Abstract
Tibial dyschondroplasia (TD) is an intractable tibiotarsal bone disorder of rapid growing avian species, which leads to huge economic losses and compromised poultry welfare. However, the exact pathogenesis and treatment of TD remain largely unknown. Based on continuous research findings, we propose the TD pathogenesis hypothesis: during skeletal development of TD chickens, due to the absence of vasculature of proximal tibial growth plates (TGP), hypertrophic chondrocytes of the TGP are unable to complete calcification in normal bone development and less dead chondrocytes in the corresponding area can be timely transported through the blood vessels. Moreover, recent studies demonstrate that the TD formation mechanism gradually tends to a large number of dead chondrocytes in the TGP region or apoptosis occur due to various factors (such as, reduction of vascular invasion and blood cells, and increased weight or mechanical force of the tibia), while the reduction of blood vessels is insufficient to remove these chondrocytes and eventually leads to the TD formation. Recognizing the possible role of the blood vessels in the incidence of TD and can propose that the improvement in vasculature might be a novel therapeutic approach for ending TD in chickens.
key words: blood vessels, bone development, chondrocyte, mechanical force, tibial dyschondroplasia
INTRODUCTION
Tibial dyschondroplasia (TD) is an intractable tibiotarsal bone disorder in fast-growing poultry, particularly broilers and turkeys resulting in chondrocyte death of tibiotarsal growth plate due to the insufficient or untimely blood supply. Its pathological features have reached a consensus by dull white non-vascular and non-mineralized growth plates from epiphyseal growth plate to the proximal tibiotarsal bone that result in bone deformation and leg lameness (Gibson et al., 1995; Pines et al., 1998; Rath et al., 2007a; Herzog et al., 2011; Huang et al., 2017a). This tibiotarsal bone disorder leads to apparent locomotion problem with rising prevalence of 30% in broilers flock (Pelicia et al., 2012). In the production practice, accurately assess the prevalence of TD is very difficult due to its mostly sub-clinical symptoms (Groves and Muir, 2017), usually the farmers are easy to relax their vigilance this time. In fact, broilers once suffering from TD will cause leg weakness, motion reduction and even walking obstacles, are likely susceptible to fractures during the feeding process, which contribute to reduced production performance and compromised poultry welfare resulting in serious economic losses to poultry industry (Genin et al., 2012). Because of the massive economic loss of TD to poultry production worldwide, its pathogenesis and prevention have always been the focus of many scientists from all over the world.
BROILER NORMAL SKELETAL DEVELOPMENT
Through the introduction in the previous paragraph, we learned that normal bone development is very important for meat-type broilers. During the process of longitudinal bone growth, endochondral ossification is critical for longitudinal bone growth and is also initiated to achieve the bone length, and the bone is then converted to the bony tissue that occurs in the growth plates located at the ends of long bones (Xian et al., 2007). During this process, endochondral chondrocytes undergo the resting chondrocytes of the growth plate proliferation slowly, then differentiate into hypertrophic chondrocytes, ultimately chondrocytes atrophy, apoptosis, mineralization, and cartilaginous matrices are replaced by osteoblasts and bone matrix, respectively (Xian et al., 2007; Yan et al., 2016). Moreover, endochondral bone formation requires not only a cartilage template (known as the growth plate) but also vascular invasion (Kozhemyakina et al., 2015; Yan et al., 2016).
The previous study by Pines and Hurvitz (1991) has claimed that the avian has much longer columns of chondrocytes and more cells in each zone, and the more metaphyseal blood vessels penetrate deeply into the growth plate unexpectedly than that in mammalian. Blood vessels are very important for bone growth because the invading vasculature not only can trigger the calcified hypertrophic cartilage matrix and formation of the bone marrow cavity but also recruit osteoblast and osteoclast precursors that are converted to bone trabecular (Fong et al., 2009; Wuelling and Vortkamp, 2011; Yan et al., 2016).
