Abstract
Mycoplasma gallisepticum (MG) infection produces a profound inflammatory response in the respiratory tract and evade birds' immune recognition to establish a chronic infection. Previous reports documented that the flavonoid baicalin possess potent anti-inflammatory, and antioxidant activities. However, whether baicalin prevent immune dysfunction is largely unknown. In the present study, the preventive effects of baicalin were determined on oxidative stress generation and apoptosis in the spleen of chickens infected with MG. Histopathological examination showed abnormal morphological changes including cell hyperplasia, lymphocytes depletion, and the red and white pulp of spleen were not clearly visible in the model group. Oxidative stress-related parameters were significantly (P < 0.05) increased in the model group. However, baicalin treatment significantly (P < 0.05) ameliorated oxidative stress and partially alleviated the abnormal morphological changes in the chicken spleen compared to model group. Terminal deoxynucleotidyl transferase–mediated dUTP nick endlabeling assay results, mRNA, and protein expression levels of mitochondrial apoptosis-related genes showed that baicalin significantly attenuated apoptosis. Moreover, baicalin restored the mRNA expression of mitochondrial dynamics-related genes and maintain the balance between mitochondrial inner and outer membranes. Intriguingly, the protective effects of baicalin were associated with the upregulation of nuclear factor erythroid 2–related factor 2 (Nrf2)/Heme oxygenase-1 (HO-1) pathway and suppression of nuclear factor-kappa B (NF-κB) pathway in the spleen of chicken. In summary, these findings indicated that baicalin promoted mitochondrial dynamics imbalance and effectively prevents oxidative stress and apoptosis in the splenocytes of chickens infected with MG.
key words: Mycoplasma gallisepticum, spleen, baicalin, apoptosis, Nrf2/HO-1 pathway
INTRODUCTION
Mycoplasma gallisepticum (MG) causes severe inflammatory response in the birds respiratory tract results in sneezing, nasal discharge and coughing. The disease is known as chronic respiratory disease in chickens and infectious sinusitis in turkeys, which causes great economic losses in the poultry industry (Gaunson et al., 2000; Jacob et al., 2014; Roussan et al., 2015). Unlike other bacteria, MG establish a firm attachment to host cells via cytadhesion and colonizes, essential for its progression and development (Chen et al., 2001; Purswell et al., 2012). MG infects a variety of non-phagocytic cells such as fibroblasts, HeLa cells, and chicken red blood cells (RBCs). Additionally, this pathogen invade, multiply and survive in extra pulmonary tissues such as heart, blood, liver, brain, and spleen (Majumder et al., 2014). Immune system protects the body from invading pathogens and damages. Spleen is one of the immune organs which plays a key role in innate immune system (Li et al.,2017). Previous studies reported the effect of MG infection on lymphoid organs of broilers such as thymus, spleen, and bursa of fabricius, and explained that lymphocytes depletion has been noticed in these organs (Lockaby et al., 1998; Manafi et al., 2015). This means that MG infection modulated the immune response and development of immune organs. Accumulative evidences showed that excessive reactive oxygen species (ROS) generation and/or oxidative stress results in impaired immune responses in the host body and induced apoptosis in immune organs (Li et al., 2012; Gostner et al., 2015; Hu et al., 2018). However, the effect of MG infection-mediated oxidative stress and apoptosis in the spleen is still unknown.
Mitochondria are pivotal organelles for the cell survival by regulating apoptosis, providing energy and calcium buffering. Excessive generation of ROS, alteration in mitochondrial respiratory chain and imbalance in mitochondrial dynamic-related proteins cause mitochondrial dysfunction (Halliwell, 1992; Praharaj et al., 2018), playing a critical role in the pathogenesis of different diseases. Studies demonstrated that increased expression of dynamin-related protein 1 (Drp1) caused mitochondrial fragmentation (Xu et al., 2013). Besides Drp1, mitochondrial fission factor (Mff) induced mitochondrial fission, and optic atrophy 1 (Opa1), mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) proteins are responsible for mitochondrial membrane fusion, which in turn, facilitated apoptosis (Alaimo et al., 2013). Cytochrome C is released upon mitochondrial damage followed by the activation of executioners of apoptosis such as caspase 9 and caspase 3 to initiate apoptosis (Caroppi et al., 2009). In addition, BCL2 Associated X (Bax) and B-Cell Lymphoma-2 (Bcl2) proteins are also involved in mitochondrial-dependent apoptosis. Bcl2 inhibits apoptosis by preventing the release of cytochrome C from mitochondria (Yip and Reed, 2008). It would be enthralling to investigate whether MG infection induce apoptosis and its underlying mechanism in the spleen of chickens that will help in exploring new drug targets for the prevention of MG infection.
Recently, plant-derived natural flavonoid compounds are becoming more and more popular due to their excellent pharmacological properties. Researchers reported that flavonoids possess anti-inflammatory, anti-tumor, anti-hepatotoxic, antioxidant, antimicrobial, anti-allergic and analgesic properties (Tian et al., 2019). Among them, one such flavonoid is baicalin extracted from the root of Scutellariae radix, has been proved to possess pharmacological effects against a variety of ailments including infection, inflammation, oxidant, and immune dysregulation (Hsu et al., 2016). The chemotherapeutic properties of baicalin may be attributed to its ability to modulate various transcription factors that are involved in various signaling pathways (Gong et al., 2017). Previous studies showed that baicalin suppressed the transcription factor nuclear factor-kappa B (NF-κB), a critical regulator of inflammation (Cheng et al., 2017). Importantly, NF-κB overexpression exacerbate the inflammation reaction through excessive production of pro-inflammatory mediators leading to immune dysregulation (Byun et al., 2013). Therefore, suppression of NF-κB pathway could prevent immune impairment in the spleen of chickens during MG infection. In addition, nuclear factor erythroid 2–related factor 2 (Nrf2) is a critical regulator of cellular redox balance to maintain homeostasis inside the cells (Itoh et al., 1999). In normal conditions, Nrf2 is found in the cytoplasm bound with Kelch ECH associating protein 1 (Keap1) (Zipper and Mulcahy, 2002). Increase in oxidative stress or exposure to electrophilic agents react with keap1 and causes the nuclear translocation of Nrf2. Nrf2 binds to the antioxidant response element (ARE) in the nucleus leading to the transcriptional activation of several antioxidant and detoxifying genes (Kensler et al., 2007). Numerous studies reported that baicalin exerts beneficial effects in part through the activation of Nrf2/HO-1 signaling pathway activation (Zhang et al.,2012). However, the effect of baicalin on NF-κB and Nrf2/HO-1 signaling pathway in the spleen of chicken is still unknown. Thus, the inhibition of NF-κB pathway and activation of Nrf2/HO-1 signaling pathway could be a novel pharmacological approach for the prevention of MG-induced oxidative stress and apoptosis. The present study was aimed to investigate the preventive effects of baicalin against oxidative stress and apoptosis induced by MG infection in the chicken spleen.
MATERIALS AND METHODS
Ethical Statement
All the experimental procedures and guidelines were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (SYXK (Hei) 2012–2067) in the present study.
Chemicals and Reagents
Baicalin (purity ≥ 98.0%) was bought from Huifeng Animal Health Co., Ltd. (Heilongjiang, China). Methanol, Ethanol (99.0%), glycine, isopropanol and chloroform are the common reagents used in the study.
