Summary
Obesity and its related metabolic disorders, such as diabetes and cardiovascular disease, are major risk factors for morbidity and mortality in the world population. In this context, supplementation with the probiotic strain Bifidobacterium animalis subsp. lactis BPL1 (CECT8145) has been shown to ameliorate obesity biomarkers. Analyzing the basis of this observation and using the pre‐clinical model Caenorhabditis elegans, we have found that lipoteichoic acid (LTA) of BPL1 is responsible for its fat‐reducing properties and that this attribute is preserved under hyperglycaemic conditions. This fat‐reducing capacity of both BPL1 and LTA‐BPL1 is abolished under glucose restriction, as a result of changes in LTA chemical composition. Moreover, we have demonstrated that LTA exerts this function through the IGF‐1 pathway, as does BPL1 strain. These results open the possibility of using LTA as a novel postbiotic, whose beneficial properties can be applied therapeutically and/or preventively in metabolic syndrome and diabetes‐related disorders.
This study provides a novel beneficial biological role of LTA from Bifidobacterium genera not previously described for probiotic LTA. Our findings stablish new evidences for the potential use of LTA from BPL1 as a new postbiotic, having therapeutic and/or preventive application in metabolic syndrome and diabetes‐related disorders.

Introduction
The obesity pandemic is escalating worldwide, with almost two billion adults currently overweight, a third of whom are obese, and up to 2.8 million obesity‐related deaths annually (following WHO guidelines). The current lack of preventive measures that are effective in the long term, or broadly applicable therapeutic approaches, triggers the need for solutions that will rely on policy interventions but also on individual, behavioural and dietary changes to manage and mitigate the burden of obesity, overweight and related comorbidities (Bluher, 2019).
In this context, the growing evidence of a potential causative role of the gut microbiome in the pathophysiology of obesity and its related metabolic disorders, such as diabetes and cardiovascular disease, has led research into the manipulation of microbiota composition and function together with improvement of host metabolism via dietary supplementation (Muscogiuri et al., 2019). Indeed, a number of probiotic strains, defined by the WHO as live microorganisms conferring health benefits to their hosts, have been shown to ameliorate obesity biomarkers, including body mass index, waist circumference and abdominal visceral fat in human clinical studies of overweight and obese subjects (Koutnikova et al., 2019; Pedret et al., 2019; Suzumura et al., 2019; Michael et al., 2020). However, little is known of the exact mechanisms of action by which these strains exert their effect (Lebeer et al., 2018). Indeed, to date the intervention of key probiotic effector molecules, so‐called postbiotics such as specific pili, S‐layer proteins, muropeptides or exopolysaccharides are unreported in the context of the microbial modulation of obesity.
Among microbe‐associated molecular patterns, lipoteichoic acids (LTAs) have been recognized to mediate the interaction with host pattern recognition receptors and to trigger signalling pathways (Lebeer et al., 2010). These adhesion macroamphiphilic components, externally exposed across Gram‐positive bacteria cell walls, have been so far generally considered as immune‐stimulatory principles (Sarkar and Mandal, 2016). Moreover, LTA shows wide structural diversity among taxonomic groups, generally formed by a hydrophilic polyglycerol‐phosphate (GroP) backbone substituted with alanine and/or sugars and covalently linked to hydrophobic glycolipids of cell membranes (Claes et al., 2012; Shiraishi et al., 2016). While these complex glycolipids act as virulence factors in pathogens such as Staphylococcus aureus, LTA from probiotics like Lactobacillus strains has been described as immunomodulatory agents (Morath et al., 2005; Sarkar and Mandal, 2016; Shiraishi et al., 2016).
Interestingly, bifidobacteria have long been known to produce atypical LTA structures, better named as lipoglycans, formed by galacto‐furanan chains with glycerophosphate substitutions anchored to the cell membrane by a glycolipid, instead of sugar substituted polyglycerol phosphate chains (Fischer et al., 1987; Iwasaki et al., 1990; Percy and Grundling, 2014; Colagiorgi et al., 2015). Unlike LTA produced by lactobacilli, which have been shown to interact with TRL2 type receptors, bifidobacterial LTAs have scarcely been studied in terms of host interaction and neither have bifidobacterial LTA‐mediated biological activities been demonstrated in vivo (Lebeer et al., 2012; Sarkar and Mandal, 2016).
Previously, the reduction of body adiposity by the probiotic strain BPL1 (Bifidobacterium animalis subsp. lactis CECT 8145) or its heat‐treated form has been demonstrated by our group in pre‐clinical studies using the nematode Caenorhabditis elegans, Zücker rats and Wistar rats under a cafeteria diet (Martorell et al., 2016; Caimari et al., 2017; Carreras et al., 2018). Moreover, the effects of both BPL1 and its heat‐treated form have been positively assessed in human volunteers with abdominal obesity (Pedret et al., 2019). However, the responsible molecule for these beneficial effects remained unidentified and uncharacterized.
Here, we describe the isolation of LTA from BPL1 and demonstrate that this postbiotic compound is able to reduce fat deposition in C. elegans through the IGF‐1 pathway. This is the first description of the beneficial effect of a bifidobacterial LTA showing the molecular target and pathways underlying its mechanism of action.
Results
Preliminary experiments were conducted to identify the subcellular location of BPL1 functional molecule(s). Having discarded the functionality of other fractions from BPL1 cultures (such as culture supernatant and DNA), while finding evidence of efficacy of BPL1 cell envelope extracts, our working hypothesis was that the fat‐reducing effect of the probiotic strain BPL1 (B. animalis subsp. lactis CECT 8145) (both in its alive and its heat‐treated form) could be due to its LTA.
Lipoteichoic acid (LTA) from BPL1 strain exerts fat‐reducing effects in vivo
LTA is an important component of the cell wall of Gram‐positive bacteria that is anchored to cell membranes by lipidic moieties (Schneewind and Missiakas, 2014; Shiraishi et al., 2016). LTA is also known to be involved in adhesion of probiotics to intestinal cells (Granato et al., 1999).
Although LTA has been described in some cases as a pro‐inflammatory molecule, several studies have reported the use of LTA for the treatment and/or prevention of human diseases and/or syndromes. For instance, some works describe LTAs from Bacillus or Lactobacillus strains as anti‐inflammatory and immune modulators (Lebeer et al., 2012; Gao et al., 2016; Mizuno et al., 2020). More recently, LTA from Lactobacillus paracasei was shown to boost mucin production and reduce leaky gut and inflammation when analysed in mice (Wang et al., 2020). However, to our knowledge, there are no previous reports describing the relationship between LTA and fat‐reducing effects, nor in vivo functional characterization of bifidobacterial LTAs.