As broiler age increases and bone development, hypertrophic chondrocytes of the tibial growth plate (TGP) cannot fully form calcification; however, the vast majority of hypertrophic chondrocytes will apoptosis in the terminally differentiating chondrocytes in hypertrophic regions that precede endochondral bone formation (Gibson, 1998; Rath et al., 1998). The apoptotic chondrocytes are characterized by nuclear pyknosis and nuclear lysis of cell morphology with intact cell membranes (Huang et al., 2018a; Zhang et al., 2019a). Surely, more cell death or apoptosis of chondrocytes is present in the growth plate when the bone joint is subjected to excessive mechanical force or when the load on the leg is increasing (Huang et al., 2017b). The organs in the animal body have powerful repair functions, and the bones are no exception. A small number of dead chondrocytes can be timely transported through the blood vessels for no accumulation in the tibia growth plate area showing in Figure 1 (Rath et al., 2007a). Therefore, slow-growing broilers generally do not have the occurrence of TD due to lighter leg weight and less dead chondrocytes in the TGP.
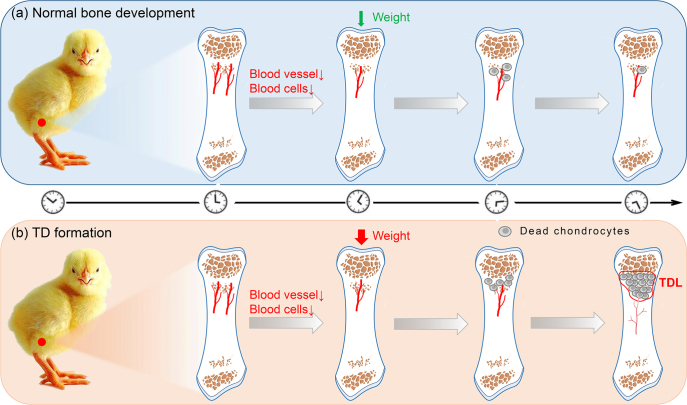
Figure 1.
Hypothetical model of tibial dyschondroplasia formation. a) Terminal differentiation of chondrocytes involves an ordered progression of cell states through proliferation, hypertrophic differentiation, to cell death. The figure shows examples of dead chondrocytes can be cleared by the blood vessels in the growth plate zone. b) Increased tibia bone weight or joint mechanical force leads to massive chondrocyte death or apoptosis. The figure shows examples of a greater number of dead chondrocytes cannot be cleared timely by less adequate blood vessels in the growth plate area. TDL, tibial dyschondroplasia lesion.
HYPOTHESIS OF TD PATHOGENESIS
Fast Growth and Greater Leg Mechanical Load
Too fast growth and greater weight gain increase the burden on the poultry's legs. In the poultry industry, the meat-type chicken is a fast-growing breed of poultry. Among them, Arbor Acre (AA) broilers are most commonly and its body weight (BW) can be as high as 2,000 g at 5 wk old (Huang et al., 2018a). In comparison to the AA chicken breeds, Tibetan chickens (TBC) grow slowly and the weight of the TBC is only about 1,000 g, which physiological characteristics of TBC are highly related to their long-term living in the special hypoxic environment of Qinghai-Tibet Plateau (Huang et al., 2017c). Semenza (2012) has also pointed out that oxygen (O2) inadequate supply for animals could compromise physiological performance, biological functions, and even growth capacity. Remarkably, any leg disorders have never been reported on TBC so far, which may be related to its slow growth rate. But broiler chickens are very susceptible to leg disease and up to 30% of broilers suffer from tibiotarsal bone disorder leads to apparent locomotion problem (Praul et al., 2000; Pelicia et al., 2012).