Strain rlow of mg and Culture Conditions
Strain Rlow of MG was used in these experiments provided by Harbin Veterinary Research Institute (Chinese Academy of Agricultural Science, Heilongjiang, China). The culture conditions for growing MG were kept the same as mentioned in our previous study (Lu et al., 2017). Briefly, modified Hayflicks medium containing 0.05% Penicillins, 0.1% Nicotinamide adenine dinucleotide (NAD), 10% freshly prepared yeast extract, 20% fetal bovine serum and 0.05% thallium acetate. At mid-exponential phase of MG, a color change was observed from phenol red to orange. Chickens were challenged at a density of 1 × 109 color change unit per milliliter (CCU/ml) for the pathogens.
Chickens and Treatments
120 one-day-old white leghorn chickens were bought from Chia Chau chicken farm situated in Harbin (China). Chickens were reared for one week to adapt to experimental conditions prior to experiments. Ad libitum feed and fresh drinking water were provided to chickens. After 1 wk, chickens were divided into 4 experimental groups in 3 replicates. Each group were randomly assigned 10 chickens. Experimental groups including (A) Control group (normal chickens) (B) Model group (MG infected group) (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). Chickens were challenged with MG strain Rlow (1 × 109 CCU/mL) in the bilateral air sacs in the thoracic region as reported previously (Xiao et al., 2014). After 3 d of post challenge, baicalin solution (0.5 ml) was given orally (Cheng et al., 2017), once in a day at a dose of 450 mg/kg. After 7 d of baicalin post treatment, chickens were humanely sacrificed to avoid pain and suffering of chickens and spleen were collected for further experimental analyses.
Measurement of Antioxidant Activities
Spleen samples from all experimental groups were examined for malonaldehyde (MDA, Cat no. A003-1) and nitric oxide (NO, Cat no. A012) content, and catalase (CAT, Cat no. A007-1), inducible nitric oxide synthase (iNOS, Cat no. A014-1), superoxide dismutase (SOD, Cat no. A001-1), glutathione peroxidase (GSH-Px, Cat no. A006), gamma glutamyl transferase (γ-GT, Cat no. C017) activities were measured on a spectrophotometer (UV190PC, Shanghai Drawell Scientific Instrument Co., Ltd. China). Spleen samples were prepared at 4°C in nine-fold volume of physiological normal saline solution in a chilled homogenizer as mentioned previously (Li et al., 2017; Muhammad et al., 2018). After, the solution was centrifuged (1000 × g for 10 min), supernatant was collected and assayed for the contents or enzyme activities. All the above mentioned kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Histopathological Examination of Chicken Spleen
For histopathological examination, 10% buffered formalin were used for fixing spleen samples for 12 h. Following dehydration with graded ethanol, samples were processed for paraffin wax and cut into sections (5 µm thickness). Then, the sections were mounted on slides, stained with hematoxylin and eosin dye and observed under a light microscope (Nikon E100, Japan, 40X magnification).
Terminal Deoxynucleotidyl Transferase–Mediated dUTP Nick Endlabeling Assay
Terminal deoxynucleotidyl transferase–mediated dUTP nick endlabeling (TUNEL) assay was employed to detect apoptotic cells in the chicken spleen. The specimens were first fixed in formalin, dehydrated in graded ethanol and embedded in paraffin wax. The sections were mounted on glass slides, and apoptotic cells were detected by an apoptosis cell detection kit (Beyotime biotechnology, Jiangsu, China) according to the instructions of the manufacturer. Hydrogen peroxide was used to inhibit endogenous peroxidase activity following treatment with proteinase K. The slides were incubated at 37°C for 1 h with terminal TdT/nucleotide mixture and rinsed in phosphate buffer solution (PBS). After nuclear labelling developed with diaminobenzidine and horseradish peroxidase, the slides were counterstained with hematoxylin and examined under a fluorescence microscope.
Determination of Cytokine Activities
The splenic tissues were first washed in normal saline to get rid of excess debris and homogenized in normal saline solution. It was then centrifuged at 1000 × g for 10 min in PBS and the supernatant was collected in new ependorff tubes. Cytokines activities were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer instructions. The samples were loaded in duplicate along with a blank control sample in 96 well plate and run on iMARKTM microplate reader (Bio-Rad Co., Ltd. Shanghai, China).
Protein Extraction and Western Blotting
Protein was extracted from spleen samples using radioimmunoprecipitation assay and a protease inhibitor phenylmethyl sulfonyl fluoride as described earlier (Muhammad et al., 2018). Proteins were separated on SDS-PAGE (10 to 15%) after equal loading and transferred to membranes made of nitrocellulose. Membranes were blocked with 5% non-fat dry milk in TBST for 1 h and incubated with specific primary antibodies overnight. The membranes were again washed 3 times with TBST 10 min each time and incubated with secondary anti-mouse or anti-rabbit IgG peroxidases for 1 h at room temperature. After, bound immune-complexes were visualized with enhance chemiluminescence (ECL, Biosharp Life Sciences, China) reagent. Image J software (National Institute of Health, Bethesda, Maryland) was used to analyze the blots.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Trizol reagent (catalog no. 15,596–018, Thermo Fisher Scientific, Carlsbad, California) was used to extract total RNA from spleen samples. RNA quality was evaluated by absorbance at 260/280 ratio (Wang et al., 2016). First strand cDNA was synthesized using Prime Script™ RT reagent Kit with gDNA Eraser kit purchased from Takara, Dalian, China. Quantitative Real-time polymerase chain reaction (qRT-PCR) was performed with a kit obtained from Takara, Dalian, China (catalog no. RR820A) in a Roche LightCycler instrument (Shanghai, China) and mRNA expression of the target genes (primers shown in Table 1) were evaluated according to 2−△△Ct method (Livak and Schmittgen, 2001), and β-actin was used as internal control gene for normalization.
Table 1.
Primers for target genes in RT-PCR.
