Abstract
Subclinical necrotic enteritis (NE) is primarily caused by the gram-positive bacterium, Clostridium perfringens (Cp). The trend towards removal of in-feed antimicrobials and subsequent increased emergence of infection in poultry has resulted in a wide interest in better understanding of the mechanism behind this disease. The virulence of NE, to a large extent, depends on the virulence of Cp strains. Thus, this study was to assess how 2 different strains of Cp affect performance and gut characteristics of broiler chickens. Ross 308 male broilers (n = 468) were assigned to a 2 × 3 factorial arrangement of treatments with antibiotics (Salinomycin at 72 ppm and zinc bacitracin at 50 ppm −, or +) and challenge (non-challenge, Cp EHE-NE18, or Cp WER-NE36). Oral administration of Eimeria oocysts (day 9) followed by inoculation with 1 mL 108 CFU Cp strains (day 14 and 15) were used to induce NE. Broiler performance was analyzed at day 10, 24, and 35. On day 16, intestinal lesion score and intestinal pH were evaluated and samples of cecal content were analyzed for bacterial counts and short-chain fatty acid concentrations (SCFA). Birds in both challenged groups showed higher feed conversion ratio (FCR), lower weight gain (P < 0.001), increased lesion scores in the jejunum (P < 0.01), and reduced pH in the ileum and cecum (P < 0.01), compared to the non-challenged birds. They also showed decreased numbers of Bacillus spp. (P < 0.001), and Ruminococcus spp. (P < 0.01) in the cecal content. On day 35, the NE36 challenged birds had a lower weight gain (P < 0.001) and higher FCR (P < 0.001) compared to the NE18 challenged birds. Interestingly, cecal Lactobacillus and lactate were increased by the NE challenge (P < 0.001), and to a greater extent in birds challenged with NE36 compared to the NE18 strain (P < 0.001). This study suggests that Cp strains varying in virulence produce different levels of disease in broiler chickens through modulating the gut environment, intestinal microbiota, and SCFA profile to different extents.
key words: Necrotic enteritis, Clostridium perfringens, virulence, microflora, chicken
INTRODUCTION
Necrotic enteritis (NE), an enteric disease caused by the gram-positive anaerobic bacterium, Clostridium perfringens (Cp), is an economically devastating disease in the broiler industry. The costs of this disease in the global poultry industry is estimated to be approximately US$6 billion per annum in production losses (Wade and Keyburn, 2015; Moore, 2016). This bacterium is present in the intestinal tract of healthy chickens, but toxin producing strains can cause a range of histotoxic infections, enteritis, liver and kidney damage, dermatitis, and gas gangrene (Uzal et al., 2014). Among those, NetB producing strains can infect chickens leading to NE outbreak, either in clinical or subclinical form (Keyburn et al., 2008; Wu et al., 2010). Traditionally, antibiotics have been widely used to control this disease; but the ban in Europe and recent voluntarily phase-out of antibiotics in animal feed in many other countries have made controlling the disease a challenging task.
Extracellular toxins can be produced by different Cp strains that are classified as A to G toxinotypes, based on the toxins they produce. All types of the Cp strains produce α-toxin (Smedley et al., 2004; Uzal et al., 2010); however, the strains that produce NetB toxin are classified as belong to toxinotype G (Rood et al., 2018). The NetB toxin is recognized as a pore-forming toxin (Savva et al., 2013) and coded by plasmid genomes (Keyburn et al., 2008). It has been reported that the majority of strains isolated from healthy birds do not carry the NetB gene (Lacey et al., 2016), whereas birds infected with NE usually host NetB producing Cp (Keyburn et al., 2010). Although NetB is renowned for causing NE, other genomic regions have also been identified as contributors towards the virulence of Cp strains (Parreira et al., 2017). The mechanism of virulence of Cp on instigating NE is still largely unknown (Lacey et al., 2016; Prescott et al., 2016) but it has been widely recognized that different strains possess different levels of virulence.
The present study compared the effect of 2 different strains of Cp with divergent virulence levels on the severity of resulting NE in broiler chickens. The 2 Cp toxinotype G strains selected to challenge birds in the study were EHE-NE18 (NE18) and WER-NE36 (NE36). It has been reported that both NE18 and NE36 strains contain NetB gene and are isolates from NE infected chickens in the field (Lacey et al., 2018). It is also been recognized that NE36 shows higher virulence compared to NE18 (Keyburn et al., 2013). Differences have been identified between NE18 and NE36, such as their ability to bind to specific collagens in the extracellular matrix (Wade et al., 2015). Also, according to their genomic sequences, these 2 strains belong to 2 different pathogenic clades of NE-causing Cp strains (Lacey et al., 2018). However, their impact on the chickens with regard to their performance, gut microbial dynamics, and metabolite profile have not been depicted especially under subclinical challenge. We hypothesize that the different Cp strains would induce differing levels of NE severity in chickens, through compromised gut health.
MATERIALS AND METHODS
All procedures of this study were reviewed and approved by the Animal Ethics Committee of the University of New England (17/024). All procedures involving the birds, including health, care, and use of laboratory animals, were fulfilled within the Code of Practice for the Use of Animals for Scientific Purposes issued by the Australian Bureau of Animal Health (NHMRC, 2013).
Experimental Procedures, Design, and Diets
Four hundred sixty-eight male broiler chicks (Ross 308) were obtained from Baiada hatchery in Tamworth, NSW, Australia. Birds were weighed and randomly assigned to 36-floor pens with hardwood shavings used as bedding materials. Room temperature was set at 33°C during the first 3 D of the trial and decreased 3°C every week until 24°C was reached at day 21. The lighting, relative humidity, and temperature followed Ross 308 strain guidelines (Aviagen, 2014). The experiment used a completely randomized design with a 2 × 3 factorial arrangement of treatments with antibiotics (−,/+) and challenge (non-challenged/NE18/NE36). The dietary treatments included a control diet (no additive) and this diet supplemented with antibiotics (Salinomycin at 72 ppm and zinc bacitracin at 50 ppm), fed in pellets form for 3 phases, starter (day 1 to 10), grower (day 11 to 24), and finisher (day 25 to 35). The diet was based on wheat and soybean meal and formulated to meet the nutrient requirements recommended by Evonik Industries (Amino Chick 2.0). Diet composition and the analyzed nutrients contents are presented in Table 1. Control diet (no additive) and control diet supplemented with antibiotics were formulated and mixed for the study. All birds had ad libitum access to feed and water throughout the study.