The previous study on broilers found that the growth rate of BW is more rapid than that of tibial weight, which implies an increase in the weight of the broiler's legs as the age increases (Huang et al., 2018a). Moreover, the excessive increase in BW of broiler chickens is mainly reflected in the increase of chest muscles. The increase of chest muscles causes the center of gravity of broilers to move in the direction of the front of the body, thereby the biomechanics of leg bones of broiler also changed (Figure 2). In a study of the effects of mechanical force on chondrocytes Huang et al. (2017b) pointed out that mechanical force can cause osteoarthritis-like pathological change and chondrocyte apoptosis of male Sprague–Dawley rats. Therefore, it can be seen that the growth rate of broiler weight plays a certain role in leg disease. Surely, once the broiler suffers from a leg disease, the BW of birds was significantly lighter due to lameness affecting food intake.
Figure 2.
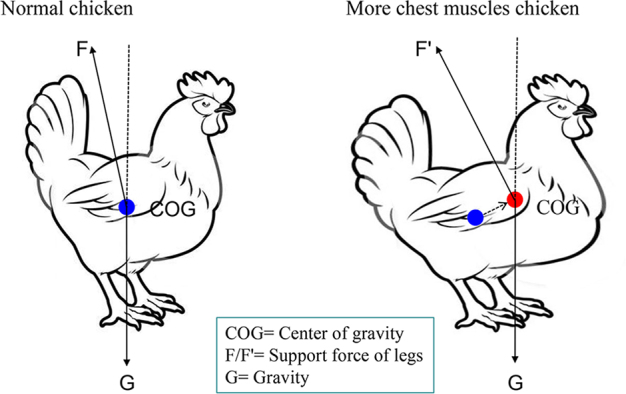
The model of broiler leg bones suffers biomechanical changes. Too fast growth and greater weight gain increase the burden on the broiler's legs and change the standing posture of chickens, which will cause the center of gravity to move forward, change support force of legs (F→F′), thereby increase legs' biomechanical force.
Insufficient Blood Cells Supply and Blood Vessel Distribution in the TGP
Thousands of cells in the animal body require the supply of O2 and nutrients to differentiate and mature, and the red blood cells (RBC) in the blood vessels play a transport function in this process. Therefore, changes in blood-related parameters should be a very critical means of assessing the physiological functions of animals (Huang et al., 2017a; Wang et al., 2018). It has been reported that the bone marrow of humans and animals is an important site for erythropoiesis and its injury suppresses the production of RBC (Tsiftsoglou et al., 2009; Medina et al., 2017; Seibert et al., 2017). In fact, the hemoglobin (Hb) comes from RBC ensures the supply of O2 and nutrients, and the removal of harmful metabolites. During erythropoiesis, insufficient or lower iron content in the bone marrow can cause a decrease in Hb content per RBC (Seibert et al., 2017). A study on blood parameters by Huang et al. (2017a) indicated that the total RBC counts, Hb levels and Hct (hematocrit) levels of broiler chickens suffering from TD were significantly reduced. Additionally, the distribution of blood vessels in the TGP region is also markedly decreased. Meanwhile, Wang et al. (2018) also had a similar finding that reduction of RBCs in the blood is affected during the TD occurrence and indicated that its change is associated with the expression of apoptosis-related genes in chicken erythrocytes. Based on the above information, we speculate that the reduction of blood vessels in the leg bones further exacerbates the lack of blood cells in the tibiae of broiler chickens suffer from TD. The histopathological changes of bone marrow and the differentiation of erythroblasts, and the role of erythropoietin in the kidney in this process deserve further attention and research.
The vasculature is known to be indispensable and critical for the bone tissue in endochondral bone development, formation, maintenance and repair (Ben Shoham et al., 2016). Moreover, bone tissue is highly vascularized and its blood vessels serve as a transport route for various blood cells (Prisby Rhonda, 2017). Besides supplying O2 and some essential nutrients, and removal of harmful metabolites for bone metabolism, bone blood vessels also deliver systemic hormones and precursor cells, and provide angiogenic and angiocrine signals for bone remodeling and homeostasis controlling (Eghbali-Fatourechi et al., 2005; Rivron et al., 2012; Kusumbe et al., 2014; Prisby Rhonda et al., 2017). Therefore, an extraordinary requisite for biological development of bone is adequate vascular supply, while the reduction of bone vessels is bound to affect both physiological and pathological processes of the bone formation.