| Target gene | Primers (from 5′ to 3′) | Length |
|---|---|---|
| Bax | Forward 5′-ACTCTGCTGCTGCTCTCCTCTC-3′ | 174 |
| Reverse 5′-ATCCACGCAGTGCCAGATGTAATC-3′ | ||
| Caspase-3 | Forward 5′-TACCGGACTGTCATCTCGTTCAGG-3′ | 166 |
| Reverse 5′-ACTGCTTCGCTTGCTGTGATCTTC-3′ | ||
| Caspase-8 | Forward 5′-GGAAGCAGTGCCAGAACTCAGAAG-3′ | 174 |
| Reverse 5′-TTGTTGTGGTCCATGCACCGATAG-3′ | ||
| Caspase-9 | Forward 5′-CCGAAGGAGCAAGCACGACAG-3′ | 121 |
| Reverse 5′-CATCTAGCATGTCAGCCAGGTCAC-3′ | ||
| P53 | Forward 5′-GGAGATGGAACCATTGCTGGAACC-3′ | 113 |
| Reverse 5′-GCTCCTGCCAGTTGCTGTGATC-3′ | ||
| Bcl2 | Forward 5′-GAGTTCGGCGGCGTGATGTG-3′ | 92 |
| Reverse 5′-TTCAGGTACTCGGTCATCCAGGTG-3′ | ||
| Cytochrome C | Forward 5′-CCTAATCGCCGTGGCCTTCTTAAC-3′ | 163 |
| Reverse 5′-GGAGGAGGTAGATGGTCGGATTGG-3′ | ||
| Nrf2 | Forward 5′-GATGTCACCCTGCCCTTAGA-3′ | 124 |
| Reverse 5′-TCGTTCCATTTGTTCCTTCTG-3′ | ||
| HO-1 | Forward 5′-TCATTGGCAAGAAGCATCCAGAGC-3′ | 176 |
| Reverse 5′-GAACTTGGTGGCGTTGGAGACTC-3′ | ||
| iNOS2 | Forward 5′-GAAGTGGTATGCTCTGCCTGCTG-3′ | 115 |
| Reverse 5′-GTCTCGCACTCCAATCTCTGTTCC-3′ | ||
| NF-κB | Forward 5′-CACATGGTGGTGACCGCCAATAG-3′ | 194 |
| Reverse 5′-GTGCCATCGTATGTAGTGCTGTCC-3′ | ||
| TNF-α | Forward 5′-TGATCGTGACACGTCTCTGC-3′ | 88 |
| Reverse 5′-CAACCAGCTATGCACCCCAG-3′ | ||
| IL-6 | Forward 5′-TTCACCGTGTGCGAGAACAGC-3′ | 80 |
| Reverse 5′-CAGCCGTCCTCCTCCGTCAC-3′ | ||
| IL-1β | Forward 5′-AGCAGCCTCAGCGAAGAGACC-3′ | 90 |
| Reverse 5′-GTCCACTGTGGTGTGCTCAGAATC-3′ | ||
| Mff | Forward 5′-GGCTCCTCAGAATGCTGACCTTG-3′ | 91 |
| Reverse 5′-CACTACAATCCGCTCTGGAACCTG-3′ | ||
| Mfn1 | Forward 5′-CCTGCTGCAACTCCAGAGAACAC-3′ | 115 |
| Reverse 5′-TCACTCCGCCAACAACGATGATG-3′ | ||
| Mfn2 | Forward 5′-AGCTGGCTGCGTACATCAATGAG-3′ | 150 |
| Reverse 5′-GCCTTGCCAACACTTCACTAATGC-3′ | ||
| Opa1 | Forward 5′-TATGTGAAACGGGCCACTGT-3′ | 85 |
| Reverse 5′-ATGGTCCACACCAGCCAAAA-3′ | ||
| NQO1 | Forward 5′-TCGCCGAGCAGAAGAAGATTGAAG-3′ | 191 |
| Reverse 5′-GGTGGTGAGTGACAGCATGGC-3′ | ||
| GSTA2 | Forward 5′-GGAGTCAATCCGGTGGCTGTTAG-3′ | 163 |
| Reverse 5′-GGCTCTGCTCTGCACCATCTTC-3′ | ||
| β-actin | Forward 5′-CAACACAGTGCTGTCTGGTGGTAC-3′ | 199 |
| Reverse 5′-CTCCTGCTTGCTGATCCACATCTG-3′ |
Data Analysis
All the analysis of data was performed by statistical package for social sciences (SPSS window version 21.0, Chicago, Illinois) software and one‐way analysis of variance was applied to determine the statistical significance among data at a P value < 0.05 followed by LSD test. The experiments were performed in triplicates (n = 3) and the data were expressed as mean ± SD. All the graphs were made by GraphPad prism (window version 6.01, San Diego, California).
RESULTS
Baicalin Alleviated Oxidative Stress in Spleen Tissues
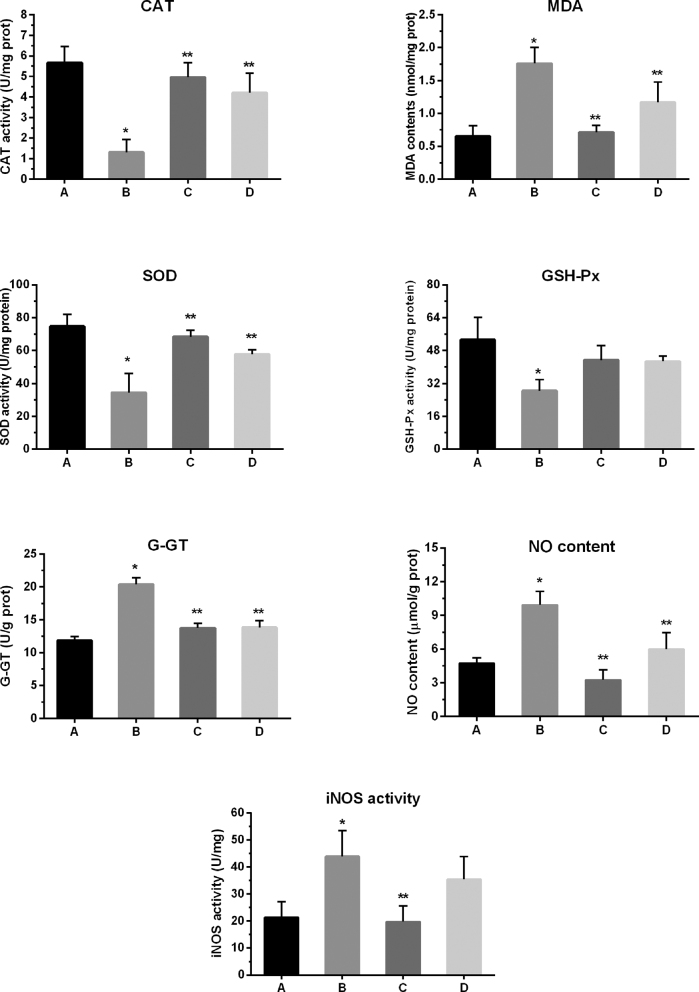
Oxidative stress-related parameters (Figure 1) were observed in the chicken spleen infected with MG. SOD, GSH-Px and CAT activities were significantly (P < 0.05) reduced in the model group, and iNOS and G-GT activities were significantly (P < 0.05) enhanced in the model group compared to control and baicalin alone group (Figure 1). Similarly, NO and MDA content were significantly (P < 0.05) increased in the model group. However, compared to model group, baicalin treatment significantly restored the normal level of these enzymes and alleviated oxidative stress in the spleen of chickens. Intriguingly, it is worthy to mention that baicalin alone treatment has no significant effect on these enzymes compared to control group.
Figure 1.
Oxidative stress-related parameters were assessed in the spleen of chicken. Experimental groups are represented as (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). All the bar graphs represent mean results ± SD (n = 3). Statistical significance were represented as ∗P < 0.05 vs. control group, ∗∗P < 0.05 vs. model group.
Histopathological Assessment of Spleen Tissues
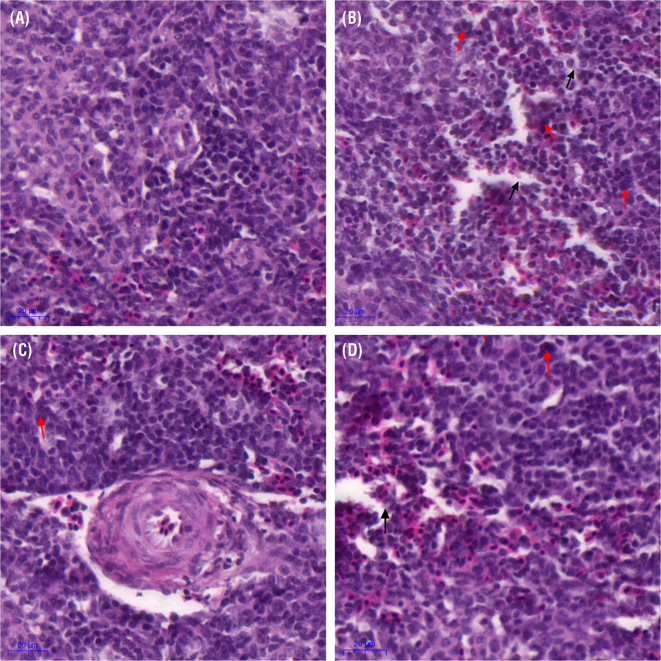
Histopathological observation (Figure 2) showed morphological changes in the tissue sections of spleen in the model group compared to control and baicalin alone group. Abnormal morphology including lymphocyte reduction, cells hyperplasia, and red, white pulp were not clearly visible in the model group (Figure 2 B). Spleen tissue micrographs from control (Figure 2 A) and baicalin alone group (Figure 2 C) showed normal morphology and appearance. However, the observed abnormal morphological signs and structural deterioration partially disappear with baicalin treatment in the spleen of chickens infected with MG (Figure 2 D).
