goblet cells number in CPM-injected broilers were elevated by dietary supplementation with 900 mg/kg APS or 900, 600 mg/kg IAPS (P < 0.05). The CPM-induced decrease in jejunum index, the duodenal VH and the jejunal IgA-producing cells number were only improved in the 900 mg/kg IAPS group (P < 0.05). Furthermore, the number of IELs and IgA-producing cells in duodenum, VH, V/C, the number of goblet cells, and mRNA expression of IL-2 and IL-10 in jejunum were higher in the 900 mg/kg IAPS group than those in the 900 mg/kg APS group (P < 0.05). In summary, IAPS possessed stronger immunomodulatory effect than APS at the same supplementation level. Therefore, gamma irradiation can be used as an alternative treatment to enhance the immunomodulatory activity of APS.
key words: broiler, irradiated Astragalus polysaccharides, intestinal development, intestinal mucosal immunity
INTRODUCTION
In poultry industry, broiler chickens are exposed to stressors, infectious diseases, and nutrient deficiencies, which impair the specific and non-specific immunity and make organism more sensitive to pathogens, consequently resulting in immunosuppression (Fan et al., 2013; Cheng et al., 2016). Aside from the damage on immune function, immunosuppression state also results in decreased growth performance by reducing BW and feed conversion (He et al., 2007). Numerous reports have shown that Chinese herbal medicines and their effective components can significantly enhance animal immune function (Qiu et al., 2007) and intestinal mucosal integrity (Han et al., 2016) with the advantages of producing fewer side effects, being widely available and high efficacy (Chen et al., 2014). Astragalus polysaccharides (APS), as one of the main active ingredients of Astragalus membranaceus (Fisch.) Bunge (family Fabaceae), have been reported to exhibit immune enhancement both in vivo and in vitro, such as promoting T and B lymphocytes proliferation (Fan et al., 2012; Li et al., 2019), increasing the serum IgG, IgM, and IgA levels (Wu, 2018; Li et al., 2019), as well as improving the development of immune organs (Li et al., 2009) and the intestinal mucosal immunity (Li et al., 2018b).
Polysaccharides are large-molecular-weight polymers with specific spatial structures. Biological activities of polysaccharides are influenced by molecular weight, glycosidic bond of the main chain and tertiary structure (Sung et al., 2009). γ irradiation is an ionic, non-thermal process that uses as a storage and functional modification agent in polymers research and application (Methacanon et al., 2011). It is also considered as one of the physical modification methods of nature polysaccharides by cleavage of the glycosidic bonds (Hussain et al., 2014). In comparison with other modification methods, such as acidic hydrolysis and enzymatic treatments, administration of γ irradiation is more reproducible, high-yield, and environment-friendly (Byun et al., 2008). In a recent in vitro study, we found that γ irradiation modification with a proper dose (25 kGy) enhanced immunomodulatory activity of APS (Ren et al. 2018). However, whether the γ-irradiated APS (IAPS) can be used as a potential immune-enhancing agent needs further investigation to assess its immunomodulatory activity in vivo.
Cyclophosphamide (CPM) is an immunosuppressive agent that reduces immunomodulation-related cytokines and antibodies, consequently impairing both innate immunity and adaptive immunity (Fu et al., 2018). As the biggest immune organ in the body, intestine is not only responsible for digestion and absorption of nutrients, but also as a barrier as part of the immune system (Shao et al., 2013). The intestinal mucosa, which represent the first line for immune response, are composed of immune cells and molecules irregular throughout lamina propria and organized lymphatic tissues (Zhao et al., 2009). We thus hypothesized that IAPS administration through oral route would influence the intestinal health of broilers via interaction directly with the intestinal mucosal immunity. Therefore, the aim of this study was to assess the protective effects of IAPS on the development of small intestine and intestinal mucosal immunity of immunosuppressive broilers induced by CPM.
MATERIALS AND METHODS
Preparation of IAPS
APS was purchased from Tianjin Sainuo Pharmaceutical Co., Ltd. (Tianjin, China), and the polysaccharides content determined according to Masuko et al. (2005) was 87.64%. The APS samples were packed in 3 identical polyethylene bags (200 g/bag).
γ irradiation treatment was carried out using a BFT-IV cobalt-60 source irradiator at an ambient temperature of 25 ± 0.5°C in XiYue Irradiation Technology Co., Ltd. (Nanjing, China). The irradiation dose in this study was 25 kGy, with a rate of 4 Gy/s. The source strength was 2 × 106 Ci. After irradiation, 100 g of IAPS from each bag were weighed, mixed and stored at −20°C for animal feeding experiment.
Birds and Experimental Design
The animal protocol for the present study was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 384 one-day-old Arbor Acres broiler chicks (Shengnong Co., Ltd., Jiangsu, China) based on similar initial BW (45.00 ± 0.45 g) were randomly allocated into 6 groups with 8 cages per group and 8 chickens per cage (100 × 60 × 40 cm). These 6 groups included: 1) control group, 2) CPM group, 3) APS900 group, 4) IAPS900 group, 5) IAPS600 group, and 6) IAPS300 group. Broilers in the control group and CPM group were fed with the basal diets. Birds in the APS900, IAPS900, IAPS600, and IAPS300 groups were fed with the same basal diets supplementation with 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively. The experiment lasted for 21 d. On days 16, 18, and 20 of the experiment, all birds in the control group were pectoral intramuscularly injected with 0.5 mL sterile saline (0.75%, wt/vol), and the birds in other treatment groups were pectoral intramuscularly injected with 0.5 mL CPM (40 mg/kg of BW) (Yang et al., 2011). The sterile distilled water was autoclaved at 121°C for 15 min. The solutions were sterilized by filtration through a 0.22-μm membrane filter.
All broilers were allowed free access to feed and water in a temperature-controlled room at Nanjing Kangxin Poultry Industry Company (Nanjing, China) during the 21-d experiment. The birds were raised under white light with a light schedule of 23 h light and 1 h dark (23L:1D; darkness duration, 2300 to 2400 h) from days 0 to 21. The temperature of the experimental room was set at 33°C at the age of 1 to 4 d and then reduced by 2 or 3°C per week to a final temperature of 26°C. The ingredient composition and nutrient levels for the starter diets are presented in Table 1. The diets were formulated to meet or exceed the nutrient requirements of the National Research Council (1994) and were devoid of antibiotics.
