Abstract
Rifapentine is undergoing development for the treatment of pulmonary tuberculosis. This study was conducted to characterize the single-dose pharmacokinetics of rifapentine and its 25-desacetyl metabolite and to assess the effect of food on the rate and extent of absorption in participants infected with human immunodeficiency virus (HIV). Twelve men and four women, mean age, 38.6 ± 6.9 years, received a single 600-mg oral dose of rifapentine in an open-label, randomized two-way, complete crossover study. Each volunteer received rifapentine following a high-fat breakfast or during a fasting period. Serial blood samples were collected for 72 h and both rifapentine and its metabolite were assayed by a validated high-performance liquid chromatography method. Pharmacokinetics of rifapentine and 25-desacetylrifapentine were determined by noncompartmental methods. Mean (± the standard deviation) maximum concentrations of rifapentine in serum and areas under the curve from time zero to infinity following a high-fat breakfast were 14.09 ± 2.81 and 373.63 ± 78.19 μg/ml, respectively, and following a fasting period they were 9.42 ± 2.67 and 256.10 ± 86.39 μg · h/ml, respectively. Pharmacokinetic data from a previously published healthy volunteer study were used for comparison. Administration of rifapentine with a high-fat breakfast resulted in a 51% increase in rifapentine bioavailability, an effect also observed in healthy volunteers. Although food increased the exposure of these patients to rifapentine, the infrequent dosing schedule for the treatment of tuberculosis (e.g., once- or twice-weekly dosing) would be unlikely to lead to accumulation. Additionally, autoinduction has been previously studied and has not been demonstrated with this compound, unlike with rifabutin and rifampin. Rifapentine was well tolerated by HIV-infected study participants. The results of our study suggest that no dosage adjustments may be required for rifapentine in HIV-infected patients (Centers for Disease Control and Prevention classification A1, A2, B1, or B2) undergoing treatment for tuberculosis.
Rifapentine, a new rifamycin derivative, inhibits RNA synthesis by interacting with bacterial DNA-dependent RNA polymerase (13). Rifapentine has demonstrated activity against medically important mycobacterium species, including the Mycobacterium avium complex (MAC) and M. tuberculosis, both in vitro and in vivo (5, 6, 9, 12). Patients suffering from AIDS are commonly afflicted with mycobacterial infections secondary to various immune system deficiencies, requiring prophylaxis or treatment with a variety of anti-infective agents, including rifamycin derivatives.
The pharmacokinetic profile of rifapentine has been previously characterized in healthy volunteers (1, 2, 4, 7, 8, 10, 11, 14–16). It should be noted that four of these rifapentine pharmacokinetic studies (4, 14–16) were conducted by using a rifapentine formulation that is available only in the Republic of China. Following oral administration of single doses, rifapentine was absorbed slowly with peak concentrations in plasma (Cmax) observed 4 to 5 h after dose administration. An important pharmacokinetic feature of rifapentine is the longer terminal elimination half-life (t1/2) of 13 to 14 h, compared with 2 to 3 h for rifampin, which may allow extended dosing intervals in patients suffering from mycobacterial infections. The primary elimination pathway for rifapentine was characterized as desacetylation to an active metabolite, 25-desacetylrifapentine, followed by fecal and renal excretion of both the parent drug and the metabolite. Less than 17% of the oral dose of rifapentine was excreted in the urine (11). Single-dose pharmacokinetics of rifapentine are reasonably predictive of multiple-dose pharmacokinetics, due in part to the lack of autoinduction and the infrequency of drug administration with the multiple-dose regimen used for tuberculosis (e.g., once or twice weekly) (7).
The intent of this study was to characterize the single-dose pharmacokinetics of rifapentine and its active metabolite, 25-desacetylrifapentine, in participants seropositive for human immunodeficiency virus (HIV) following dosing in both fasted and fed states and to compare the data obtained to those previously published on healthy volunteers (8).
(This research was presented, in part, at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy in 1997 [10].)
MATERIALS AND METHODS
Study design and patient population.