Figure 2.
Spleen photomicrographs stained with hematoxylin and eosin (scale bar = 20 mm) are shown in the Figure 2 (n = 3). Black arrows represent lymphocytes shedding, and red arrows show mononuclear hyperplasia. Experimental groups including (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg).
Suppression of Proinflammatory Cytokines and NF-κB by Baicalin
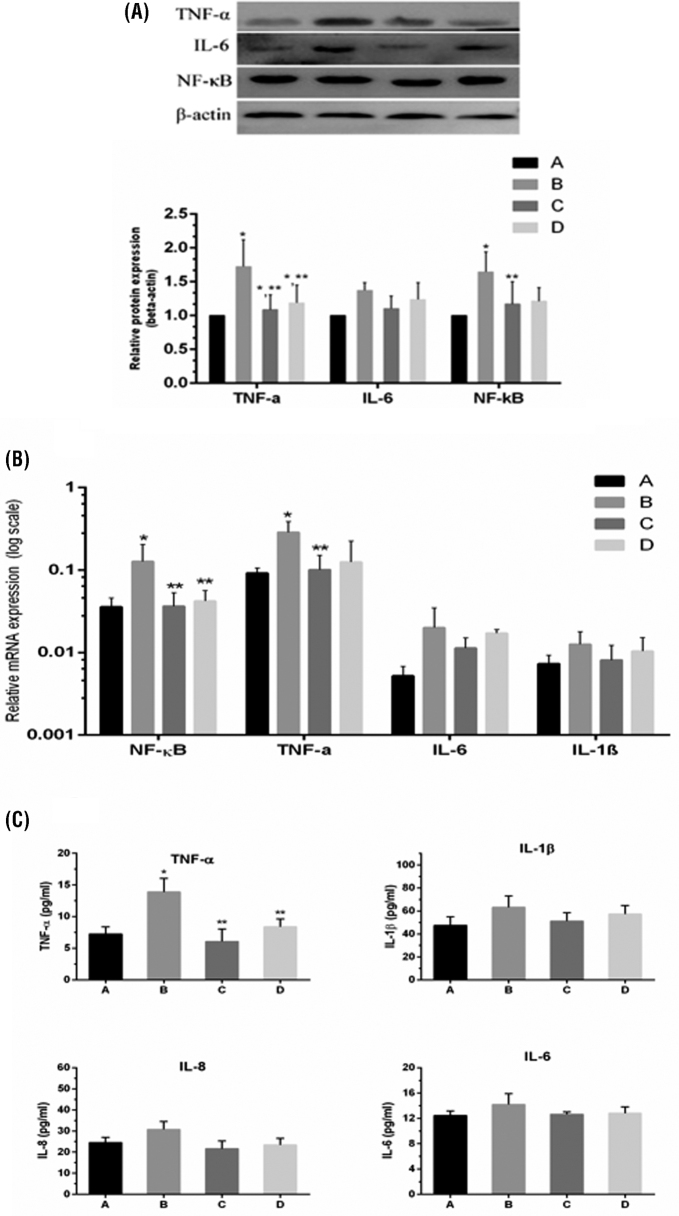
The mRNA and protein expression level of proinflammatory cytokines and NF-κB are shown in Figure 3 (A, B). NF-κB, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1β mRNA expression (Figure 3 B) were significantly (P < 0.05) enhanced in the model group compared to control and baicalin alone group. Protein expression results (Figure 3 A) of NF-κB, TNF-α, and IL-6 showed the same trend as mRNA in the model group and significantly (P < 0.05) increased in the model group compared to control and baicalin alone group. Meanwhile, the mRNA and protein expression level of NF-κB, and proinflammatory cytokines were reduced (P < 0.05) with baicalin treatment in comparison to model group. Proinflammatory cytokine activities (Figure 3 C) were increased in the model group compared to control and baicalin alone group. While, baicalin treatment significantly (P < 0.05) alleviated the increased expression of these cytokines in the spleen.
Figure 3.
Panel (A) shows the protein expression of NF-κB, TNF-α and IL-6, panel (B) shows the mRNA expression of NF-κB, TNF-α, IL-6 and IL-1β and panel (C) shows enzyme activities of cytokines TNF-α, IL-6, IL-8, and IL-1β in the spleen tissues of chicken. Whereas, experimental groups represented as (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). Statistical significance were represented as ∗P < 0.05 vs. control group, ∗∗P < 0.05 vs. model group. All the values shows mean results ± SD (n = 3).
