Drinking culture has high significance in both China and the world, whether in the entertainment sector or in social occasions; according to the World Health Organization's 2018 Global Alcohol and Health Report, about 3 million people died from excessive drinking in 2016, accounting for 5.3% of the total global deaths that year. Oxidative stress and inflammation are the most common pathological phenomena caused by alcohol abuse (Snyder et al., 2017). Scutellarin, a kind of flavonoid, is one of the main active ingredients extracted from breviscapine. It exerts anti-inflammatory, antioxidant, and vasodilation effects, and has been used to treat cardiovascular diseases and alcoholic liver injury. Although scutellarin can effectively alleviate multi-target organ injury induced by different forms of stimulation, its protective effect on alcoholic brain injury has not been well-defined. Therefore, the present study established an acute alcohol mice brain injury model to explore the effect of scutellarin on acute alcoholic brain injury. The study was carried out based on the targets of oxidative stress and inflammation, which is of great significance for the targeted therapy of clinical alcohol diseases.
Alcohol exposure will promote the production of reactive oxygen species (ROS) (Hathroubi et al., 2018). ROS are the products of normal cell metabolism and the basis for maintaining cell homeostasis. A limited concentration of ROS participates in the inflammation and immune responses, enhancing the learning and memory capabilities. However, when ROS accumulate excessively in the brain, the antioxidant enzymes including copper/zinc superoxide dismutase (SOD), glutathione system, and catalase (CAT) will be largely consumed, and the imbalance of oxidants and antioxidants triggers oxidative stress. The brain, as an advanced organ that governs various behaviors of the limbs, has high oxygen consumption and low antioxidant capacity, and it is rich in unsaturated fatty acids. These factors make the brain organ extremely susceptible to oxidative stress. What is more, the production of ROS also leads to the oxidation of lipids containing carbon–carbon double bonds in the membrane lipid bilayer. Therefore, lipid peroxidation is accompanied by oxidative stress. Because of the high content of malondialdehyde (MDA) during lipid peroxidation, it is generally considered as a biomarker of this process (Mas-Bargues et al., 2021).
The production of inflammatory cytokines is promoted by ROS (Li et al., 2013). The excessive accumulation of ROS caused by alcohol abuse can trigger inflammation, which leads to organ damage, such as that of the heart and the brain. Interleukin-1β (IL-1β) is a kind of interleukin cytokine, which is produced in response to stimulation in the early stage of inflammation and then induces the production of other inflammatory mediators, exacerbating the inflammatory response. IL-6 is a central proinflammatory cytokine. Tumor necrosis factor-α (TNF-α) is also an important proinflammatory cytokine, which can stimulate the release of IL-1β and cyclooxygenase (COX). Alfonso-Loeches et al. (2014) found that alcohol stimulation led to neuroinflammation and brain damage by activating Toll like receptor-4 (TLR4)/IL-1 receptor Ⅰ (IL-1R I) in astrocytes, which induced the secretion of IL-1β, TNF-α, and IL-6 and the production of inflammatory mediators inducible nitric oxide synthase (iNOS) and COX-2.
Oxidative stress and inflammation are two phenomena that have received much attention in recent years. Based on these two targets, this study established a model of alcoholic brain injury, and explored whether the anti-inflammatory and antioxidant activity of scutellarin can relieve the associated brain damage. This work applied scutellarin treatment in an acute brain injury model for the first time, providing an effective method for the prevention of alcoholic brain injury worldwide, which has great future significance.
We established an alcoholic brain injury model using specific pathogen-free (SPF) BALB/cmale mice (6 weeks old, weighing 18‒22 g), purchased from Pizhou Dongfang Breed Co., Ltd. (Pizhou, China). The mice were randomly divided into five groups with six individuals in each group, including the negative control group, the alcohol group (diluted to 50% (volume fraction) in water), and the scutellarin treatment groups with low, middle, and high doses (10, 25, and 50 mg/kg body weight (BW)+50% (volume fraction) ethanol, 12 mL/kg BW, respectively). Equal volumes of distilled water or scutellarin were injected once daily for 3 d intraperitoneally, and alcohol was administered by oral gavage 12 h after the last injection. The mice were fasted for 12 h after ethanol treatment and then euthanized for further experiments.
