Abstract
The present study evaluated the efficacy of ethanol treatment (0, 30, 50, or 70%) alone or in combination with ultrasound (37 kHz, 380 W) for the reduction of natural indigenous mesophilic aerobic bacteria (MAB), coliforms, and inoculated Salmonella Typhimurium on chicken skin. Bacterial cells with loose, intermediate, or tight attachment to chicken skin were recovered by shaking in an incubator (200 rpm) for 5 min, stomaching for 1 min, or blending for 1 min, respectively. Chicken skins were inoculated with a suspension (7 log CFU/mL) of S. Typhimurium. Ethanol reduced the number of MAB, coliforms, and S. Typhimurium on the chicken skin in a concentration-dependent manner, whereas ultrasound treatment without ethanol was ineffective. A combination of 70% ethanol with ultrasound treatment was the most effective in reducing S. Typhimurium populations with loose, intermediate, and tight attachment (reduction by 2.86 log CFU/g, 2.49 log CFU/g, and 1.63 log CFU/g, respectively). However, chicken skin treated with 50% ethanol alone or with a combination of >50% ethanol and ultrasound showed significant changes in Hunter color values (a* and b*) and texture (shear force) (P > 0.05). On the other hand, a combination of 30% ethanol and ultrasound yielded the best results, leading to a reduction of S. Typhimurium by a >1.0 log CFU/g, but did not alter the color or texture of chicken skin. Thus, a combination of 30% ethanol and ultrasound appears to be the optimum treatment for reduction of microbial contamination in production and distribution of skin-on chicken products, and enhance poultry safety without decreasing food quality.
key words: Salmonella Typhimurium, chicken skin, ultrasound, ethanol, quality
INTRODUCTION
Contamination of poultry and poultry products with pathogenic microorganisms has been of considerable concern to both consumers and the poultry industry. Salmonella, one of the most prevalent microorganisms in poultry, is a serious public health concern, being a major cause of food-borne gastroenteritis, especially in immune-compromised individuals (El-Gazzar and Marth, 1992; Rabsch et al., 2001). According to the Foodborne Diseases Active Surveillance Network (FoodNet) reports, Salmonella serotypes continue to be the leading causes of large foodborne outbreaks in the United States (Henao et al., 2010; Nyachuba, 2010). Of the 25,656 cases of infection (per 100,000) reported in the United States, 5,893 were hospitalized and 120 died, and non-typhoidal Salmonella spp. was identified as the second primary causative microbe (18.3%) after the Campylobacter during 2018 (Tack et al., 2019). Although extensive food regulations and monitoring systems have been implemented in the food industry, meat and poultry products continue to be associated with consumer concern regarding food safety. According to the European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) report, 9,061 human cases have been associated with 1,067 salmonellosis outbreaks in the EU and egg, poultry, poultry products, and cheese are the main sources of salmonellosis (EFSA and ECDC, 2017). In 2014, the predominant serovar in food was S. Infantis, followed by S. Typhimurium, S. Enteritidis, and S. Dublin in the European region. All these serovars were reported to be the most frequent among human cases in Europe (Lelièvre et al., 2019). Most reports of human Salmonella infections in the United States involve one of the 3 serotypes, S. Enteritidis (2.6 per 100,000 population), S. Newport (1.6), and S. Typhimurium (1.5) during 2015 to 2018 (Tack et al., 2019). Among these, S. Typhimurium is the most predominant serotype associated with the consumption of poultry and poultry products, with the findings supported by laboratory confirmation.
As poultry is a major food source worldwide regardless of religion or racial and cultural barriers, global poultry consumption has been continuously increasing (FAO, 2010, 2013). In the poultry industry, microbial contamination on the surface of and within deep channels in poultry skin has been identified, occurring during a variety of poultry processing stages including scalding, defeathering, evisceration, and chilling (Hafez et al., 1997; Ono and Yamamoto, 1999; Buhr et al., 2005; McKee, 2012). Thomas and McMeekin (1980) reported that a greater variety of microorganisms were found on poultry skin immersed in water than on unprocessed poultry skin. Various microorganisms can contaminate poultry via tears in the skin generated during feather plucking. Although most chicken is chemically treated prior to packaging, microorganisms that are strongly attached to the skin often cannot be eliminated completely, thus potentially leading to cross-contamination (Ko et al., 2005; Zhang et al., 2011). Microorganisms on poultry skin cannot be easily removed by gentle rinsing (Hinton and Cason, 2008), and the number of bacterial cells detected with intermediate or tight attachment increases due to stomaching (Notermans and Kampelmacher, 1975). Therefore, several microorganisms remain on poultry skin after processing (Hannah et al., 2011). Lee et al. (2014) have reported that naturally occurring microbes are more likely to have intermediate or tight attachment to the skin than loose attachment.