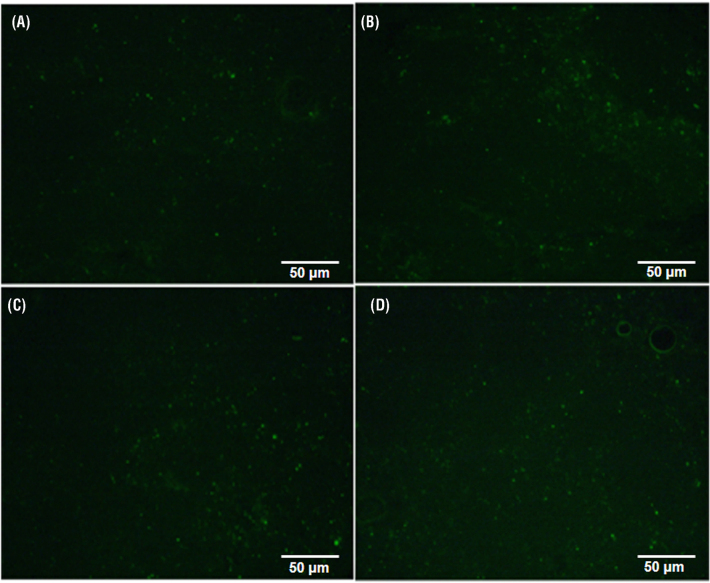
Baicalin Attenuated Apoptosis in the Spleen
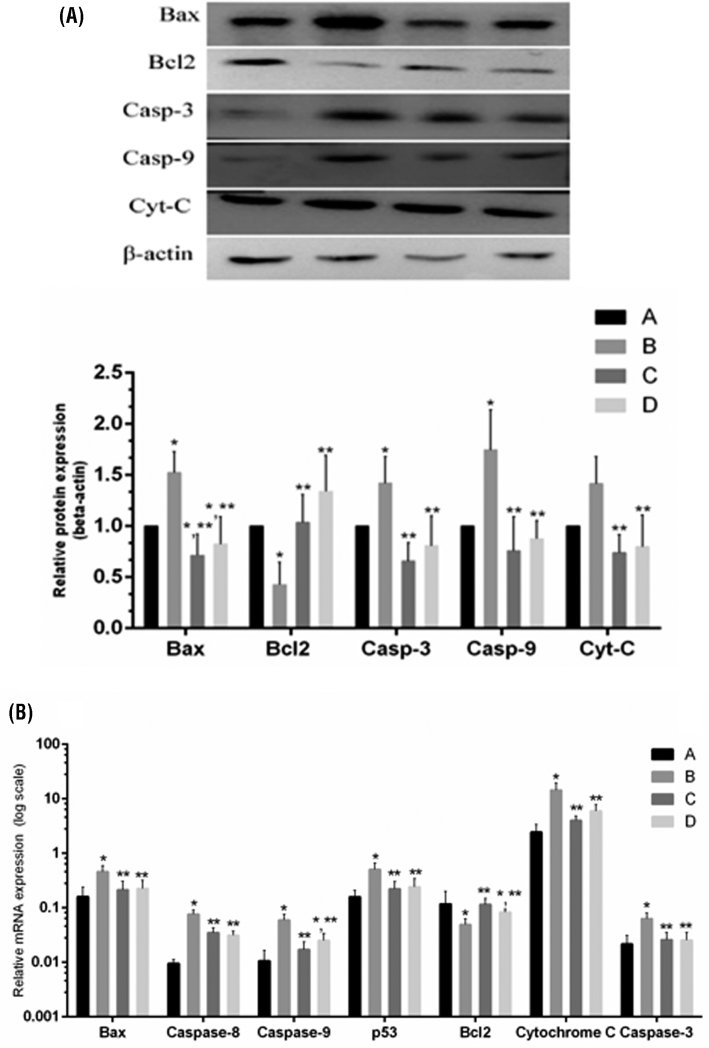
Level of apoptosis (Figures 4 and 5) was measured in the spleen of chickens to evaluate the preventive effects of baicalin against MG-induced immune impairment. The mRNA and protein expression level (Figure 4) of mitochondria-related apoptosis genes showed significant (P < 0.05) upregulation in the model group compared to control and baicalin alone group with the exception of Bcl2. Bcl2 level significantly decreased in the model group both at mRNA and protein expression level. More importantly, significant (P < 0.05) decrease has been noted in mRNA and protein expression level of these enzymes with baicalin treatment compared to model group. In addition, anti-apoptotic gene (Bcl2) mRNA and protein levels were significantly (P < 0.05) enhanced with baicalin treatment in comparison with model group. In addition, TUNEL results (Figure 5) showed extensive positive-stained nuclei in the model group compared to control and baicalin alone treated group. Meanwhile, baicalin treatment significantly reduced positive-stained nuclei in the spleen of chickens infected with MG as compared to model group.
Figure 4.
The mRNA and protein expression of mitochondrial-related apoptosis genes. Panel (A) shows protein expression level and panel (B) shows the mRNA expression of mitochondrial apoptosis related genes. Whereas, experimental groups consisting of (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). Statistical significance were represented as ∗P < 0.05 vs. control group, ∗∗P < 0.05 vs. model group. All the values shows mean results ± SD (n = 3).
Figure 5.
Apoptosis were measured by TUNEL assay at day 7 post-treatment after infection. Positive stained nuclei (apoptotic cells) were observed in the spleen of chickens (scale = 50 µm). Whereas, experimental groups consisting of (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg).
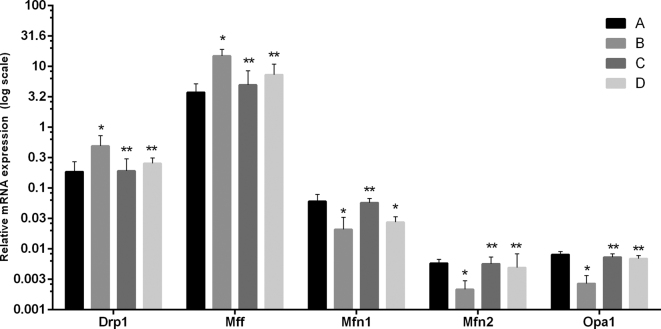
Effect of Baicalin and MG Infection on Mitochondrial Dynamics in the Chicken Spleen
Figure 6 represents the level of mRNA expression level of mitochondrial dynamics of 4 experimental groups. MG infection significantly altered the mRNA expression level of these genes in comparison to control and baicalin alone group. The expression level of Drp1 and Mff were significantly enhanced in the model group. While, Mfn1, Mfn2, and Opa1 mRNA expression level were significantly (P < 0.05) decreased in the model group. Meanwhile, baicalin treatment significantly prevented the altered mRNA expression level of these genes.
Figure 6.
The effect of MG infection and/or baicalin on mitochondrial dynamics related genes mRNA expression were assessed in the spleen tissues. Whereas, experimental groups including (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). Statistical significance were represented as ∗P < 0.05 vs. control group, ∗∗P < 0.05 vs. model group. All the bar graphs shows mean results ± SD (n = 3).
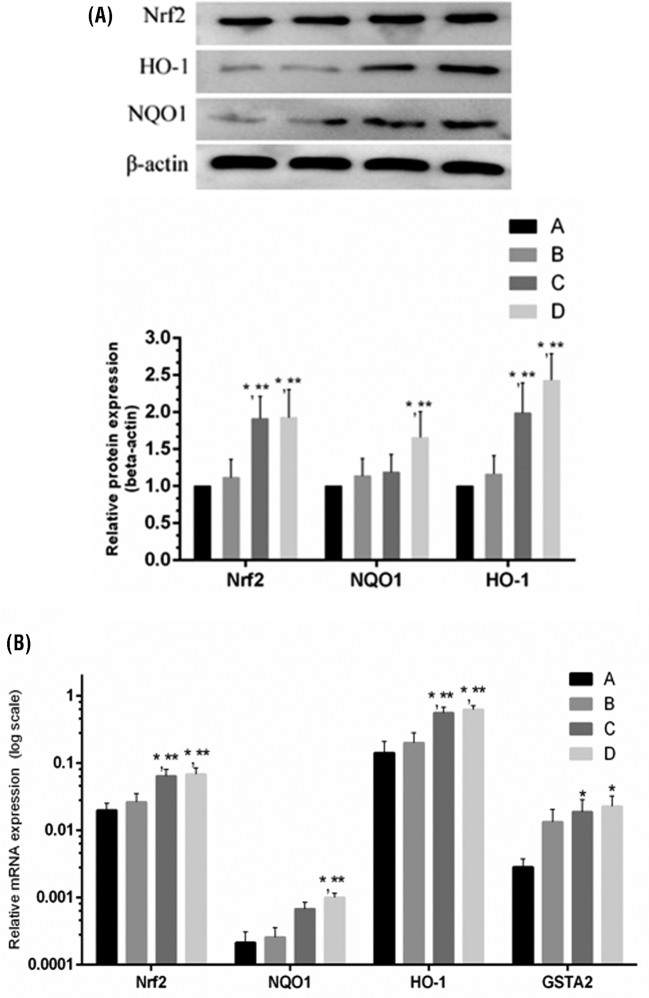
Effect of Baicalin and MG Infection on the Expression of Nrf2/HO-1 Signaling Pathway
The mRNA and protein expression level of Nrf2 and its downstream genes are represented in Figure 7. Interestingly, Nrf2 mRNA and protein expression level increased (P > 0.05) in the model group compared to control group. Similarly, NAD(P)H: quinone oxidoreductase 1 (NQO1), HO-1 and Glutathione S-transferase-A 2 (GSTA2) mRNA also increased in the model group compared to control group. However, the increase in mRNA expression level of these genes is not statistically significant compared to control group. Meanwhile, baicalin treatment significantly (P < 0.05) enhanced Nrf2 and its downstream genes mRNA and protein expression level compared to control and model group.