Table 1.
Ingredient composition and calculated nutrient levels of the basal diets.
| 1 to 21 d | |
|---|---|
| Ingredient (%) | |
| Corn | 57.61 |
| Soybean meal | 31.00 |
| Corn gluten meal1 | 3.29 |
| Soybean oil | 3.11 |
| Dicalcium phosphate | 2.00 |
| Limestone | 1.20 |
| Salt | 0.30 |
| L-Lysine HCl | 0.34 |
| DL-Methionine | 0.15 |
| Premix2 | 1.00 |
| Calculated Nutrient levels (%) | |
| Metabolizable energy (MJ/kg) | 12.56 |
| Crude protein | 21.00 |
| Calcium | 1.00 |
| Total phosphorus | 0.70 |
| Available phosphorus | 0.46 |
| Lysine | 1.20 |
| Methionine | 0.50 |
| Methionine + cysteine | 0.85 |
The crud protein content was 60%.
Premix provided per kilogram of diet: vitamin A, 12,000 IU; vitaminD3, 2500 IU; vitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; choline chloride, 400 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Sample Collection
At the age of 21 d, birds were group weighed by cage for the analysis of the mean BW for each replication. Then, one bird from each cage with similar weight close to the average weight of their cage was selected and weighted. All birds (total 48 birds) were stunned electrically (50 V, alternating current, 400 Hz for 5 s each one), and then immediately slaughtered via exsanguination of the left carotid artery. The duodenum (from ventriculus to pancreo-biliary ducts), jejunum (from pancreo-biliary ducts to yolk stalk) and ileum (from yolk stalk to ileocecal junction) were collected. For morphological analysis, approximately 1-cm middle portion of the 3 sections of small intestine were excised, flushed with cold sterile saline (0.75%, wt/vol) and fixed in 4% paraformaldehyde at least for 24 h. Afterwards, the contents of 3 sections of small intestine were individually squeezed out and weighted for calculated the intestine index according to the formula: the small intestine index (g/kg) = the weight of small intestine (g)/BW (kg). After cut open, the mucosa of jejunum was aseptically scraped from the luminal surface using a clean glass microscope slide, minced, snap-frozen in liquid nitrogen, and then stored at −80°C for mRNA extraction.
Histological Observation of Small Intestine
Intestinal samples were dehydrated in a graded ethanol series (50, 70, 80, 90, 95, and 100% ethanol), clarified with fresh xylene, and then embedded into paraffins. About 5 μm intestinal cross sections were prepared and stained with common haematoxylin-eosin (HE) staining (Chen et al., 2015) for morphology measurement. The images of stained samples were captured using an Olympus DP12 CCD digital camera (Olympus Optical Co. Ltd., Tokyo, Japan) under a light microscope at 40× magnification (Olympus BX41, Olympus Optical Co. Ltd.). Then, the villus height (VH; from the tip of the villi to the villi crypt opening) and the crypt depth (CD; from the base of the crypt to the level of the crypt opening) were determined by the Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA), the ratio of VH to crypt depth (VH/CD) was then calculated. At least 6 well-oriented, intact villus-crypt units of intestinal (duodenum, jejunum and ileum) cross section from one section per chicken were examined. The average value of the 6 structures per chicken was used.
Immunocytes Counts in Small Intestine
Histological sections (5 μm) were prepared using the same protocol described above. For intraepithelial lymphocytes (IELs) counts, tissue slides were stained with HE method. As for goblet cells, tissue slides were prepared and stained with glycogen periodic acid-Schiff according to Wang et al. (2009). All sections were observed under a light microscope at 400× amplification (Scope A1, Carl Zeiss Co. Ltd., Jena, Germany). For different part of small intestine (duodenum, jejunum and ileum), the numbers of IELs and goblet cells at 5 different fields of intestinal villi from one section in each bird were counted for the statistical analysis of the data, and expressed as number of cells per 100 columnar epithelial cells.
Measurement of IgA -Producing Cells Using Immunohistochemistry
Expression of IgA-producing cells in the tissue samples was performed by immuno-histochemical analysis. The histological sections (5 μm) were prepared using the same protocol as described above. The number of IgA-producing cells was revealed using the avidin-biotin complex immunohistochemical method. The staining procedure was based on the methods of Wang et al. (2009). The mouse anti-chicken IgA monoclonal antibody (Cat no. 8330–01; Southern Biotechnology Inc., Birmingham, AL) and was diluted to 10 μg/mL with PBS (0.01 M, pH 7.4). The number of IgA-producing cells in the intestinal lamina propria was counted using a light microscope (Scope A1, Carl Zeiss Co. Ltd.) at 200× amplification. Five different fields per section in each chicken were chosen randomly and analyzed. The results were expressed as the number of cells/mm2.
RNA Extraction and Real-Time Quantitative PCR Analysis
The total RNA was extracted from the frozen mucosa samples using the Trizol reagent (Takara Biotechnology Co. Ltd., Dalian, China) according to the manufacturer's protocol. Then, total RNA was treated with DNase I (Takara Biotechnology Co. Ltd.) to remove DNA and reverse transcribed to cDNA (20 μL reaction system for maximum use of 500 ng of total RNA) using a PrimeScript RT master mix kit (Takara Biotechnology Co. Ltd.). The reverse-transcription (RT) reactions were incubated for 15 min at 37°C, followed by 5 s at 85°C to inactivate the RT enzyme. The RT products (cDNA) were stored at −20°C.
The mRNA expressions of selected genes including interleukin-2 (IL-2), interleukin-10 (IL-10), lipopolysaccharide induced TNF factor (LITAF), and interferon γ (IFN-γ) were evaluated by the Real-Time Quantitative PCR (RT-qPCR). The RT-qPCR mixture system was performed using the CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR Premix Ex Taq kits (Takara Biotechnology Co. Ltd.). The reaction volume was 20 μL, containing 10 μL of SYBR Premix Ex Taq II (2×), 0.4 μL of ROX Reference Dye II (50×), 0.4 μL of a forward primer (10 μM), 0.4 μL of a reverse primer (10 μM), 1 μL of cDNA, and 7.8 μL sterilized double-distilled water. The pairs of specific primers for target genes and the housekeeping gene are presented in Table 2 and were synthesized by Invitrogen (Invitrogen Life Technologies, Shanghai, China). The thermal cycling conditions were used as follows: 95°C for 5 min; 40 cycles at 95°C for 10 s, 60°C for 30 s, 72°C for 30 s; and 72°C for 5 min. The cycle threshold (Ct) values were normalized to the expression level of β -actin. All samples were run in triplicate, and the expression of target gene relative to β -actin was calculated using the 2−ΔΔCt method as previously described (Livak and Schmittgen, 2001).