To test this, we isolated and purified LTA from BPL1 strain overnight cultures grown in MRS‐Cys. Isolation and purification of LTA were conducted by butanol extraction of the insoluble fraction of BPL1 cellular lysates, followed by hydrophobic exchange chromatography, as previously described to obtain high‐quality LTA (Morath et al., 2001; Draing et al., 2006; Gründling and Schneewind, 2007; Kho and Meredith, 2018). A single elution peak was obtained by phosphate assay (Fig. S1A). The fractions forming the peak were pooled and used as purified LTA. The purity of LTA with respect to potential cross‐contaminants, cell‐wall material and nucleic acid was determined by specific analysis. Endotoxin contamination was excluded through an LAL assay, and endotoxin content was < 5 EU mg−1 in the lyophilized LTA. Nucleic acids contamination was determined by measuring UV absorption at 260 nm and 280 nm. DNA/RNA accounted for less than 1.5% wt/wt. Peptidoglycan (PG) contamination was excluded by analysis of derived muropeptides after mutanolysin treatment in which no peaks of PG fragments were detected. In addition, N‐acetylmuramic acid (MurNAc) or the amino acids ornithine, lysine and serine, known to be present in the PG of BPL1 (previously determined by 1D‐ and 2D‐TLC (thin‐layer chromatography) of the total hydrolysate of the PG of BPL1, data not shown), were not detected by gas or liquid chromatography of the total hydrolysate of purified LTA. To further characterize purified LTA, a sample was stained through Alcian blue/silver staining following SDS‐PAGE (Fig. S1B), and analysed using MALDI‐TOF mass spectrometry and nuclear magnetic resonance (Fig. S1D), revealing a compound with an estimated molecular mass distribution in the range of 8–10 kDa.
The fat‐reducing potential of LTA was tested in the model organism C. elegans. This nematode stores lipids in hypodermic and intestinal cells, easily detected by staining with Nile red. Molecular mechanisms involved in lipid synthesis, degradation and transport are conserved between C. elegans and mammals (Ashrafi et al., 2003; Jones and Ashrafi, 2009). Evaluation of the nematode’s fat content by fluorescence quantification in a spectrofluorometer indicated a significant reducing effect of LTA at doses ranging from 50 to 0.1 µg ml−1. Particularly, 10 µg ml−1 was the lowest dose providing the highest fat‐reducing effect (26.2% of fat reduction) (Fig. 1A). Higher doses of LTA (20 and 50 µg ml−1) exert similar reducing effect than 10 µg ml− 1 (Fig. 1A). In order to validate these results, lipid content was assessed by fluorescence microscopy using Nile red staining. Nematodes fed with of BPL1 cells, heat‐treated cells (HT‐BPL1) or LTA‐BPL1 (using the effective dose of 10 µg ml−1) showed reduced fluorescence intensity compared with control‐fed nematodes, as observed by microscope imaging (Fig. 1B).
Fig. 1.
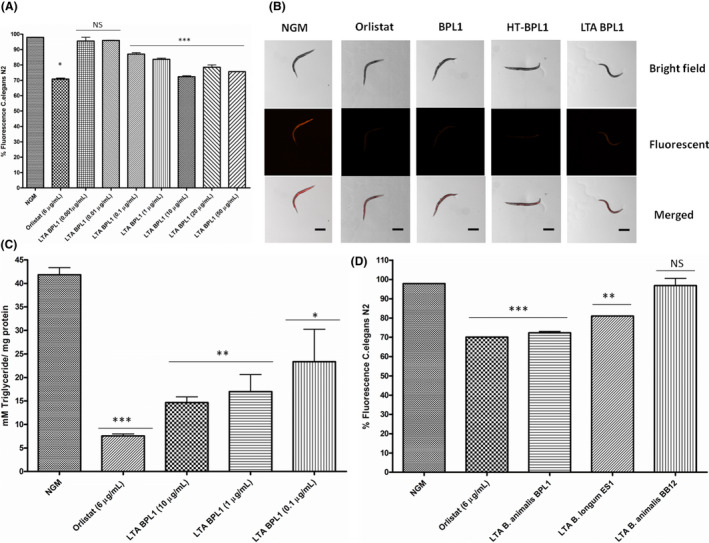
Lipoteichoic acid (LTA) from BPL1 strain demonstrates fat‐reducing activity. A. Fat‐reducing effect of purified LTA at different doses. B. Representative images of Nile red staining of lipid content in live young adult C. elegans in a wild‐type N2 animal under fluorescence microscopy. Nematodes were fed with BPL1 cells, heat‐treated BPL1 cells (HT‐BPL1) or LTA (10 µg ml‐1). Scale bar 250 µm. Original image taken by the authors for this paper with a Nikon‐SMZ18 Fluorescence Stereomicroscope. C. Quantification of triglyceride content (mM TG/mg protein) in C. elegans fed with purified LTA from BPL1. D. Analysis of fat‐reducing activity of LTAs from B. longum ES1 and B. animalis subsp. lactis BB12. For a, b percentage of fluorescence in bacterial and LTA‐fed nematodes (wild‐type strain N2) is represented. Nile red was quantified at young adult stage. Orlistat (6 µg ml‐1) was used as positive control. Data are mean ± SD. and were calculated from two biological independent experiments. For A, B, C, D *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
As triglycerides (TGs) are the main constituents in lipid droplets stored in C. elegans, and lipid accumulation has been associated with increase in TG content in this nematode (Zhang et al., 2011), we further quantified TGs levels in nematodes fed with three LTA effective doses. Orlistat, the anti‐obesity drug with a well‐documented efficacy in weight reduction and weight maintenance (Kiortsis et al., 2005), was included as positive control. Results indicated a significant reduction in total TG content in animals fed with BPL1‐LTA (10 µg ml−1; P < 0.01; 1 and 0.1 µg ml−1; P < 0.05) (Fig. 1C). In the case of Orlistat, a positive effect on TGs reduction was observed, in accordance with previous reports suggesting improvement of serum TGs levels in overweight patients (Kiortsis et al., 2005). These results support the total fat reduction observed, and are consistent with the fat‐reducing effect of the probiotic strain BPL1 (and its heat‐treated form).
In order to further investigate whether the fat‐reducing effect of LTA isolated from BPL1 was a strain‐dependent property, LTAs from other bifidobacterial strains were isolated and purified following the previously described protocols. LTAs from B. longum ES1 and B. animalis subsp lactis BB12 were functionally evaluated for its fat‐reducing capacity in C. elegans. These strains were selected as previous studies indicated that the cells provided high and low fat‐reducing effect respectively (Martorell et al., 2016). Results showed that ES1‐LTA significantly reduced nematode’s fat content, but in a lesser extent than BPL1‐LTA (Fig. 1D). However, LTA purified from BB12 lacks the fat‐reducing activity.
Glucose limiting conditions affects BPL1‐LTA functionality
There is evidence showing that growth conditions can modulate some constituents of LTA (Neuhaus and Baddiley, 2003; Brown et al., 2013; Mistretta et al., 2019). In fact, it is known that glucose is a precursor in the biosynthesis of many envelope components as, for example, PG and teichoic and lipoteichoic acids (van Heijenoort, 2007; Colagiorgi et al., 2015). Therefore, we hypothesized that a limitation of glucose might provoke modification of LTA content and/or composition. To confirm our hypothesis, we assessed whether glucose restriction in the BPL1 culture medium affected LTA functionality and, consequently, the functionality of the strain. LTA was purified from BPL1 cells cultured in MRS‐Cys in glucose‐limiting conditions, vs standard MRS‐Cys medium and evaluated for their ability to reduce fat in C. elegans. LTA extracted from BPL1 cells grown in standard glucose medium was active, whereas LTA isolated from BPL1 grown in restricted glucose medium was non‐functional (Fig. 2A). Accordingly, BPL1 cells cultured in MRS‐Cys, grown in glucose restriction, failed to reduce fat in C. elegans, whereas BPL1 cells cultured in MRS‐Cys with excess glucose, maintained their functionality.