Figure 7.
The effect of MG infection and/or baicalin on transcription factor Nrf2 and its downstream genes (HO-1, NQO1, GSTA2). Panel (A) shows the protein expression levels of Nrf2, NQO1 and HO-1 and panel (B) shows the mRNA expression level of Nrf2, NQO1, HO-1, and GSTA2. Whereas, experimental groups consist of (A) Control group (B) Model group (C) Baicalin alone treated group (450 mg/kg) and (D) Model group treated with baicalin (450 mg/kg). Statistical significance were represented as ∗P < 0.05 vs. control group, ∗∗P < 0.05 vs. model group. All the bar graphs shows mean results ± SD (n = 3).
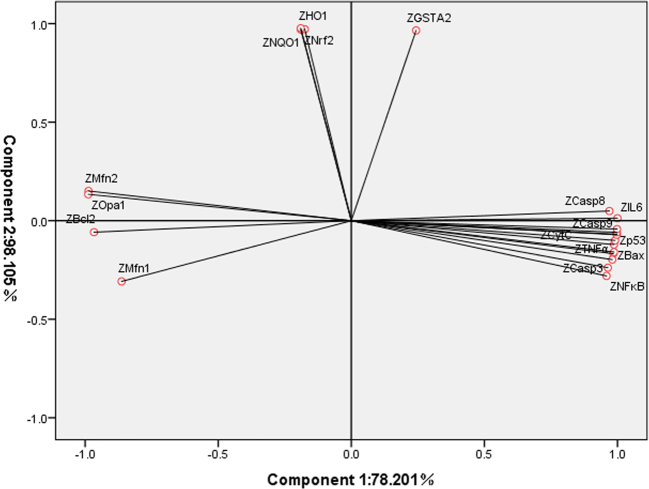
Principal Component Analysis
Principal component analysis was carried out to determine the key factors involved in individual variations, which define the most important parameters with the advantage of compressing the data. Using Principal Component Analysis (Figure 8), the data is divided into two principal components, the first principal component (78.201%) and second principal component (98.105%), respectively, except Bcl2. Mfn1, Mfn2, Opa1, Nrf2, NQO1, and HO-1 are positively correlated with principal component 1, but only Casp-8, Mfn2, Opa1, IL-6, Nrf2, NQO1, HO-1, and GSTA2 are positively correlated with principal component 2. The rotating component matrix obtained through principal component analysis is shown in Table 2.
Figure 8.
The rotating components in space obtained from principal component analysis. The first and second principle components were 78.201 and 98.105%, respectively.
Table 2.
Rotating component matrix.
| Component | Bax | Casp-3 | Casp-8 | Casp-9 | p53 | Bcl2 | Cyt-C | Drp1 | Mff | Mfn2 | Opa1 | NF-κB | TNF-α | IL-6 | IL-1β | Nrf2 | NQO1 | HO-1 | GSTA2 | iNOS2 | Mfn1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.987 | 0.964 | 0.970 | 0.998 | 0.991 | −0.967 | 0.997 | 0.984 | 0.997 | −0.987 | −0.988 | 0.959 | 0.980 | 1.00 | 0.999 | −0.190 | −0.175 | −0.190 | 0.243 | 0.987 | −0.863 |
| 2 | −0.121 | −0.239 | 0.049 | −0.069 | −0.100 | −0.058 | −0.072 | −0.168 | −0.059 | 0.151 | 0.134 | −0.280 | −0.197 | 0.013 | −0.042 | 0.970 | 0.972 | 0.977 | 0.977 | −0.159 | −0.308 |
DISCUSSION
Baicalin has been used as a traditional medicine in East Asia for several decades (Ishimaru et al., 1995). A wide variety of pharmacological properties of baicalin such as anti-cancer, anti-pruritic and anti-inflammatory effects had been reported in literature (Lin and Shieh, 1996). Nowadays, natural products have been extensively used against bacterial diseases with the benefits of avoiding antibiotic resistance and harmful side effects (Jang et al., 2014). Baicalin is one of the natural flavonoids that showed potential therapeutic effects against bacterial diseases (Fujita et al., 2005). In addition, it shows synergistic effects with a variety of other antibiotics (Cai et al., 2016). A previous study demonstrated that baicalin prevents from mycoplasma pneumonia infection (Meng et al., 2013). (Garmyn et al., 2017) reported the efficacy of tiamulin alone or in combination with chlortetracycline against MG infection in chickens. However, the immunomodulatory effects of baicalin against MG infection in the chicken spleen is still not reported. In the present study, we investigated the preventive effects of baicalin against MG infection induced immune impairment involving oxidative stress and apoptosis in the chicken spleen. Histological and ultrastructural observation showed that baicalin treatment partially ameliorated the pathological changes in the spleen. Intriguingly, MG infection produced oxidative stress which is the possible cause of these abnormal pathological changes and structural deterioration in the spleen of chickens. Oxidative stress-related enzyme activities were subsequently altered in the spleen tissues, confirmed an increase in oxidative stress in the chickens infected with MG. More importantly, baicalin treatment significantly alleviated oxidative stress compared to model group. These findings are in consistence with previous studies that baicalin prevented from oxidative stress (Wu et al., 2018). In addition, we noticed a profound level of apoptosis in the spleen of model group as compared to control and baicalin alone treated group. TUNEL assay results showed a number of positive stained nuclei in the model group. Subsequently, the mRNA and protein expression level of apoptosis-related genes were significantly changed. It has been suggested from these results that baicalin significantly attenuated apoptosis and could prevent immune impairment in the spleen of chickens infected with MG. Previous reports demonstrated that mitochondrial apoptosis is also associated with mitochondrial dynamics genes (Dabrowska et al., 2015). In the present study, we noted an increase in mRNA expression level of Drp1 and Mff genes in the model group, responsible for mitochondrial fragmentation and fission. While, mitochondrial membrane fusion-related genes such as Opa1, Mfn1 and Mfn2 mRNA expression level were significantly downregulated in the model group. These findings are in agreement with earlier studies (Halliwell 1992; Alaimo et al., 2013; Xu et al., 2013; Jin et al., 2017; Praharaj et al., 2018), demonstrating that the alteration in mitochondrial dynamics results in mitochondrial dysfunction leading to apoptosis. In addition, our data showed that baicalin treatment significantly promoted the expression level of mitochondrial dynamics and prevents mitochondrial dysfunction during MG infection. However, further studies are needed to investigate the effect of baicalin on mitochondrial respiratory chain complex associated with energy metabolism in the spleen of chickens.