Chemical or physical treatment or their combinations (McClements 1995; Piyasena et al., 2003; James et al., 2006) have been used to reduce microbial contamination in chicken skin, which represents a major reservoir of pathogenic microorganisms and is hazardous because it is often consumed with the meat (Ko et al., 2005; Zhang et al., 2011). Various methods of chemical disinfection including treatment with acidified sodium chlorite, bromine, chlorine dioxide, organic acid, peracetic acid, trisodium phosphate, monochloramine, or electrolyzed water (Bilgili, 2009) have been employed in the poultry industry to eliminate microbial organisms. In particular, ethanol has been extensively used as a disinfectant and a preservative in food products (Barker and Park, 2001; Kalathenos and Russell, 2003) since the 1970s. Conventionally, 70% ethanol is used as a surface disinfectant. Various studies have found that ethanol treatment is effective in microbial reduction. A study reported that 20% ethanol had no effect against Bacillus cereus, Staphylococcus aureus, or Escherichia coli but 30% ethanol did (Jang et al., 2003). Most companies that manufacture noodles in Korea use ethanol treatment to prolong the shelf-life of wet noodles (Kim et al., 2011).
Ultrasound treatment has been used as a physical treatment method to reduce pathogenic microorganisms in food processing and produce healthy, safe, and high-quality foods. Ultrasound leads to powerful cavitation, which can detach microorganisms from food surfaces and disrupt their lipid membranes; the frequency of ultrasound commonly used in the food industry ranges from 20 kHz to 10 MHz (Leighton, 1994; Scouten and Beuchat, 2002; Seymour et al., 2002; Piyasena et al., 2003). Ultrasound has been shown to effectively reduce microorganisms when combined with temperature, pH, or chemical treatment such as chlorination (Earnshaw et al., 1995; McClements, 1995; Piyasena et al., 2003; Sagong et al., 2011). São José and Vanetti (2012) reported that combinations of commercial sanitizers (hydrogen peroxide, sodium dichloroisocyanurate, peracetic acid, and chlorine dioxide) with ultrasound were effective in removing mesophilic aerobic bacteria (MAB) and S. Typhimurium from cherry tomatoes. Ultrasound has also been shown to be more effective in spinach disinfection when used in combination with a disinfectant than alone (Zhou et al., 2009). Therefore, the use of ultrasound for decontamination in the poultry industry is justifiable (Bolder, 1997).
However, a combination of ethanol and ultrasound in treatment of chicken skin has not yet been evaluated. The purpose of the present study was to investigate the effectiveness of 30, 50, or 70% ethanol in combination with ultrasound (37 kHz, 380 W) for removal of MAB, coliforms, and S. Typhimurium with loose, intermediate, or tight attachment to chicken skin.
MATERIALS AND METHODS
Bacterial Strains and Inoculum Preparation
S. Typhimurium isolated from poultry was used. The strain was transferred from a stock culture and stored at –70°C in tryptic soy broth (TSB; Difco Laboratories, Detroit, MI, USA) containing 50% glycerol (Fisher Scientific, Itasca, IL). For activation, the strain was subcultured at least twice at 37°C for 24 h in 10 mL of TSB. The cells were centrifuged at 12,000× g for 10 min at 4°C. The cell pellet was suspended in phosphate buffered saline (PBS; Oxoid, Basingstoke, UK) after one wash to yield a final cell concentration of 7 log CFU/mL for inoculation. Bacteria were counted by plating on xylose lysine deoxycholate agar (XLD; Difco Laboratories, Detroit, MI, USA) containing 25 μg/mL of nalidixic acid (NA; Sigma Aldrich Co. St. Louis, MO, USA) and 25 μg/mL of novobiocin (NO; Sigma Aldrich Co. St. Louis, MO, USA) and incubated at 37°C for 24 h.
Sample Preparation and Inoculation
Chicken skin was obtained from a local market (Anseong, Korea) and stored at 4°C prior to the experiment. The chicken skin was cut into uniform 10 g pieces using sterile stainless steel scissors and used immediately. To remove background microorganisms from the chicken skin in S. Typhimurium inoculation experiments, samples were treated with UV light (Sankyo UV Co. Ltd., Seoul, Korea) at 1,000 μW s/cm2 for 5 min and then rinsed once with sterile distilled water for 2 min. Experiments involving natural indigenous MAB or coliforms did not involve UV treatment. Samples were dried on a clean bench for 10 min, and the skin surfaces were inoculated with 0.5 mL of S. Typhimurium suspension for 10 min. The samples were stored at 4°C for 1 h to allow the S. Typhimurium to become attached, and then rinsed in sterile distilled water for 20 s to remove non-attached cells. Uninoculated skins were used to evaluate coliforms and MAB.
Disinfection Treatments
Ethanol at 30, 50, or 70% (Biosesang, Sungnam, Korea) was used as chemical treatment to remove S. Typhimurium, MAB, and coliforms from chicken skin. All disinfectant solutions were manufactured before use and applied at room temperature. Ultrasound (P 300 H model, 230 V, Hucom System Co., Elmasonic, Kolpingstr, Singen, Germany) was used as physical treatment to detach the S. Typhimurium, MAB, and coliforms from the surface of the chicken skin. Before the ultrasound treatment, the ultrasound chamber was filled with 12 L of distilled water, and then inoculated samples were placed in sterile glass beakers (250 mL) containing 90 mL of chemical disinfectant and exposed to ultrasound at 37 kHz and 380 W for 5 min. Levels of S. Typhimurium, MAB, and coliforms were measured. Skin samples with non-treated ethanol and ultrasound served as control for this study. All experiments were repeated 3 times.