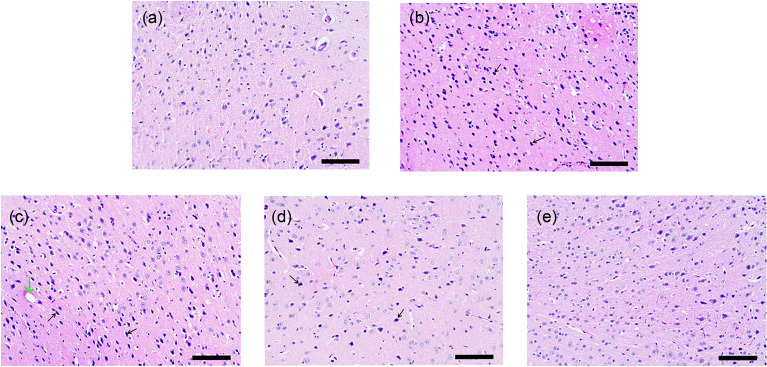
In order to observe whether scutellarin can alleviate brain damage caused by alcohol, the cerebrum tissue samples were stained by hematoxylin and eosin (H&E) staining. As shown in Fig. 1, in the alcohol group, the morphology and structure of cerebrum tissue cells were severely altered, with almost all neurons contracting and showing hyperchromatism. The nucleus and cytoplasm were indistinguishable. Compared with the alcohol group, the samples in the low- and medium-dose scutellarin treatment displayed no obvious change, accompanied by capillary swelling and decreased neurons, while the high-dose scutellarin treatment group showed sharply reduced neuron shrinkage and exhibited shallow staining.
Fig. 1. Scutellarin alleviates pathological damage caused by acute alcohol exposure. Scutellarin was injected intraperitoneally 3 d in advance as a pre-treatment drug. The low, medium, and high doses were 10, 25, and 50 mg/kg, respectively. After 12 h of intragastric administration, the cerebrum tissues were taken for H&E analysis to observe the morphological changes of the structures in the control group (a), alcohol group (b), low-dose group (c), medium-dose group (d), and high-dose group (e). Scale bar=100 μm. The black arrow indicates pyknotic neuron, and the green arrow indicates swollen capillary. H&E: hematoxylin and eosin.
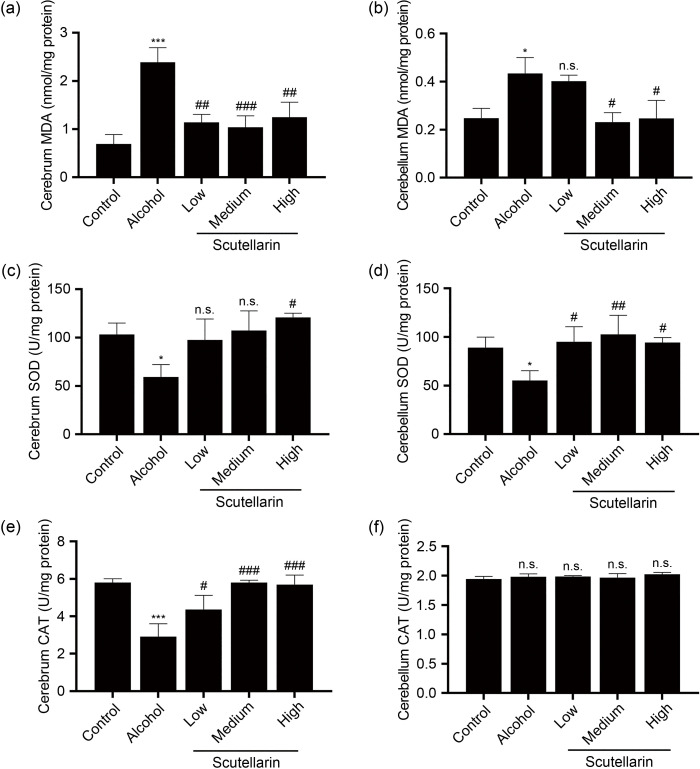
In order to explore how scutellarin affects oxidative stress in the brain caused by alcohol, this work tested the activity of related antioxidant enzymes and the content of MDA, a marker of lipid peroxidation in brain tissue. As shown in Fig. 2a, compared with the control group, the MDA content in the cerebrum of the alcohol-treated group was significantly increased. After scutellarin pre-treatment, the MDA content was significantly lower than that in the alcohol group. However, in the cerebellum, different phenomena were observed: only the middle and high doses led to significant differences compared with the alcohol group, while the low doses resulted in no difference from the alcohol group (Fig. 2b). The determination of the activity of antioxidant enzymes SOD and CAT showed that the activity of these two antioxidant enzymes in the two brain tissues was significantly reduced in the alcohol group (Figs. 2c–2e). In the cerebrum tissue, only high-dose scutellarin could increase the SOD activity, while medium- and low-dose scutellarin had no effect on the SOD activity. Interestingly, compared with the alcohol group, all doses of scutellarin increased the SOD activity in the cerebellum (Figs. 2c and 2d). However, for CAT activity, the alcohol group exhibited no effect on the cerebellum, and pre-treatment with scutellarin had no effect on the cerebellum CAT activity (Fig. 2f).