The study was designed as an open-label, randomized, two-way crossover trial involving 16 HIV-infected participants. Prospective HIV-infected participants belonging to Centers for Disease Control and Prevention (CDC) classification system category A1, A2, B1, or B2 (based on the 1993 CDC HIV classification system and expanded AIDS surveillance definition) were considered eligible for enrollment (3). HIV testing consisted of repeated reactive screening by enzyme-linked immunosorbent assay and specific antibody identification by a supplemental test (Western blotting). Candidates were between 19 and 55 years of age and within 15% of the ideal body weight and weighed at least 40 kg. A complete medical history was recorded, and prospective participants underwent pre- and poststudy physical examinations, urine toxicology screening, hepatitis B serology testing, 12-lead electrocardiography, and serum pregnancy testing. Pre- and poststudy laboratory evaluations were also conducted and included hematological testing, urinalysis, and blood chemistry profiling. Participants were excluded if, upon prestudy evaluation, it was determined that clinically significant findings existed following physical, cardiac, or laboratory examinations. The comparator group consisted of 20 healthy, young (18 to 45 years old) male volunteers who had participated in a previous, single-dose (600 mg) rifapentine pharmacokinetic study (8) similar in design.
Drug administration.
Participants were randomized to receive both of the following treatments separated by a 14-day washout period: treatment A, 600 mg of rifapentine (four 150-mg tablets) with a high-fat breakfast; treatment B, 600 mg of rifapentine (four 150-mg tablets) following a 10-h fasting state. The meal consisted of approximately 850 calories from 33 g of protein, 55 g of fat, and 58 g of carbohydrates. During the course of the study, the participants refrained from the use of other medications.
Blood sample collection.
Blood samples for plasma drug concentration determinations were collected prior to drug administration and at 2, 4, 5, 6, 7, 8, 10, 12, 18, 24, 36, 48, and 72 h after oral administration of rifapentine. Each sample collection was immediately preceded by the drawing from the venous access site of a 3-ml sample which was discarded. Blood samples (5 ml) were collected from an indwelling catheter placed in the antecubital vein of the forearm. Samples were drawn into heparinized, evacuated specimen collection tubes. Samples were centrifuged at 2,000 × g under refrigerated conditions (0 to 4°C) for 10 min, and the plasma was subsequently stored at −20°C prior to shipment to Phoenix International Life Sciences, Inc., Montreal, Quebec, Canada, for plasma drug concentration determination.
Analytical procedures.
Rifapentine and 25-desacetylrifapentine concentrations in plasma were determined by a validated high-performance liquid chromatography method by Phoenix International Life Sciences, Inc. The validated assay standard curve ranges were 0.5 to 60 and 0.4 to 50 μg/ml for rifapentine and 25-desacetylrifapentine, respectively. The batch-to-batch mean accuracy percentages for the quality control samples of rifapentine and 25-desacetylrifapentine were 89.7 to 101.1% and 90.9 to 100.8%, respectively. The respective mean percent coefficients of variation (precision) for the quality control samples were 2.1 to 7.3% and 2.2 to 10.5%.
Pharmacokinetic analysis.
Rifapentine pharmacokinetic parameters were determined by using noncompartmental methods from plasma drug concentration-versus-time data. The area under the plasma rifapentine concentration-time curve from time zero to 72 h (AUC0–72) was determined by using the linear trapezoidal rule, and the AUC from time zero to infinity (AUC0–∞) was estimated by dividing the last plasma drug concentration by the terminal elimination rate constant (λz). λz was estimated by the linear least-squares regression of the log plasma drug concentration-time data from the terminal elimination phase. The t1/2 was calculated from the equation t1/2 = 0.693/λz. Peak plasma drug concentrations (Cmax) and times to Cmax (Tmax) were obtained by visual inspection of the concentration-versus-time profiles for each participant. Oral clearance (CLpo) was calculated by dividing the dose by the plasma AUC0–∞.
Statistical analysis.