Table 2.
Primer sequences used for RT-qPCR analysis.
| Genes1 | Primer sequence (5′-3′) | Amplicon size (bp) | Genbank number |
|---|---|---|---|
| IL-2 | F: GCTAATGACTACAGCTTATGGAGCA | 187 | NM 000594 |
| R: TGGGTCTCAGTTGGTGTGTAGAG | |||
| IL-10 | F: GCGCTTCTACACAGATGAGGT | 179 | NM 0010,0,4414.2 |
| R: CGAACGTCTCCTTGATCTGC | |||
| LITAF | F: AGACCAGATGGGAAGGGAATGAA | 219 | XM 01,529,4125.1 |
| R: GAAGAGGCCACCACACGACAG | |||
| IFN-γ | F: ATGTAGCTGACGGTGGACCT | 193 | NM 205,149.1 |
| R: ACGCCATCAGGAAGGTTGTT | |||
| β- actin | F: ATCCGGACCCTCCATTGTC | 120 | NM 205,518.1 |
| R: AGCCATGCCAATCTCGTCTT |
IL-2, interleukin-2; IL-10, interleukin-10; LITAF, lipopolysaccharide induced TNF factor; IFN-γ, Interferon γ.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) using SPSS statistical software (Version 20.0 for windows, SPSS Inc., Chicago, IL, USA). Differences among treatments were examined using Duncan's multiple range tests. Unless otherwise stated, all results were presented by mean values and the standard error of the mean (SEM). Differences were considered significant as P < 0.05.
RESULTS
BW and Intestine Index
CPM stimulation decreased the BW and jejunum index of 21-d broilers compared to the control group (P < 0.05; Table 3). Both APS and IAPS treatments had no effect on the BW of 21-d broilers compared with the CPM group (P > 0.05). IAPS900 treatment elevated the jejunum index compared with the CPM treatment (IAPS900: 14.16 g/kg·BW; CPM: 13.02 g/kg·BW;P < 0.05). No differences were observed in relative weight of the duodenum and ileum among all treatments (P > 0.05).
Table 3.
Effect of APS and IAPS on the BW and intestine index of CPM immunosuppressed broilers.
| Treatments1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | CPM | APS900 | IAPS900 | IAPS600 | IAPS300 | SEM | P value |
| BW (kg/bird) | 0.726a | 0.663b | 0.688a,b | 0.692a,b | 0.687a,b | 0.672b | 0.006 | 0.047 |
| Duodenum index (g/kg·BW) | 8.04 | 7.30 | 7.36 | 7.77 | 7.65 | 7.34 | 0.099 | 0.188 |
| Jejunum index (g/kg·BW) | 14.65a | 13.02b | 13.65a,b | 14.16a | 13.70a,b | 13.57a,b | 0.152 | 0.036 |
| Ileum index (g/kg·BW) | 10.29 | 9.72 | 10.20 | 10.24 | 10.22 | 9.98 | 0.182 | 0.953 |
Different letters in the mean value of the same row indicate a significant difference (P < 0.05). The results are represented as the mean value ± SEM (n = 8).
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively; CPM, cyclophosphamide; APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS.
Intestinal Morphology
As shown in Table 4, CPM stimulation reduced the VH and V/C, and increased the CD both in the duodenum and jejunum compared with the control group, respectively (P < 0.01). APS900, IAPS900, and IAPS300 treatments increased the VH and V/C in jejunum and decreased the CD in duodenum compared to the CPM group (P < 0.05). The birds in IAPS900 group showed higher VH and V/C of the duodenum and lower CD of the jejunum than those in the CPM group (P < 0.05). Moreover, the VH and V/C of jejunum in the IAPS900 group were higher than those in the APS900 group (VH: 1332.43 μm 1256.59 μm; V/C: 5.20 vs. 4.81; P < 0.05). Supplementation with APS or IAPS had no effect on ileum morphology compared to the CPM group (P > 0.05).
Table 4.
Effect of APS and IAPS on the intestine morphology of CPM immunosuppressed broilers.
| Treatments2 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items1 | Control | CPM | APS900 | IAPS900 | IAPS600 | IAPS300 | SEM | P value |
| Duodenum | ||||||||
| VH (μm) | 1879.99a | 1724.85c | 1761.52b,c | 1809.72b | 1786.02b,c | 1743.55c | 10.92 | <0.001 |
| CD (μm) | 175.65c | 209.86a | 186.28b,c | 182.76b,c | 184.65b,c | 204.68a,b | 3.420 | 0.016 |
| V/C | 10.78a | 8.63c | 9.20b,c | 9.68b | 9.35b,c | 9.02b,c | 0.136 | <0.001 |
| Jejunum | ||||||||
| VH (μm) | 1357.03a | 1180.53c | 1256.59b | 1332.43a | 1330.08a | 1219.94b | 10.77 | <0.001 |
| CD (μm) | 247.50c | 270.10a | 262.23a-c | 253.38b,c | 257.06a-c | 267.72a,b | 2.217 | 0.018 |
| V/C | 5.31a | 4.41c | 4.81b | 5.20a | 5.16a | 4.44c | 0.069 | <0.001 |
| Ileum | ||||||||
| VH (μm) | 900.19 | 808.24 | 851.00 | 868.68 | 858.75 | 831.89 | 9.593 | 0.101 |
| CD (μm) | 174.35 | 183.99 | 181.22 | 176.72 | 181.03 | 182.81 | 1.225 | 0.170 |
| V/C | 5.18a | 4.41b | 4.67b | 4.84a,b | 4.82a,b | 4.61b | 0.069 | 0.024 |
Different letters in the mean value of the same row indicate a significant difference (P < 0.05). The results are represented as the mean value ± SEM (n = 8).
VH, villus height; CD, crypt depth; V/D, the ratio of villus height to crypt depth.
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively; CPM, cyclophosphamide; APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS.