Fig. 2.
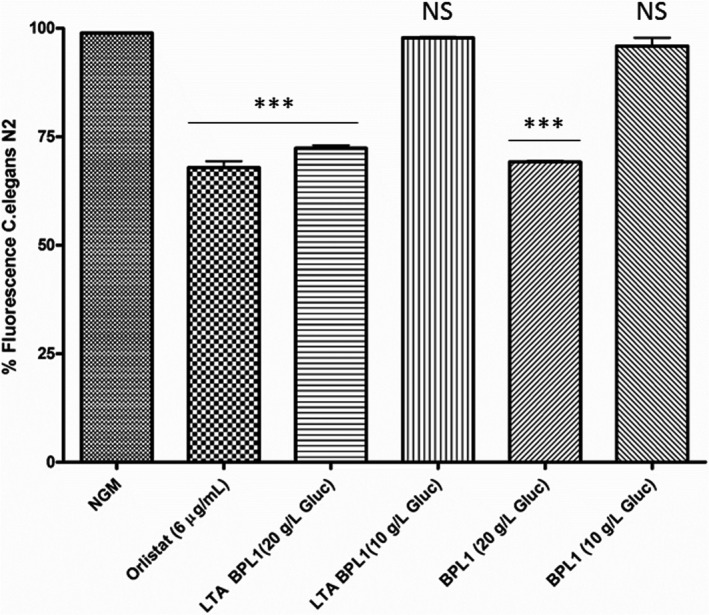
LTA from BPL1 functionality is modified by glucose. Purified LTA obtained from BPL1 in standard MRS‐Cys (20 g l−1) and from BPL1 cells grown in low glucose MRS‐Cys (10 g l−1). BPL1 cells grown in excess or restriction of glucose were included. ***P < 0.001; NS: not significant.
Because no significant changes in LTA yield vs dried biomass were detected in glucose limitation, we further hypothesized that changes in LTA composition in the strain could be impacting functionality. To confirm this, chemical composition of purified LTAs obtained from BPL1 cultures growth in MRS‐Cys with 10 or 20 g l−1 of glucose was analysed. Briefly, the amount of galactose, glycerol, glycerol‐P and phosphorous was determined in purified LTA samples. As shown in Table 1, the relative content of glycerol, and glycerol‐phosphate, versus glucose, was found to be higher in the LTA sample purified from BPL1 grown in MRS‐Cys 20 g l−1 glucose than those from cells grown in glucose limitation, supporting that chemical changes in BPL1‐LTA are responsible for the resulting functionality.
Table 1.
Compositional analysis of BPL1‐LTA in different growth conditions
| Source of LTA | Molar ratio to glucose | % Fat reduction | ||
|---|---|---|---|---|
| Galactose | Glycerol | Glycerol‐P | ||
|
B. animalis subsp lactis BPL1 MRS‐Cys (20 g l‐1 glucose) |
0.8 | 2.2 | 12.6 | 26.9 |
|
B. animalis subsp lactis BPL1 MRS‐Cys (10 g l‐1 glucose) |
0.6 | 0.7 | 1.1 | 2.0 |
Fat‐reducing effect of BPL1‐LTA requires the insulin‐like signalling pathway (IGF‐1) and is preserved under hyperglycaemic conditions
Having demonstrated the efficacy of LTA from the BPL1 strain in fat reduction, we investigated the mechanisms underlying this functional effect, to verify that BPL1 cells and LTA from BPL1 indeed share the same molecular targets. We have previously reported that fat‐reducing and antioxidant activities of BPL1 cells are dependent on the IIS pathway (Martorell et al., 2016). In C. elegans, the insulin‐like signalling pathway (IGF‐1) regulates ageing, immunity and lipid metabolism (Garsin et al., 2003; Zheng and Greenway, 2012). This pathway is conserved across nematodes and mammals, including humans (Morris et al., 1996; Kimura et al., 1997; Lin et al., 1997; Barbieri et al., 2003), and has been a key area of research in obesity. Downstream insulin signalling pathway, DAF‐16, codes for the Forkhead family of transcription factors (FOXO) and plays a central role in mediating the molecular mechanisms triggered by the pathway (Zheng and Greenway, 2012). Due to the evolutionary conservation of the IIS pathway, study of compounds targeting this pathway in C. elegans is likely to shed light on its function in higher organisms and humans. To investigate the role of the IIS pathway in LTA‐mediated fat reduction, DAF‐2 (insulin receptor)/DAF‐16, the key regulators in the IIS pathway were evaluated. The fluorescence assays showed that the DAF‐16 mutation (daf‐16 (mgDf50)) abolished the LTA‐mediated fat‐reducing effect (Fig. 3A). This was also the case for BPL1 and HT‐BPL1 cells. This result strongly suggests that fat reduction induced by BPL1 and its LTA is DAF‐16 dependent. DAF‐2 is the human insulin receptor homologue upstream DAF‐16 in the IIS pathway, and as in humans, mutations in the insulin receptor increase fat accumulation, showing an obese phenotype (Zheng and Greenway, 2012). Our results show that feeding DAF‐2 mutant (daf‐2 (e1370)) worms with LTA and also with BPL1 or HT‐BPL1 cells do not produce a fat‐reducing phenotype, indicating that LTA‐mediated regulation of DAF‐16 is dependent on DAF‐2, and therefore, LTA effect requires the insulin/IGF‐1‐like signalling pathway (IIS) (Fig. 3A).
Fig. 3.