Fig. 2. Effects of scutellarin on the antioxidant enzyme activity and MDA content in the brain tissues due to acute alcohol exposure. The supernatants of cerebrum and cerebellum homogenate were used to determine the activity of antioxidant enzymes SOD and CAT, and the content of MDA. Among them, (a), (c), and (e) represent the levels of MDA, SOD, and CAT in the cerebrum, respectively, while (b), (d), and (f) represent the levels of MDA, SOD, and CAT in the cerebellum, respectively. Data were presented as mean±SD (n=6). * P<0.05, *** P<0.001, vs. the control group. # P<0.05, ## P<0.01, ### P<0.001, vs. the alcohol group. n.s.: no significance (P>0.05). SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde; SD: standard deviation.
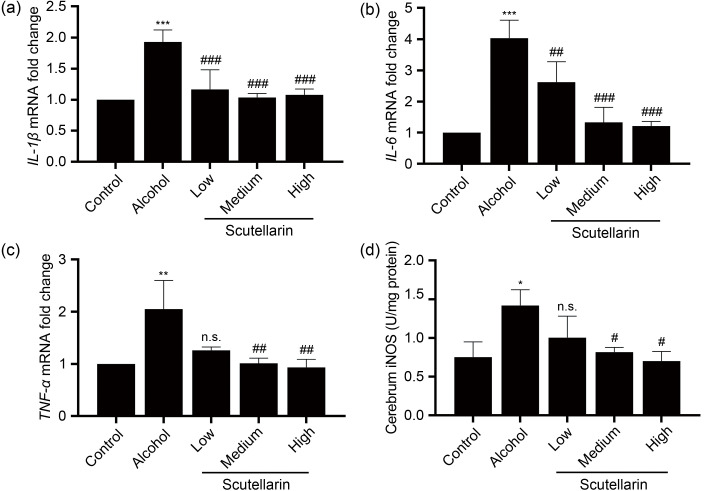
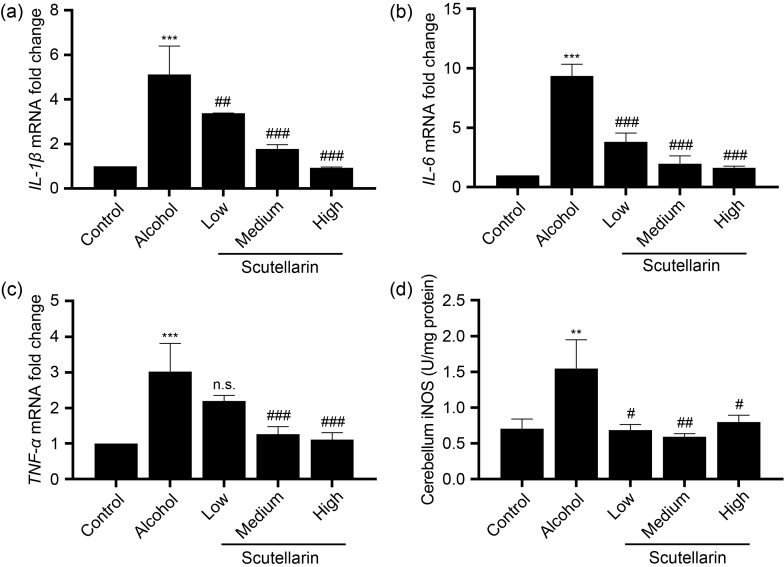
In order to validate whether scutellarin can alleviate the inflammation caused by alcohol exposure, we detected the messenger RNA (mRNA) expression levels of key inflammatory factors. As shown in Fig. 3, compared with the control group, the expression levels of IL-1β, IL-6, TNF-α, and the activity of iNOS in the cerebrum of acute alcohol-treated mice were significantly increased. Low-dose scutellarin treatment had no effect on the reduction of inflammatory factors TNF-α or iNOS. Other doses of scutellarin could reduce the increases in the expression of proinflammatory-related factors caused by acute alcohol exposure. In the low-dose scutellarin group, in addition to the significantly reduced expression of iNOS, the expression of other inflammatory factors in the cerebellum was consistent with that in the cerebrum (Fig. 4).