The Number of IELs in Intestine
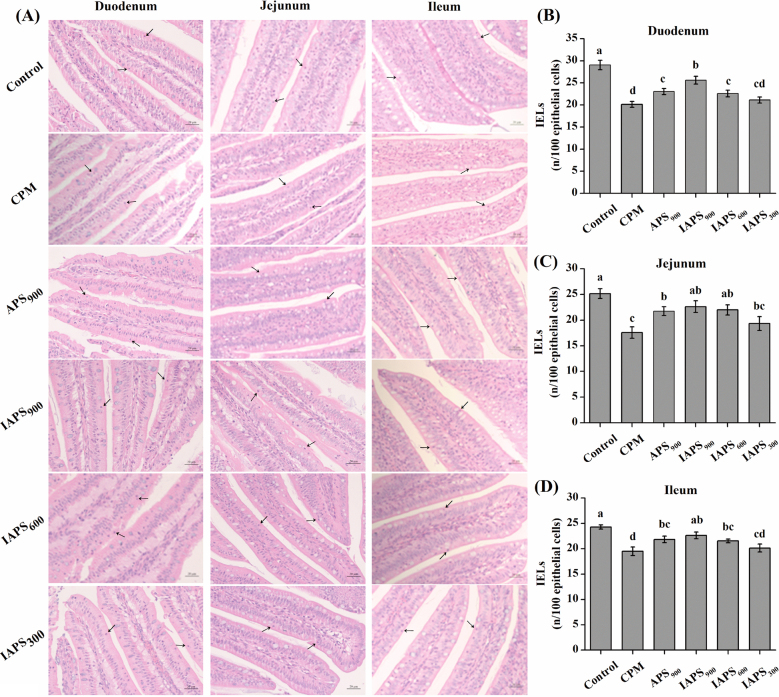
In sections of the small intestine samples, the IELs dispersed as single cells within the epithelial cell layer that surrounds the intestinal lumen in the basal region of the epithelium in duodenum and jejunum (Figure 1 A). The number of IELs in the duodenum, jejunum and ileum were decreased in CPM group than those in the control group (P < 0.05; Figure 1 B, C and D). Compared with the CPM treatment, APS900, IAPS900, and IAPS600 treatments increased the number of IELs in all the 3 sections of intestine (P < 0.05; Figure 1 B, C and D). In addition, the number of IELs in the duodenum was higher in IAPS900 treatment than that in the APS900 treatment (IAPS900: 25.67/100 epithelial cells; APS900: 22.98/100 epithelial cells;P < 0.05; Figure 1 B).
Figure 1.
Effect of APS and IAPS on the number of intraepithelial lymphocytes (IELs) in intestine of CPM immunosuppressed broilers. The results are represented as the mean value ± SE of each treatment (n = 8). Means without a common letter significantly differ (P < 0.05). Control, broilers were fed a basal diet and injection with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively. APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS; CPM, cyclophosphamide.
The Number of Goblet Cells in Intestine
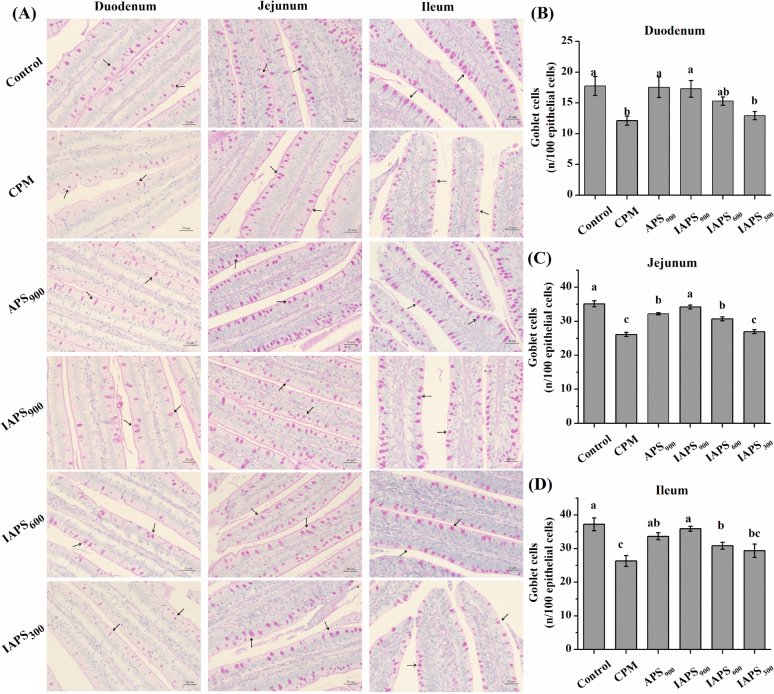
Goblet cells were mainly distributed among columnar cells and presented a typical goblet shape (Figure 2 A). Compared with the control group, the CPM treatment decreased the number of goblet cells in the duodenum, jejunum and ileum (P < 0.05; Figure 2 B, C, and D). Compared with the CPM group, birds in the APS900, IAPS900, and IAPS600 groups showed higher number of goblet cells both in the jejunum and ileum (P < 0.05; Figure 2 C and D). The number of goblet cells in the duodenum was higher in APS900 and IAPS900 groups than that in the CPM group (P < 0.05; Figure 2 B). Furthermore, IAPS900 treatment increased the number of jejunal goblet cells compared to the APS900 treatment (P < 0.05).
Figure 2.
Effect of APS and IAPS on the number of goblet cells in intestine of CPM immunosuppressed broilers. The results are represented as the mean value ± SE of each treatment (n = 8). Means without a common letter significantly differ (P < 0.05). Control, broilers were fed a basal diet and injection with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively. APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS; CPM, cyclophosphamide.