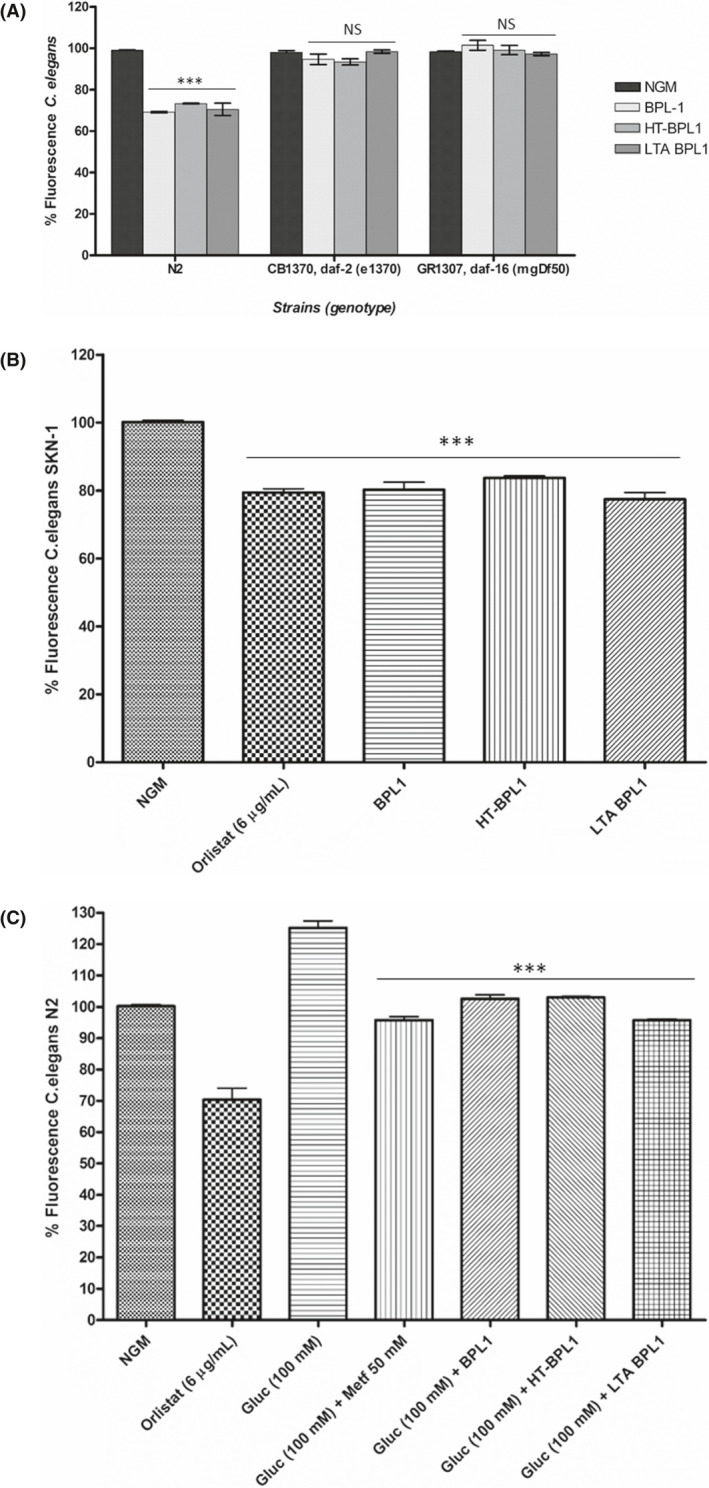
Lipoteichoic acid (LTA) from BPL1 strain requires the Insulin‐like signalling pathway (IGF‐I) to exert its fat‐reducing effect, and has functional activity in hyperglycaemic conditions. A. Feeding worms with LTA in mutant daf‐2 and daf‐16 strains; the same as with the live BPL1 cells and the heat‐treated BPL1 cells. B. Fat content in C. elegans mutant for SKN‐1 transcription factor treated with LTA from BPL1. C. Effect of treatments on a C. elegans hyperglycaemic model. Nematodes of wild‐type strain N2 grown in high glucose (100 mM). Metformin was used as positive control. For A, B, C percentage of fluorescence in LTA‐fed nematodes (wild‐type strain N2, GR1307, daf‐16 (mgDf50), CB1370, daf‐2 (e1370), or LG333 Skn‐1 (zu 135)). Nile red staining was quantified at young adult stage. Orlistat (6 µg ml−1) was used as positive control. Data are mean ± SD. and were calculated from two independent biological experiments. ***P < 0.001, NS: not significant.
Different authors have reported the activation of p‐38 mitogen‐activated protein kinases pathway (p38 MAPK pathway) by probiotic strains. In this sense, there are previous reports of improved lifespan, locomotion and reproductive viability achieved by feeding C. elegans with the Bifidobacterium longum subsp. infantis ATCC 15697T (Komura et al., 2013). The prolongevity effect occurred when cell‐wall fractions where used, via activation of the SKN‐1/p‐38 MAPK pathway, but not through the activation of DAF‐16 system. In our study, we observed a different response both with BPL1 and HT‐BPL1 that is independent of SKN‐1, as fat‐reducing effects were still observed in a C. elegans mutant deficient in the orthologue of mammalian Nrf transcription factor (Inoue et al., 2005) (Fig. 3B). Similarly, LTA also reduces fat content in C. elegans mutant of SKN‐1 transcription factor (Fig. 3B), suggesting that SKN‐1 is not required for its function. Interestingly, these results indicate that LTA from BPL1 follows a different mechanism to that reported for LTA from L. paracasei, which modulates p38‐MAPK pathway to reduce age‐related inflammation (Wang et al., 2020).
The insulin signalling pathway is also involved in glucose transport (Das, 1999; Zheng and Greenway, 2012), playing a role in glucose homoeostasis and insulin sensitivity (Hong et al., 2017). Therefore, it is a target pathway for the study of diabetes (or obesity‐related diabetes) and the compounds targeting this pathway emerge as potential therapeutics, such as metformin (Anisimov, 2013). Diabetes mellitus is a metabolic disorder that affects a growing number of the world's population and is characterized by elevated blood glucose levels, resulting in low insulin or insulin resistance. Due to the chronic elevated levels of glucose, diabetic patients have increased risk to develop disorders like type 2 diabetes, obesity, nephropathy and atherosclerosis (Brownlee, 2001). In C. elegans, glucose has been shown to shorten lifespan by upregulating IGF‐1 pathway (IIS) activity or increasing reactive oxygen species (ROS) (Schlotterer et al., 2009), and because of that, it has been suggested that C. elegans is a good model system to evaluate glucose toxicity and to develop more efficient diabetes therapies. This nematode has been used as model to study the hyperglycaemic characteristic of diabetes (Zhu et al., 2015), with results showing a shortened lifespan in high‐glucose‐treated worms by upregulation of IGF‐1 pathway (IIS). Furthermore, glucose increases fat accumulation in worms, as it does in mammals (Zheng and Greenway, 2012), and glucose‐fed nematodes exhibit an increased triglyceride content and enlarged body size (Alcantar‐Fernandez et al., 2018). Moreover, glucose may affect the stress response (Schulz et al., 2007).
We have used high‐glucose‐fed nematodes to model diabetes and evaluate the efficacy of LTA. Taking into account that glucose is a known precursor of TGs and that are the main components of lipid droplets in the nematode, we evaluated the effect of glucose (100 mM) on nematode fat content. Our results showed that fat content increased by 20%, in nematodes fed on high‐glucose NGM, in agreement with other reports (Fig. 3C). Metformin (biguanide), a drug used in the management of type‐2 diabetes, was included as positive control. Furthermore, we evaluated whether LTA could reduce fat content in a diabetic obese C. elegans model. Hyperglycaemic nematodes fed with LTA showed a significant reduction in body fat content (Fig. 3C). This effect was also recorded in nematodes fed with BPL1 and HT‐BPL1 cells (Fig. 3C). Very recently, we have published the results of a clinical trial of children with Prader–Willi treated with the BPL1 probiotic strain (Amat‐Bou et al., 2020). Results of this trial clearly indicated a positive effect of BPL1 on insulin levels and HOMA index (Homeostatic Model Assessment for Insulin Resistance). All these results show the potential of BPL1 cells and its heat‐treated form, HT‐BPL1, as ingredients for therapeutic and/or preventive uses in diabetes‐related obesity. Furthermore, and for the first time, the efficacy of LTA is shown in an in vivo hyperglycaemic model, highlighting its relevance not only as a functional signalling molecule but also as a postbiotic with proven beneficial effects.
Discussion
Overall, our findings reveal a previously unrecognized role for LTA as a new lipid modulator exhibiting fat‐reducing properties in the pre‐clinical model of C. elegans. As mentioned above, different roles have been described for probiotic LTA, particularly LTA from lactic acid bacteria, as for example host adhesion mediation and immunomodulation (Lebeer et al., 2012; Shiraishi et al., 2016; Kim et al., 2017). However, no effect of LTA on obesity and hyperglycaemia has been described yet. Further analysis should be undertaken to define putative additional relationship of LTA effect with longevity and oxidative stress, both regulated by the insulin pathway that plays an important role in obesity and metabolic syndrome.