Enumeration of Microorganisms
Microbial analysis was performed as described in Zhang et al. (2013). Briefly, samples (10 g) were treated with chemical or physical disinfection or both; the samples were placed in 90 mL of 0.1% peptone water (PW, Oxoid, Basingstoke, Hampshire, England) in a sterile glass beaker and shaken at 200 rpm for 5 min in a shaking incubator (VS-101Si, Vision Science, Daejeon, Korea) at room temperature. The recovered microorganisms were classified as cells with loose attachment. Rinsed chicken skin was transferred to Whirl-Pak bags (Nasco, Fort Atkinson, WI) containing 90 mL of 0.1% PW and stomached for 1 min in a stomacher (Stomacher, SH-IIM, Elmex, Tokyo, Japan). The recovered microorganisms were classified as cells with intermediate attachment. Finally, stomached chicken skin was transferred to a sterile bottle containing 90 mL of PW. Using a blender (SMX-760 J, Shinil, Seoul, Korea), the sample was blended for 1 min. The recovered microorganisms were classified as cells with tight attachment. Serial 10-fold dilutions of the rinsed, stomached, or blended samples were plated on xylose lysine deoxycholate agar (XLD, Difco Laboratories, Detroit, MI, USA) containing 25 μL/mL of NA and 25 μm/mL of NO, tryptic soy agar (TSA, Difco Laboratories, Detroit, MI, USA), or violet red bile agar (VRBA, Difco Laboratories, Detroit, MI, USA) and the numbers of S. Typhimurium, MAB, and coliforms, respectively, were counted following incubation.
Color and Texture Measurement
After the combination treatments, differences in the color and texture of all treated samples were measured to assess any changes in the quality of the chicken skin. Chicken skin color was measured using a color difference meter (UltraScan PRO, Hunterlab Co., USA) at 5 locations on each sample and expressed as lightness (L*), redness (a*), and yellowness (b*). As described by Salim et al. (2012), sample texture was measured by stretching the chicken skin using a texture analyzer (TAHDi/500, TAHD Co.) at a test speed of 1.00 mm/s and trigger force of 0.04903 N. This force was required to tear the skin samples and samples slipped out from the clamp were omitted. All experiments were performed 3 times.
Field Emission Scanning Electron Microscopy
Field emission scanning electron microscopy (FE-SEM, Sigma, Carl Zeiss, Germany) was performed as described in Lee et al. (2014). FE-SEM was conducted to observe changes in the number of S. Typhimurium on the surface of chicken skin after treatment with 70% ethanol, ultrasound, or their combination. The results were compared with controls treated with sterile distilled water. The samples were dipped into a suspension of S. Typhimurium (8 log CFU/mL) for 10 min. The chicken skin was dried for 1 h on a clean bench. Then, the chicken skin was gently washed with PBS (PBS, pH 7.2, Oxoid, Basingstoke, UK), fixed overnight with 2% glutaraldehyde (Sigma, St. Louis, MO, USA) and post-fixed in 2% osmium tetroxide (OsO4, Sigma, St Louis, MO, USA) for 1 h followed by washing in PBS for 15 min to remove the fixation solution. Next, the samples were dehydrated using a graded ethanol series (50, 60, 70, 80, 90, and 100). The final treatment with 100% ethanol was performed 3 times. All ethanol steps were performed for 15 min in duplicate. The samples were then successively dehydrated with 25, 50, 75, and 100% hexamethyldisilazane (Sigma Aldrich, MO) in ethanol for 15 min. The chicken skin samples were then dried in a freeze dryer for 3 D, coated with gold palladium, and observed under FE-SEM. The FE-SEM microscope was operated at an acceleration voltage of 2 kV at a 5-mm working distance.
Statistical Analysis
The experimental data were analyzed with ANOVA using the software Statistical Analysis System (SAS), version 9.2 (SAS Institute, Cary, NC, USA). Average values and significance were determined using Duncan's multiple-range test, and results were considered significantly different at P < 0.05.
RESULTS AND DISCUSSION
Autochthonous Flora on Chicken Skin
In many countries, chicken meat including breasts, legs, and wings are commonly consumed at home and produced in the food service industry. Chicken meat in Korea is generally distributed in packaged and refrigerated products via supermarkets, or without plastic packaging in some traditional markets. It is plausible that contamination of chicken meat and skin is likely to occur during storage in retail markets due to a number of pathogenic bacteria. Table 1 shows the number of natural indigenous MAB and coliforms found on chicken skin without treatment. No significant differences (P > 0.05) were observed between the number of MAB with intermediate (6.84 log CFU/g) attachment and those with tight (6.72 log CFU/g) attachment; however, the number of MAB with loose attachment was significantly different from both the above, at 5.87 log CFU/g (P < 0.05). The number of coliforms with loose, intermediate, or tight attachment to chicken skin was 3.51, 3.77, and 3.36 log CFU/g, respectively, and had no significant differences (P > 0.05). The naturally existing MAB and coliforms with intermediate attachment were the most numerous, at 6.84 and 3.77 log CFU/g respectively, compared to those with loose or tight attachment. Lee et al. (2014) reported that naturally existing MAB and coliforms had more cells with intermediate (6.69 and 5.06 log CFU/g) or tight (6.59 and 5.59 log CFU/g) attachment those with loose (5.84 and 4.61 log CFU/g) attachment. Microorganisms can proliferate on chicken skin, which can be damaged during production processes including defeathering, scalding, plucking, and subsequent stages (Notermans and Kampelmacher, 1975; Thomas and McMeekin, 1980).