The Number of IgA-Producing Cells in Intestine
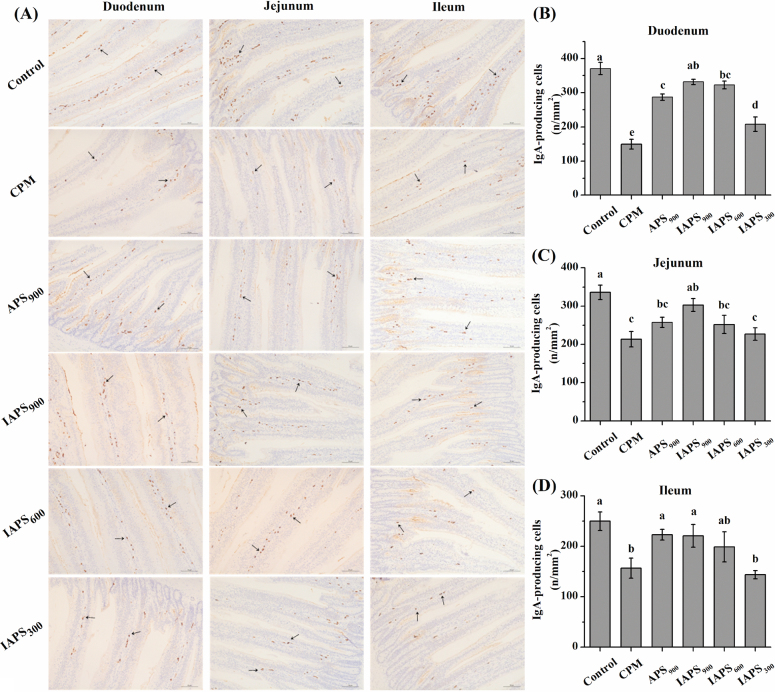
The IgA-producing cell, which was recognized as a nucleus surrounded by a ring of yellow-brown cytoplasm, mainly distributed in the area of the mucosal lamina propria of the intestine (Figure 3 A). Compared with the control group, CPM stimulation decreased the number of IgA-producing cells in all 3 sections of small intestine (P < 0.05; Figure 3 B, C and D). Dietary addition with APS at 900 mg/kg or IAPS at all dosage increased the number of IgA-producing cells in the duodenum compared with the CPM treatment (P < 0.05; Figure 3 A and B). The number of IgA-producing cells in ileum was higher in APS900 and IAPS900 groups than that in the CPM group (P < 0.05; Figure 3 D). The number of jejunal IgA-producing cells was only elevated in the IAPS900 group in comparison to the CPM treatment (IAPS900: 303.05/mm2; CPM: 213.32/mm2; P < 0.05; Figure 3 C). Moreover, the number of IgA-producing cells in duodenum was higher in the IAPS900 group than that in the APS900 group (IAPS900:331.63/mm2; APS900: 287.11/mm2; P < 0.05; Figure 3 A).
Figure 3.
Effect of APS and IAPS on the number of IgA-producing cells in intestine of CPM immunosuppressed broilers. The results are represented as the mean value ± SE of each treatment (n = 8). Means without a common letter significantly differ (P < 0.05). Control, broilers were fed a basal diet and injection with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively. APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS; CPM, cyclophosphamide.
Relative mRNA Expression of IL-2, IL-10, LITAF, and IFN-γ in Jejunum
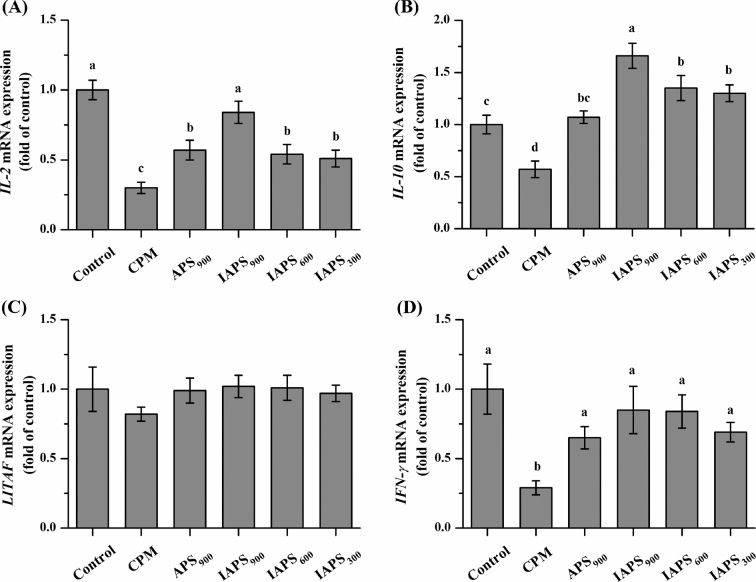
Figure 4 shows the relative mRNA expression of some key cytokines in the jejunum. The mRNA expression of IL-2, IL-10, and IFN-γ were downregulated after CPM injected (P < 0.05; Figure 4 A, B, and D). Dietary addition with APS at 900 mg/kg or IAPS at all dosage upregulated the mRNA expression of IL-2, IL-10, and IFN-γ compared with the CPM treatment (P < 0.05; Figure 4 A, B and D), whereas no difference was observed on LITAF mRNA expression among all treatments (P > 0.05; Figure 4 C). In addition, the mRNA expression of IL-2 and IL-10 were higher in IAPS900 group than those in the APS900 group (P < 0.05; Figure 4 A and B).
Figure 4.
Effect of APS and IAPS on the mRNA expression of IL-2 (A), IL-10 (B), LITAF (C) and IFN-γ (D) in jejunum of CPM immunosuppressed broilers. The results are represented as the mean value ± SE of each treatment (n = 8). Means without a common letter significantly differ (P < 0.05). Control, broilers were fed a basal diet and injection with saline; CPM, CPM-injected broilers were fed a basal diet; APS900, IAPS900, IAPS600, and IAPS300, CPM-injected broilers were fed a basal diet containing 900 mg/kg APS, and 900, 600, or 300 mg/kg IAPS, respectively. APS, Astragalus polysaccharides; IAPS, 60 Co γ-ray irradiated APS; CPM, cyclophosphamide; IL-2, interleukin-2; IL-10, interleukin-10; LITAF, lipopolysaccharide induced TNF factor; IFN-γ, Interferon γ.
DISCUSSION
Poultry immunosuppression leads to the damage of immune system, reduced growth performance, even increased mortality rate (Fussell, 1998). CPM shows an adverse effect on the intestinal mucosal barrier by shortening the intestinal villi, reducing lymphocytes counts, and disrupting the intestinal flora, which results in intestinal epithelial damage and immunosuppression (Wang et al., 2013; Fu et al., 2018). Small intestine has a frequent contact with viruses, bacteria, and other exogenous matters, and the surface mucosa of the intestine plays a critical role in preventing the invasion of harmful substances (Huang et al., 2017). The systematic immune system could be modulated by initiating the intestinal mucosal immune system (Li et al., 2018a). Therefore, protection of small intestinal mucosa is important to the relief of immunosuppressive status. In this study, we investigated the protective effects of IAPS on the development and the immunity of intestine mucosa of immunosuppressed broilers induced by CPM.