Once broiler suffering from TD, increasing evidences can be observed by histopathology stained with hematoxylin and eosin showing a clearly reduction in vascular distribution and pale in the hypertrophic chondrocyte zone of the proximal TGP, which further suppressed bone growth and development (Rath et al., 2007a; Herzog et al., 2011; Huang et al., 2017a, 2018a). Previous study by Rath et al. (2007a) demonstrated that the occurrence of TD in broilers is due to the disruption of genes encoding vascular endothelial growth factor (VEGF) receptors, leading to a large number of endothelial cells death, which seriously suppresses the bone vascularization and the removal of dead chondrocytes, thereby the accumulation of dead chondrocytes leads to TD lesion formation. Moreover, improving the VEGF signaling pathway by some drugs, such as tetramethylpyrazine, can alleviate TD and increase blood vessel distribution in the TGP (Mehmood et al., 2018). Then, we can't help thinking about why broilers are prone to TD. A study on this question by statistical calculation gives an answer that the growth rate of angiogenesis or vascular invasion in the hypertrophic chondrocyte zone of proximal TGPs is slower than that of tibial weight and BW in the early growth stage of AA broilers, which implies bone blood vessel is below the required level of bone metabolism (Huang et al., 2018a).
Apoptotic Chondrocytes are Largely Retained in the TGP
During the bone growth and development of broilers, the vast majority of hypertrophic chondrocytes will go through apoptosis in the terminally differentiating chondrocytes in hypertrophic regions. Increasing evidence has confirmed that chondrocytes from the TGP zone undergo apoptosis during the development and formation of TD (Praul et al., 1997; Rath et al., 1998; Wang et al., 2018) and the vascular invasion of the hypertrophic chondrocyte zone in the proximal TGPs decreased rapidly in chickens with TD (Rath et al., 2007a; Tian et al., 2009; Huang et al., 2018a, b; Figure 3). According to previous studies, these apoptotic cells can be detected and observed by using different laboratory techniques including histopathology, terminal deoxynucleotide transferase-mediated nick end labeling, biochemical measurement of DNA fragmentation and flow cytometry (Praul et al., 1997; Rath et al., 1998). It is worth emphasizing that apoptosis has been suggested to be the mode of cell death in the terminally differentiating chondrocytes in hypertrophic regions that precede endochondral bone formation and has been shown in chondrocytes of hypertrophic regions of chicken growth plates (Rath et al., 1998). Moreover, the more apoptotic chondrocytes will appear on the growth plate when the bone joint is subjected to excessive mechanical force or the load on the leg is increasing (Huang et al.,2017b).
Figure 3.
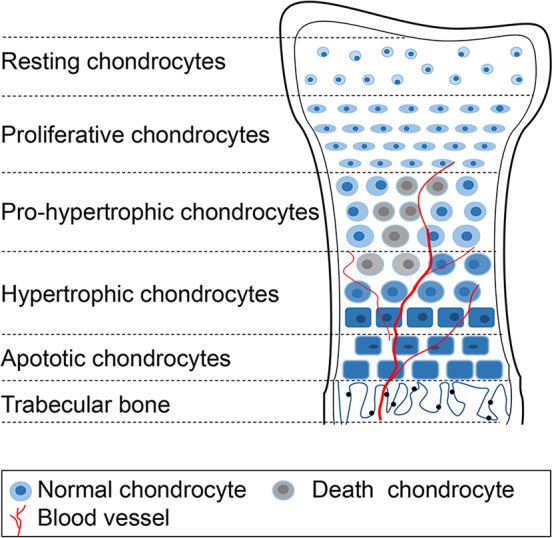
Death chondrocytes accumulated in the hypertrophic area of the tibial growth plate of tibial dyschondroplasia (TD) chicken. The figure shows an example of dead chondrocytes, which cannot be cleared timely in the pro-hypertrophic- and hypertrophic- zone of TD chicken due to the reduction of blood vessels.