Table 1.
Composition and nutrient content of feed.
| Ingredient, % | Starter | Grower | Finisher |
|---|---|---|---|
| Wheat | 30.0 | 44.7 | 34.8 |
| Sorghum | 31.0 | 20.0 | 30.0 |
| Soybean meal | 27.1 | 19.1 | 19.0 |
| Canola meal solvent | 2.00 | 5.00 | 4.5 |
| Meat and bone meal | 4.60 | 5.00 | 5.00 |
| Canola oil | 2.44 | 3.90 | 4.80 |
| Limestone | 0.67 | 0.58 | 0.52 |
| Dical Phos 18P/21Ca | 0.65 | 0.43 | 0.34 |
| Salt | 0.11 | 0.12 | 0.13 |
| Na bicarb | 0.16 | 0.13 | 0.12 |
| UNE VM1 | 0.09 | 0.09 | 0.09 |
| UNE TM2 | 0.10 | 0.10 | 0.10 |
| Choline Cl 70% | 0.04 | 0.04 | 0.02 |
| L-lysine HCl 78.4 | 0.41 | 0.35 | 0.32 |
| DL-methionine | 0.31 | 0.24 | 0.25 |
| L-threonine | 0.19 | 0.15 | 0.15 |
| Calculated nutrients | |||
| ME kcal/kg | 3,025 | 3,150 | 3,200 |
| Crude protein % | 23.0 | 22.5 | 21.4 |
| Isoleucine % | 0.98 | 0.87 | 0.87 |
| Digestible Arg % | 1.31 | 1.16 | 1.15 |
| Digestible Lys % | 1.27 | 1.21 | 1.06 |
| Digestible Met % | 0.60 | 0.61 | 0.54 |
| Digestible Thr % | 0.83 | 0.73 | 0.72 |
| Linoleic 18:2% | 1.87 | 2.00 | 1.95 |
Vitamin concentrate (DSM Nutritional Products, Wagga Wagga, NSW, Australia) supplied per kilogram of diet: retinol, 12,000 IU; cholecalciferol, 5,000 IU; tocopheryl acetate, 75 mg, menadione, 3 mg; thiamine, 3 mg; riboflavin, 8 mg; niacin, 55 mg; pantothenate, 13 mg; pyridoxine, 5 mg; folate, 2 mg; cyanocobalamin, 16 μg; biotin, 200 μg; cereal-based carrier, 149 mg; mineral oil, 2.5 mg.
Trace mineral concentrate supplied per kilogram of diet: Cu (sulfate), 16 mg; Fe (sulfate), 40 mg; I (iodide), 1.25 mg; Se (selenate), 0.3 mg; Mn (sulfate and oxide), 120 mg; Zn (sulfate and oxide), 100 mg; cereal-based carrier, 128 mg; mineral oil, 3.75 mg.
Necrotic Enteritis Challenge
On day 9, challenged groups were inoculated with 1 mL per os field strain of Eimeria (Eimeria Pty Ltd, Ringwood, Vic, Australia). Each dose of inoculum consisted of 5,000 sporulated oocysts each of Eimeria maxima and Eimeria acervulina and 2,500 sporulated oocysts of Eimeria brunetti in 1 mL of 1% (w/v) sterile phosphate-buffered saline (PBS). Non-challenged groups were inoculated with sterile PBS as a control. Primary poultry isolates of Cp strains EHE-NE18 and WER-NE36 containing the toxin NetB (Keyburn et al., 2008) were obtained from CSIRO Livestock Industries, Geelong, Australia. The challenge inocula were freshly prepared by growing the bacterial strains separately in 100 mL of sterile thioglycolate (USP alternative, Oxoid, Australia) with added starch (10 g/L) and pancreatic digest of casein (5 g/L); this was incubated overnight at 39°C. Stock cultures of Cp strains were later subcultured in thioglycolate broth followed by cooked meat media (Oxoid, Australia). On day 14 and 15, birds in the challenged group were gavaged with 1 mL per os 108 CFU/mL of Cp with their respective strains, whereas the non-challenged birds were gavaged with sterile thioglycolate broth (Rodgers et al., 2015).
Performance Measurements, Sample Collection, pH Evaluation, and Lesion Scoring
Pen and feed weights were measured on day 0, 10, 24, and 35 and mortality was recorded daily. Average body weight (BW) gain and feed intake (FI) were recorded and FCR was calculated, taking into account bird mortality. Any mortality in the challenge period (day 14 to 16) underwent necropsy to determine if the cause of death is due to NE infection or not.
On day 16, a total of 2 birds from each pen were randomly selected and weighed. All intestinal sections (duodenum, jejunum, and ileum) were excised for lesion scoring, digesta collection, and pH measurements. Intestinal pH values were measured by inserting an EcoScan 5/6 pH meter (Envirosensors spear tip pH probe, Australia) probe into the frontal ileum and cecum sections, respectively. For SCFA analysis, ileal and cecal digesta samples were collected separately into sterile 50 mL containers and stored at −20°C until analysis. Approximately 1 mL of the contents was also placed in sterile 2-mL Eppendorf tubes, snap-frozen in liquid nitrogen and stored at −20°C for DNA extraction. The total length of the small intestine was scored for lesions based on a 0 to 4 scoring system reported by Broussard et al. (1986) where 0 refers to a healthy appearance and 4 a blood-filled intestine with ruptures on the epithelial layer. Two experienced personnel performed lesion scoring with no knowledge of the treatment allocation of the birds.