In general, the development of digestive organs is associated with the digestive and absorptive ability of nutrients, thus affecting the developmental status of broilers. Small intestine is one of the most important digestive organs, and small intestinal mucosa with normal structure is necessary for optimal growth as well as nutrient digestion and absorption (Gao et al., 2018). In the current study, broilers administration with CPM at 40 mg/kg of BW showed lower BW and the relative weight of jejunum of 21-d broilers. The direct reason may be due to the reduced VH and V/H, and the increased CD of jejunum and duodenum. VH, CD, and V/C are measured as an indicator of the gut health and the development of intestine of broilers (Shao et al., 2013). Lower VH leads to reductions in the villus surface area, thereby resulting in the decrease of the absorptive ability (Khambualai et al., 2009). Decreased intestinal V/C represents a lower number of mature and functional enterocytes (Cairo et al., 2018). The observations of our study suggested that CPM induced damages to the small intestinal mucosa, which likely impaired the mucosal barrier and the development of intestine. It is reported that oral administration of APS could significantly decrease duodenum CD and increase the jejunum VH and V/D of chickens (Li et al., 2018b). Similar to previous study, we found that APS900, IAPS900 and IAPS300 treatments increased the VH and V/C in the jejunum and decreased the CD of duodenum compared to the CPM group, suggesting both APS and IAPS could restore part of the villus loss or damage associated with CPM. As we expected, CPM-induced decreases in the BW and the relative weight of jejunum were improved by addition of IAPS at 900 mg/kg. Moreover, the VH and V/C of the jejunum in the IAPS900 group were higher than those in the APS900 group, indicating that IAPS had a better effect on the intestinal development than APS. The reason was probably due to the γ irradiation resulted in the decrease in molecular weight and viscosity, and the increase in solubility of APS (Ren et al., 2018), thereby improving the biological activity of polysaccharides in the small intestine.
Immunocompetent cells provide the first contact with exogenous pathogens or antigens, which are the important structure in intestinal mucosal barrier (Wu et al., 2018). As one type of the immunocompetent cells of the intestinal mucosal barrier, IELs are located between intestinal epithelial cells in the epithelium above the basement membrane, which can offer effective protection against virus (Sun et al., 2013). Stimulation of IELs is characterized by secretion of cytokines (such as IL-2, IL-10, and IFN-γ) for immune defense and lead to the promotion of cellular immune mechanisms (Qiu and Yang, 2013). Goblet cells are highly polarized secretory cells that typically scattered among columnar cells. These specialized epithelial cells play an important role in regulating the intestinal immune functions by synthesizing and releasing mucins (Chen et al., 2015). Goblet cells depletion results in mucous layers defective, which ultimately lead to increased bacterial adhesion to the surface epithelium and the enhanced susceptibility to intestinal pathogenic microbes (Shao et al., 2013). Therefore, the number of immunocompetent cells in intestine is a great indicator to evaluate the status of immune function in local intestinal mucosa.
The current study demonstrated CPM treatment obviously reduced the number of IELs and goblet cells in all 3 sections of small intestinal mucosa, which probably result in the reduction of the intestinal immune function. These results are agreement with our observation on intestinal morphology, in which a reduced VH was found after CPM treatment. Similar result was reported by Wang et al. (2013), who found that injection of CPM significantly decreased the number of IELs and goblet cells in the jejunum of mice. Administration of APS is helpful to elevate the number of jejunal IELs (Wang et al., 2015). In this study, APS900, IAPS900, and IAPS300 treatments significantly increased intestinal IELs number of the immunosuppressive broilers, suggesting that both APS and IAPS could promote the intestinal mucosal immune response by improving the maturation and proliferation of IELs. Not only IELs, the CPM-induced decrease of intestinal goblet cells number was significantly elevated by dietary supplementation with 900 mg/kg APS, or 900, 600 mg/kg IAPS. More importantly, birds in the IAPS900 group showed higher jejunal goblet cells counts and duodenal IELs counts compared with the APS900 group, indicating that the protective effect of IAPS was superior to APS. According to the report of Schols et al. (2009), the mucosal immune response is initiated by polysaccharides absorption through M cells, which are present in Peyer's patches. Especially, the permeability of macromolecules across the M cell was size dependent, and whenever it is small, it can pass easier (Liang et al., 2001). Our previous study found that the molecular weight of IAPS was significantly lower than APS, and the number of small fragments increased after γ irradiation (Ren et al., 2018). Thereby, IAPS could penetrate the barrier through M cells more easily and stimulate the mucosal immune response.
Cytokines, mainly secreted by T lymphocytes, exert pleiotropic effects on cell-mediated immune response (Wu et al., 2015). CPM is known to suppress the immune response by decreasing the level of cytokines (eg. IFN-γ, IL-6) of broilers (Fan et al., 2013). Our data shows that the mRNA expression of IL-2, IL-10 and IFN-γ in jejunum were significantly downregulated in the CPM-treated birds, which demonstrated that the cell-regulated immune response in the intestinal mucosa could be impaired by CPM stimulation. IL-2 is mainly produced by IELs in intestinal epithelia, which induces the proliferation and differentiation of T cells, B cells, as well as NK cells (Qiu and Yang, 2013). IFN-γ secreted by T helper (Th) 1 cells plays a great role in native and acquired immunity against the invasion of virus and intracellular bacteria (Wu et al., 2018). It is reported that the expression of cytokines (eg. interleukin-6, IFN-γ) can be induced by APS (Wei et al., 2016). In this study, the higher IL-2 and IFN-γ mRNA expression in jejunum of APS and IAPS groups as compared to the CPM group, indicating that both APS and IAPS could improve the cell-mediated immunity in intestine mucosa through the activation of innate immune response mechanisms moderately. The authors also noted that the mRNA expression of anti-inflammatory cytokines IL-10 in jejunum was also significantly upregulated by administration of APS and IAPS treatments compared to the CPM group. High level of IL-10 mRNA expression may promote B lymphocyte proliferation and antibody synthesis and the stimulation of humoral immune mechanisms in the gut-associated lymphoid tissue (Jarosz et al., 2017). Upregulation of both inflammatory and anti-inflammatory cytokines in the APS900 and IAPS900 groups might be explained by the immunological balance effects between Th1 and Th2 cytokines and ensure homeostasis of the organism and protects it against an excessive immune response. It should be emphasized that the mRNA expression of IL-2 and IL-10 in the IAPS900 group was higher than that in APS900 group, which indicated that IAPS has a stronger immunostimulatory effect than APS. According to the result of Li et al. (2018b), oral administration of APS can be recognized by immune receptor of toll-like receptor 4 (TLR4) acting as a specific antigen and then the immune system will be activated and moderately induce the expression of cytokines. Previous study reported by Methacanon et al. (2011) found that polysaccharides with smaller molecules have better chance in binding to more receptors. Based on the results of the aforementioned literatures and our previous study (Ren et al., 2018), it is speculated that the IAPS with lower molecular weight and higher solubility relative to APS may be conducive to form more biologically active polysaccharides, and consequently activating TLR4 to induce the mRNA expression of cytokines. On the other hand, the higher IELs counts in broilers treated with 900 mg/kg IAPS as compared to the broilers treated with 900 mg/kg IAPS can also contribute to upregulate mRNA expression of IL-2 and IL-10.