Articular cartilage, one of its main functions is to carry mechanical loads. Previous studies have found that mechanical stress is an important factor regulating the proliferation and differentiation of cartilage, and indicated that appropriate mechanical stress stimulation can promote the proliferation and differentiation of chondrocytes, while excessive mechanical stress can inhibit the activity of chondrocytes and promote their apoptosis (Gibson et al., 1995; Huang et al., 2017b). Zhang et al. (2019b) showed that chondrocyte apoptosis by endoplasmic reticulum stress signaling pathway regulation can lead to the occurrence of femoral head necrosis in broilers.
Apoptosis is one of the 4 basic cell death modes including apoptosis, senescent death, necrosis and stress-induced cell death in animals and has been shown in chondrocytes of hypertrophic zones in the TGP of chickens (Rath et al., 1998; Shi et al., 2018). Moreover, Rath et al. (1998) by terminal deoxynucleotide transferase-mediated nick end labeling-fluorescent/propidium iodide (PI) staining of growth plate pointed out that normal growth plates showed no significant apoptosis of chondrocytes from hypertrophic and chondrolyzing zones. However, TD chickens had excessive chondrocytes undergo condensation and apoptosis in transition zone cartilage in TD chickens under similar conditions. At the same time, apoptotic changes of TD chicken were also observed on the capillary vessels causing a significant reduction in the vascular distribution in TGP, which resulted in insufficient blood vessels responsible for removing the apoptotic cells in normal tissues contributing to the pathogenesis of TD (Rath et al.,1998; Huang et al., 2018a; Figure 1 b).
Besides, the role of autophagy in growth plate chondrocytes is also concerned. Leach and Monsonego-Ornan (2007) pointed out that the regulation mechanisms of specific genes and gene products involved in autophagy and endoplasmic reticulum stress may be related to the development of the TD lesion. It is noted that the absence of autophagy can promote apoptosis (Pei et al., 2016). Therefore, increasing autophagy may be a potential strategy to inhibit apoptosis, which may improve the occurrence of TD.
CONCLUSIONS
Endochondral bone formation requires normal growth of the growth plate and proper vascular invasion. Once the growth plate chondrocytes are abnormal in the process of differentiation, maturation, and calcification, and the insufficient supply of blood vessels, which can cause abnormal development of the bones, and even lead to the occurrence of leg diseases. In some experiments, cartilages and hypertrophic zone of the TGPs from chickens with TD were observed to have a decreased number of blood vessels and angionecrosis (Tian et al., 2013; Huang et al., 2017a), which raises the possibility that the abnormal of differentiation, maturation apoptotic chondrocytes cannot be transported out timely. Similar studies have been reported in the previous study that endothelial cell death compromise vascularization, cartilage remodeling, and the removal of dead chondrocytes leading to TD lesions (Rath et al., 2007). Therefore, increasing research shows that the TD formation mechanism gradually tends to a large number of dead chondrocytes in the TGP region or apoptosis, while the reduction of blood vessels is insufficient to remove these chondrocytes and eventually leads to the TD formation in broiler chicken.
Although a great deal of data describing the role of blood vessels in tibia development of this disease have been collected, there have been reports that genetic selection, copper-deficient diets, calcium and phosphorus metabolism disorders, and dietary dithiocarbamates are also associated with TD lesion formation (Rosselot et al., 1994; Ledwaba and Roberson, 2003; Leach and Monsonego-Ornan, 2007; Rath et al., 2007b; Kapell et al., 2017). However, what is the cause and pathogenesis of TD lesion formation in chickens and how to prevent and minimize its deleterious effects on the poultry industry remains the challenge for future research.
