Abstract
2′,6′-Dihydroxy-4′-methoxychalcone (DMC) was purified from the dichloromethane extract of Piper aduncum inflorescences. DMC showed significant activity in vitro against promastigotes and intracellular amastigotes of Leishmania amazonensis, with 50% effective doses of 0.5 and 24 μg/ml, respectively. Its inhibitory effect on amastigotes is apparently a direct effect on the parasites and is not due to activation of the nitrogen oxidative metabolism of macrophages, since the production of nitric oxide by both unstimulated and recombinant gamma interferon-stimulated macrophages was decreased rather than increased with DMC. The phagocytic activity of macrophages was functioning normally even with DMC concentrations as high as 80 μg/ml, as seen by electron microscopy and by the uptake of fluorescein isothiocyanate-labeled beads. Ultrastructural studies also showed that in the presence of DMC the mitochondria of promastigotes were enlarged and disorganized. Despite destruction of intracellular amastigotes, no disarrangement of macrophage organelles were observed, even at 80 μg of DMC/ml. These observations suggest that DMC is selectively toxic to the parasites. Its simple structure may well enable it to serve as a new lead compound for the synthesis of novel antileishmanial drugs.
Leishmaniasis is a disfiguring and sometimes fatal protozoan disease, affecting more than 12 million people worldwide (4), for which there is still no safe vaccine (15, 16). Recently, a dramatic increase in the rate of Leishmania infections in human immunodeficiency virus patients (1, 2), together with the development of drug resistance by the parasites (5), has worsened this problem. Despite the tremendous progress made in the understanding of the biochemistry and molecular biology of the parasite, the first-choice treatment for the several forms of leishmaniasis still relies on daily intramuscular injections of pentavalent antimonials developed more than 50 years ago (9). Pentavalent antimonials are potentially toxic and often ineffective, and the second-choice drugs, such as amphotericin B and pentamidine, may be even more toxic (17, 30). Therefore, the search for novel, effective, and safe therapeutic compounds has become a priority.
Plants still provide unparalleled chemical diversity and bioactivity (34), which has led to the development of hundreds of pharmaceutical drugs. Indeed, the majority of drugs used clinically for the treatment of infectious agents are derived from natural products. The antimalarial agents quinine, chloroquine, and artemisinin, and the antiamoebic agent emetin, are good examples of successful plant-derived drugs (8, 21). For leishmaniasis, various plant-derived compounds with activity against the parasites have been described (reviewed in reference 19), although none so far has been developed and approved for clinical use, perhaps due to toxicity for mammalian systems. As part of a program where antileishmanial compounds are sought in a great variety of Brazilian plants preselected according to their chemical composition, we describe here the selective effects of an active chalcone purified from Piper aduncum (Piperaceae) against Leishmania amazonensis, a causative agent of cutaneous leishmaniasis.
MATERIALS AND METHODS
Plant extraction and purification of DMC.
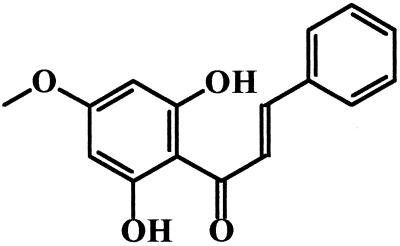
P. aduncum was collected in the outskirts of Rio de Janeiro State, Brazil, during the summer. The inflorescences (150 g) were dried, powdered, and then submitted to successive extractions with hexane, dichloromethane, and methanol at room temperature. Each extract was evaporated to dryness under reduced pressure before antipromastigote activity-guided fractionation (see below). 2′,6′-Dihydroxy-4′-methoxychalcone (DMC) was purified as described previously (27). Briefly, the dichloromethane extract (7 g) was chromatographed on a silica gel column by using mixtures of hexane-ethyl acetate and ethyl acetate-methanol, yielding 90 mg of a pure substance, identified by gas chromatography-mass spectrometry and nuclear magnetic resonance as DMC (Fig. 1).
FIG. 1.
Structure of DMC.
Mice.
BALB/c mice originally purchased from Jackson Laboratory (Bar Harbor, Maine) were bred and maintained at our own facilities. Male mice were used at 8 to 10 weeks of age.
Parasites.
The L. amazonensis isolate LV/79 (MPRO/BR/72/M 1841) was used. The parasites were routinely isolated from mouse lesions and maintained as promastigotes at 26°C in Dulbecco-modified minimum essential medium (DMEM; Sigma Chemical Co., St. Louis, Mo.) containing 10% heat-inactivated fetal calf serum (HIFCS; Microbiológica, Rio de Janeiro, Brazil), 100 μg of streptomycin/ml, and 100 U of penicillin/ml (referred to below as complete medium). Subcultures were made in the late-log phase of growth, and parasites were used no later than at the fourth passage.
Antipromastigote activity.