In addition, the functionality of probiotic strain BPL1, and in turn of BPL1‐LTA, has been shown to be abolished when the microorganism is cultured in glucose limiting conditions. This has been correlated with a change in the composition of the molecule. This is, to our knowledge, the first report to describe the modulation over functionality in a bifidobacterial probiotic strain, attending to processing conditions, and related to the composition of the molecule responsible for the functional effect. Due to the high complexity of LTA, further analysis will be necessary to fully elucidate the chemical structure of LTA from BPL1 and gain a better understanding of the structure–function relationship.
Few reports summarize findings on the beneficial health properties of probiotic LTA (Lebeer et al., 2012; Gao et al., 2016), and thus, our study is not only the first one to show a novel beneficial biological role of LTA from genus Bifidobacterium, but also the first one that demonstrates the involvement of LTA in fat‐reducing activity using C. elegans as a host model.
Our results illustrate the potential of LTA obtained from BPL1 probiotic strain as a new postbiotic, having therapeutic and/or preventive application in metabolic syndrome and diabetes‐related disorders. Such applications include promising uses as an ingredient in human nutrition, including food and beverages, nutritional supplements and also medical foods. Current evidence on the health benefits of postbiotics is steadily growing, but their future acceptance will require solid studies to elucidate the mechanisms underlying the functionality of the isolated molecules. Here, we show that LTA from BPL1 requires the IGF‐1 pathway to exert its fat‐reducing function, establishing the basis for a more in‐depth study on the use of this postbiotic as adjuvant in treatment of metabolic syndrome.
Experimental procedures
C. elegans strains and maintenance
Caenorhabditis elegans N2 wild‐type strain (Bristol) and the mutant strains GR1307, daf‐16 (mgDf50), CB1370, daf‐2 (e1370), and LG333 Skn‐1 (zu 135) were provided by Caenorhabditis Genetic Center (CGC), University of Minnesota (USA).
The nematode strains were routinely propagated on Nematode Growth Medium (NGM) plates with Escherichia coli strain OP50 as a food source at 20°C. Worms were synchronized by isolating eggs from gravid adults at 20°C, and eggs were hatched in NGM plates. In the experiments for fat quantification, the worms were fed with the different compounds from egg to adult stage.
To induce hyperglycaemic conditions, nematodes were grown in NGM plates supplemented with 100 mM of glucose until reaching the young adult stage (Zhu et al., 2015).
Bacterial strains and culture conditions
Bifidobacterium animalis subsp. lactis BPL1 (CECT 8145) and Bifidobacterium longum ES1 (CECT 7347) were isolated from faeces of healthy babies under breast‐milk feeding as described elsewhere (Izquierdo et al., 2008; Martorell et al., 2016). The commercial strain Bifidobacterium animalis subsp. lactis BB12 was also included in the study. For standard cultivation, all the bifidobacterial strains were grown in MRS medium [Peptone from casein, tryptic digest 10 g l−1; meat extract 10 g l−1; yeast extract 10 g l−1; D‐glucose 20 g l−1; K2HPO4 2 g l−1; di‐ammonium hydrogen citrate 2 g l−1; sodium acetate 5 g l−1; MgSO4 0.2 g l−1; MnSO4 0.05 g l−1; Tween 80 1 g l−1) supplemented with cysteine (Sigma, 0.05% wt/vol)], MRS‐Cys, for 18 h at 37°C in an anaerobiosis atmosphere generated by GasPakTM EZ Anaerobe Container System (BD). In order to achieve glucose limitation, BPL1 cells were cultured in MRS‐Cys with a starting concentration of 10 g l−1 glucose. Glucose consumption kinetics were determined and correlated with bacterial growth, to validate that residual glucose in supernatant was limiting in MRS‐10, whereas glucose remained above 5 g l−1 in MRS‐20 both in exponential and stationary phases.
Escherichia coli OP50 strain was cultured in LB broth (Bacto‐tryptone 10 g l−1; Bacto‐yeast 5 g l−1; NaCl 5 g l−1) for 18 h at 37°C and used in seeding NGM plates.
To test the influence of glucose contained in the culture medium on the functional effect of LTA from BPL1, glucose concentration was modified in the MRS‐Cys medium. BPL1 was grown in MRS‐Cys with different concentrations of D‐glucose (20, 15 and 10 g l−1), maintaining the other ingredients constant (see recipes above). After 18 h at 37°C in an anaerobic atmosphere generated by GasPakTM EZ Anaerobe Container System (BD), bacteria cultured under these glucose conditions were tested in the C. elegans fat reduction assay. Cells were harvested by centrifugation and washed three times with saline solution and used for LTA isolation and purification.
Fat reduction assays in C. elegans
The C. elegans fat content under the different treatments (cells or purified LTA) was measured using the Nile red (Sigma, St. Louis, MO, USA) staining method, following the protocol previously described (Martorell et al., 2016). In the case of cells, both alive and heat‐inactivated (108 cells/plate) were added to the NGM surface. The dye was added to the surface of NGM plates seeded with OP50 at a final concentration of 0.05 µg ml−1. Positive control of the assay was NGM plates with E. coli OP50 with anti‐obesity drug Orlistat (6 µg ml−1) (Kiortsis et al., 2005). Synchronized worms were incubated in these plates for three days until reaching young adult stage. After this period, nematodes were transferred to M9 buffer and the fluorescence was measured using an FP‐6200 system (JASCO Analytical Instrument, Easton, MD, USA) with a λex = 480 nm and λem = 571 nm. Two experiments were performed per condition to analyse a total of 120 nematodes per condition/treatment.
Triglyceride (TG) quantification
Total TGs were quantified in nematodes fed with LTA from BPL1 using the Triglyceride Quantification Kit (Biovision, Mountain View, CA, USA). Age‐synchronized worms were cultured in NGM plates already seeded with E. coli OP50 or NGM plates supplemented with purified LTA (10, 1 and 0.1 μg ml−1). The anti‐obesity drug Orlistat was included as positive control. Orlistat is an inhibitor of intestinal lipases that are responsible for the breakdown of dietary triglycerides into fatty acids and monoglyceride, reducing fat absorption. In addition, they reduce serum TG levels and lipoprotein‐associated phospholipase A2 (PA2) (Kiortsis et al., 2005; Filippatos et al., 2007). Worms at young adult stage were then collected and washed using M9 buffer. After worm settling, supernatant was removed, and 400 μl of the triglyceride assay buffer was added to worm pellet. Worms were sonicated with a digital sonifier (Branson Ultrasonics Corp., Danbury, CT, USA) using four pulses of 30 s at 10% power. Protein content of each condition was measured using BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Samples were slowly heated twice at 90°C for 5 min in a thermomixer (ThermoFisher, Vantaa, Finland) to solubilize all TG in the solution. After brief centrifugation, aliquots (50 μl per well) were used for the triglyceride assay following the manufacturer instructions. Four different biological replicates were included for each condition in four independent experiments.
Microscopy analysis
A fluorescent stereomicroscope was used to visualize the Nile red‐stained lipid droplets of nematodes under different treatments. Populations of worms incubated from egg to young adult stage in NGM plates with Nile red (0.05 µg ml−1) in the same conditions tested in regular C. elegans fat reduction assay (BPL1 cells, HT‐BPL1 cells and LTA‐BPL1). Age‐synchronized worms were transferred to a new agarose 1% (wt/v) plates, and fluorescence was measured in a Fluorescence Stereomicroscope (Nikon‐SMZ18, Konan, Minato‐ku, Tokyo, Japan), equipped with NIS‐ELEMENT image software. A total of 30 worms were analysed per condition. Orlistat (6 µg ml−1) was used as positive control.