Ethical Approval
This study was performed in strict accordance with the Regulations of the Chinese National Research Council (1994) and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
ACKNOWLEDGMENTS
We are grateful to the financial support from the Outstanding Talents of Henan Agricultural University (No. 30500421) for supporting this work.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
REFERENCES
- Ben Shoham A., Rot C., Stern T., Krief S., Akiva A., Dadosh T., Sabany H., Lu Y., Kadler K.E., Zelzer E. Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology. Development. 2016;143:3933–3943. doi: 10.1242/dev.139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G., Lamsam J., Fraser D., Nagel D., Riggs B.L., Khosla S. Circulating osteoblast-lineage cells in humans. N. Engl. J. Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- Fong L., Tan K., Tran C., Cool J., Scherer M.A., Elovaris R., Coyle P., Foster B.K., Rofe A.M., Xian C.J. Interaction of dietary zinc and intracellular binding protein metallothionein in postnatal bone growth. Bone. 2009;44:1151–1162. doi: 10.1016/j.bone.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Genin O., Hasdai A., Shinder D., Pines M. The effect of inhibition of heat-shock proteins on thiram-induced tibial dyschondroplasia. Poult. Sci. 2012;91:1619–1626. doi: 10.3382/ps.2012-02207. [DOI] [PubMed] [Google Scholar]
- Gibson G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc. Res. Tech. 1998;43:191–204. doi: 10.1002/(SICI)1097-0029(19981015)43:2<191::AID-JEMT10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Gibson G.J., Kohler W.J., Schaffler M.B. Chondrocyte apoptosis in endochondral ossification of chick sterna. Dev. Dyn. 1995;203:468–476. doi: 10.1002/aja.1002030409. [DOI] [PubMed] [Google Scholar]
- Groves P.J., Muir W.I. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal. 2017;11:112–120. doi: 10.1017/S1751731116001105. [DOI] [PubMed] [Google Scholar]
- Herzog A., Genin O., Hasdai A., Shinder D., Pines M. Hsp90 and angiogenesis in bone disorders–lessons from the avian growth plate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R140–R147. doi: 10.1152/ajpregu.00134.2011. [DOI] [PubMed] [Google Scholar]
- Huang S., Tong X., Rehman M.U., Wang M., Zhang L., Wang L., Li J., Yang S. Oxygen supplementation ameliorates tibial development via stimulating vascularization in Tibetan chickens at high altitudes. Int. J. Biol. Sci. 2017;13:1547–1559. doi: 10.7150/ijbs.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Wang M., Rehman M.U., Zhang L., Tong X., Shen Y., Li J. Role of angiopoietin-like 4 on bone vascularization in chickens exposed to high-altitude hypoxia. J. Comp. Pathol. 2018;161:25–33. doi: 10.1016/j.jcpa.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Huang S.C., Rehman M.U., Lan Y.F., Qiu G., Zhang H., Iqbal M.K., Luo H.Q., Mehmood K., Zhang L.H., Li J.K. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1alpha/VEGF/VEGFR signaling pathway in chickens. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-09664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.C., Zhang L.H., Zhang J.L., Rehman M.U., Tong X.L., Qiu G., Jiang X., Iqbal M., Shahzad M., Shen Y.Q., Li J.K. Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-22109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.W., Zhou M., Wang Q., Zhu M.J., Chen S., Li H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch. Oral Biol. 2017;84:125–132. doi: 10.1016/j.archoralbio.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Kapell D.N.R.G., Hocking P.M., Glover P.K., Kremer V.D., Avendaño S. Genetic basis of leg health and its relationship with body weight in purebred turkey lines. Poult. Sci. 2017;96:1553–1562. doi: 10.3382/ps/pew479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E., Lassar A.B., Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R.M., Jr, Monsonego-Ornan E. Tibial dyschondroplasia 40 years later. Poult. Sci. 2007;86:2053–2058. doi: 10.1093/ps/86.10.2053. [DOI] [PubMed] [Google Scholar]