The inhibition of promastigote growth in vitro was assessed by a modification of the method described by Yang and Liew (38). Briefly, promastigotes were incubated at 26°C in Schneider Drosophila medium (Gibco, Paisley, United Kingdom) plus 5% HIFCS in the presence of various concentrations of the dichloromethane extract, DMC, or medium alone in 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark). After 48 h, 1 μCi of [3H]thymidine (Sigma Chemical Co.) was added to each well. Parasites were harvested 6 h later with a dot blot apparatus as described previously (32), and [3H]thymidine incorporation was measured in a β-scintillation counter. All cultures were performed in triplicate, and the results were expressed as percent inhibition in relation to controls cultured in medium alone.
Antiamastigote activity.
Resident macrophages were harvested from the peritoneal cavities of normal BALB/c mice in ice-cold DMEM. The cells were plated at 2 × 106/ml (0.4 ml/well) in Lab-Tek 8-chamber slides (Nunc, Naperville, Ill.) and incubated at 37°C under an atmosphere of 4% CO2 for 1 h. Nonadherent cells were removed by washing with prewarmed phosphate-buffered saline (PBS). Stationary-phase L. amazonensis promastigotes were added at a 4:1 parasite/macrophage ratio, and the cultures were incubated for a further 4 h. The cell monolayers were washed three times with prewarmed PBS to remove free parasites, and 0.4 ml of DMC in complete medium at different concentrations was added in duplicate for a further 48 h. The cultures were then fixed with absolute methanol, stained with Giemsa stain, and examined under light microscopy. The number of intracellular amastigotes was determined by counting at least 100 macrophages per sample, and the results were expressed as percent inhibition in relation to controls without DMC. The 50% effective dose (ED50) was determined by logarithm regression analysis.
Phagocytic activity.
Resident mouse peritoneal macrophages were harvested as described above and plated in 24-well tissue culture plates (Nunc) at 106/well. After removal of nonadherent cells, 0.5 ml of DMC was added at various concentrations. After 48 h of incubation at 37°C and 5% CO2, the cultures were washed once with prewarmed PBS, and 5 × 107 fluorescein isothiocyanate (FITC)-labeled latex beads (diameter, 1 μm; Polysciences, Warrington, Pa.) were added for a further 4 h. At the end of the incubation time, the cells were washed four times with prewarmed PBS to remove free beads and lysed by two cycles of freeze-thawing in distilled water, and the fluorescence in the lysate was measured by plate fluorometry (Fluoroskan II; Labsystems Oy, Helsinki, Finland). Negative controls were macrophages fixed with 1% formalin prior to addition of the beads.
Nitric oxide production.
Resident mouse peritoneal macrophages were plated and incubated at 4 × 105/well in 96-well flat-bottom plates with DMC at several concentrations in the presence or absence of 10 U of murine recombinant gamma interferon (rIFN-γ)/ml. After 48 h, NO production was measured by assessing the nitrite content of culture supernatants by the method described by Ding et al. (12). Briefly, 100 μl of fresh Griess reagent [1% sulfanilamide p-aminobenzene sulfonamide, 5% H3PO4, 0.1% n-(1-naphthyl)ethylenediamine dihydrochloride; Sigma] was added to equal volumes of culture supernatants. After 10 min of incubation at room temperature, the optical density at 570 nm was measured. The nitrite concentration was determined by using NaNO2 diluted in DMEM as the standard and DMEM plus Griess reagent alone as the blank. Addition of DMC did not alter the blank values.
Ultrastructural studies.
Electron microscopy studies were carried out to examine the effect of DMC on the ultrastructures of both promastigote and amastigote forms. For promastigotes, 5-ml suspensions of 106 parasites/ml of complete medium were incubated for 48 h at 26°C in the presence or absence of 50 μg of DMC/ml in 25-cm2 tissue culture flasks (Nunc). The cells were then washed with PBS, centrifuged, and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h. For intracellular amastigotes, 5-ml suspensions of 2 × 106 resident peritoneal exudate cells/ml were plated in 25-cm2 tissue culture flasks and macrophages were infected as described above. The infected cells were incubated with 40 or 80 μg of DMC/ml or with complete medium alone for 48 h. The macrophages were washed twice with prewarmed PBS, fixed as for promastigotes, and collected by gentle scraping. Both promastigotes and infected macrophages were postfixed with 1% OsO4 and 0.8% potassium ferricyanide, then washed with PBS, included in agar, dehydrated with acetone, and embedded in Epon. Fine sections were made with an ultramicrotome LKB Ultratome V, stained with uranyl acetate and lead citrate, and examined in a Zeiss EM10C electron microscope.
RESULTS
Inhibition of parasite growth.