Cecal Bacterial Enumeration
The DNA was extracted from the frozen cecal samples following the method described by Kheravii et al. (2018). Briefly, approximately 60 mg of frozen cecal samples were added to 300 mg of glass beads (0.1 mm) in a 2 mL Eppendorf tube. A QIAxtractor DNA Reagents, and QIAxtractor DNA plasticware kits (Qiagen, Inc., Doncaster, VIC, Australia) were used for the DNA extraction. Samples were lysed with 300 µ L of Qiagen Lysis Buffer with cells disrupted by shaking the tubes in a bead beater mill (Retsch GmbH & Co, Haan, Germany) for 5 min at 30/S frequency. Samples were then placed in a heating block for 2 h at 55°C followed by 5 min centrifuge at 20,000 × g. The DNA was extracted using an X-tractor gene automated DNA extraction system (Corbett Life Science, Sydney, Australia). Reagents (DXB, DXW, DXF, and DXE) were placed in their specific locations in the robotics machine together with 200 µ L of the lysate transferred automatically into loading block. Then 400 μL of the binding buffer (DXB) was added to the 200 µ L lysate and incubated for 6 min, and then 500 µ L of the lysed samples were transferred into capture columns and vacuumed at 30 kPa for 3 min. Following this 600 µ L DXW was transferred to the capture columns and vacuumed for 30 kPa for 2 min, 600 µ L DXF was transferred to the columns and vacuumed at 35 kPa for 1 min, and DNA was dried by vacuuming again at 25 kPa for 5 min. Finally, an elution block was used to elute the extracts by addition of 60 µ L DXE and the samples were vacuumed at 30 kPa for 2 min. The resulting DNA samples were measured on a Nanodrop 8000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) for assessment of quantity and purity. DNA with ratios of A260/A280 being >1.8 were considered of high purity and were stored at −20°C.
The extracted cecal DNA was diluted 20 times in nuclease-free water and the quantitative real-time polymerase chain reaction (PCR) was performed to quantify 6 bacterial groups with a real-time PCR system Rotorgene 6000 (Corbett, Sydney, Australia). The SYBRGreen containing Mix (SensiMix SYBR No-Rox, Bioline, Sydney, Australia) was used for Bacillus, Bacteroides, Bifidobacteria, Lactobacilli, and Ruminococcus and for Cp, SensiFAST Probe SYBR No-ROX (Bioline, Sydney, Australia) was used. The specific 16S rRNA primers used for these groups of bacteria are shown in Table 2.
Table 2.
Primers used for the qPCR analysis of different bacteria groups.
| Target group | Primer/probe sequence | Amplicon length (bp) | Annealing temp. (C°) | Reference |
|---|---|---|---|---|
| Bacillus spp. | F-GCA ACG AGC GCA ACC CTT GA | 92 | 63 | (Zhang et al., 2015) |
| R-TCA TCC CCA CCT TCC CC GGT | ||||
| Bacteroides spp. | F-GAG AGG AAG GTC CCC CAC | 108 | 63 | (Layton et al., 2006) |
| R-CGC TAC TTG GCT GGT TCA G | ||||
| Bifidobacterium spp. | F-GCG TCC GCT GTG GGC | 106 | 63 | (Requena et al., 2002) |
| R-CTT CTC CGG CAT GGT GTT G | ||||
| Clostridium perfringens | F- GCA TAA CGT TGA AAG ATG G | 120 | 60 | (Wise and Siragusa, 2007) |
| R- CCT TGG TAG GCC GTT ACC C | ||||
| TaqMan probe: 5′-FAM-TCA TCA TTC AAC CAA AGG AGC AAT CC-TAMRA-3′ | ||||
| Lactobacillus spp. | F-CAC CGC TAC ACA TGG AG | 186 | 63 | (Wise and Siragusa, 2007) |
| R-AGC AGT AGG GAA TCT TCC A | ||||
| Ruminococcus spp. | F-GGC GGC YTR CTG GGC TTT | 157 | 63 | (Ramirez-Farias et al., 2008) |
| R-CCA GGT GGA TWA CTT ATT GTG TTA A |
Cecal SCFAs
The method described by Jensen et al. (1995) was used for the SCFA analysis. In brief, approximately 1 g of cecal digesta was weighed and 1 mL of internal standard (0.01 Methyl-butyric acid) was added. The solution was vortexed and centrifuged for 20 min at 15,000 × g at 5°C. An aliquot of 1 mL supernatant was transferred to 8 mL vials, and 0.5 mL of concentrated HCl (36%) and 2.5 mL of ether were added. The mixture was vortexed, centrifuged at 1,000 × g for 15 min in 5°C. From the resulting supernatant, 400 µ L of it was then mixed with 40 µ L N-tert-butyldimethylsilyl-N-methyl trifluoroacetamide. Samples were then vortexed and heated (at 80°C) for 20 min. The vials were then kept in room temperature for 48 h before being analyzed on a Varian CP3400 CX gas Chromatograph (Varian Analytical Instruments, Palo Alto, CA, USA). SCFA concentrations are expressed as μmol/g digesta.
Data Analysis
All the data derived were checked for normal distribution prior to conducting statistical analyses. The statistical analysis was carried out with the GLM of SPSS statistics 24 to assess the main effects (challenge and antibiotics) and interactions. Intestinal lesion scoring data was analyzed by the non-parametric Kruskal–Wallis test as the data was not normally distributed. Tukey's mean separation test was used to make pairwise comparisons among treatments when interaction was significant. Statistical significance was declared at P < 0.05. Correlations between parameters were examined using the Pearson's product moment correlation coefficient test.
RESULTS
Bird Performance
Growth performance of broilers was affected by the strain of Cp and the presence of antibiotics at day 24 and 35 of the trial (Table 3). Antibiotics improved BW at day 24 (P < 0.01) in all groups. A challenge × antibiotic interactions was observed for BW (P < 0.05) at day 35, where antibiotics improved BW in both challenged groups (NE18 and NE36) but did not significantly affect the non-challenged birds. At day 35, antibiotic supplementation as a main effect increased FI regardless of the challenge (P < 0.01), while the challenge significantly decreased FI at day 24 (P < 0.001) and 35 (P < 0.05) in both challenged groups (NE18 and NE36), compared to those in the non-challenged groups. On day 24 and 35, birds challenged with NE36 showed higher FCR (P < 0.001) compared to birds challenged with NE18 and both infected groups had higher FCR (P < 0.001) compared to the non-challenged birds. However, antibiotics treated birds tended to have a decreased FCR (P = 0.074). No significant effect of antibiotic supplementation and challenge was observed in the first 10 D of the experiment (P > 0.05). No interactions between antibiotic and challenge were observed for FI and FCR in any period of the experiment (P > 0.05).
Table 3.