LTA purification and analysis
The bacteria underwent butanol extraction and hydrophobic interaction chromatography as described previously (Kho and Meredith, 2018). Briefly, bacteria were recovered from overnight grown cultures, by centrifugation at 12 000 g 15 min and washed twice with sodium citrate 50 mM pH 4.7. The bacterial pellet was suspended in sodium citrate 50 mM pH 4.7 and disrupted in a PANDA PLUS 2000 homogenizer (GEA) at 1000 bar. Insoluble cellular material was collected by centrifugation at 12 000 g 90min, suspended in sodium citrate 50 mM pH 4.7 and extracted for 45 min 37°C with an equal volume of 1‐butanol. The aqueous phase containing LTA was retrieved, freeze‐dried, dissolved in sodium citrate 50 mM pH 4.7 and loaded onto a hydrophobic interaction chromatography column (HiTrap Octyl FF).
Lipoteichoic acid was purified with a linear 15–65% 1‐propanol gradient in sodium citrate 50 mM pH 4.7. Fractions containing LTA were determined by a phosphate assay as described elsewhere (Draing et al., 2006). Phosphate positive samples were pooled and dialysed (Fig. S1).
Purified LTA was analysed by GC‐Q‐MS, after acid hydrolysis. Briefly, LTA was dissolved at 1 g l−1 in HCl 2 M, and acid hydrolysis conducted at 100°C for 2 h. D‐glucose, D‐galactose, glycerol and glycerol‐3‐phosphate were determined as their trimethylsilyl derivatives (Fiehn et al., 2000). GC was conducted in an Agilent 7820A gas chromatographer using a GC column DB5‐MS, coupled to a 5977B mass detector, and identification by comparison with Agilent Fiehn GC/MS Metabolomics RTL Library.
Biochemical characterization of LTAs
Lipoteichoic acid molecules were characterized by matrix‐assisted laser desorption ionization–time of flight (MALDI‐TOF) mass spectrometry in the proteomics facility of SCSIE University of Valencia (this proteomics laboratory is a member of Proteored PRB3 and is supported by grant PT17/0019 of the PE I + D+I 2013–2016, funded by ISCIII and ERDF). Briefly, 1 µl of a sample and 1 µl of a matrix solution (10 mg ml−1 CHCA in 70% ACN, 0.1% TFA) were spotted onto the sample plate. After drying, the sample was analysed with a mass spectrometer (5800 MALDI TOFTOF, ABSciex) in reflector mode, a mass range of 1660–1500 m/z, with lase intensity of 6000.
Lipoteichoic acid was further analysed by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). The molecular weight of LTA was shown in the SDS‐PAGE gel imaged using the cationic dye Alcian blue coupled with modified silver staining for enhanced sensitivity (Kho and Meredith, 2018).
The structure of the LTA was assayed by 1H nuclear magnetic resonance (NMR) spectrometry. Briefly, 1H‐NMR was performed on a Bruker AVANCE III 700 Ultrashield spectrometer (Bruekr BioSpin, Rheinstetten, Germany) operating at a 1H frequency of 700.13 MHz, and equipped with a 5 mm TCI (cryoprobe) with Z‐gradient. The acquisition pulse sequence used were those from Bruker Topspin 3.6 with water presaturation and 2 s recycle time. Spectra were referenced using the TSP signal at 0 ppm.
The purity of the LTA was determined by measuring its endotoxin content with the Limulus amebocyte lysate assay (Lonza Bioscience, Basel, Switzerland) since the assay is insensitive to LTA (Morath et al., 2001). DNA and RNA contaminations were determined by measuring ultraviolet (UV) absorption at 260 and 280 nm (NanoDrop Spectrophotometer; Thermo Fisher Scientific). Sugar component of PG backbone N‐acetylmuramic acid was determined by liquid chromatography analysis carried out in an Alliance 2695 HPLC System (Waters Corporation, Milford, MA, USA)) coupled to a refractive index detector (model 2414; Waters Corporation) and an ion‐exchange column (Aminex HPX‐87H; Bio‐Rad, Hercules, CA, USA). Amino acids ornithine, lysine and serine were determined by GC‐Q‐MS, after acid hydrolysis of purified LTA (HCl 2 M, 10°C, 2 h) and trimethylsilyl derivatization (Fiehn et al., 2000). GC was conducted in a gas chromatographer (model 7820A; Agilent, Santa Clara, CA, USA) using a GC column DB5‐MS, coupled to a 5977B mass detector, and identification by comparison with Agilent Fiehn GC/MS Metabolomics RTL Library.
For enzymatic hydrolysis of PG, sample of purified LTA was treated (0.04 mg ml 37°C, 4 h) with mutanolysin (SIGMA). After filtration (Amicon Ultra‐0.5; Merck Millipore, Darmstadt, Germany), soluble muropeptides were reduced by using sodium borohydride at a final concentration of 5 mg ml−1. The reaction was stopped after 20 min by lowering the pH to 2–4 with phosphoric acid. Fragments were separated with HPLC and a reversed phase octadecyl silica (ODS) C18 column from Phenomenex (Torrance, CA, USA). Elution was conducted at 30°C as follows: linear gradient of 0 to 100% buffer B [50 mM sodium phosphate pH5.10 with 15% (v/v) methanol] over a period of 120 min after 10 min in buffer A (50 mM sodium phosphate pH4.33) with a flow rate of 0.5 ml per minute. Eluted compounds were detected by monitoring Abs 206 nm (Schaub and Dillard, 2017).
Statistical analysis
Results are given as the mean ± standard deviation. Data on fat deposition in C. elegans were analysed by one‐way ANOVA test, using a Tukey’s multiple comparison post‐test. To compare effects among different C. elegans strains, two‐way ANOVA test was used. Differences between groups in cell adhesion assays were analysed by Student’s t‐test. All the statistical analyses were performed with GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA, USA), setting the level of statistical significance at 5%.
Conflict of interest
None declared.
Funding Information
The authors gratefully acknowledge Prof. Dr. Jose Luis Garcia and Dr. Ping Zhong for their thorough revision of the manuscript, and Dr. Blake Hammann, from ADM Research, for his support in NMR testing.
Authors’ contributions
P. M., M. T. and D. R. conceived the experiments, design of the study and analysis of results. F. B. conducted the C. elegans experiments. M. E. performed the biochemical analysis. M. B. and V. N. performed the LTA extractions and purifications. B. Á. and V. N. performed the cell culture experiments. F. B. and S. L. prepared the figures and the statistical analysis of data. P. M. and M. T. wrote the manuscript. D. R., M. E., B. Á., E. C. and F. B. contribute to the final manuscript revision.
Supporting information
Fig. S1. Purification and characterization of lipoteichoic acid (LTA). A. Screening for phosphate content of hydrophobic interaction chromatography fractions after N‐butanol extraction of BPL1 cell‐wall material. B. SDS‐PAGE and Alcian blue/silver staining. C. MALDI‐TOF mass spectrum. D. 1H‐NMR spectrum.