Table 1.
Populations (log CFU/g) of loosely, intermediately, and tightly attached mesophilic aerobic bacteria and coliform on chicken skin.
| Item | Loosely | Intermediately | Tightly |
|---|---|---|---|
| Mesophilic aerobic bacteria | 5.87 ± 0.15b | 6.84 ± 0.20a | 6.72 ± 0.32a |
| Coliform | 3.51 ± 0.27 | 3.77 ± 0.26 | 3.36 ± 0.41 |
Mean values within the same row with no common superscripts were different (P < 0.05).
Effect of Treatments on Autochthonous Flora
The effect of ethanol and ultrasound treatment on MAB and coliforms with loose, intermediate, or tight attachment to chicken skin are shown in Tables 2 and 3. Chicken skin treated with 0% ethanol (water treatment) had MAB and coliforms, respectively at 4.48 and 2.58 log CFU/g with loose attachment, 5.04 and 3.52 log CFU/g with intermediate attachment, and 5.22 and 3.31 log CFU/g with tight attachment (data not shown). Single treatment with 30% ethanol produced no significant (P > 0.05) reduction in any of the above 3 types of MAB or coliforms. However, a single treatment with 50 or 70% ethanol caused significant differences (P < 0.05) in the reductions of all 3 types of MAB and coliforms on chicken skin. The reduction in MAB numbers due to 50 or 70% ethanol treatment was 1.03 and 1.57 log CFU/g, respectively, in those with loose attachment, 0.80 and 0.98 log CFU/g, respectively, in those with intermediate attachment, and 0.57 and 0.69 log CFU/g, respectively, in those with tight attachment. In the case of coliforms, treatment with 50 or 70% ethanol alone caused decreases of 0.93 and 1.02 log CFU/g, respectively, in those with loose attachment, which were significantly different (P < 0.05) from the decreases in the numbers of coliforms with intermediate (0.72 and 0.88 log CFU/g, respectively) or tight (0.53 and 0.65 log CFU/g, respectively) attachment. The reduction in the numbers of MAB and coliforms increased in an ethanol concentration-dependent manner from 30 to 70%, with 70% being the most effective (Table 2 and 3). Cho and Park (2012) reported that treatment with 10% ethanol reduced the numbers of total mesophilic bacteria and coliforms to 2.43 and 2.37 log CFU/g, respectively in cabbage. Piernas and Guiraud (1998) observed that treatment with 70% ethanol for 10 min reduced the number of total mesophilic bacteria to 3.50 log CFU/g in rice sprouts. However, the present study found that treatment with a single chemical antimicrobial agent could not remove microorganisms with intermediate or tight attachment to chicken skin as effectively as those with loose attachment. Many studies have been focused on the application of hurdle technology in rapid and efficient decontamination in the food industry including in poultry processing (McKee, 2012; Ahn et al., 2013).
Table 2.
Reduction efficacy (log CFU/g) of ethanol alone and ethanol/ultrasound against loosely, intermediately, and tightly attached mesophilic aerobic bacteria on chicken skin.
| Treatments | Ethanol (%) | Loosely | Intermediately | Tightly |
|---|---|---|---|---|
| Without US | 30 | 0.67 ± 0.17d | 0.59 ± 0.06c,d | 0.44 ± 0.04b,c |
| 50 | 1.03 ± 0.03c,x | 0.80 ± 0.01b,c,y | 0.57 ± 0.11b,c,z | |
| 70 | 1.57 ± 0.27b,x | 0.98 ± 0.25b,y | 0.69 ± 0.04b,c,z | |
| With US1 | 0 | 0.13 ± 0.05e | 0.28 ± 0.05d | 0.22 ± 0.08c |
| 30 | 1.38 ± 0.18b,c,x | 0.85 ± 0.09b,c,x,y | 0.72 ± 0.10b,y | |
| 50 | 2.60 ± 0.01a,x | 1.50 ± 0.26a,y | 1.34 ± 0.04a,y | |
| 70 | 2.66 ± 0.08a,x | 1.63 ± 0.01a,y | 1.43 ± 0.23a,y |
Mean values within the same column with no common superscripts were different (P < 0.05).
Mean values within the same row with no common superscripts were different (P < 0.05).
US: ultrasound treatment (frequencies of 37 kHz, 380 W, 5 min).
Table 3.