Fig. 3. Effects of scutellarin on the proinflammatory factor mRNA levels and iNOS activity in the cerebrum tissues caused by alcohol exposure. (a‒c) The mRNA extracted from the tissues was reverse-transcribed into cDNA, and the mRNA expression levels of IL-1β (a), IL-6, (b), and TNF - α (c) in the cerebrum were measured using qPCR assays. (d) The activity of iNOS in the supernatants of cerebrum homogenates was determined by a biochemical kit. Data were presented as mean±SD (n=6). * P<0.05, ** P<0.01, *** P<0.001, vs. the control group. # P<0.05, ## P<0.01, ### P<0.001, vs. the alcohol group. n.s.: no significance (P>0.05). mRNA: messenger RNA; cDNA: complementary DNA; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF - α: tumor necrosis factor-α; qPCR: quantitative polymerase chain reaction; iNOS: inducible nitric oxide synthase; SD: standard deviation.
Fig. 4. Effects of scutellarin on the proinflammatory factor mRNA levels and iNOS activity in the cerebellum caused by alcohol exposure. (a‒c) The cDNA template was obtained by reverse transcription using mRNA extracted from the tissues, and the mRNA expression levels of IL-1β (a), IL-6 (b), and TNF - α (c) in cerebellar tissues were detected by qPCR. (d) The activity of iNOS in the supernatants of cerebellum tissue homogenate was determined by a biochemical kit. Data were presented as mean±SD (n=6). ** P<0.01, *** P<0.001, vs. the control group. # P<0.05, ## P<0.01, ### P<0.001, vs. the alcohol group. n.s.: no significance (P>0.05). mRNA: messenger RNA; cDNA: complementary DNA; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF - α: tumor necrosis factor-α; qPCR: quantitative polymerase chain reaction; iNOS: inducible nitric oxide synthase; SD: standard deviation.
Alcohol abuse has become so frequent around the world that alcohol-related injuries, such as accidental and pathological injuries, account for a large proportion of total deaths each year. Although alcoholic brain injury is fairly common, no commercial drugs have been developed for treatment. Therefore, this study aimed to determine the preventive and protective effects of scutellarin on acute alcohol exposure by establishing an acute alcoholic mice brain injury model.
The molecular mechanisms of oxidative stress and inflammation induced by alcohol stimulation may be related to the activation of several signaling pathways. The nuclear factor erythroid-2-related factor (Nrf2) signaling pathway is a representative signaling pathway of oxidative stress. Nrf2, as an important transcription factor for nuclear transcription to regulate cellular oxidative stress response, if stimulated by certain agents (such as ROS imbalance stimulants), will not bind stably with Kelch-like epichlorohydrin (ECH)-associated protein l (Keap-1) in the cytoplasm and will be transferred to the nucleus, combine with antioxidant response elements, and enhance the expression of downstream antioxidants. A previous study showed that babaodan could reduce the contents of ROS and MDA, upregulate the expression of antioxidant enzyme heme oxygenase-1 (HO-1) protein, and alleviate ethanol-induced acute brain injury through Nrf2 activation (Yu et al., 2019). Exercise has protective function via upregulating the decreased activity of the antioxidant enzymes SOD and CAT in the heart and liver induced by acute alcohol stimulation by promoting the activation of the Nrf2/Keap-1/HO-1 pathway (Fan et al., 2021). As a powerful antioxidant, scutellarin could increase the mRNA expression of HO-1 and nicotinamide adenine (phosphate) dinucleotide (NAD(P)H) quinone oxidoreductase 1 (NQO1), while inhibiting Nrf2 could significantly reverse the protective effect of scutellarin on the hypoxia/reoxygenation-related injury of liver cells (Wu and Jia, 2019). In addition, scutellarin could reduce the content of MDA in the hippocampus, and it enhanced the antioxidant and defense components, such as SOD, CAT, and glutathione (GSH), and alleviated cognitive defects induced by lipopolysaccharide in rats (Baluchnejadmojarad et al., 2018). Based on the target of oxidative stress, it was speculated that scutellarin might play a similar antioxidant effect in the pathological model of alcoholic brain injury in this study, while Fig. 2 confirmed this speculation. Furthermore, the protective effect of scutellarin on the brain may also depend on the regulation of the Nrf2 pathway, which needs to be determined by deeply exploring the protective mechanism of scutellarin against acute alcohol brain injury in the future. The brain tissue inflammation caused by alcohol stimulation may be related to the activation of the nuclear factor-κB (NF-κB) pathway. NF-κB, a heterodimer composed