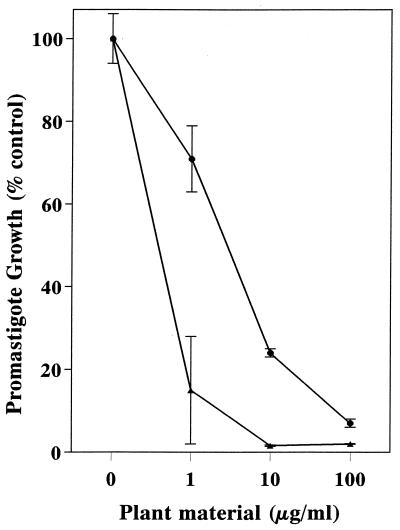
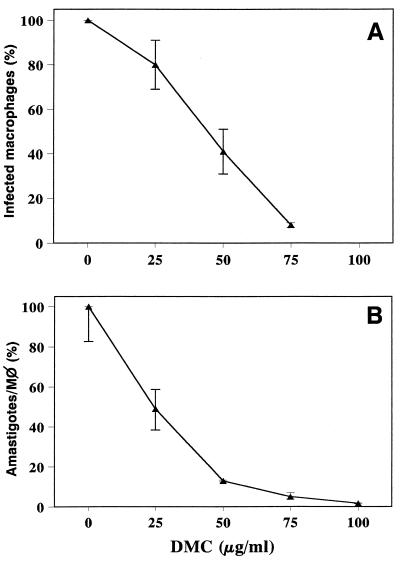
The dichloromethane extract from P. aduncum inflorescences actively decreased the viability of promastigotes of L. amazonensis in vitro, as monitored by reduction in DNA synthesis following a 48-h parasite culture in the presence of the extract. The crude extract inhibited promastigote growth with an ED50 of 2.2 μg/ml (Fig. 2). Preliminary experiments demonstrated that inflorescences were superior to leaves and stems and that dichloromethane was better than hexane and methanol in extracting the active substance(s). Antipromastigote activity-guided fractionation led to the purification of DMC, which showed an ED50 of 0.5 μg/ml (Fig. 2). Purified DMC was also tested on intracellular amastigotes, and three independent experiments showed that whereas the percentage of infected macrophages was reduced by half at 40 μg of DMC/ml (Fig. 3A), the parasite load was reduced by half at 24 μg/ml (the ED50) (Fig. 3B). We observed no apparent toxic effect of DMC on macrophages by light microscopy, such as rounding or detachment from the substrate, even at 100 μg/ml.
FIG. 2.
Effect of the crude dichloromethane extract of P. aduncum and the isolated DMC against promastigotes of L. amazonensis. Promastigotes were cultured for 48 h in the presence of the indicated concentrations of dichloromethane extract (●) or DMC (▴). [3H]thymidine incorporation for controls in medium alone, taken as 100%, was 11,700 cpm. Data are means ± standard deviations (SD).
FIG. 3.
Effect of DMC against intracellular amastigotes. Macrophages were infected with L. amazonensis and cultivated for 48 h in the presence of the indicated concentrations of DMC. (A) Percentage of infected macrophages. (B) Parasite load (number of amastigotes/macrophage) as a percentage of that in controls, taken as 100%. Controls averaged 12 amastigotes/macrophage. Each experimental point is the mean from two independent cultures ± SD.
Effect of DMC on macrophage phagocytosis.
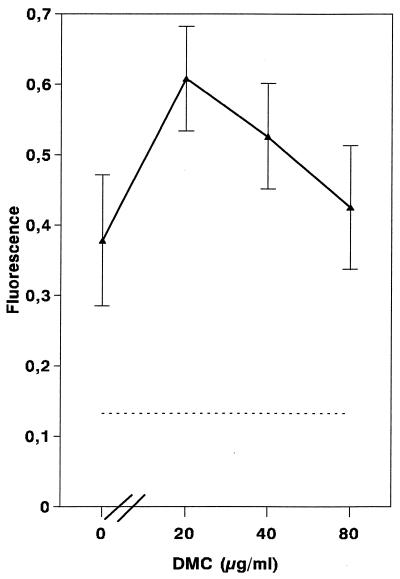
To assess whether DMC affects macrophage phagocytic activity, and hence viability, macrophage monolayers were incubated for 48 h in the presence of increasing concentrations of DMC and then were allowed to phagocytose FITC-labeled latex beads. Figure 4 shows that the phagocytic activity in the presence of higher (80-μg/ml) concentrations of DMC was the same as that of untreated cells. These results, together with the observations that DMC at 100 μg/ml does not affect the proliferative response of lymph node cells to concanavalin A (measured by the incorporation of [3H]thymidine) or the viability of lymph node cells or murine mastocytoma cells (as measured by trypan blue dye exclusion and 51Cr release assays, respectively [data not shown]), indicate that DMC is selectively toxic to the parasites but not to mammalian cells.
FIG. 4.
Effect of DMC on the phagocytic activity of macrophages. Macrophages were preincubated for 18 h in the presence of the indicated concentrations of DMC, washed, and then allowed to phagocytose FITC-labeled latex beads for a further 4 h. After washing, the fluorescence in the cell monolayers was measured. Dashed line, background fluorescence in macrophage cultures fixed with formalin and treated in the same way. Data are means ± SD (n = 3).