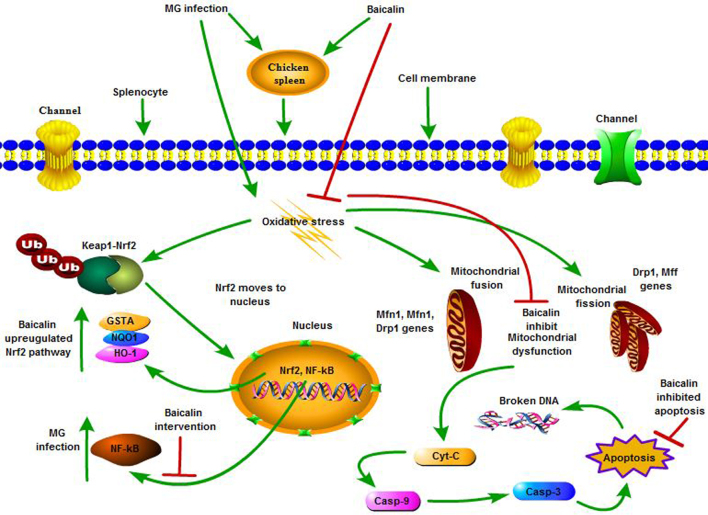
Previous studies demonstrated that upregulation of Nrf2 pathway protects the body from oxidative stress and injuries (Wang et al., 2018), and Nrf2 is a key therapeutic drug target in case of oxidative stress and various other diseases (Zhang and Gordon, 2004). Antioxidant enzymes including GST, HO-1, and NQO1 prevent cells from oxidative stress (Banerjee et al., 1999). Recently, the upregulation of these antioxidant enzymes by chemical or natural products is a common strategy to provide protection to cells in case of diseases as well as cancer (Hwang et al., 2011). In addition, iNOS plays a crucial role in inflammatory response often induced by oxidative stress via NF-κB pathway (Chung et al., 2007). Therefore, the molecular inhibition of iNOS through the modulation of NF-κB is considered to be a key target in the spleen of chickens during MG infection. Our results showed that baicalin significantly inhibited the expression of NF-κB pathway. It could be speculated that baicalin alleviated oxidative stress-mediated alteration in cytokines expression. In addition, baicalin significantly upregulated the transcription factor Nrf2 and its downstream genes such as GST, HO-1 and NQO1 both at mRNA and protein level. The data provided an evidence that the cytoprotective action of baicalin is partially attributed to its ability to induce cytoprotective genes during MG infection, and Nrf2 promoted defense system against MG infection-mediated immune impairment in the chicken spleen. These results are in line with previous findings (Kim et al., 2010; Shen et al., 2017) that baicalin inhibited NF-κB and upregulated Nrf2 signaling pathway to attenuate oxidative stress and confers cytoprotection. Moreover, further molecular and mechanistic studies are needed to scrutinize the underlying mechanism of baicalin behind the regulation of NF-κB and Nrf2 signaling pathway. In conclusion, baicalin efficiently prevented oxidative stress and apoptosis in the chicken spleen during MG infection (refer to schematic diagram, Figure 9). In addition, baicalin promoted the balance among mitochondrial dynamics and protect mitochondrial dysfunction. Overall, these findings suggested that baicalin protect immune dysfunction through the activation of Nrf2/HO-1 signaling pathway, and suppressed NF-κB and proinflammatory cytokines in the spleen of chickens. Nevertheless, further studies are required to investigate the crosstalk between these pathways to better understand the preventive mechanism of baicalin against various infections.
Figure 9.
The schematic diagram showing the preventive effects of baicalin against MG infection-mediated oxidative stress and apoptosis in the spleen of chickens. Red arrows shows inhibition, while green arrows shows upregulation or increased expression.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31772801 and 31802241) and Post-doctoral Research Foundation in Heilongjiang Province (LBH-Q18019). The first author “Muhammad Ishfaq” thanks to teacher “Xiangyu Guo” of the Agriculture Economics Department (Northeast Agricultural University, Harbin, China) for his encouragement and support during my study in China.
Conflict of interest statement
All authors declared that there are no potential conflicts of interests.
Contributor Information
Liangjun Ding, Email: dlj2019@neau.edu.cn.
Jichang Li, Email: lijichang@neau.edu.cn.
REFERENCES
- Alaimo A., Gorojod R.M., Miglietta E.A., Villarreal A., Ramos A.J., Kotler M.L. Manganese induces mitochondrial dynamics impairment and apoptotic cell death: a study in human Gli36 cells. Neurosci. Lett. 2013;554:76–81. doi: 10.1016/j.neulet.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Banerjee B.D., Seth V., Bhattacharya A., Pasha S.T., Chakraborty A.K. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol. Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Byun E.B., Sung N.Y., Byun E.H., Song D.S., Kim J.K., Park J.H., Song B.S., Park S.H., Lee J.W., Byun M.W., Kim J.H. The procyanidin trimer C1 inhibits LPS-induced MAPK and NFkappaB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2013;15:450–456. doi: 10.1016/j.intimp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Cai W., Fu Y., Zhang W., Chen X., Zhao J., Song W., Li Y., Huang Y., Wu Z., Sun R., Dong C., Zhang F. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016;16:181. doi: 10.1186/s12866-016-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppi P., Sinibaldi F., Fiorucci L., Santucci R. Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr. Med. Chem. 2009;16:4058–4065. doi: 10.2174/092986709789378206. [DOI] [PubMed] [Google Scholar]
- Chen H.J., Yu S.Q., Shen X.Y., Chen D.Q., Qiu X.S., Song C.P., Ding C. The Mycoplasma gallisepticum alpha-enolase is cell surface-exposed and mediates adherence by binding to chicken plasminogen. Microb. Pathog. 2001;51:285–290.. doi: 10.1016/j.micpath.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Cheng P., Wang T., Li W., Muhammad I., Wang H., Sun X., Yang Y., Li J., Xiao T., Zhang X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.Y., Park J.H., Kim M.J., Kim H.O., Hwang J.K., Lee S.K., Park K.K. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-kappa B. Carcinogenesis. 2007;28:1224–1231. doi: 10.1093/carcin/bgm005. [DOI] [PubMed] [Google Scholar]
- Dabrowska A., Venero J.L., Iwasawa R., Hankir M.K., Rahman S., Boobis A., Hajji N. PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging. 2015;7:629–647. doi: 10.18632/aging.100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Shiota S., Kuroda T., Hatano T., Yoshida T., Mizushima T., Tsuchiya T. Remarkable synergies between baicalein and tetracycline and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005;49:391–396. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- Garmyn A., Vereecken M., Degussem K., Depondt W.W., Haesebrouck F., Martel A. Efficacy of tiamulin alone or in combination with chlortetracycline against experimental Mycoplasma gallisepticum infection in chickens. Poult. Sci. 2017;96:3367–3374. doi: 10.3382/ps/pex105. [DOI] [PubMed] [Google Scholar]
- Gaunson J.E., Philip C.J., Whithear K.G., Browning G.F. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology. 2000;146:1223–1229. doi: 10.1099/00221287-146-5-1223. [DOI] [PubMed] [Google Scholar]
- Gong W.Y., Zhao Z.X., Liu B.J., Lu L.W., Dong J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017;126:844–852. doi: 10.1016/j.ejmech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- Gostner J.M., Becker K., Ueberall F., Fuchs D. The good and bad of antioxidant foods: an immunological perspective. Food. Chem. Toxicol. 2015;80:72–79. doi: 10.1016/j.fct.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hsu Y.M., Yu B., Tseng C.S., Chang C.H., Chen D.S., Su C.H., Chen Y.S. Preventive activities of Scutellariae Radix, Gardeniae Fructus, and probiotics in Salmonella enterica serovar Typhimurium infection in chickens. Anim. Feed. Sci. Technol. 2016;214:121–129. [Google Scholar]