of p65/p50 or p52/v-rel reticuloendotheliosis viral oncogene homolog B (RelB), is an important transcription factor that can regulate a variety of biological activities, including cell proliferation, apoptosis, and inflammation (Liu et al., 2020). Under normal circumstances, NF-κB in the cytoplasm is inactive, and its dimer complex can combine with the inhibitor of NF-κB (IkB) to form a new trimeric complex. Once the NF-κB pathway is activated, the IκB protein is phosphorylated, dissociating it from the trimer, and the dimer of NF-κB rapidly moves from the cytoplasm to the nucleus, triggering an inflammatory cascade. For example, TNF-α stimulation triggered osteoarthritis by significantly upregulating the mRNA expression of inflammatory factors IL-1β, IL-6, and the activity of iNOS, and increasing the phosphorylated protein contents of IκBα and NF-κB p65, which in turn led to osteoarthritis (Wang et al., 2019). Grape-leaf extract could alleviate alcohol-induced liver injury by inhibiting the activation of the NF-κB pathway and the expression of proinflammatory factors IL-6 and TNF-α (Amen et al., 2020). Besides, Zhang et al. (2014) demonstrated that microRNA (miR)-339-5p regulated the brain inflammation induced by the alcohol-induced overexpression of IL-1β, IL-6, and TNF-α by inhibiting the NF-κB pathway. Therefore, it is conjectured that scutellarin could play a similar anti-inflammatory effect in alcoholic brain injury in this study, and Figs. 3 and 4 confirmed this assumption. Whether the anti-inflammatory roles of scutellarin in acute alcohol brain injury are regulated by the NF-κB pathway needs further clarification. In short, brain damage caused by acute alcohol stimulation, regardless of what signal pathway is activated, is closely related to inflammation and oxidative stress. Therefore, clarifying the roles of scutellarin on these two targets in acute alcohol brain injury is of great significance for subsequent research on specific signaling pathways.
Scutellarin has been widely used in clinical practice due to its high efficiency and safety, such as its anti-inflammatory and antioxidant activity in cerebral ischemia perfusion injury and angina pectoris. In addition, the anti-tumor activity of scutellarin has been reported in a large number of clinical studies. For example, scutellarin inhibited the growth of xenograft tumors from patients with esophageal cancer by directly targeting protein kinase B (PKB or Akt) (Wang et al., 2015), and it has also been used to inhibit the metastasis of glioma cells. Scutellarin could be combined with Western drugs in clinical practice, such as glucocorticoids in the treatment of sudden sensorineural hearing loss (Zheng et al., 2020) and enalapril in the treatment of kidney damage in diabetic rats (Xu et al., 2013). Although scutellarin has many benefits, it faces the problem of low bioavailability. The main reasons for this may include its low oral solubility, limited membrane permeability, and the impact of first pass elimination in the gastrointestinal tract. In recent years, researchers have attempted to improve the oral bioavailability of scutellarin using nanoparticles, liposome nanocomposite particles, phospholipid complexes, and other agents (Wang et al., 2017).
In summary, our research results suggest that acute alcohol exposure causes oxidative stress and inflammation; however, these two phenomena can be alleviated by scutellarin by upregulating antioxidant enzyme activity and downregulating the expression of inflammatory factors. Scutellarin is therefore considered to be a promising agent for the prevention of acute alcoholic brain injury, as well as a potential candidate for the prevention and treatment of other clinical diseases.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Funding Statement
This work was supported by the Basic Science (Natural Science) Research Project of Higher Education of Jiangsu Province (No. 21KJB230001), the Open Foundation of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (No. HY202101), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author contributions
Tianmeng ZHANG: research design, writing original draft, and editing. Kun WANG: conceptualization, software, formal analysis, and visualization. Hui FAN, Xiao ZHANG, and Feixue LIU: investigation and data curation. Qiankun YANG, Xin FENG, Yi CHEN, and Daoyang TENG: methodology. Panpan ZHAO and Jingquan DONG: data curation, resources, and project administration.
Compliance with ethics guidelines
Tianmeng ZHANG, Kun WANG, Hui FAN, Qiankun YANG, Xiao ZHANG, Feixue LIU, Xin FENG, Yi CHEN, Daoyang TENG, Panpan ZHAO, and Jingquan DONG declare that they have no conflict of interest.