Effect of DMC on nitric oxide production by macrophages.
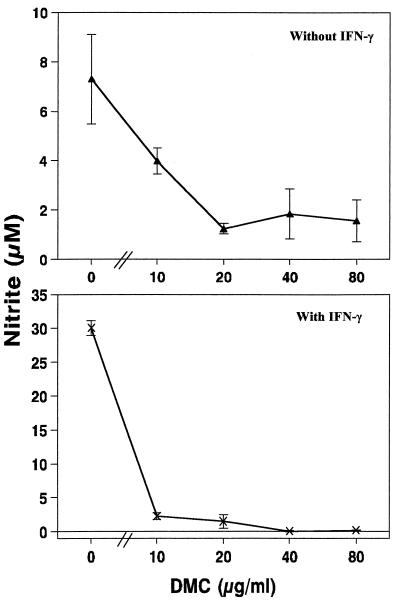
To investigate whether the apparent increase in phagocytic activity observed in Fig. 4 reflected a generalized activation state of macrophages that could include microbicidal mechanisms, we measured the production of NO upon incubation with DMC. When resident macrophages were incubated for 48 h in the presence of increasing concentrations of DMC, a steady decrease in spontaneous NO production was observed (Fig. 5). The capacity of macrophages to produce NO was abolished by DMC even in the presence of 10 U of rIFN-γ/ml, which by itself produced a fivefold increase in NO synthesis, and was almost completely abrogated by the presence of 10 μg of DMC/ml (Fig. 5). These results suggest that the antiamastigote activity of DMC is not due to activation of this powerful leishmanicidal mechanism evolved by the macrophages (12, 22–24) but may rather be due to the direct effect of DMC on the parasites.
FIG. 5.
Effect of DMC on the production of nitric oxide by macrophages. Macrophages were cultivated for 48 h in the presence of the indicated concentrations of DMC in the absence (top) or presence (bottom) of 50 U of rIFN-γ/ml. The nitrite concentration in the cell supernatants was measured with Griess reagent. Data are means ± (n = 3).
Electron microscopy studies.
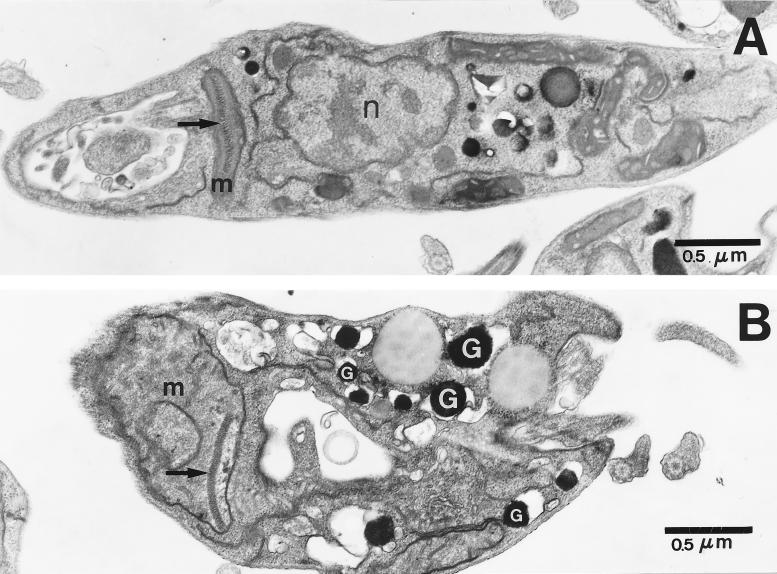
To better evaluate the subcellular changes induced by DMC on the parasites, either free promastigotes or intracellular amastigotes were cultured for 48 h in the presence of different concentrations of DMC and then processed for electron microscopy. For promastigotes, treatment with 50 μg of DMC/ml induced an increase in the size and number of electron-dense granules (Fig. 6B) compared to those in untreated controls (Fig. 6A). The promastigote forms displayed enlarged and more-diffuse mitochondrion profiles with a loss of matrix and crista patterns, indicating damage to that organelle.
FIG. 6.
Ultrastructural effects of DMC on promastigotes. Promastigotes were incubated in medium alone (A) or with 50 μg of DMC/ml (B) for 48 h. (A) Section showing the normal aspect of the nucleus (n) and mitochondrion (m) containing the kinetoplast (arrow). (B) A promastigote exhibiting several electron-dense granules (G) and a dense mitochondrion (m) with a swollen matrix and loss of cristae.