Acknowledgements
The authors gratefully acknowledge Prof. Dr. Jose Luis Garcia and Dr. Ping Zhong for their thorough revision of the manuscript, and Dr. Blake Hammann, from ADM Research, for his support in NMR testing.
Microb. Biotechnol. (2022) 15(00), 805–816
References
- Alcantar‐Fernandez, J. , Navarro, R.E. , Salazar‐Martinez, A.M. , Perez‐Andrade, M.E. , and Miranda‐Rios, J. (2018) Caenorhabditis elegans respond to high‐glucose diets through a network of stress‐responsive transcription factors. PLoS One 13: e0199888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat‐Bou, M. , Garcia‐Ribera, S. , Climent, E. , Piquer‐Garcia, I. , Corripio, R. , Sanchez‐Infantes, D. , et al. (2020) Effects of Bifidobacterium animalis subsp. lactis (BPL1) Supplementation in children and adolescents with Prader‐Willi syndrome: a randomized crossover trial. Nutrients 12: 3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov, V.N. (2013) Metformin: do we finally have an anti‐aging drug? Cell Cycle 12: 3483–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi, K. , Chang, F.Y. , Watts, J.L. , Fraser, A.G. , Kamath, R.S. , Ahringer, J. , and Ruvkun, G. (2003) Genome‐wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272. [DOI] [PubMed] [Google Scholar]
- Barbieri, M. , Bonafe, M. , Franceschi, C. , and Paolisso, G. (2003) Insulin/IGF‐I‐signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab 285: E1064–E1071. [DOI] [PubMed] [Google Scholar]
- Bluher, M. (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15: 288–298. [DOI] [PubMed] [Google Scholar]
- Brown, S. , Santa Maria, J.P. Jr , and Walker, S. (2013) Wall teichoic acids of gram‐positive bacteria. Annu Rev Microbiol 67: 313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee, M. (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820. [DOI] [PubMed] [Google Scholar]
- Caimari, A. , del Bas, J.M. , Boqué, N. , Crescenti, A. , Puiggròs, F. , Chenoll, E. , et al. (2017) Heat‐killed Bifidobacterium animalis subsp. lactis CECT 8145 increases lean mass and ameliorates metabolic syndrome in cafeteria‐fed obese rats. J Funct Foods 38: 251–263. [Google Scholar]
- Carreras, N.L. , Martorell, P. , Chenoll, E. , Genoves, S. , Ramon, D. , and Aleixandre, A. (2018) Anti‐obesity properties of the strain Bifidobacterium animalis subsp. lactis CECT 8145 in Zucker fatty rats. Benef Microbes 9: 629–641. [DOI] [PubMed] [Google Scholar]
- Claes, I.J. , Segers, M.E. , Verhoeven, T.L. , Dusselier, M. , Sels, B.F. , De Keersmaecker, S.C. , et al. (2012) Lipoteichoic acid is an important microbe‐associated molecular pattern of Lactobacillus rhamnosus GG. Microb Cell Fact 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiorgi, A. , Turroni, F. , Mancabelli, L. , Serafini, F. , Secchi, A. , van Sinderen, D. , and Ventura, M. (2015) Insights into teichoic acid biosynthesis by Bifidobacterium bifidum PRL2010. FEMS Microbiol Lett 362: fnv141. [DOI] [PubMed] [Google Scholar]
- Das, U.N. (1999) GLUT‐4, tumor necrosis factor, essential fatty acids and daf‐genes and their role in insulin resistance and non‐insulin dependent diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 60: 13–20. [DOI] [PubMed] [Google Scholar]
- Draing, C. , Pfitzenmaier, M. , Zummo, S. , Mancuso, G. , Geyer, A. , Hartung, T. , and von Aulock, S. (2006) Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae . J Biol Chem 281: 33849–33859. [DOI] [PubMed] [Google Scholar]
- Fiehn, O. , Kopka, J. , Trethewey, R.N. , and Willmitzer, L. (2000) Identification of uncommon plant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal Chem 72: 3573–3580. [DOI] [PubMed] [Google Scholar]
- Filippatos, T.D. , Gazi, I.F. , Liberopoulos, E.N. , Athyros, V.G. , Elisaf, M.S. , Tselepis, A.D. , et al. (2007) The effect of orlistat and fenofibrate, alone or in combination, on small dense LDL and lipoprotein‐assocciated phospholipase A2 in obese patients with metabolic syndrome. Atherosclerosis 193: 428–437. [DOI] [PubMed] [Google Scholar]
- Fischer, W. , Bauer, W. , and Feigel, M. (1987) Analysis of the lipoteichoic‐acid‐like macroamphiphile from Bifidobacterium bifidum subspecies pennsylvanicum by one‐ and two‐dimensional 1H‐ and 13C‐NMR spectroscopy. Eur J Biochem 165: 647–652. [DOI] [PubMed] [Google Scholar]
- Gao, Q. , Gao, Q. , Min, M. , Zhang, C. , Peng, S. , and Shi, Z. (2016) Ability of Lactobacillus plantarum lipoteichoic acid to inhibit Vibrio anguillarum‐induced inflammation and apoptosis in silvery pomfret (Pampus argenteus) intestinal epithelial cells. Fish Shellfish Immunol 54: 573–579. [DOI] [PubMed] [Google Scholar]
- Garsin, D.A. , Villanueva, J.M. , Begun, J. , Kim, D.H. , Sifri, C.D. , Calderwood, S.B. , et al. (2003) Long‐lived C. elegans daf‐2 mutants are resistant to bacterial pathogens. Science 300: 1921. [DOI] [PubMed] [Google Scholar]
- Granato, D. , Perotti, F. , Masserey, I. , Rouvet, M. , Golliard, M. , Servin, A. , and Brassart, D. (1999) Cell surface‐associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte‐like Caco‐2 cells. Appl Environ Microbiol 65: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründling, A. , and Schneewind, O. (2007) Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus . Proc Natl Acad Sci USA 104: 8478–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H. , Cui, Z.Z. , Zhu, L. , Fu, S.P. , Rossi, M. , Cui, Y.H. , and Zhu, B.M. (2017) Central IGF1 improves glucose tolerance and insulin sensitivity in mice. Nutr Diabetes 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, H. , Hisamoto, N. , An, J.H. , Oliveira, R.P. , Nishida, E. , Blackwell, T.K. , and Matsumoto, K. (2005) The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN‐1 in oxidative stress response. Genes Dev 19: 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki, H. , Araki, Y. , Ito, E. , Nagaoka, M. , and Yokokura, T. (1990) Structure of macroamphiphiles from several Bifidobacterium strains. J Bacteriol 172: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo, E. , Medina, M. , Ennahar, S. , Marchioni, E. , and Sanz, Y. (2008) Resistance to simulated gastrointestinal conditions and adhesion to mucus as probiotic criteria for Bifidobacterium longum strains. Curr Microbiol 56: 613–618. [DOI] [PubMed] [Google Scholar]
- Jones, K.T. , and Ashrafi, K. (2009) Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis Model Mech 2: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho, K. , and Meredith, T.C. (2018) Salt‐Induced stress stimulates a lipoteichoic acid‐specific three‐component glycosylation system in Staphylococcus aureus . J Bacteriol 200: e00017–00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.W. , Kang, S.‐S. , Woo, S.‐J. , Park, O.‐J. , Ahn, K.B. , Song, K.‐D. , et al. (2017) Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates Poly I:C‐Induced IL‐8 production in porcine intestinal epithelial cells. Front Microbiol 8: 1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K.D. , Tissenbaum, H.A. , Liu, Y. , and Ruvkun, G. (1997) daf‐2, an Insulin receptor‐like gene that regulates longevity and diapause in Caenorhabditis elegans . Science 277: 942. [DOI] [PubMed] [Google Scholar]