The secretory IgA (sIgA) has been considered to serve as a major antibody isotype in intestinal mucosal immunity that can prevent pathogens and toxins from invading epithelial surface and maintain the homeostasis of intestine (Mantis et al., 2011). The IgA-producing cells are the main component of intestinal mucosal humoral immunity contributing to sIgA production, which are mainly distributed in the intestinal lamina propria (Zhai et al., 2011). In this study, the number of IgA-producing cells in small intestine was lower in the CPM group than that in the control group, suggesting that CPM had adverse effect on the intestinal mucosal immunity. Dietary supplementation with 900 mg/kg APS or IAPS at 900, 600, 300 mg/kg increased the number of IgA-producing cells in duodenum, indicating that both APS and IAPS are beneficial for regulating the intestinal immune response. APS had no effect on the number of jejunal IgA-producing cells of CPM-injected broilers, whereas a considerable increase was found between IAPS900 group and CPM group. Furthermore, the number of duodenal IgA-producing cells was higher in IAPS900 group than that in the APS900 group, suggesting that IAPS was more efficacious on promoting intestinal mucosal humoral immunity than APS.
In conclusion, the present study demonstrated that dietary supplementation with 900 mg/kg APS or IAPS at 900, 600 mg/kg IAPS showed protective and reparative effects to alleviate CPM-induced damages of the intestinal mucosa by promoting the intestinal morphology, increasing the number of immunocompetent cells and upregulating the mRNA expression of some cytokines in jejunal mucosa. Furthermore, IAPS possessed a more effective immunoregulatory activity than APS in immunosuppressed broilers. Therefore, gamma irradiation can be used as an alternative treatment to enhance the immunomodulatory activity of APS.
ACKNOWLEDGMENTS
This work was financed by the National Key Research and Development Program of China (2017YFD0500505), the National Natural Science Foundation of China (31601957), the Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents, and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS2018282).
REFERENCES
- Byun E.H., Kim J.H., Sung N.Y., Choi J.I., Lim S.T., Kim K.H., Yook H.S., Byun M.W., Lee J.H. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008;77:781–786. [Google Scholar]
- Cairo P.L.G., Gois F.D., Sbardella M., Silveira H., de Oliveira R.M., Allaman I.B., Cantarelli V.S., Costa L.B. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J. Sci. Food. Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen X., Qiu S., Hu Y., Jiang C., Wang D., Fan Q., Zhang C., Huang Y., Yu Y., Yang H., Liu C., Gao Z., Hou R., Li X. Effects of epimedium polysaccharide-propolis flavone oral liquid on mucosal immunity in chickens. Int. J. Biol. Macromol. 2014;64:6–10. doi: 10.1016/j.ijbiomac.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xie J., Hu M.Y., Tang J., Shao Z.F., Li M.H. Protective effects of γ-aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J. Anim. Plant Sci. 2015;25:78–87. [Google Scholar]
- Cheng K., Song Z.H., Zheng X.C., Zhang H., Zhang J.F., Zhang L.L., Zhou Y.M., Wang T. Effects of dietary vitamin e type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2016;96:1159–1166. doi: 10.3382/ps/pew336. [DOI] [PubMed] [Google Scholar]
- Fan Y, Hu Y., Wang D., Liu J., Zhang J., Zhao X., Liu X., Liu C., Yuan J., Ruan S. Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohydr. Polym. 2012;88:68–74. [Google Scholar]
- Fan Y., Lu Y., Wang D., Liu J., Song X., Zhang W., Zhao X., Nguyen T.L., Hu Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell Immunol. 2013;281:37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Fu Y.P., Feng B., Zhu Z.K., Feng X., Chen S.F., li L.X., Yin Z.Q., Haung C., Chen X.F., Zhang B.Z., Jia R.Y., Song X., Lv C., Yue G.Z., Ye G., Liang X.X., He C. L, Yin L.Z., Zou Y.F. The polysaccharides from Codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules. 2018;23 doi: 10.3390/molecules23071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussell L.W. Poultry industry strategies for control of immunosuppressive diseases. Poult. Sci. 1998;77:1193–1196. doi: 10.1093/ps/77.8.1193. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L‐arginine on the development of digestive organs, intestinal function and post‐hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. 2018;102:e166–e175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Han M., Song P., Huang C., Rezaei A., Farrar S., Brown M.A., Ma X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget. 2016;7:80313–80326. doi: 10.18632/oncotarget.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Yang X., Guo Y. Effects of different dietary oil sources on immune function in cyclophosphamide immunosuppressed chickens. Anim. Feed Sci. Technol. 2007;139:186–200. [Google Scholar]
- Huang X., Nie S., Xie M. Interaction between gut immunity and polysaccharides. Crit. Rev. Food Sci. Nutr. 2017;57:2943–2955. doi: 10.1080/10408398.2015.1079165. [DOI] [PubMed] [Google Scholar]
- Hussain P.R., Wani I.A., Suradkar P.P., Dar M.A. Gamma irradiation induced modification of bean polysaccharides: impact on physicochemical, morphological and antioxidant properties. Carbohydr. Polym. 2014;110:183–194. doi: 10.1016/j.carbpol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Jarosz L., Marek A., Gradzki Z., Kwiecien M., Zylinska B., Kaczmarek B. Effect of feed supplementation with zinc glycine chelate and zinc sulfate on cytokine and immunoglobulin gene expression profiles in chicken intestinal tissue. Poult. Sci. 2017;96:4224–4235. doi: 10.3382/ps/pex253. [DOI] [PubMed] [Google Scholar]