Reduction efficacy (log CFU/g) of ethanol alone and ethanol/ultrasound against loosely, intermediately, and tightly attached coliform on chicken skin.
| Treatments | Ethanol (%) | Loosely | Intermediately | Tightly |
|---|---|---|---|---|
| Without US | 30 | 0.57 ± 0.17d | 0.39 ± 0.05d | 0.32 ± 0.43c |
| 50 | 0.93 ± 0.24c,d,x | 0.72 ± 0.01c,y | 0.53 ± 0.18b,z | |
| 70 | 1.02 ± 0.44b,c,x | 0.88 ± 0.14b,c,y | 0.65 ± 0.11a,b,y | |
| With US1 | 0 | 0.16 ± 0.37e | 0.15 ± 0.01e | 0.12 ± 0.07d |
| 30 | 1.04 ± 0.01b,c,x | 0.80 ± 0.08b,c,y | 0.52 ± 0.06b,z | |
| 50 | 1.80 ± 0.07a,b,x | 1.05 ± 0.14a,b,y | 0.94 ± 0.20a,z | |
| 70 | 1.91 ± 0.12a,x | 1.27 ± 0.15a,y | 0.99 ± 0.12a,z |
Mean values within the same column with no common superscripts were different (P < 0.05).
Mean values within the same row with no common superscripts were different (P < 0.05).
US: ultrasound treatment (frequencies of 37 kHz, 380 W, 5 min).
The effect of ultrasound treatment alone did not result in significant differences (P > 0.05) in bacterial numbers in the present study. Sams and Feria (1991) reported that ultrasound treatment reduced aerobic microorganisms by 0.8 log CFU/cm2 on chicken legs. Most studies have shown that antimicrobial activity of ultrasound alone is relatively low and is only effective under specific conditions (Cao et al., 2010: Fulya et al., 2015: O'Donnell et al., 2010). However, the present study found that a combination of 30 or 50% ethanol and ultrasound significantly (P < 0.05) reduced the numbers of MAB with loose attachment (by 1.38 and 2.60 log CFU/g respectively), intermediate attachment (by 0.85 and 1.50 log CFU/g, respectively), or tight attachment (by 0.72 and 1.34 log CFU/g, respectively). Likewise, a combination of 30% or 50% ethanol and ultrasound reduced the number of coliforms with loose attachment (by 1.04 and 1.80 log CFU/g respectively), intermediate attachment (0.80 and 1.05 log CFU/g), or tight attachment (0.52 and 0.94, log CFU/g) to chicken skin. There were no significant differences between 50 and 70% ethanol in combination with ultrasound (P > 0.05). These results indicated that increase in ethanol concentration beyond 50% did not result in an additional antimicrobial effect.
Effect of Treatment on S. Typhimurium
The effects of ethanol and ultrasound treatment on S. Typhimurium with loose, intermediate, or tight attachment to chicken skin are shown in Table 4. The numbers of S. Typhimurium on chicken skin treated with 0% ethanol (water treatment) were 5.93 log CFU/g with loose attachment, 5.45 log CFU/g with intermediate attachment, and 4.40 log CFU/g with tight attachment (data not shown). Treatment with 30% ethanol reduced S. Typhimurium with loose, intermediate, or tight attachment by 0.59, 0.42, and 0.40 log CFU/g, respectively, which were not significant differences (P > 0.05). Ingram (1989) reported that ethanol can freely penetrate bacterial membranes. Ethanol inhibits the cross-linking of peptidoglycans via a decomposition mechanism. Treatment with 50 or 70% ethanol caused significant differences (P < 0.05) in the numbers of S. Typhimurium with intermediate or tight attachment, and loose or intermediate attachment, respectively, compared to control. As with the previous results, the antimicrobial effect increased as the ethanol concentration increased. Treatment with 30, 50, or 70% ethanol significantly (P < 0.05) reduced S. Typhimurium with loose, intermediate, or tight attachment to chicken skin (Table 4). Phongphakdee and Nitisinprasert (2015) also found that 70, 50, or 30% ethanol treatment significantly reduced the numbers of S. Enteritidis, S. Typhimurium, and E. coli O157; therefore, these concentrations of ethanol may be useful as antimicrobial treatment to reduce Gram-negative bacteria. However, such high levels of ethanol might result in undesirable odor or other quality problems in chicken skin (Lachenmeier, 2008).
Table 4.
Reduction efficacy (log CFU/g) of ethanol alone and ethanol/ultrasound against loosely, intermediately, and tightly attached S. Typhimurium on chicken skin.
| Treatments | Ethanol (%) | Loosely | Intermediately | Tightly |
|---|---|---|---|---|
| Without US | 30 | 0.59 ± 0.25d | 0.42 ± 0.11d | 0.40 ± 0.05e |
| 50 | 1.26 ± 0.33b,x | 1.09 ± 0.32c,x | 0.79 ± 0.01d,y | |
| 70 | 1.38 ± 0.26b,x | 1.22 ± 0.15c,y | 1.15 ± 0.17c,y | |
| With US1 | 0 | 0.89 ± 0.08c,x | 0.55 ± 0.11d,y | 0.53 ± 0.05e,y |
| 30 | 1.58 ± 0.02b,x | 1.36 ± 0.03c,y | 1.15± 0.24c,z | |
| 50 | 2.86 ± 0.16a,x | 1.82 ± 0.12b,y | 1.43 ± 0.29b,z | |
| 70 | 2.86 ± 0.16a,x | 2.49 ± 0.16a,y | 1.63 ± 0.07a,z |
Mean values within the same column with no common superscripts were different (P < 0.05).