All experiments were approved by the Ethics Committee of Jiangsu Ocean University, Lianyungang, China (No. 2020220670). Laboratory animals were provided with humanitarian care, and the number of animals used and the pain caused to experimental animals were minimized.
References
- Alfonso-Loeches S, Ureña-Peralta JR, Morillo-Bargues MJ, et al. , 2014. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci, 8: 216. 10.3389/fncel.2014.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen Y, Sherif AE, Shawky NM, et al. , 2020. Grape-leaf extract attenuates alcohol-induced liver injury via interference with NF-κB signaling pathway. Biomolecules, 10(4): 558. 10.3390/biom10040558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluchnejadmojarad T, Zeinali H, Roghani M, 2018. Scutellarin alleviates lipopolysaccharide-induced cognitive deficits in the rat: insights into underlying mechanisms. Int Immunopharmacol, 54: 311-319. 10.1016/j.intimp.2017.11.033 [DOI] [PubMed] [Google Scholar]
- Fan RH, Zhang Y, Botchway BOA, et al. , 2021. Resveratrol can attenuate astrocyte activation to treat spinal cord injury by inhibiting inflammatory responses. Mol Neurobiol, 58: 5799-5813. 10.1007/s12035-021-02509-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathroubi S, Loera-Muro A, Guerrero-Barrera AL, et al. , 2018. Actinobacillus pleuropneumoniae biofilms: role in pathogenicity and potential impact for vaccination development. Anim Health Res Rev, 19(1): 17-30. 10.1017/S146625231700010X [DOI] [PubMed] [Google Scholar]
- Li XY, Fang P, Mai JT, et al. , 2013. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol, 6: 19. 10.1186/1756-8722-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FE, Li L, Lu W, et al. , 2020. Scutellarin ameliorates cartilage degeneration in osteoarthritis by inhibiting the Wnt/β-catenin and MAPK signaling pathways. Int Immunopharmacol, 78: 105954. 10.1016/j.intimp.2019.105954 [DOI] [PubMed] [Google Scholar]
- Mas-Bargues C, Escrivá C, Dromant M, et al. , 2021. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch Biochem Biophys, 709: 108941. 10.1016/j.abb.2021.108941 [DOI] [PubMed] [Google Scholar]
- Snyder B, Shell B, Cunningham JT, et al. , 2017. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep, 5(9): e13258. 10.14814/phy2.13258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li YF, Gao SC, et al. , 2015. Breviscapine injection improves the therapeutic effect of Western medicine on angina pectoris patients. PLoS ONE, 10(6): e0129969. 10.1371/journal.pone.0129969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JN, Tan JY, Luo JH, et al. , 2017. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J Nanobiotechnol, 15: 18. 10.1186/s12951-017-0251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Li JY, Li F, et al. , 2019. Scutellarin suppresses cartilage destruction in osteoarthritis mouse model by inhibiting the NF-κB and PI3K/AKT signaling pathways. Int Immunopharmacol, 77: 105928. 10.1016/j.intimp.2019.105928 [DOI] [PubMed] [Google Scholar]
- Wu HY, Jia L, 2019. Scutellarin attenuates hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis and oxidative stress through regulating Keap1/Nrf2/ARE signaling. Biosci Rep, 39(11): BSR20192501. 10.1042/BSR20192501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Zhang W, Zhang P, et al. , 2013. Superior renoprotective effects of the combination of breviscapine with enalapril and its mechanism in diabetic rats. Phytomedicine, 20(10): 820-827. 10.1016/j.phymed.2013.03.027 [DOI] [PubMed] [Google Scholar]
- Yu Y, Tian ZQ, Liang L, et al. , 2019. Babao Dan attenuates acute ethanol-induced liver injury via Nrf2 activation and autophagy. Cell Biosci, 9: 80. 10.1186/s13578-019-0343-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wei GK, Di ZY, et al. , 2014. miR-339-5p inhibits alcohol-induced brain inflammation through regulating NF-κB pathway. Biochem Biophys Res Commun, 452(3): 450-456. 10.1016/j.bbrc.2014.08.092 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Shen Y, Xia L, et al. , 2020. Glucocorticoid and breviscapine combination therapy versus glucocorticoid alone on sudden sensorineural hearing loss in patients with different audiometric curves. Adv Ther, 37(12): 4959-4968. 10.1007/s12325-020-01513-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.