Performance of chickens in response to the challenge with 2 strains of Clostridium perfringens and supplementation of antibiotics during 0 to 35 D of age.
| Performance |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 to 10 |
0 to 24 |
0 to 35 |
||||||||
| Challenge | Antibiotics | BW | FI | FCR | BW | FI | FCR | BW | FI | FCR |
| Non-challenge | No | 284 | 284 | 1.010 | 1,366 | 1,705 | 1.247 | 2,529a | 3,363 | 1.330 |
| Yes | 282 | 282 | 1.005 | 1,356 | 1,697 | 1.251 | 2,551a | 3,436 | 1.347 | |
| NE-182 | No | 284 | 285 | 0.998 | 1,103 | 1,431 | 0.998 | 2,258c,d | 3,216 | 1.424 |
| Yes | 292 | 291 | 1.027 | 1,193 | 1,527 | 1.027 | 2,450a,b | 3,356 | 1.373 | |
| NE-363 | No | 293 | 293 | 1.023 | 1,052 | 1,479 | 1.023 | 2,157d | 3,173 | 1.475 |
| Yes | 290 | 291 | 1.017 | 1,180 | 1,528 | 1.017 | 2,327b,c | 3,340 | 1.435 | |
| Main effects | ||||||||||
| Antibiotic1 | ||||||||||
| No | 288 | 287 | 1.009 | 1,174b | 1,538 | 1.323a | 2,315 | 3,249b | 1.403 | |
| Yes | 287 | 289 | 1.012 | 1,243a | 1,590 | 1.292b | 2,443 | 3,380a | 1.385 | |
| Challenge | ||||||||||
| Non-challenged | 283 | 289 | 1.007 | 1,361a | 1,701a | 1.250c | 2,540 | 3,396a | 1.338c | |
| NE18 | 288 | 291 | 1.002 | 1,148b | 1,479b | 1.308b | 2,354 | 3,289b | 1.398b | |
| NE36 | 290 | 284 | 1.025 | 1,116b | 1,503b | 1.362a | 2,242 | 3,257b | 1.455a | |
| P- value | ||||||||||
| Antibiotic | 0.693 | 0.693 | 0.666 | 0.010 | 0.162 | 0.032 | <0.001 | 0.002 | 0.074 | |
| Challenge | 0.411 | 0.409 | 0.091 | 0.001 | 0.001 | 0.001 | <0.001 | 0.016 | < 0.001 | |
| Antibiotic × challenge | 0.610 | 0.609 | 0.754 | 0.084 | 0.453 | 0.170 | 0.013 | 0.636 | 0.091 | |
Salinomycin (72 ppm) and zinc bacitracin (50 ppm).
Clostridium perfringens strains (108 CFU/mL).
Means sharing the same superscripts are not significantly different from each other at P < 0.05. BW: Body Weight gain (g/bird), FI: Feed intake (g/bird), FCR: Feed conversion ratio.
Lesion Score, Intestinal pH, and Mortality
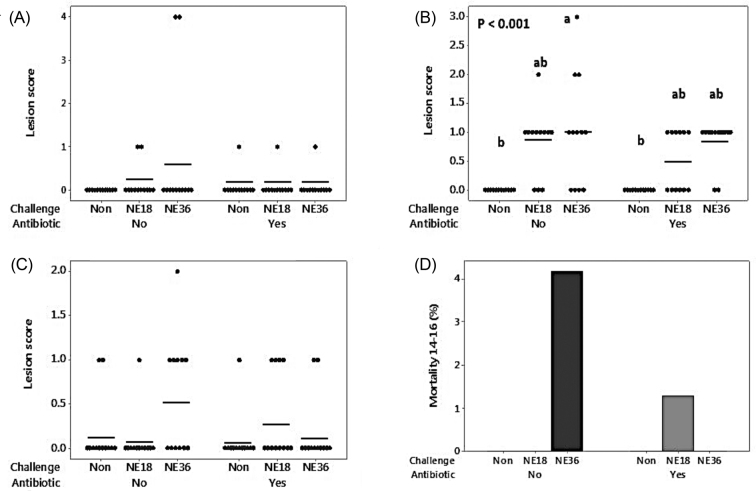
Figure 1 shows gross lesions in 3 sections of the intestines. The NE36 challenged birds fed with control diets produced significantly higher lesions in the jejunum compared to non-challenged groups (P < 0.01). The other sections of intestine investigated were not significantly affected by either challenge or antibiotics (P > 0.05). During day 14 to 16, a total of 4 mortality were recorded and postmortem examination was performed. One bird from the NE18 challenged group supplemented with antibiotics died at day 15 from NE with damaged intestine. At day 16, a total of 3 birds died from the NE36 challenged groups with no supplemented antibiotics. One bird had an enlarged pale liver that was an untypical NE sign. The other 2 birds had no lesion or other NE-associated signs. The birds had no digesta in the intestine which is the indication that these 2 birds suffered from anorexia. These dead birds were particularly small and weak compared to other birds in the treatment. Statistically, no significant difference was observed in mortality during the challenge period (P > 0.05). Cecal and ileal pH was reduced (P < 0.001 and 0.01, respectively) in both groups of challenged birds (Table 4).
Figure 1.
Individual and average intestinal lesion scores of all sampled birds (day 16) challenged with 2 strains of Clostridium perfringens (NE18 and NE36). (A) lesion score in duodenum; (B) lesion score in jejunum; (C) lesion score in ileum; (D) Mortality of birds at day 14 to 16.
Table 4.
Intestinal pH in responses to the challenge with Clostridium perfringens strains NE18 and NE36 and antibiotic supplementation in chickens at day 16.
| pH |
||
|---|---|---|
| Main effects | Cecum | Ileum |
| Antibiotic1 | ||
| No | 6.40 | 5.81 |
| Yes | 6.44 | 5.86 |
| Challenge | ||
| Non-challenge | 6.55a | 5.97a |
| NE182 | 6.35b | 5.80b |
| NE363 | 6.36b | 5.74b |
| P-value | ||
| Antibiotic | 0.338 | 0.387 |
| Challenge | 0.001 | 0.004 |
| Antibiotic × challenge | 0.481 | 0.926 |
Salinomycin (72 ppm) and zinc bacitracin (50 ppm).
Clostridium perfringens strains (108 CFU/mL).
Means sharing the same superscripts are not significantly different from each other at P < 0.05.