- Kiortsis, D.N. , Filippatos, T.D. , and Elisaf, M.S. (2005) The effects of orlistat on metabolic parameters and other cadiovascular risk factors. Diabetes Metab 31: 15–22. [DOI] [PubMed] [Google Scholar]
- Komura, T. , Ikeda, T. , Yasui, C. , Saeki, S. , and Nishikawa, Y. (2013) Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans . Biogerontology 14: 73–87. [DOI] [PubMed] [Google Scholar]
- Koutnikova, H. , Genser, B. , Monteiro‐Sepulveda, M. , Faurie, J.M. , Rizkalla, S. , Schrezenmeir, J. , and Clement, K. (2019) Impact of bacterial probiotics on obesity, diabetes and non‐alcoholic fatty liver disease related variables: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open 9: e017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Bron, P.A. , Marco, M.L. , Van Pijkeren, J.P. , O'Connell Motherway, M. , Hill, C. , et al. (2018) Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol 49: 217–223. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Claes, I.J.J. , and Vanderleyden, J. (2012) Anti‐inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol 20: 5–10. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Vanderleyden, J. , and De Keersmaecker, S.C. (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171–184. [DOI] [PubMed] [Google Scholar]
- Lin, K. , Dorman, J.B. , Rodan, A. , and Kenyon, C. (1997) daf‐16: An HNF‐3/forkhead family member that can function to double the life‐span of Caenorhabditis elegans . Science 278: 1319–1322. [DOI] [PubMed] [Google Scholar]
- Martorell, P. , Llopis, S. , Gonzalez, N. , Chenoll, E. , Lopez‐Carreras, N. , Aleixandre, A. , et al. (2016) Probiotic strain Bifidobacterium animalis subsp. lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans . J Agric Food Chem 64: 3462–3472. [DOI] [PubMed] [Google Scholar]
- Michael, D.R. , Jack, A.A. , Masetti, G. , Davies, T.S. , Loxley, K.E. , Kerry‐Smith, J. , et al. (2020) A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well‐being. Sci Rep 10: 4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta, N. , Brossaud, M. , Telles, F. , Sanchez, V. , Talaga, P. , and Rokbi, B. (2019) Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Sci Rep 9: 3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, H. , Arce, L. , Tomotsune, K. , Albarracin, L. , Funabashi, R. , Vera, D. , et al. (2020) Lipoteichoic acid is involved in the ability of the immunobiotic strain Lactobacillus plantarum CRL1506 to modulate the intestinal antiviral innate immunity triggered by TLR3 activation. Front Immunol 11: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath, S. , von Aulock, S. , and Hartung, T. (2005) Structure/function relationships of lipoteichoic acids. J Endotoxin Res 11: 348–356. [DOI] [PubMed] [Google Scholar]
- Morath, S. , Geyer, A. , and Hartung, T. (2001) Structure‐function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus . J Exp Med 193: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J.Z. , Tissenbaum, H.A. , and Ruvkun, G. (1996) A phosphatidylinositol‐3‐OH kinase family member regulating longevity and diapause in Caenorhabditis elegans . Nature 382: 536–539. [DOI] [PubMed] [Google Scholar]
- Muscogiuri, G. , Cantone, E. , Cassarano, S. , Tuccinardi, D. , Barrea, L. , Savastano, S. , and Colao, A. (2019) Gut microbiota: a new path to treat obesity. Int J Obes Suppl 9: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus, F.C. , and Baddiley, J. (2003) A continuum of anionic charge: structures and functions of D‐alanyl‐teichoic acids in gram‐positive bacteria. Microbiol Mol Biol Rev 67: 686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedret, A. , Valls, R.M. , Calderón‐Pérez, L. , Llauradó, E. , Companys, J. , Pla‐Pagà, L. , et al. (2019) Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond) 43: 1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy, M.G. , and Grundling, A. (2014) Lipoteichoic acid synthesis and function in gram‐positive bacteria. Annu Rev Microbiol 68: 81–100. [DOI] [PubMed] [Google Scholar]
- Sarkar, A. , and Mandal, S. (2016) Bifidobacteria‐Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res 192: 159–171. [DOI] [PubMed] [Google Scholar]
- Schaub, R. , and Dillard, J. (2017) Digestion of peptidoglycan and analysis of soluble fragments. Bio Protoc 7. doi: 10.21769/bioprotoc.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotterer, A. , Kukudov, G. , Bozorgmehr, F. , Hutter, H. , Du, X. , Oikonomou, D. , et al. (2009) C. elegans as model for the study of high glucose‐ mediated life span reduction. Diabetes 58: 2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind, O. , and Missiakas, D. (2014) Lipoteichoic acids, phosphate‐containing polymers in the envelope of Gram‐positive bacteria. J Bacteriol 196: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, T.J. , Zarse, K. , Voigt, A. , Urban, N. , Birringer, M. , and Ristow, M. (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6: 280–293. [DOI] [PubMed] [Google Scholar]
- Shiraishi, T. , Yokota, S. , Fukiya, S. , and Yokota, A. (2016) Structural diversity and biological significance of lipoteichoic acid in Gram‐positive bacteria: focusing on beneficial probiotic lactic acid bacteria. Biosci Microbiota Food Health 35: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura, E.A. , Bersch‐Ferreira, A.C. , Torreglosa, C.R. , da Silva, J.T. , Coqueiro, A.Y. , Kuntz, M.G.F. , et al. (2019) Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta‐analyses of randomized trials. Nutr Rev 77: 430–450. [DOI] [PubMed] [Google Scholar]
- van Heijenoort, J. (2007) Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol Mol Biol Rev 71: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Ahmadi, S. , Nagpal, R. , Jain, S. , Mishra, S.P. , Kavanagh, K. , et al. (2020) Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3–5 ameliorates aging‐related leaky gut, inflammation and improves physical and cognitive functions: from C. elegans to mice. GeroScience 42: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Bakheet, R. , Parhar, R.S. , Huang, C.H. , Hussain, M.M. , Pan, X. , et al. (2011) Regulation of fat storage and reproduction by Krüppel‐like transcription factor KLF3 and fat‐associated genes in Caenorhabditis elegans . J Mol Biol 411: 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , and Greenway, F.L. (2012) Caenorhabditis elegans as a model for obesity research. Int J Obesity 36: 186–194. [DOI] [PubMed] [Google Scholar]
- Zhu, G. , Yin, F. , Wang, L. , Wei, W. , Jiang, L. , and Qin, J. (2016) Modeling type 2 diabetes‐like hyperglycemia in C. elegans on a microdevice. Integr Biol 8: 30–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Purification and characterization of lipoteichoic acid (LTA). A. Screening for phosphate content of hydrophobic interaction chromatography fractions after N‐butanol extraction of BPL1 cell‐wall material. B. SDS‐PAGE and Alcian blue/silver staining. C. MALDI‐TOF mass spectrum. D. 1H‐NMR spectrum.