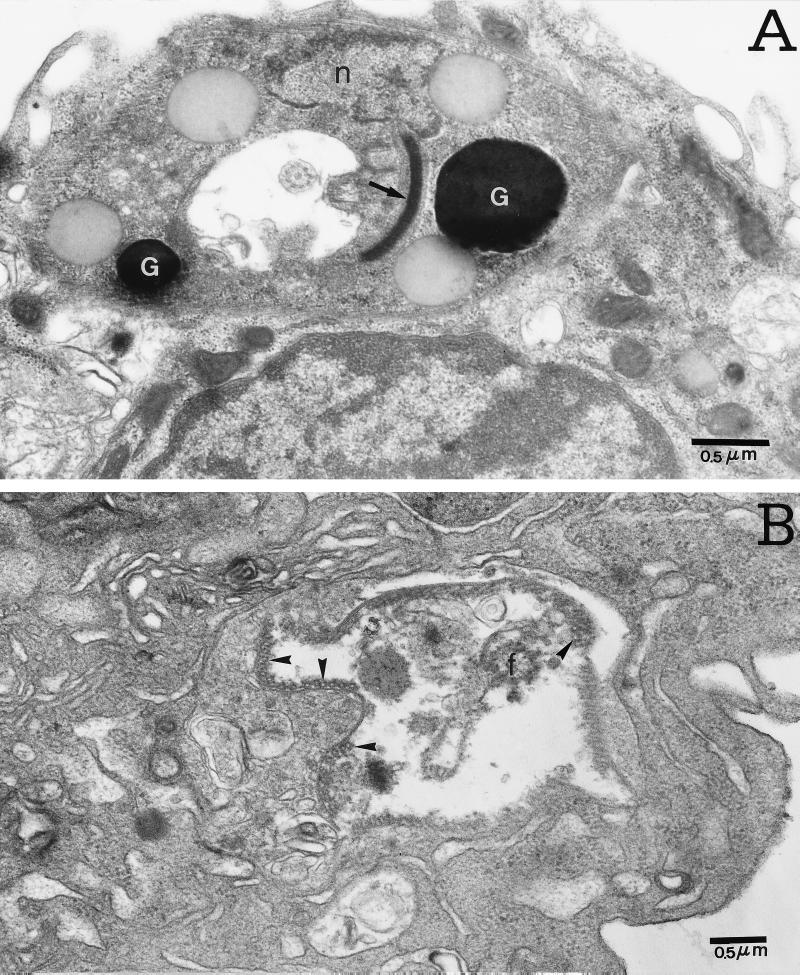
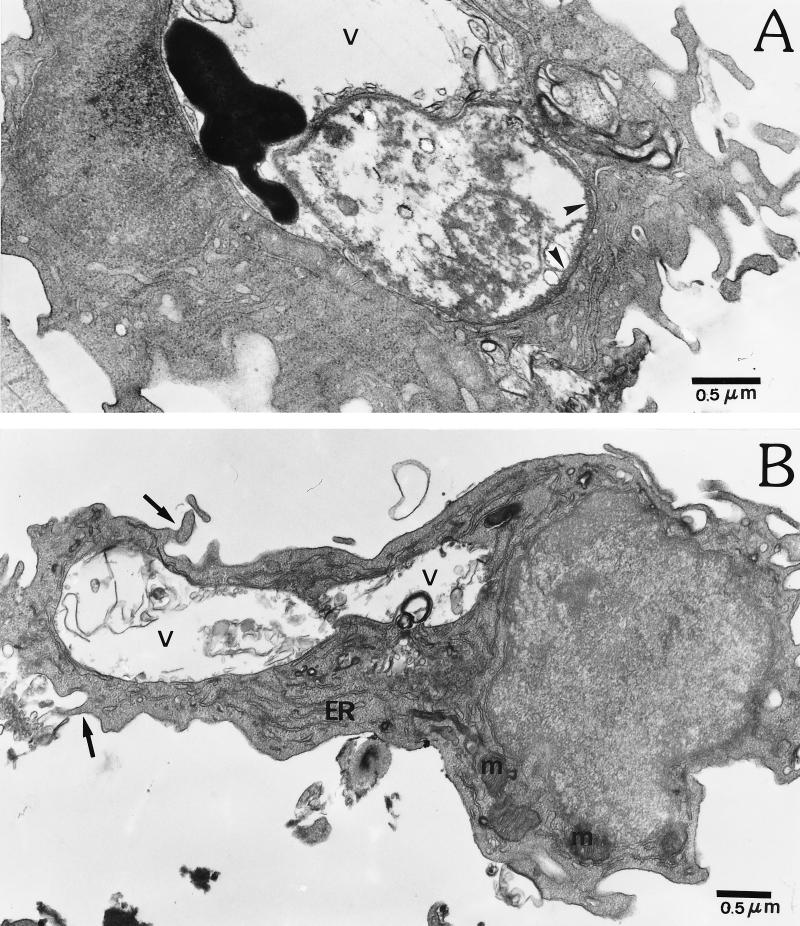
A typical intracellular amastigote from untreated cultures is shown in Fig. 7A, where the normal size and electron density of the nucleus and kinetoplast are seen. Clear changes can be seen when the cultures are treated with 40 μg of DMC/ml (Fig. 7B); a vacuole containing degraded parasite organelles such as membrane-associated subpellicular microtubules and flagella was found. Treatment of infected macrophages with a higher concentration of DMC (80 μg/ml) (Fig. 8) produced greater parasite destruction, such that no intact parasites could be found. Figure 8A shows the presence of subpellicular microtubules of the parasite membrane and a highly electron-dense material inside the parasitophorous vacuole. At 80 μg of DMC/ml, most macrophages, such as that represented in Fig. 8B, showed well-preserved mitochondria, abundant endoplasmic reticulum, and vacuoles containing degraded material, possibly amastigotes. Plasma membrane projections are also seen. This observation is consistent with the enhanced phagocytic activity demonstrated in Fig. 4 for latex beads.