Gut Microflora Changes in Responses to Cp Challenge
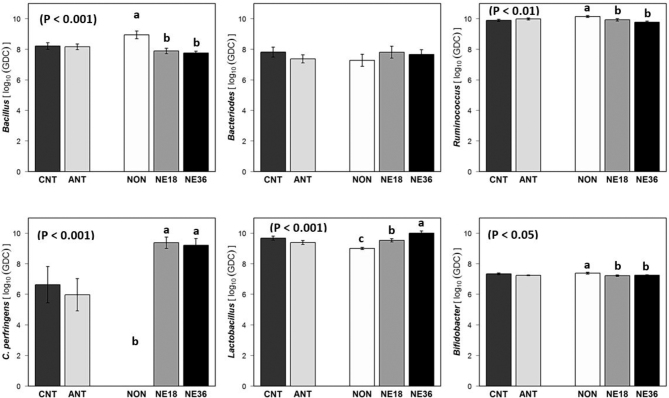
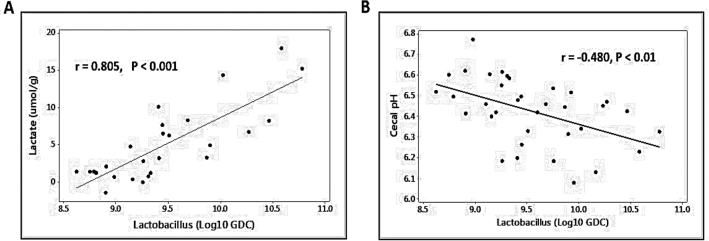
Challenged birds had lower Bacillus (P < 0.001),Ruminococcus (P < 0.01), and Bifidobacterium (P < 0.05) counts at day 16 (Figure 2). However, both challenged groups had higher Cp (P < 0.01) and Lactobacillus (P < 0.001) counts compared to the non-challenged birds. No significant difference in cecal Bacteroides was observed between different treatments. The number of Lactobacilli in the ceca tended to be lower in birds fed with the antibiotic supplemented diets compared to those without (P = 0.057). No significant antibiotic × challenge was observed for any bacterial species measured (P > 0.05). A positive correlation (r = −0.480, P < 0.01) was observed between cecal Lactobacillus numbers and cecal pH which is illustrated in Figure 3 B.
Figure 2.
Bacterial levels (Log10 copies of respective bacterial genome) in cecal content of birds in response to challenge with NE18 and NE36 strains of Clostridium perfringens and antibiotic supplementation at day 16. GDC: Genomic DNA copies/g; CNT: No antibiotic supplementation; ANT: Antibitotic supplementation (Salinomycin at 72 ppm and zinc bacitracin at 50 ppm); NON: Non-challenged; NE18: birds challenged with NE18 strain; NE36: birds challenged with NE36 strain.
Figure 3.
(A) A strong positive correlation between cecal Lactobacillus level and lactate concentration in cecum contents; (B) A negative correlation between cecal Lactobacillus level and cecal pH.
Cecal SCFAs and Correlations with Cecal Bacterial Population
The SCFA concentrations in cecal digesta (μmol/g) are shown in Table 5. Broilers challenged with NE36 strain had increased concentrations of lactate (P < 0.001) and propionate (P < 0.01) compared to those challenged with the NE18 strain and the control birds. Both NE challenged groups had higher isobutyrate (P < 0.01) levels compared to the non-challenged birds. Isovalerate concentrations were significantly higher in NE18 challenged birds compared to the non-challenged groups. Furthermore, decreased concentrations of acetate (P < 0.05) and butyrate (P < 0.01) were observed in the challenged bird. Supplementation of antibiotics reduced propionate (P < 0.05) and lactate (P < 0.05) concentrations. There was no significant challenge × antibiotic interaction observed for any SCFA measured.
Table 5.
Concentration of short-chain fatty acids (μmol/g) in cecal content in responses to the challenge with Clostridium perfringens strains NE18 and NE36 and antibiotic supplementation in chickens at day 16.
| Cecal SCFA (μmol/g) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Main effect | Acetate | Propionate | Isobutyrate | Butyrate | Isovalerate | Valerate | Lactate | Succinate |
| Antibiotic1 | ||||||||
| No | 88.7 | 7.14a | 0.96 | 17.2 | 0.36 | 1.90 | 6.29a | 7.07 |
| Yes | 91.3 | 4.86b | 0.80 | 19.5 | 0.23 | 1.88 | 3.26b | 8.07 |
| Challenge | ||||||||
| Non-challenged | 101.0a | 4.55b | 0.60b | 24.6a | 0.15b | 1.24c | 1.35b | 6.43 |
| NE182 | 83.4b | 5.36b | 1.02a | 16.1b | 0.40a | 1.84b | 3.38b | 8.30 |
| NE363 | 85.4b | 8.07a | 1.02a | 14.6b | 0.35a,b | 2.60a | 10.7a | 8.92 |
| P-value | ||||||||
| Antibiotic | 0.628 | 0.011 | 0.149 | 0.300 | 0.106 | 0.922 | 0.010 | 0.802 |
| Challenge | 0.021 | 0.005 | 0.002 | 0.002 | 0.027 | 0.001 | 0.001 | 0.368 |
| Antibiotic × challenge | 0.916 | 0.102 | 0.088 | 0.921 | 0.544 | 0.652 | 0.333 | 0.269 |
Salinomycin (72 ppm) and zinc bacitracin (50 ppm).
Clostridium perfringens strains (108 CFU/mL).
Means sharing the same superscripts are not significantly different from each other atP < 0.05.
Correlations between bacterial populations and SCFA concentrations or cecal pH were tested and the resuts are shown in Table 6. A strong positive correlation between lactate concentration and C. perfringnes (P < 0.01) and Lactobacillus (P < 0.001) population was observed. Acetate and butyrate, on the other hand, showed negative correlation with C. perfringnes (P < 0.01 and 0.001, respectively) and Lactobacillus (P < 0.05 and 0.01, respectively). Positive correlations were present for propionate (P < 0.05), valerate (P < 0.001), isovalerate, and isobutyrate (P < 0.01) with C. perfringens, and valerate (P < 0.05), isovalerate, and isobutyrate (P < 0.01) were negatively correlated with Bacillus. Furthermore, succinate and isobutyrate were negatively correlated with Bifidobacteria (P < 0.01 and 0.01, respectively) and lactate, propionate, valerate, and isovalerate negatively correlated with Ruminococuus (P < 0.05, 0.05, 0.05, and 0.01, respectively). Cecal pH was negatively correlated with only C. perfringnes (P < 0.001) and Lactobacillus (P < 0.01).
Table 6.