Comparisons between treatments were done by analysis of natural-log-transformed data. A three-way analysis of variance using PROC MIXED in SAS with terms for subject, treatment, and period was performed for each parameter. From this analysis, estimated treatment differences and 90% confidence intervals for treatment differences were calculated. The log-transformed results were transformed back to the original scale to obtain treatment ratios and 90% confidence intervals for the ratio of treatment means.
Safety assessment.
Safety measures included prestudy and poststudy vital sign (heart rate, respiration rate, temperature, and blood pressure) measurements, 12-lead electrocardiography, physical examinations, and clinical laboratory testing. Any adverse event observed by the investigator or reported by the subject during the study period was recorded.
RESULTS
Twelve men and four women were enrolled in the study and received the study drug (mean age ± standard deviation [SD], 38.6 ± 6.9 years; mean weight ± SD, 74.3 ± 12.6 kg). The median CD4 count was 307 (range, 225 to 489) cells/mm3. The CDC classification breakdown is as follows: A1, 2 patients; A2, 10 patients; B1, 0 patients; B2, 5 patients. One participant not included in the analysis was enrolled and withdrew consent prior to receiving the study drug. In addition, following the successful completion of one study period, one participant tested positive for tetrahydrocannabinol prior to receiving rifapentine during the second study period and was immediately eliminated from the study. This participant’s data from the first study period were used in the final analysis of data. The comparator group consisted of 20 healthy, nonsmoking men between the ages of 18 and 45 years (mean age ± SD, 25.7 = 8.2 years) and weighing within 10% of the ideal body weight (8).
Pharmacokinetics.
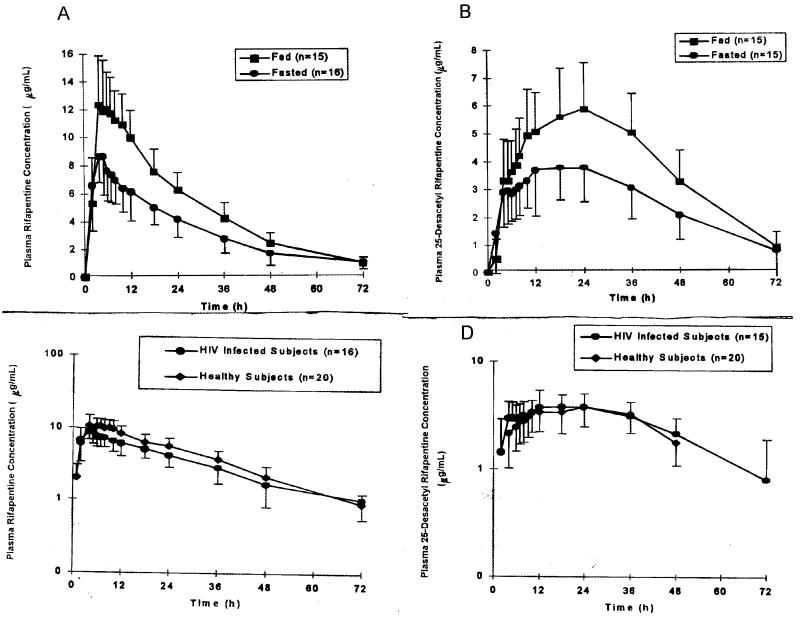
Plasma rifapentine and 25-desacetylrifapentine pharmacokinetic data for HIV-infected subjects and healthy young males are presented in Table 1. The mean plasma concentration-versus-time curves for rifapentine and 25-desacetylrifapentine in both the fasted and fed states in HIV-infected volunteers are illustrated in Fig. 1A and B. The mean comparative plasma drug concentration-versus-time curves of rifapentine and 25-desacetylrifapentine in HIV-infected volunteers and in healthy young men are presented in Fig. 1C and D.
TABLE 1.