FIG. 7.
Ultrastructural effects of DMC on intracellular amastigotes. Infected macrophages were cultured in medium alone (A) or with 40 μg of DMC/ml (B) for 48 h. (A) A normal amastigote nucleus (n), a kinetoplast (arrow), and a few electron-dense granules (G) are shown. (B) Disrupted amastigote identified by subpellicular microtubules (arrowheads) and flagellum (f) inside a vacuole of a representative macrophage.
FIG. 8.
Electron micrograph of infected macrophage cultures treated with 80 μg of DMC/ml. (A) Section of an infected macrophage showing a vacuole (v) containing a large, dense material; subpellicular microtubules (arrowheads); and debris from a disrupted amastigote. (B) Section of a macrophage with normal mitochondrion profiles (m), endoplasmic reticulum (ER), membrane projections (arrows), and large vacuoles (v) containing cellular debris.
DISCUSSION
Chalcones are flavonoids present in a variety of plant species. A range of biological effects, such as antibacterial, antitumor, antifungal (3, 29), antiviral (11), and antiplasmodial (20) activities, have been ascribed to them. This paper describes for the first time the activity of DMC against both promastigote and amastigote forms of L. amazonensis. The antileishmanial activity of a different chalcone, the licochalcone A isolated from the roots of Glycyrrhiza spp. (Fabaceae), has been described for Leishmania major and Leishmania donovani (6, 7, 39). In those studies, the ED50 against the amastigotes of both species was 4 μg/ml, a dose sixfold smaller than found here for DMC against L. amazonensis (24 μg/ml). However, the efficacies of those two chalcones cannot be compared due to differences in experimental protocols, and more importantly, also in drug susceptibility among parasite species. L. amazonensis may be more refractory than other Leishmania species to different drugs (28) and to the toxic effects of nitric oxide (31).
Generally, the antiamastigote activity of a drug may be selective and direct against the parasite, or it may act indirectly, by activating macrophage microbicidal mechanisms, such as the production of NO, which is considered the most important macrophage leishmanicidal mechanism (14, 23, 26, 36). For instance, activation of the NO synthase pathway is involved in the protective effect of the leaf extract of the plant Kalanchoe pinnata against infection of BALB/c mice with L. amazonensis (10), an effect that is reversible with NO inhibitors and that may also be responsible for the observed immunosuppressive effect of that plant (33). In the present work, the antileishmanial effect of DMC seems to result from direct action on the parasite rather than from activation of NO production by the macrophages, as evidenced by its inhibitory action on axenic promastigotes (Fig. 2 and 6B). The activity of a drug against axenic promastigotes or axenic amastigotes is not per se indicative of antileishmanial action, as the drug may not reach the parasitophorous vacuole, may be metabolically converted into different products by the macrophages (35), or may simply be nonselectively cytotoxic. We observed by optical and by electron microscopy that effective antiamastigote concentrations of DMC (Fig. 3) inflicted no significant damage on macrophages. The evidence for selectivity against the parasites was reinforced by the observation that other mammalian cell types, such as murine T cells and the mastocytoma cell line P815, showed no signs of cytotoxicity with concentrations of DMC as high as 100 μg/ml (data not shown). The phagocytic function of macrophages operated normally in the presence of DMC (Fig. 4). Plasma membrane projections were seen, indicating that the drug maintained macrophage phagocytic activity (Fig. 8B) and vitality at concentrations which were toxic against the parasites. However, the capacity of macrophages to spontaneously produce NO in culture was ablated with low concentrations of DMC (Fig. 5). The antioxidative effect of DMC was strong enough to inhibit IFN-γ-induced NO production (Fig. 5), indicating that the antiamastigote effect of DMC is not due to the induction of toxic nitrogen intermediates by macrophages and may rather be direct and selective against the parasite.
We observed that DMC strongly affects the mitochondria of promastigotes at 50 μg/ml (Fig. 6B). Other leishmanicidal drugs, such as paromomycin (25) and licochalcone A (7), also affect the parasite mitochondrion. In fact, the ultrastructural alterations induced by licochalcone A in promastigotes and amastigotes of L. donovani were similar to those observed in this study for DMC and L. amazonensis. However, unlike licochalcone A (39), DMC did not affect the macrophage mitochondrion at higher concentrations (80 μg/ml), suggesting that despite the higher ED50 required for the leishmanicidal activity discussed above, use of DMC may be safer for mammalian cells.