Mean values within the same row with no common superscripts were different (P < 0.05).
US: ultrasound treatment (frequencies of 37 kHz, 380 W, 5 min).
The effect of ultrasound resulted in significant differences (P < 0.05) in the numbers of S. Typhimurium with loose (0.89 log CFU/g), intermediate (0.55 log CFU/g), or tight (0.59 log CFU/g) attachment compared to control. Kwak et al. (2011) reported that the numbers of E. coli O157:H7 and S. Typhimurium were reduced by 0.33 to 0.95 log CFU/g with single ultrasound (40 kHz) treatment for 20 min. The effect of combinations of 30, 50, or 70% ethanol with ultrasound resulted in significant differences (P < 0.05) in the numbers of S. Typhimurium with any of the 3 different types of attachment. As observed previously, the combination of 70% ethanol and ultrasound resulted in the most S. Typhimurium reduction (1.63–2.86 log CFU/g), followed by ethanol alone (1.15–1.38 log CFU/g). Piyasena et al. (2003) reported that the bacteria are killed due to cavitation created via changes in pressure caused by the ultrasonic waves. Cavitation occurs when air bubbles are generated in a liquid due to a reduction in pressure. Thus, microbes with intermediate or tight attachment to chicken skin can be eliminated due to cavitation. Studies on the combination of chemical agents with ultrasound have demonstrated pathogen inactivation (São José and Vanetti, 2012; Sagong et al., 2013). Sagong et al. (2011) reported that numbers of S. Typhimurium, Listeria monocytogenes, and E. coli O157:H7 on lettuce were reduced (0.8–1.0 log CFU/g) by combined treatment with ultrasound (40 kHz) and 2% organic acids (malic acid, lactic acid, and citric acid). Lee et al. (2014) also reported that the combination of NaOCl (200 ppm) and ultrasound (37 kHz, 380 W) reduced the numbers of MAB, coliforms, and S. Typhimurium with loose (0.75, 0.43, and 0.83 log CFU/g respectively), intermediate (0.38, 0.35, and 0.99 log CFU/g respectively), or tight (0.47, 0.41, and 0.54 log CFU/g respectively) attachment. In the present study, the combination of 30% ethanol and ultrasound reduced the number of S. Typhimurium to a similar extent as 70% ethanol alone. Taken together, this study showed that a combination of ultrasound and ethanol treatment was the most effective in reducing microbial load on chicken skin.
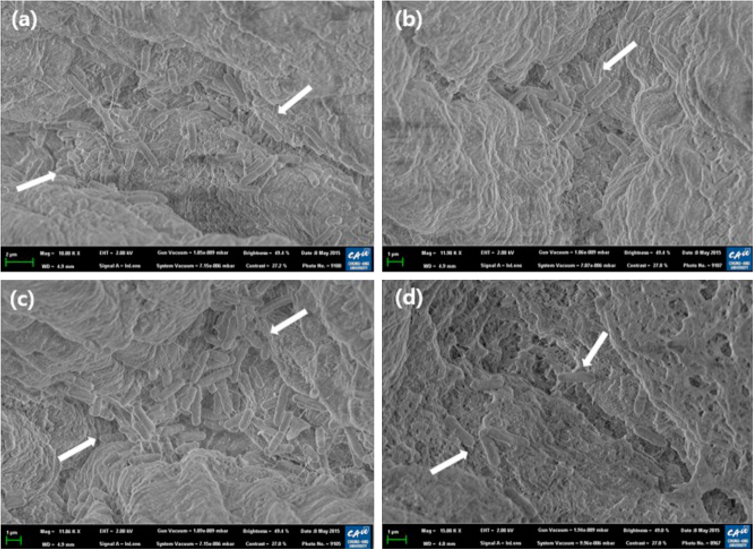
Field Emission Scanning Electron Microscopy
Using FE-SEM, the numbers of S. Typhimurium on the flat surfaces (Figure 1) and in the crevices (Figure 2) of chicken skin were visualized. The FE-SEM micrographs show the effect of sterile distilled water (Figure 1 a and 2 a), ethanol (Figure 1 b and 2 b), ultrasound (Figure 1 c and 2 c), or a combination of ethanol and ultrasound (Figure 1 d and 2 d) on reducing the numbers of S. Typhimurium. The images showed that treatment with water (control) or ultrasound alone resulted in retention of a large number of S. Typhimurium on the chicken skin. Lee et al. (2014) reported FE-SEM data showing that ultrasound or water treatment had no effect on numbers of S. Typhimurium on chicken skin, regardless of attachment type. Noriega et al. (2011) reported that it is challenging to remove pathogens from crevices or folded sections with a single treatment. Ultrasound was shown to physically detach microorganisms from the skin surface and thus enhance the action of NaOCl on Salmonella and E. coli O157:H7 on alfalfa seeds (Demirdoven and Baysal, 2009). In the present study, compared with sterile distilled water (control, Figure 1 a), flat-surface samples treated with a combination of 70% ethanol and ultrasound (Figure 2 d) showed less dense microorganism populations. A combination of 70% ethanol and ultrasound (Figure 2 d) was more effective in reducing microbe density compared to ethanol treatment alone (Figure 2 c). Similarly, samples treated with a combination of ethanol and ultrasound (Figure 2 d) showed a lower density of microorganisms in the crevices of chicken skin than those treated with sterile distilled water (control) (Figure 2 a), ethanol (Figure 2 b), or ultrasound (Figure 2 c) alone. Thus, FE-SEM demonstrated that combined treatment with 70% ethanol and ultrasound reduced S. Typhimurium on chicken skin more effectively than treatment with 70% ethanol alone.