- Ledwaba M., Roberson K. Effectiveness of twenty-five-hydroxycholecalciferol in the prevention of tibial dyschondroplasia in Ross cockerels depends on dietary calcium level. Poult. Sci. 2003;82:1769–1777. doi: 10.1093/ps/82.11.1769. [DOI] [PubMed] [Google Scholar]
- Medina S., Xu H., Wang S.C., Lauer F.T., Liu K.J., Burchiel S.W. Low level arsenite exposures suppress the development of bone marrow erythroid progenitors and result in anemia in adult male mice. Toxicol. Lett. 2017;273:106–111. doi: 10.1016/j.toxlet.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Li K., Wang L., Rehman M.U., Nabi F., Iqbal M.K., Luo H., Shahzad M., Li J. Effect of tetramethylpyrazine on tibial dyschondroplasia incidence, tibial angiogenesis, performance and characteristics via hif-1α/vegf signaling pathway in chickens. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-20562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicia K., Aparecido I.M., Garcia E.A., Molino A.B., Santos G.C., Berto D.A., Vieira Filho J.A.I., Murakami E.S.M., Montenegro A.T., Silva A.M. Evaluation of a radiographic method to detect tibial dyschondroplasia lesions in broilers. Rev. Bras. Cienc. Avic. 2012;14:129–135. [Google Scholar]
- Pei J., Deng J., Ye Z., Wang J., Gou H., Liu W., Zhao M., Liao M., Yi L., Chen J. Absence of autophagy promotes apoptosis by modulating the ROS-dependent RLR signaling pathway in classical swine fever virus-infected cells. Autophagy. 2016;12:1738–1758. doi: 10.1080/15548627.2016.1196318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M., Hurwitz S. The role of the growth plate in longitudinal bone growth. Poult. Sci. 1991;70:1806–1814. doi: 10.3382/ps.0701806. [DOI] [PubMed] [Google Scholar]
- Pines M., Knopov V., Genina O., Hurwitz S., Faerman A., Gerstenfeld L.C., Leach R.M. Development of avian tibial dyschondroplasia: gene expression and protein synthesis. Calcif. Tissue Int. 1998;63:521–527. doi: 10.1007/s002239900568. [DOI] [PubMed] [Google Scholar]
- Praul C.A., Gay C.V., Leach R.M., Jr. Chondrocytes of the tibial dyschondroplastic lesion are apoptotic. Int. J. Dev. Biol. 1997;41:621–626. [PubMed] [Google Scholar]
- Praul C.A., Ford B.C., Gay C.V., Pines M., Leach R.M. Gene expression and tibial dyschondroplasia. Poult. Sci. 2000;79:1009–1013. doi: 10.1093/ps/79.7.1009. [DOI] [PubMed] [Google Scholar]
- Prisby Rhonda D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 2017;235:R77–R100. doi: 10.1530/JOE-16-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath N.C., Huff W.E., Huff G.R. Thiram-induced changes in the expression of genes relating to vascularization and tibial dyschondroplasia. Poult. Sci. 2007;86:2390–2395. doi: 10.3382/ps.2007-00219. [DOI] [PubMed] [Google Scholar]
- Rath N.C., Huff W.E., Bayyari G.R., Balog J.M. Cell death in avian tibial dyschondroplasia. Avian Dis. 1998;42:72–79. [PubMed] [Google Scholar]