Comparison of plasma rifapentine and 25-desacetylrifapentine pharmacokinetic data for HIV-infected participants and healthy, young male volunteers
| Drug and parameter | Result for groupa:
|
Paired groups, comparison ratio (90% CI)b | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Rifapentine | |||||
| Cmax (μg/ml) | 11.76 (27) | 9.42 (28) | 17.57 (19) | 14.09 (20) | C-A, 152 (136–168)c; D-B, 153 (134–176)c |
| Tmax (h) | 5.00 (41) | 4.82 (43) | 6.58 (27) | 5.34 (40) | C-A, 137 (109–173); D-B, 113 (91–141)d |
| AUC0–∞ (μg · h/ml) | 319 (27) | 256 (34) | 499 (30) | 374 (21) | C-A, 154 (142–168)c; D-B, 151 (132–172)c |
| CLpo (liter/h) | 2.02 (28) | 2.62 (36) | 1.30 (27) | 1.68 (25) | C-A, 65 (60–71)c; D-B, 66 (58–76)c |
| t1/2 (h) | 15.94 (26) | 17.63 (29) | 15.11 (19) | 16.47 (18) | C-A, 94 (87–102); D-B, 95 (86–105) |
| 25-Desacetylrifapentine | |||||
| Cmax (μg/ml) | 4.03 (30) | 4.45 (33) | 5.91 (23) | 6.25 (27) | C-A; 149 (132–169); D-B, 150 (128–175) |
| Tmax (h) | 19.25 (44) | 15.61 (69) | 21.68 (32) | 19.61 (29) | C-A; 117 (97–141); D-B, 172 (114–259) |
| AUC(0–∞) (μg · h/ml) | 177 (33) | 215 (39) | 267 (23) | 294 (30) | C-A, 154 (138–172); D-B, 151 (125–182) |
| t1/2 (h) | 14.29 (24) | 18.32 (26) | 13.27 (21) | 14.17 (24) | C-A, 95 (81–112); D-B, 76 (66–88) |
Groups: A, 20 fasted healthy subjects; B, 16 fasted HIV-infected subjects; C, 20 fed healthy subjects; D, 15 HIV-infected subjects.
CI, confidence interval.
P < 0.001.
P = 0.326.
FIG. 1.
(A and B) Mean plasma drug concentrations in both the fed and fasted states. (A) Rifapentine in HIV-infected volunteers. (B) 25-Desacetylrifapentine in HIV-infected volunteers. (C) Comparison of mean plasma rifapentine concentrations in HIV-infected volunteers and healthy subjects. (D) Comparison of mean plasma 25-desacetylrifapentine concentrations in HIV-infected volunteers and healthy subjects.
Rifapentine was quantifiable in plasma for 48 h after drug administration in all subjects and for up to 72 h in 12 of 15 subjects when given with food. The AUC0–72 accounted for over 90% of the total AUC0–∞ for 25 of 31 profiles, indicating that the sampling scheme was appropriate for the AUC0–∞ calculations.
Plasma rifapentine concentrations peaked 4.8 to 5.3 h after dosing, on average, in the HIV-infected subjects. The disposition of rifapentine in HIV-infected subjects was monophasic, with a mean terminal t1/2 of 16.5 to 17.6 h. AUC0–∞ and Cmax increase of 51 and 53% were observed when rifapentine was given with a high-fat breakfast, respectively. However, there was no statistically significant difference between the rates of rifapentine absorption in the fasted and fed states (P = 0.326).
The 25-desacetyl metabolite formed slowly, with peak plasma drug concentrations observed 15.6 to 19.6 h after rifapentine administration. The plasma drug concentration-versus-time profile of 25-desacetylrifapentine mimicked the profile of rifapentine with a t1/2 of 14.2 to 18.3 h. Similar concentrations of rifapentine and 25-desacetylrifapentine in plasma were observed 24 h after dosing. However, higher concentrations of 25-desacetylrifapentine, compared with the parent compound rifapentine, in plasma persisted at the final sample collection time at 72 h after dosing.