Figure 1.
Field emission scanning electron microscopy images of Salmonella Typhimurium on chicken skin (flat surface) treated with 50% ethanol and ultrasound at room temperature (arrows; 15,000 × arrows; 15,000) h (a) S. Typhimurium on chicken skin treated with sterile distilled water (control) (b) S. Typhimurium on chicken skin treated with ethanol alone (c) S. Typhimurium on chicken skin treated with ultrasound alone (d) S. Typhimurium on chicken skin treated with a combination of ethanol and ultrasound.
Figure 2.
FE-SEM images of Salmonella Typhimurium in the crevices of chicken skin treated with 50% ethanol and ultrasound at room temperature. (a) S. Typhimurium on chicken skin treated with sterile distilled water (control) (arrows; 11,000 × terile distille (b) S. Typhimurium on chicken skin treated with ethanol alone (arrows; 11,000 × arrows; 11,000) (c) S. Typhimurium on chicken skin treated with ultrasound alone (arrows; 11,000 × arrows; 11,000 (d) S. Typhimurium on chicken skin treated with a combination of ethanol and ultrasound (arrows; 15,000 × arrows; 15,000.
Color and Texture
The color and texture of poultry meat are important factors associated with consumer concern (Karaoglu et al., 2004; Sharma et al., 2013). Weak skin is more sensitive to mechanical tearing and can easily reduce the quality and shelf life of meat (Fletcher and Thomason,1980; Salim et al., 2012). The present study found no significant differences (P > 0.05, Table 5) in Hunter color L* values due to any of the treatments. Further, compared to control (water treatment), single ultrasound treatment did not cause a significant difference in Hunter color L*, a*, or b* values and texture. Lee et al. (2014) found that chicken skin treated with ultrasound (37 kHz, 380 W) alone showed no changes in color or texture. Ultrasound has shown potential advantages as a novel and alternative technique to maintain meat quality and microbial safety, in addition to prolonging shelf-life during the poultry processing (Bhat et al., 2011). Ultrasound treatment did not affect the quality of chicken skin in the present study. Therefore, ultrasound could be a useful technique in the poultry industry in combination with other decontamination techniques such as chemical treatments. Results of the present study also showed that as ethanol concentration increased, Hunter color a* values decreased and Hunter color b* values increased (P < 0.05, Table 5). In a previous study, the quality of chicken skin was sensorially and instrumentally verified, and the results showed that chicken skin became yellower and harder following treatment with increasing concentrations of ethanol, compared to skin treated with lower ethanol concentrations or with no ethanol at all (Lee et al., 2014). Sensory evaluation by panelists was not conducted in the present study. However, the control (water-treated) chicken samples were characterized as having more chicken flavor than ethanol-treated chicken samples. Further, compared to the control chicken skin, ethanol-treated chicken samples were composed of more white meat or dark meat. In addition, shear force increased as ethanol concentration increased (P < 0.05, Table 5), indicating that the hardness of the chicken skin increased. Thus, treatment with 50% or higher ethanol concentrations changed the color and texture of chicken skin.
Table 5.
Color parameters (L*, a*, and b*)1 and shear force values (kg/cm2) for chicken skin treated with ethanol alone and ethanol/US.2
| Treatments | Ethanol (%) | L* | a* | b* | Shear force (kg/cm3) |
|---|---|---|---|---|---|
| Control | 83.77 ± 0.373 | 4.28 ± 0.49a | 13.35 ± 1.30d | 0.33 ± 2.83c | |
| Without US | 30 | 84.98 ± 0.71 | 3.65 ± 0.30b,c | 14.84 ± 1.19a | 0.33 ± 4.72c |
| 50 | 84.39 ± 0.92 | 2.27 ± 0.80b,c | 16.55 ± 0.30b,c | 0.39 ± 4.79a–c | |
| 70 | 84.86 ± 0.73 | 2.78 ± 0.08b | 16.15 ± 0.58b,c | 0.43 ± 1.74a | |
| With US2 | 0 | 84.21 ± 1.38 | 3.97 ± 0.69a | 14.19 ± 0.55c,d | 0.33 ± 2.64c |
| 30 | 84.65 ± 0.34 | 3.28 ± 0.18b | 14.47 ± 0.64c,d | 0.34 ± 3.40b,c | |
| 50 | 84.00 ± 0.93 | 2.07 ± 0.62b,c | 16.25 ± 0.60b | 0.37 ± 1.11b,c | |
| 70 | 84.19 ± 1.03 | 2.09 ± 0.70c | 16.91 ± 0.47a | 0.40 ± 2.19a | |
The means and standard deviations were calculated based on 10 replicates (color) and 10 replicates (texture).