- Hu X., Chi Q., Wang D., Chi X., Teng X., Li S. Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotoxicol. Environ. Saf. 2018;164:201–209. doi: 10.1016/j.ecoenv.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Hwang Y.P., Choi J.H., Yun H.J., Han E.H., Kim H.G., Kim J.Y., Park B.H., Khanal T., Choi J.M., Chung Y.C., Jeong H.G. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food. Chem. Toxicol. 2011;49:93–99. doi: 10.1016/j.fct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Ishimaru K., Nishikawa K., Omoto T., Asai I., Yoshihira K., Shimomura K. Two flavone 21-glucosides from Scutellaria baicalensis. Phytochemistry. 1995;40:279–281. doi: 10.1016/0031-9422(95)00200-q. [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes. Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Branton S.L., Evans J.D., Leigh S.A., Peebles E.D. Effects of live and killed vaccines against Mycoplasma gallisepticum on the performance characteristics of commercial layer chickens. Poult. Sci. 2014;93:1403–1409. doi: 10.3382/ps.2013-03748. [DOI] [PubMed] [Google Scholar]
- Jang E.J., Cha S.M., Choi S.M., Cha J.D. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral. Biol. 2014;59:1233–1241. doi: 10.1016/j.archoralbio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Jin X, Xu Z., Zhao X., Chen M., Xu S. The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere. 2017;180:259–266. doi: 10.1016/j.chemosphere.2017.03.130. [DOI] [PubMed] [Google Scholar]
- Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Moon Y.J., Lee S.M. Protective Effects of Baicalin against Ischemia/Reperfusion Injury in Rat Liver. J. Nat. Prod. 2010;73:2003–2008. doi: 10.1021/np100389z. [DOI] [PubMed] [Google Scholar]
- Li W.J., Li L., Zhen W.Y., Wang L.F., Pan M., Lv J.Q., Wang F., Yao Y.F., Nie S.P., Xie M.Y. Ganoderma atrum polysaccharide ameliorates ROS generation and apoptosis in spleen and thymus of immunosuppressed mice. Food. Chem. Toxicol. 2017;99:199–208. doi: 10.1016/j.fct.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Li W.J., Nie S.P., Peng X.P., Liu X.Z., Li C., Chen Y., Xie M.Y. Ganoderma atrum polysaccharide improves age-related oxidative stress and immune impairment in mice. J. Agric. Food. Chem. 2012;60:1413–1418. doi: 10.1021/jf204748a. [DOI] [PubMed] [Google Scholar]
- Lin C.C., Shieh D.E. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am. J. Chin. Med. 1996;24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lockaby S.B., Hoerr F.J., Lauerman L.H., Kleven S.H. Pathogenicity of mycoplasma synoviae in broiler chickens. Vet. Pathol. 1998;35:178–190. doi: 10.1177/030098589803500303. [DOI] [PubMed] [Google Scholar]
- Lu Z., Xie D., Chen Y., Tian E., Muhammad I., Chen X., Miao Y., Hu W., Wu Z., Ni H., Xin J., Li Y., Li J. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum-infected RAW264.7 cells. Mol. Immunol. 2017;87:161–170. doi: 10.1016/j.molimm.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Majumder S., Zappulla F., Silbart L.K. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-kB dependent pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M., Pirany N., Noor Ali M., Hedayati M., Khalaji S., Yari M. Experimental pathology of T-2 toxicosis and mycoplasma infection on performance and hepatic functions of broiler chickens. Poult. Sci. 2015;94:1483–1492. doi: 10.3382/ps/pev115. [DOI] [PubMed] [Google Scholar]
- Meng Y., Huo J., Lu W., Wang X., Zhang J., Wang W. Modulation of P1 and EGF Expression by Baicalin. Int. J. Mol. Sci. 2013;14:146–157. doi: 10.3390/ijms14010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., Cheng P., Hussain M.A., Zhang X. Dual role of dietary curcumin through attenuating AFB1-Induced oxidative stress and liver injury via modulating liver Phase-I and Phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 2018;9:554. doi: 10.3389/fphar.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praharaj P.P., Naik P.P., Panigrahi D.P., Bhol C.S., Mahapatra K.K., Patra S., Sethi G., Bhutia S.K. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: its implication in cancer therapeutics. Cell. Mol. Life. Sci. 2018;76:1641–1652. doi: 10.1007/s00018-018-2990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purswell J.L., Evans J.D., Leigh S.A., Collier S.D., Olanrewaju H.A., Kim E.J., Pharr G.T., Peebles E.D., Branton S.L. Mycoplasma gallisepticum transmission: comparison of commercial F-strain vaccine versus layer complex-derived field strains in a tunnel ventilated house. Poult. Sci. 2012;91:3072–3079. doi: 10.3382/ps.2012-02619. [DOI] [PubMed] [Google Scholar]
- Roussan D.A., Khawaldeh G., Shaheen A. A survey of Mycoplasma gallisepticum and Mycoplasma synovaie with avian influenza H9 subtype in meat-type chicken in Jordan between 2011–2015. Poult. Sci. 2015;94:1499–1503. doi: 10.3382/ps/pev119. [DOI] [PubMed] [Google Scholar]
- Shen K., Feng X., Pan H., Zhang F., Xie H., Zheng S. Baicalin ameliorates experimental liver cholestasis in mice by modulation of oxidative stress, inflammation, and NRF2 transcription factor. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/6169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Zhang P., Yang J., Zhang Z., Wang H., Guo Y., Liu M. The protective effect of the flavonoid fraction of Abutilon theophrasti Medic. leaves on LPS-induced acute lung injury in mice via the NF-κB and MAPK signalling pathways. Biomed. Pharmacother. 2019;109:1024–1031. doi: 10.1016/j.biopha.2018.10.197. [DOI] [PubMed] [Google Scholar]
- Wang H., Muhammad I., Li W., Sun X., Cheng P., Zhang X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ. Toxicol. Pharmacol. 2018;59:94–104. doi: 10.1016/j.etap.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Wang J., Yi M., Chen X., Muhammad I., Liu F., Li R., Li J., Li J. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain. Neurotoxicol. Teratol. 2016;55:32–37. doi: 10.1016/j.ntt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Wu Y., Wang F., Fan L., Zhang W., Wang T., Du Y., Bai X. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-kappa b and p38 MAPK signaling pathways. Biomed. Pharmacother. 2018;97:1673–1679. doi: 10.1016/j.biopha.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Xiao X., Zhao D.H., Yang X., Shi W., Deng H., Ma J., Zhang S., Liu Y.H. Mycoplasma gallisepticum and Escherichia coli mixed infection model in broiler chickens for studying valnemulin pharmacokinetics. J. Vet. Pharmacol. Ther. 2014;37:99–102. doi: 10.1111/jvp.12065. [DOI] [PubMed] [Google Scholar]
- Xu S., Pi H., Chen Y., Zhang N., Guo P., Lu Y., He M., Xie J., Zhong M., Zhang Y., Yu Z., Zhou Z. Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity. Cell Death Dis. 2013;4:e540. doi: 10.1038/cddis.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip K.W., Reed J.C. Bcl‐2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gordon G.B. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol. Cancer. Ther. 2004;3:885–893. [PubMed] [Google Scholar]
- Zhang Z., Cui W., Li G., Yuan S., Xu D., Hoi M.P., Lin Z., Dou J., Han Y., Lee S.M. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J. Agric. Food. Chem. 2012;60:8171–8182. doi: 10.1021/jf301511m. [DOI] [PubMed] [Google Scholar]
- Zipper L.M., Mulcahy R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]