- Khambualai O, Yamauchi K., Koge K., Kashimura J. Morphology of the intestinal mucosa and growth performance of chickens fed diets containing sugar cane extract. J. Anim. Feed Sci. 2009;18:322–334. [Google Scholar]
- Li S.P., Zhao X.J., Wang J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Li Y., Lei X., Yin Z., Guo W., Wu S., Yang X. Transgenerational effects of paternal dietary Astragalus polysaccharides on spleen immunity of broilers. Int. J. Biol. Macromol. 2018;115:90–97. doi: 10.1016/j.ijbiomac.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Li Y., Lei X., Guo W., Wu S., Duan Y., Yang X. Transgenerational endotoxin tolerance-like effect caused by paternal dietary Astragalus polysaccharides in broilers' jejunum. Int. J. Biol. Macromol. 2018;111:769–779. doi: 10.1016/j.ijbiomac.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Li S., Ren L., Zhu X., Li J., Zhang L., Wang X., Gao F., Zhou G. Immunomodulatory effect of γ-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Liang E., Kabcenell A.K., Coleman J.R., Robson J., Ruffles R., Yazdanian M. Permeability measurement of macromolecules and assessment of mucosal antigen sampling using in vitro converted M cells. J. Pharmacol. Toxicol. Methods. 2001;46:93–101. doi: 10.1016/s1056-8719(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S., Lee Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Methacanon P., Weerawatsophon U., Tanjak P., Rachtawee P., Prathumpai W. Interleukin-8 stimulating activity of low molecular weight β-glucan depolymerized by γ-irradiation. Carbohydr. Polym. 2011;86:574–580. [Google Scholar]
- National Research Council . 9th rev. ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Qiu Y., Yang H. Effects of intraepithelial lymphocyte-derived cytokines on intestinal mucosal barrier function. J. Interferon Cytokine Res. 2013;33:551–562. doi: 10.1089/jir.2012.0162. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Hu Y.L., Cui B.A., Zhang H.Y., Kong X.F., Wang D.Y. Immunopotentiating effects of four chinese herbal polysaccharides administered at vaccination in chickens. Poult. Sci. 2007;86:2530–2535. doi: 10.3382/ps.2007-00076. [DOI] [PubMed] [Google Scholar]
- Ren L., Wang X., Li S., Li J., Zhu X., Zhang L., Gao F., Zhou G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018;120:641–649. doi: 10.1016/j.ijbiomac.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Schols H.A., Visser R.G.F., Voragen A.G.J., Schols H.A., Visser R.G.F., Voragen A.G.J. In: recent studies on structures and intestinal immunity modulating activities of pectins and pectic polysaccharides from medicinal herbs. Yamada H., Kiyohara H., Matsumoto T., editors. 2009. Pectins & Pectinases; pp. 293–303. Tokyo, Japan. [Google Scholar]
- Shao Y., Guo Y., Wang Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with salmonellaenterica serovar Typhimurium. Poult. Sci. 2013;92:1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Sun Q., Shang Y., She R., Jiang T., Wang D., Ding Y., Yin J. Detection of intestinal intraepithelial lymphocytes, goblet cells and secretory IgA in the intestinal mucosa during Newcastle disease virus infection. Avian Pathol. 2013;42:541–545. doi: 10.1080/03079457.2013.845292. [DOI] [PubMed] [Google Scholar]
- Sung N.Y., Byun E.H., Kwon S.K., Song B.S., Choi J.I., Kim J.H., Byun M.W., Yoo Y.C., Kim M.R., Lee J.W. Immune-enhancing activities of low molecular weight β-glucan depolymerized by gamma irradiation. Radiat. Phys. Chem. 2009;78:433–436. [Google Scholar]
- Wang D., Ma W., She R., Sun Q., Liu Y., Hu Y., Liu L., Yang Y., Peng K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009;88:967–974. doi: 10.3382/ps.2008-00533. [DOI] [PubMed] [Google Scholar]
- Wang W., Lu J.B., Wang C., Wang C.S., Zhang H.H., Li C.Y., Qian G.Y. Effects of Sargassum fusiforme polysaccharides on antioxidant activities and intestinal functions in mice. Int. J. Biol. Macromol. 2013;58:127–132. doi: 10.1016/j.ijbiomac.2013.03.062. [DOI] [PubMed] [Google Scholar]
- Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. Effect of Astragalus polysaccharide, and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol. 2015;76:188–194. doi: 10.1016/j.ijbiomac.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wei W., Xiao H.T., Bao W.R., Ma D.L., Leung C.H., Han X.Q., Ko C.H., Lau C.B.S., Wong C.K., Fung K.P., Leung P.C., Bian Z.X., Han Q.B. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharmacol. 2016;179:243–252. doi: 10.1016/j.jep.2015.12.060. [DOI] [PubMed] [Google Scholar]
- Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Wang X., Huang J. Toxicological effects of nickel chloride on the cytokine mRNA expression and protein levels in intestinal mucosal immunity of broilers. Environ. Toxicol. 2015;30:1309–1321. doi: 10.1002/tox.22001. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Wu Y., Jiang H., Zhu E., Li J., Wang Q., Zhou W., Qin T., Wu X., Wu B., Huang Y. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 2018;107:1151–1161. doi: 10.1016/j.ijbiomac.2017.09.092. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Zhao H., Luo Y., Lu C., Lin N., Xiao C., Guan S., Guo D.A., Liu Z., Ju D., He X., Lu A. Enteric mucosal immune response might trigger the immunomodulation activity of Ganoderma lucidum polysaccharide in mice. Planta Med. 2009;76:223–227. doi: 10.1055/s-0029-1186055. [DOI] [PubMed] [Google Scholar]