Pearson correlations between cecal SCFA concentrations or pH and bacterial populations (log10 GDC1).
| Acetate | Lactate | Propionate | Butyrate | Valerate | Succinate | Isovalerate | Isobutyrate | Cecal pH | |
|---|---|---|---|---|---|---|---|---|---|
| Bacillus | 0.058 | −0.015 | −0.270 | 0.481** | −0.420* | 0.046 | −0.479** | −0.495** | 0.321 |
| Bacteroides | −0.187 | 0.008 | 0.211 | −0.214 | 0.068 | −0.078 | 0.257 | 0.174 | 0.097 |
| Ruminococcus | 0.196 | −0.537* | −0.381* | 0.305 | −0.447* | −0.173 | −0.424** | −0.651 | 0.300 |
| C. perfringens | −0.486** | 0.392** | 0.427* | −0.567*** | 0.573*** | 0.206 | 0.501** | 0.603** | −0.606*** |
| Lactobacillus | −0.568* | 0.806*** | 0.423* | −0.468** | 0.520** | 0.291 | 0.046 | 0.317 | −0.480** |
| Bifidobacters | 0.195 | −0.192 | −0.192 | 0.173 | −0.174 | −0.402** | −0.292 | −0.382** | 0.239 |
Stars (*) donate the strength of the significance: P < 0.001***, 0.001 < P < 0.01**, 0.01 < P < 0.05*.
GDC: genomic DNA copy number.
DISCUSSION
In the current study, the Cp strains, NE18 and NE36, introduced different levels of NE severity in broiler chickens. Both strains produced subclinical NE; however, NE36 had a more severe impact on performance resulting in impaired FCR, and decreased BW and FI. The effect of NE36 on lesion scores in the jejunum was significantly higher than the non-challenged birds. Cecal bacterial population and concentration of some cecal SCFAs were altered by at least NE36. The results of the study accept the hypothesis that Cp strains with different virulence introduce different levels of NE severity in the chickens by compromising gut health, leading to poorer bird performance.
The introduction of a subclinical NE challenge was successful in this study, based on the observation of typical signs of subclinical NE such as depressed performance, mild lesions in the small intestine, and no NE-related mortality. The disrupted nutrient absorption and utilization inflicted by damaged intestinal mucosa due to Eimeria and Cp infections could be responsible for reduced BW and impaired FCR as has been shown before (Attia et al., 2012; Rodrigues et al., 2017; Xue et al., 2018). The reduced FI in NE challenged birds is believed to be correlated to the immune system activation, as activated cytokines can reduce FI and consequently lower BW in infected birds (Dantzer, 2004). In this study, the NE36 strain significantly reduced BW and increased FCR compared to the NE18 strain. The antibiotic × challenge interaction observed on BW confirmed that antibiotic supplementation could only positively affect BW in the presence of NE challenge. The general lack of an antibiotic effect is in agreement with other studies that have shown that well-nourished healthy broilers reared under clean and disinfected conditions have minimal response to antibiotic supplements (Toghyani et al., 2010; Erener et al., 2011). Positive effects of antibiotics could also be due to the suppressive effects of salinomycin onEimeria decreasing the intestinal damage caused by oocytes, thus reducing the chance of birds to be predisposed to NE. Nevertheless, the numeric improvement in BW and FCR and a significant increase in FI in chickens fed with antibiotics confirms the overall positive impact of antibiotic supplementation, as illustrated by a number of other studies (Ocak et al., 2008; Crisol-Martínez et al., 2017).
It is widely accepted that intestinal microflora and their metabolic activity have a significant impact on broiler health and performance. Gut microbiota are key regulators of immune functions and inflammatory responses during disease outbreaks (Prenderville et al., 2015). Thus, they play a critical role in the occurrence and severity of the disease and resilience of birds towards the infections. In the present study, it is interesting that higher levels of cecal Lactobacillus were observed in the challenged birds compared to the control. Lactobacillus is the most abundant bacterial group accounting for 98% of total bacteria in the upper gastrointestinal tract (GIT) (Gong et al., 2007; Stanley et al., 2012), and some strains of the species are widely used as probiotics to inhibit the growth of Cp through the production of organic acids and bacteriocins (Caly et al., 2015). Intuitively, one would expect that increased Lactobacillus in the intestine may indicate a healthier gut, whereas it does not seem to be the case according to the findings of the current study together with others. It is therefore proposed that the increase of Lactobacillus in challenged birds could be attributed to the following factors: (1) possible perturbations of intestinal nutrient supply by the disease challenge and complex nutritional requirements by bacteria including Lactobacillus; (2) possible boost of Lactobacillus in the intestine by the recovery of gut health of the birds following NE challenge; and (3) over-influx of upper intestinal content, i.e., in ileum, where Lactobacilli are the main bacterial population, to the ceca caused by NE challenge. The microbiota composition produced by challenge could affect the available nutrients for bacteria including Lactobacillus thus the dynamics of its population in the intestine (Stanley et al., 2012). On the other hand, Latorre et al. (2018) proposed that an increased abundance of Lactobacillus may be related to the rapid recovery of morbid birds in the challenged group. This may be the case in some circumstances as the recovery of the birds from NE infection is very fast with lesions caused by NE healing within 5 D of Cp challenge (Keerqin et al., 2017).
Intestinal microbiota modulation also changes the pattern of SCFA concentrations in the gut, which can affect intestinal function and integrity (Meimandipour et al., 2010). As mentioned earlier, an over-influx of upper GIT content to the ceca in NE challenged birds may partly answer the question as to why there was an abundance of Lactobacillus in NE challenged birds. Lactobacillus is one of the lactic acid producing bacteria and the main end product of these bacteria is lactic acid (Garvie, 1980; Alvarez-Sieiro et al., 2016) that has the ability to produce peristalsis, which in turn can increase nutrient transit rate through the intestine (Saunders and Sillery, 1982). Therefore, the upper GIT content may bring more bacteria, mainly species of Lactobacillus, into the ceca. In the present study, the NE36 challenged birds had the highest concentration of lactate, which is in accordance with the changes of Lactobacillus abundance. Additionally, among the correlations observed between bacteria population and the SCFAs concentrations Lactobacillus and Lactate showed the strongest positive correlation (Figure 3 A) that could indicate the relationship between these two parameters. Other studies have previously observed increased abundance of Lactobacillus in NE challenged birds (M'Sadeq et al., 2015; Lin et al., 2017; Latorre et al., 2018). Conversely, it has also been reported that NE challenge leads to lower abundance of Lactobacillus (Qing et al., 2017; Kheravii et al., 2018) or the overall Lactobacillus numbers remain stable (Mikkelsen et al., 2009; Wu et al., 2010; Stanley et al., 2012). Our findings, together with those from other studies (Engberg et al., 2000; Crisol-Martínez et al., 2017), support the notion of a suppressed growth rate associated with the upsurge of Lactobacillus numbers. The current study seems to suggest that the level of Lactobacillus in GIT especially in the case of NE challenge is not necessarily the indication of the health status of chickens, as one would expect. However, with the converse results presented in different studies, further research is needed to understand the relationship between NE and Lactobacillus population.