- Rath N.C., Huff W.E., Huff G.R., Kannan L. Induction of tibial dyschondroplasia by carbamate and thiocarbamate pesticides. Avian Dis. 2007;51:590–593. doi: 10.1637/0005-2086(2007)51[590:IOTDBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rivron N.C., Raiss C.C., Liu J., Nandakumar A., Sticht C., Gretz N., Truckenmüller R., Rouwkema J., van Blitterswijk C.A. Sonic Hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc. Nati. Acad. Sci. 2012;109:4413–4418. doi: 10.1073/pnas.1117627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselot G., Sokol C., Leach R. Effect of lesion size on the metabolic activity of tibial dyschondroplastic chondrocytes. Poult. Sci. 1994;73:452–456. doi: 10.3382/ps.0730452. [DOI] [PubMed] [Google Scholar]
- Seibert E., Richter A., Kuhlmann M.K., Wang S., Levin N.W., Kotanko P., Handelman G.J. Plasma vitamin C levels in ESRD patients and occurrence of hypochromic erythrocytes. Hemodial. Int. 2017;21:250–255. doi: 10.1111/hdi.12467. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhou H., Lei M., Chen L., Zellmer L., He Y., Yang W., Xu N., Liao D.J. Spontaneous cancers, but not many induced ones in animals, resemble semi-new organisms that possess a unique programmed cell death mode different from apoptosis, senescent death, necrosis and stress-induced cell death. J. Cancer. 2018;9:4726–4735. doi: 10.7150/jca.26502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W.X., Li J.K., Qin P., Wang R., Ning G.B., Qiao J.G., Li H.Q., Bi D.R., Pan S.Y., Guo D.Z. Screening of differentially expressed genes in the growth plate of broiler chickens with tibial dyschondroplasia by microarray analysis. BMC Genom. 2013;14:276. doi: 10.1186/1471-2164-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W.X., Zhang W.P., Li J.K., Bi D.R., Guo D.Z., Pan S.Y., Zhang Y.H., Qin P. Identification of differentially expressed genes in the growth plate of broiler chickens with thiram-induced tibial dyschondroplasia. Avian Pathol. 2009;38:161–166. doi: 10.1080/03079450902737789. [DOI] [PubMed] [Google Scholar]
- Tsiftsoglou A.S., Vizirianakis I.S., Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009;61:800–830. doi: 10.1002/iub.226. [DOI] [PubMed] [Google Scholar]
- Wang C.X., Niu S., Jahejo A.R., Jia F.J., Li Z., Zhang N., Ning G.B., Zhang D., Li H.Q., Ma H.L., Hao W.F., Gao W.W., Gao S.M., Li J.H., Li G.L., Yan F., Gao R.K., Zhao Y.J., Chen H.C., Tian W.X. Identification of apoptosis-related genes in erythrocytes of broiler chickens and their response to thiram-induced tibial dyschondroplasia and recombinant glutathione-S-transferase A3 protein. Res. Vet. Sci. 2018;120:11–16. doi: 10.1016/j.rvsc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Wuelling M., Vortkamp A. Chondrocyte proliferation and differentiation. Endocr. Dev. 2011;21:1–11. doi: 10.1159/000328081. [DOI] [PubMed] [Google Scholar]
- Xian C.J., Cool J.C., Scherer M.A., Macsai C.E., Fan C., Covino M., Foster B.K. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone. 2007;41:842–850. doi: 10.1016/j.bone.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Yan B., Zhang Z., Jin D., Cai C., Jia C., Liu W., Wang T., Li S., Zhang H., Huang B., Lai P., Wang H., Liu A., Zeng C., Cai D., Jiang Y., Bai X. mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation. Nat. Commun. 2016;7 doi: 10.1038/ncomms11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Mehmood K., Jiang X., Li Z., Yao W., Zhang J., Tong X., Wang Y., Li A., Waqas M., Iqbal M., Li J. Identification of differentially expressed MiRNAs profile in a thiram-induced tibial dyschondroplasia. Ecotoxicol. Environ. Saf. 2019;175:83–89. doi: 10.1016/j.ecoenv.2019.03.043. [DOI] [PubMed] [Google Scholar]
- Zhang M., Li S., Pang K., Zhou Z. Endoplasmic reticulum stress affected chondrocyte apoptosis in femoral head necrosis induced by glucocorticoid in broilers. Poult. Sci. 2019;98:1111–1120. doi: 10.3382/ps/pey474. [DOI] [PubMed] [Google Scholar]