The mechanism(s) by which DMC kills leishmaniae is still unknown. Other chalcones have been shown to inhibit glutathione reductase (40), rendering them possible candidates as inhibitors of trypanothione reductase, the enzyme responsible for the redox balance in trypanosomatids. Whether DMC affects parasite oxidative metabolism is not known yet, but this possibility should be considered, since methyl and hydroxy substituents have been shown to greatly increase the antioxidant activity of chalcones (3). In fact, the reduced capacity of macrophages to produce NO in the presence of DMC (Fig. 5) may reflect the drug’s effect on the nitrogen oxidative metabolism. Another importnat chemotherapeutic target in trypanosomatids and fungi is ergosterol synthesis, as this sterol is the main lipid component of their membranes and mammalian cells do not produce it (37). The possibility that DMC acts on this pathway should also be considered, as inhibitory activity of DMC against the fungi Candida albicans and Cryptococcus neoformans has been reported (29).
Overall, the results of this work, besides further supporting the antileishmanial activity of chalcones, present a compound of this group which is nontoxic to various mammalian cell types at concentrations that effectively kill all intracellular parasites in vitro. The wide use of P. aduncum formulations in folk medicine for the treatment of unrelated diseases, such as trachoma and stomachaches (13), and for the healing of wounds (18) is indicative that DMC may be safely used in humans. P. aduncum grows abundantly in all regions of endemicity for leishmaniasis in Brazil, including the Amazonia, where the access of the population to medical care is limited and L. amazonensis infection is very prevalent. The use of inflorescences for the preparation of DMC-rich extracts in the treatment of leishmaniasis would not damage the species, as would the use of the roots of Chinese Glycyrrhiza for the extraction of licochalcone. Another advantage of DMC is that its chemical structure is simpler than that of licochalcone (6), which may make it a less complex model for the synthesis of a novel antileishmanial chalcone.
ACKNOWLEDGMENT
We are indebted to Venício Féo da Veiga from the Setor de Microscopia Eletrônica, Instituto de Microbiologia-UFRJ, for help with the electron microscopy preparations.
REFERENCES
- 1.Albrecht H, Sobottka I, Emminger C, Jablonowski H, Just G, Stoehr A, Kubin T, Salzberger B, Lutz T, van Lunzen J. Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonendemic for Leishmania donovani. Arch Pathol Lab Med. 1996;120:189–198. [PubMed] [Google Scholar]
- 2.Alvar J. Leishmaniasis and AIDS co-infection: the Spanish example. Parasitol Today. 1994;10:160–163. doi: 10.1016/0169-4758(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 3.Anto R J, Sukumaran K, Kuttan G, Rao M N, Subbaraju V, Kuttan R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett. 1995;97:33–37. doi: 10.1016/0304-3835(95)03945-s. [DOI] [PubMed] [Google Scholar]
- 4.Ashford R W, Desjeux P, de Raadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 5.Berman J D. Human leishmaniasis: clinical, diagnostic and chemotherapeutic developments in the last ten years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Christensen S B, Blom J, Lemmich E, Nadelmann L, Fich K, Theander T G, Kharazmi A. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob Agents Chemother. 1993;37:2550–2556. doi: 10.1128/aac.37.12.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Christensen S B, Theander T G, Kharazmi A. Antileishmanial activity of licochalcone A in mice infected with Leishmania major and in hamsters infected with Leishmania donovani. Antimicrob Agents Chemother. 1994;38:1339–1344. doi: 10.1128/aac.38.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft S L. A rationale for antiparasite drug discovery. Parasitol Today. 1994;10:385–386. doi: 10.1016/0169-4758(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 9.Croft S L, Urbina J A, Brun R. Chemotherapy of human leishmaniasis and trypanosomiasis. In: Hide G, Mottram J C, Coombs G H, Holmes P H, editors. Trypanosomiasis and leishmaniasis. London, United Kingdom: CAB International; 1997. pp. 245–257. [Google Scholar]
- 10.Da Silva, S. A., G. S. S. Costa, and B. Rossi-Bergmann. The anti-leishmanial effect of Kalanchoe is mediated by nitric oxide intermediates. Parasitology, in press. [DOI] [PubMed]
- 11.Dewindt B, van Eemeren K, Andries K. Antiviral capsid-binding compounds can inhibit the adsorption of minor receptor rhinoviruses. Antivir Res. 1994;25:67–72. doi: 10.1016/0166-3542(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 12.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 13.Duke J A. Handbook of medicinal herbs. New York, N.Y: CRC Press; 1985. [Google Scholar]