Ruminococcus is also one of the most dominant bacteria prevalent in the ceca (Park et al., 2017). It plays an important role in digestion and can produce bacteriocins that may control the growth of Cp (Crost et al., 2011). In this study, the cecal Ruminococcus population decreased in the NE challenge birds. We speculate that lower numbers of this bacterium could be a sign of impaired digestion and immune defense in the gut. Ruminococcus produces butyrate as a primary end product (Takahashi et al., 2016). The role of this fatty acid in energy metabolism and intestinal epithelial cell proliferation is well documented (Rinttilä and Apajalahti, 2013); it can increase arterial blood flow that is linked to improved nutrient absorption (Whitehead et al., 1986), stimulates host defense peptides, and has anti-inflammatory actions on the intestine epithelium (Sunkara et al., 2011; Celasco et al., 2014). This suggests that lower levels of Ruminococcus and thus limited available butyrate in the intestine may have led to poorer performance in the challenged birds.
The extracellular toxins produced by Cp can disrupt the phospholipid bilayer in cells, causing inundation of ions, which, in turn, may cause osmotic balance change and consequential cell lysis (Yan et al., 2013). This intestinal damage will affect the ability of birds to uptake nutrients, instead of making them available for the use of microorganisms in the intestine (Choct, 2009). It is suggested that different strains of Cp affect the performance differently through modulating gut microbiota and immunity of chickens under challenge. The more virulent strains show a more severe impact on performance and negatively affected microbiota compared to the less virulent strains. However, it should be noted that these negative results can also be partially due to the coccoidal infections prior to the Cp challenge, as coccidia is capable of producing damage in the gut that can affect BW and FCR by microbiota modulation, nutrient uptake, and immune responses (Tan et al., 2014). Essentially, the administration of Eimeria spp. in the NE challenge model was to predispose birds for the effective infection by Cp. Therefore, it is expected that coccoidal infection would affect the birds negatively. However, more detailed investigations on the mechanism that underlies the virulence and how the Cp strains differ in their impact on the gut health and integrity thus the performance of the chickens is warranted.
In conclusion, the broilers infected with NE18 and NE36 showed subclinical NE, and NE36 impaired performance more severely than NE18. It has been speculated that the impact of the Cp strains on the disease and performance of the broiler chickens is due to their influence on the gut microfloral dynamics and thus the profile of bacterial metabolites such as SCFAs in the intestine. The changes of particular bacterial groups by the Cp challenge may be the indications of gut health under certain conditions. However, further data need to be collected for robust proof of how these bacterial changes affect the intestinal health of chickens.
ACKNOWLEDGMENTS
The authors thank Petrina Young of Eimeria Pty Ltd for providing Eimeria species, Robert Moore for providing Clostridium perfringens EHE-18 and WER-36 strains, and Shuyu Song for her help and guidance with the lab work.
REFERENCES
- Alvarez-Sieiro P., Montalbán-López M., Mu D., Kuipers O.P. Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 2016;100:2939–2951. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y., Ellakany H., El-Hamid A., Bovera F., Ghazaly S. Control of Salmonella enteritidis infection in male layer chickens by acetic acid and/or prebiotics, probiotics and antibiotics. Arch. Geflügelk. 2012;76:239–245. [Google Scholar]
- Aviagen . Aviagen; Huntsville, AL, USA: 2014. Ross Broiler Management Handbook. [Google Scholar]
- Broussard C.T., Hofacre C.L., Page R.K., Fletcher O.J. Necrotic enteritis in cage-reared commercial layer pullets. Avian Dis. 1986;30:617–619. [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celasco G., Moro L., Aiello C., Mangano K., Milasi A., Quattrocchi C., Di Marco R. Calcium butyrate: anti-inflammatory effect on experimental colitis in rats and antitumor properties. Biomed. Rep. 2014;2:559–563. doi: 10.3892/br.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choct M. Managing gut health through nutrition. Br. Poult. Sci. 2009;50:9–15. doi: 10.1080/00071660802538632. [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Crost E.H., Ajandouz E.H., Villard C., Geraert P.A., Puigserver A., Fons M. Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie. 2011;93:1487–1494. doi: 10.1016/j.biochi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- Erener G., Ocak N., Altop A., Cankaya S., Aksoy H.M., Ozturk E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian-Aust. J. Anim. Sci. 2011;24:1128–1135. [Google Scholar]
- Garvie E.I. Bacterial lactate dehydrogenases. Microbiol. Rev. 1980;44:106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., Yang C., Han Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 2007;59:147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- Jensen M., Cox R., Jensen B.B. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. J. Anim. Sci. 1995;61:293–304. [Google Scholar]
- Keerqin C., Morgan N., Wu S., Svihus B., Choct M. Reintroduction of microflora from necrotic enteritis-resistant chickens reduces gross lesions and improves performance of necrotic enteritis-challenged broilers. J. Appl. Poult. Res. 2017;26:449–457. [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Di Rubbo A., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Portela R.W., Sproat K., Ford M.E., Bannam T.L., Yan X., Rood J.I., Moore R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 2013;44:54. doi: 10.1186/1297-9716-44-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Yan X.X., Bannam T.L., Van Immerseel F., Rood J.I., Moore R.J. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 2010;41:21. doi: 10.1051/vetres/2009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Effect of oat hulls as a free choice feeding on broiler performance, short chain fatty acids and microflora under a mild necrotic enteritis challenge. Anim. Nut. 2018;4:65–72. doi: 10.1016/j.aninu.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J.A., Allnutt T.R., Vezina B., Van T.T.H., Stent T., Han X., Rood J.I., Wade B., Keyburn A.L., Seemann T. Whole genome analysis reveals the diversity and evolutionary relationships between necrotic enteritis-causing strains of Clostridium perfringens. BMC Genomics. 