When rifapentine was administered during fasting, the mean Cmax and AUC0–∞ of rifapentine were both 20% lower in HIV-infected subjects than in healthy, young male subjects. However, the mean Cmax and AUC0–∞ of the active metabolite 25-desacetylrifapentine in HIV-infected subjects were 10 and 21% higher, respectively, than in healthy subjects. The mean CLpo of rifapentine was 30% higher in HIV-infected subjects than in healthy males. The t1/2s of rifapentine and its metabolite 25-desacetylrifapentine were similar between the HIV-infected and healthy volunteers. Although concentrations of the parent drug in plasma were slightly lower in the HIV-infected subjects, those of its active metabolite were slightly higher than in healthy young males, indicating that rifapentine would provide equal coverage for M. tuberculosis in the HIV-infected and healthy volunteers.
Safety.
Both of the treatments were well tolerated by HIV-infected subjects. No serious adverse events occurred during the study that were attributable to rifapentine. A total of 21 adverse events occurred, 4 events (headache and nausea) were assessed by the investigator as possibly treatment related, and 17 were assessed as not treatment related.
DISCUSSION
Mycobacterial diseases continue to plague various geographic domains of the world. In certain areas of the United States and throughout many other countries of the world, tuberculosis, including multiple-drug-resistant tuberculosis, remains a therapeutic challenge to clinicians. As HIV emerged, later stages of infection (CD4 counts of <100 cells/mm3) were associated with a higher frequency of MAC infection. Both of these mycobacterial diseases are reported with increased frequency in immunocompromised patients, including those patients in various stages of HIV infection. Rifapentine appears to be at least as active in vitro as previous rifamycin compounds for the treatment of these diseases while offering an extended t1/2 that prolongs both serum and intracellular drug concentrations.
The recent introduction of protease inhibitors has created a paradigm shift, dramatically changing the treatment of HIV and AIDS. Fewer patients are progressing to the traditional advanced stages of AIDS, and therefore, reduced numbers of patients are being treated for and given prophylaxis against the more common opportunistic infections. Efforts to pursue rifapentine development were recently discontinued for MAC indications. The main reason for this was that there were not enough patients available for enrollment in efficacy trials. The development of resistance to the current protease inhibitor regimens, perpetuated by nonadherence and tolerability issues, could realign the previous paradigm of disease progression.
Since Mycobacterium species often cause infection intracellularly, it is important to also examine the intracellular drug penetration potential of new antimycobacterial therapies. Mor et al. compared the intracellular activities of rifapentine and rifampin against M. tuberculosis in an experimental model of intracellular infection (9). Once-weekly exposures of infected macrophages to rifapentine concentrations equal to that achieved following administration of a 600-mg dose resulted in a marked reduction of the mycobacterial burden that was maintained over the 4-week study period. The study also concluded that rifapentine demonstrated a greater ability to penetrate macrophages, achieving a four- to fivefold greater ratio of intracellular accumulation than rifampin.
In our study, food significantly increased the extent of absorption of rifapentine in participants seropositive for HIV (P < 0.001). In fact, the mean concentrations of both rifapentine and 25-desacetylrifapentine in plasma exceeded the MICs for at least 90% of the MAC strains tested 24 h after dosing (4 μg/ml) and for at least 90% of the M. tuberculosis strains tested (0.06 to 0.25 μg/ml) 3 days after dosing (5, 6, 9, 12). The effect of seropositivity for HIV did not appear to impact the pharmacokinetics of rifapentine, as the pharmacokinetic profiles of our participants were similar to those of healthy volunteers (8). In addition, the administration of rifapentine was well tolerated by participants seropositive for HIV, as adverse events during the study period were mild and infrequent. The single-dose pharmacokinetic profile of rifapentine compares similarly with its multiple-dose pharmacokinetic profile, suggesting that dosing modifications for HIV-seropositive patients are unnecessary (7). This is not surprising, since the infrequency of the multiple-dose regimen (administration once or twice weekly for the treatment of tuberculosis) did not result in significant accumulation and autoinduction of rifapentine metabolism did not occur.
Rifapentine offers the advantage of a compound with an extended t1/2 that provides therapeutic plasma drug concentrations against M. tuberculosis for at least 72 h after dosing. For patients with tuberculosis who require lengthy therapeutic courses with multiple and often complex medication regimens, extended-interval dosing offers enhanced compliance potential.