- 14.Green S J, Meltzer M S, Hibb J R, Nacy C. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 15.Grimaldi G J. Meetings on vaccine studies towards the control of leishmaniasis. UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) Mem Inst Oswaldo Cruz. 1995;90:553–556. [PubMed] [Google Scholar]
- 16.Handman E. Leishmania vaccines: old and new. Parasitol Today. 1997;13:236–238. doi: 10.1016/s0169-4758(97)01060-0. [DOI] [PubMed] [Google Scholar]
- 17.Hepburn N C, Siddique I, Howie A F, Beckett G J, Hayes P C. Hepatotoxicity of sodium stibogluconate therapy for American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1994;88:453–455. doi: 10.1016/0035-9203(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 18.Holdsworth D, Damasc F. Medicinal plants of Morobe Province, Papua New Guinea. Int Guide Drug Res. 1986;24:217. [Google Scholar]
- 19.Iwu M M, Jackson J E, Schuster B G. Medicinal plants in the fight against leishmaniasis. Parasitol Today. 1994;10:65–68. doi: 10.1016/0169-4758(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 20.Kharazmi A, Chen M, Theander T G, Christensen S B. Discovery of oxygenated chalcones as novel antimalarial agents. Ann Trop Med Parasitol. 1997;91:S91–S95. [Google Scholar]
- 21.Kirby G C. Medicinal plants and the control of protozoal disease, with particular reference to malaria. Trans R Soc Trop Med Hyg. 1996;90:605–609. doi: 10.1016/s0035-9203(96)90404-6. [DOI] [PubMed] [Google Scholar]
- 22.Lemesre J-L, Sereno D, Daulouede S, Veyret B, Brajon N, Vincendeau P. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically grown amastigote forms. Exp Parasitol. 1997;86:56–68. doi: 10.1006/expr.1997.4151. [DOI] [PubMed] [Google Scholar]
- 23.Liew F Y, Cox F E G. Nonspecific defense mechanism: the role of nitric oxide. Immunoparasitol Today. 1991;1991:A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 24.Liew F Y, Millot S, Parkinson C, Palmer R M J, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 25.Maarouf M, de Kouchkovsky Y, Brown S, Petit P X, Robert-Gero M. In vivo interference of paromomycin with mitochondrial activity of Leishmania. Exp Cell Res. 1997;232:339–348. doi: 10.1006/excr.1997.3500. [DOI] [PubMed] [Google Scholar]
- 26.Mauel J, Ransijn A. Leishmania spp. Mechanisms of toxicity of nitrogen oxidation products. Exp Parasitol. 1997;87:98–111. doi: 10.1006/expr.1997.4205. [DOI] [PubMed] [Google Scholar]
- 27.Moreira D L, Gimarães E F, Kaplan M A C. A chromene from Piper aduncum L. Phytochemistry. 1998;48:1075–1077. [Google Scholar]
- 28.Neal R A, Allen S, McCoy N, Olliaro P, Croft S L. The sensitivity of Leishmania species to aminosidine. J Antimicrob Chemother. 1995;35:577–584. doi: 10.1093/jac/35.5.577. [DOI] [PubMed] [Google Scholar]
- 29.Okunade A L, Hufford C D, Clark A M, Lentz D. Antimicrobial properties of the constituents of Piper aduncum. Phytother Res. 1997;11:142–144. [Google Scholar]
- 30.Olliaro P I, Bryceson D M. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today. 1993;9:323–328. doi: 10.1016/0169-4758(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 31.Romão P R T, Antoniazi S A, Lima H C, Cruz A K, Cunha F Q. Is glutathione an important virulence factor in leishmaniasis? Mem Inst Oswaldo Cruz. 1997;92(Suppl. I):219. [Google Scholar]
- 32.Rossi-Bergmann B, Noleto G. A low-cost and efficient procedure for harvesting DNA-labeled cells using a dot-blot apparatus. BioTechniques. 1994;17:678–680. [PubMed] [Google Scholar]
- 33.Rossi-Bergmann B, Costa S S, Borges M B S, Da Silva S A, Noleto G, Souza M L M, Moraes V L G. Immunosuppressive effect of the aqueous extract of Kalanchoe pinnata in mice. Phytother Res. 1994;8:399–402. [Google Scholar]
- 34.Science Medicine from plants. Science. 1990;247:513. doi: 10.1126/science.2300807. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 35.Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, Lemesre J L. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–789. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114:S91–S99. [PubMed] [Google Scholar]
- 38.Yang D M, Liew F Y. Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology. 1993;106:7–11. doi: 10.1017/s0031182000074758. [DOI] [PubMed] [Google Scholar]
- 39.Zhai L, Blom J, Chen M, Christensen S B, Kharazmi A. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob Agents Chemother. 1995;39:2742–2748. doi: 10.1128/aac.39.12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K, Yang E B, Tang W Y, Wong K P, Mack P. Inhibition of glutathione reductase by plant polyphenols. Biochem Pharmacol. 1997;54:1047–1053. doi: 10.1016/s0006-2952(97)00315-8. [DOI] [PubMed] [Google Scholar]