2018;19:379. doi: 10.1186/s12864-018-4771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J.A., Johanesen P.A., Lyras D., Moore R.J. Genomic diversity of necrotic enteritis-associated strains of Clostridium perfringens: a review. Avian Pathol. 2016;45:302–307. doi: 10.1080/03079457.2016.1153799. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F., Hernandez X., Kwon Y.M., Ricke S.C. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton A., McKay L., Williams D., Garrett V., Gentry R., Sayler G. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S.-B., Swick R.A., Choct M. Dietary acylated starch improves performance and gut health in necrotic enteritis challenged broilers. Poult. Sci. 2015;94:2434–2444. doi: 10.3382/ps/pev219. [DOI] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A., Azhar K., Hair-Bejo M., Kabeir B., Javanmard A., Muhammad Anas O., Yazid A. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Mikkelsen L., Vidanarachchi J., Olnood C., Bao Y., Selle P., Choct M. Effect of potassium diformate on growth performance and gut microbiota in broiler chickens challenged with necrotic enteritis. Br. Poult. Sci. 2009;50:66–75. doi: 10.1080/00071660802613252. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- NHMRC Australian code for the care and use of animals for scientific purposes 8th Edition. 2013. https://www.deakin.edu.au/__data/assets/pdf_file/0011/536348/australian-code-for-the-care-and-use-of-animals-for-scientific-purposes-2013.pdf
- Ocak N., Erener G., Burak Ak F., Sungu M., Altop A., Ozmen A. Performance of broilers fed diets supplemented with dry peppermint (Mentha piperita L.) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech J. Anim. Sci. 2008;53:169. [Google Scholar]
- Park S.H., Kim S.A., Rubinelli P.M., Roto S.M., Ricke S.C. Microbial compositional changes in broiler chicken cecal contents from birds challenged with different Salmonella vaccine candidate strains. Vaccine. 2017;35:3204–3208. doi: 10.1016/j.vaccine.2017.04.073. [DOI] [PubMed] [Google Scholar]
- Parreira V.R., Ojha S., Lepp D., Gohari I.M., Zhou H., Susta L., Gong J., Prescott J.F. Necrotic enteritis locus 1 diguanylate cyclase and phosphodiesterase (cyclic-di-GMP) gene mutation attenuates virulence in an avian necrotic enteritis isolate of Clostridium perfringens. Vet. Microbiol. 2017;208:69–73. doi: 10.1016/j.vetmic.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Prenderville J.A., Kennedy P.J., Dinan T.G., Cryan J.F. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38:13–25. doi: 10.1016/j.tins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Qing X., Zeng D., Wang H., Ni X., Liu L., Lai J., Khalique A., Pan K., Jing B. Preventing subclinical necrotic enteritis through Lactobacillus johnsonii BS15 by ameliorating lipid metabolism and intestinal microflora in broiler chickens. AMB Express. 2017;7:139. doi: 10.1186/s13568-017-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- Requena T., Burton J., Matsuki T., Munro K., Simon M.A., Tanaka R., Watanabe K., Tannock G.W. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 2002;68:2420–2427. doi: 10.1128/AEM.68.5.2420-2427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance1. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Svihus B., Bedford M., Gous R., Choct M. Intermittent lighting improves resilience of broilers during the peak phase of sub-clinical necrotic enteritis infection. Poult. Sci. 2017;97:438–446. doi: 10.3382/ps/pex315. [DOI] [PubMed] [Google Scholar]
- Rood J.I., Adams V., Lacey J., Lyras D., McClane B.A., Melville S.B., Moore R.J., Popoff M.R., Sarker M.R., Songer J.G. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10. doi: 10.1016/j.anaerobe.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D., Sillery J. Effect of lactate and H+ on structure and function of rat intestine. Dig. Dis. Sci. 1982;27:33–41. doi: 10.1007/BF01308119. [DOI] [PubMed] [Google Scholar]
- Savva C.G., da Costa S.P.F., Bokori-Brown M., Naylor C.E., Cole A.R., Moss D.S., Titball R.W., Basak A.K. Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens. J. Biol. Chem. 2013;288:3512–3522. doi: 10.1074/jbc.M112.430223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley J., Fisher D., Sayeed S., Chakrabarti G., McClane B. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Sunkara L.T., Achanta M., Schreiber N.B., Bommineni Y.R., Dai G., Jiang W., Lamont S., Lillehoj H.S., Beker A., Teeter R.G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., Inatomi O., Bamba S., Andoh A., Sugimoto M. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- Tan J., Applegate T.J., Liu S., Guo Y., Eicher S.D. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014;112:1098–1109. doi: 10.1017/S0007114514001846. [DOI] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Gheisari A., Ghalamkari G., Mohammadrezaei M. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita) Livest. Sci. 2010;129:173–178. [Google Scholar]
- Uzal F., Vidal J., McClane B., Gurjar A. Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxinology J. 2010;2:24. [PMC free article] [PubMed] [Google Scholar]
- Uzal F.A., Freedman J.C., Shrestha A., Theoret J.R., Garcia J., Awad M.M., Adams V., Moore R.J., Rood J.I., McClane B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014;9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wade B., Keyburn A.L., Seemann T., Rood J.I., Moore R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015;180:299–303. doi: 10.1016/j.vetmic.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Whitehead R., Young G., Bhathal P. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215) Gut. 1986;27:1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise M., Siragusa G. Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. App. Microbiol. 2007;102:1138–1149. doi: 10.1111/j.1365-2672.2006.03153.x. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Rodgers N., Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Xue G., Barekatain R., Wu S., Choct M., Swick R. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 2018;0:1–8. doi: 10.3382/ps/pex444. [DOI] [PubMed] [Google Scholar]
- Yan X.X., Porter C.J., Hardy S.P., Steer D., Smith A.I., Quinsey N.S., Hughes V., Cheung J.K., Keyburn A.L., Kaldhusdal M., Moore R.J., Bannam T.L., Whisstock J.C., Rood J.I. Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens. MBio. 2013;4:e00019. doi: 10.1128/mBio.00019-13. e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen D., Yu B., He J., Yu J., Mao X., Wang J., Luo J., Huang Z., Cheng G. Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J. Anim. Sci. 2015;93:2967–2976. doi: 10.2527/jas.2014-8820. [DOI] [PubMed] [Google Scholar]