ACKNOWLEDGMENT
This work was supported by Hoechst Marion Roussel, Inc.
REFERENCES
- 1.Birmingham A, Coleman J, Orme M L E, Park B K, Pearson N J, Short A H, Southgate P J. Antibacterial activity in serum and urine following oral administration in man of DL473 (a cyclopentyl derivative of rifampicin) Br J Clin Pharmacol. 1978;6:455P–456P. doi: 10.1111/j.1365-2125.1978.tb04626.x. [DOI] [PubMed] [Google Scholar]
- 2.Buniva G, Sassella D, Frigo G M. Proceedings of the 12th International Congress of Chemotherapy. 1983. Pharmacokinetics of rifapentine in man, abstr. 111; pp. 29–33. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1993 Revised CDC HIV classification system and expanded AIDS surveillance definition for adolescents and adults. Morbid Mortal Weekly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 4.Chan S L, Yew W W, Porter J H D, McAdam K P W J, Allen B W, Dickinson J M. Comparison of Chinese and Western rifapentines and improvement of bioavailability by prior taking of various meals. Int J Antimicrob Agents. 1994;3:267–274. doi: 10.1016/0924-8579(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson J M, Mitchison D A. In vitro activity of selected rifamycins against rifampicin-resistant M. tuberculosis and MAIS-complex mycobacteria. Tubercle. 1987;68:177–182. doi: 10.1016/0041-3879(87)90053-5. [DOI] [PubMed] [Google Scholar]
- 6.Heifets L B, Lindholm-Levy P J, Flory M A. Bactericidal activity in vitro of various rifamycins against M. avium and M. tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 7.Keung, A. C.-F., M. G. Eller, K. A. McKenzie, and S. J. Weir. Single and multiple dose pharmacokinetics of rifapentine and its active metabolite 25-desacetyl-rifapentine in man. Submitted for publication.
- 8.Keung A C-F, Miller T D, Green V I, Ames M, Eller M G, Weir S J. Bioavailability and food effect study of rifapentine in healthy adults. Pharm Res. 1995;12(Suppl.):S419. [Google Scholar]
- 9.Mor N, Simon B, Mezo N, Heifets L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob Agents Chemother. 1995;39:2073–2077. doi: 10.1128/aac.39.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens R C, Jr, Keung A C-F, Gardner S, Eller M G, Weir S J, Nicolau D P, Nightingale C H. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pharmacokinetic and food effect evaluation of rifapentine in subjects seropositive for the human immunodeficiency virus, abstr. A-2; p. 1. [Google Scholar]
- 11.Reith, K., A. C.-F. Keung, P. C. Toren, M. G. Eller, L. Cheng, and S. J. Weir. Mass balance and metabolism of 14C-rifapentine in healthy volunteers. Drug Metab. Dispos., in press. [PubMed]
- 12.Truffot C, Bismuth R, Boval C, et al. Proceedings of the 12th International Congress of Chemotherapy. 1982. The in vitro and in vivo experimental activity of cyclopentyl rifamycin (DL-473) on mycobacterium tuberculosis. Current chemotherapy and immunotherapy, abstr. 693; p. 172. [Google Scholar]
- 13.Wehrli W. Rifampin: mechanisms of action and resistance. Rev Infect Dis. 1983;5(Suppl. 3):S407–S411. doi: 10.1093/clinids/5.supplement_3.s407. [DOI] [PubMed] [Google Scholar]
- 14.Yan B, X N, Zhu L, Zhang F, et al. Absorption and elimination of cyclopentyl-rifamycin in human subjects. Chin J Tuberc Respir Dis. 1987;10:267–268. [PubMed] [Google Scholar]
- 15.Zhang Q, Cao Y, Zhang Y. Clinical pharmacokinetic study on rifapentine. Chin J Antibiot. 1994;19:456–458. [Google Scholar]
- 16.Zheng C, Yi L, Huiyan Z. Pharmacokinetics of domestic rifapentine in patients with acute pulmonary infections. Chin J Antibiot. 1987;12:333–336. [Google Scholar]