Mean values within the same column with no common superscripts were different (P < 0.05).
Color are L* (lightness), a* (redness), b* (yellowness).
US: ultrasound treatment (frequencies of 37 kHz, 380 W, 5 min).
No significance; means value within a same column are no different (P > 0.05).
CONCLUSIONS
The present study demonstrates that microorganisms with intermediate or tight attachment to chicken skin (total MAB, coliforms, and S. Typhimurium) were more resistant to chemical disinfectant than those with loose attachment. Ethanol had a concentration-dependent antimicrobial effect against MAB, coliforms, and S. Typhimurium on chicken skin, whereas ultrasound alone was not particularly effective in reducing numbers of any of the 3 groups of microbes. Combined treatment with 30, 50, or 70% ethanol and ultrasound (37 kHz, 380 W, 5 min) more effectively removed microorganisms with loose attachment than those with intermediate or tight attachment. Among all combination treatments, the combination of 70% ethanol and ultrasound was the most effective at reducing numbers of bacteria with loose, intermediate, or tight attachment; 2.66, 1.63, and 1.43 log CFU/g reduction respectively in MAB; 1.91, 1.27, and 0.99 log CFU/g reduction respectively in coliforms, and 2.86, 2.49, and 1.63 log CFU/g reduction respectively in S. Typhimurium. The combination of ethanol (30–70%) and ultrasound treatment resulted in better decontamination than ethanol alone. Changes in the color and texture of chicken skin occurred following treatment with 50% ethanol alone or a combination of >50% ethanol and ultrasound. However, the combination of 30% ethanol and ultrasound was considered to be optimal, because it produced >1 log CFU/g reduction in S. Typhimurium without changes in color or texture. The results of the present study suggest that the combination of 30% ethanol and ultrasound may be the optimum treatment for reduction of microbial contamination in skin-on chicken meat products, and thus enhance poultry safety without decreasing food quality.
ACKNOWLEDGMENTS
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2019.
REFERENCES
- Ahn D.U., Kim I.S., Lee E.J. Irradiation and additive combinations on the pathogen reduction and quality of poultry meat. Poult. Sci. 2013;92:534–545. doi: 10.3382/ps.2012-02722. [DOI] [PubMed] [Google Scholar]
- Barker C., Park S.F. Sensitization of Listeria monocytogenes to low pH, organic acids, and osmotic stress by erhanol. Appl. Environ. Microbiol. 2001;67:1594–1600. doi: 10.1128/AEM.67.4.1594-1600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgili S.F. Antimicrobials approved for poultry processing in the US. Wogs Fact Sheet. 2009. http://www.ag.auburn.edu/poul/extensionoutreach/wogs/wogsfeb09.pdf
- Bhat R., Kamaruddin N. S. B. C, Liong T., Karim A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Utrason. Sonochem. 2011;18:1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Bolder N.M. Decontamination of meat and poultry carcasses. Trends Food Sci. Technol. 1997;8:221–227. [Google Scholar]
- Buhr R.J., Bourassa D.V., Northcutt J.K., Hinto A., Jr., Ingram K.D., Cason J.A. Bacteria recovery from genetically feathered and featherless broiler carcasses after immersion chilling. Poultry Sci. 2005;84:1499–1504. doi: 10.1093/ps/84.9.1499. [DOI] [PubMed] [Google Scholar]
- Cao S., Hu Z., Pang B., Wang H., Xie H., Wu F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control. 2010;21:529–532. [Google Scholar]
- Cho S.K., Park J.H. Bacterial biocontrol of sprouts through ethanol and organic acids. Korean J. Food Nutr. 2012;25:149–155. [Google Scholar]
- Demirdoven A., Baysal T. The use of ultrasound and combined technologies in food preservation. Food Rev. Int. 2009;25:1–11. [Google Scholar]
- Earnshaw R.G., Appleyard J., Hurst R.M. Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 1995;28:197–219. doi: 10.1016/0168-1605(95)00057-7. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. 2017. [DOI] [PMC free article] [PubMed]
- El-Gazzar E.F., Marth E.H. Salomonellae, Salmonellosis, and dairy foods: a review. J. Dairy Sci. 1992;75:2327–2343. doi: 10.3168/jds.S0022-0302(92)77993-4. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L., Thomason D.M. The influence of environment and processing conditions on the physical carcass quality factors associated with oily bird syndrome. Poult. Sci. 1980;59:731–736. [Google Scholar]
- Food and Agriculture Organization (FAO) Poultry meat & Eggs, agribusiness handbook. 2010. https://www.responsibleagroinvestment.org/sites/responsibleagroinvestment.org/files/FAO_Agbiz%20handbook_Poultry_Meat.pdf
- Food and Agriculture Organization (FAO) FAOSTAT. 2013. http://faostat3.fao.org/home/index.html#SEARCH_DATA
- Fulya T., Gulden B.K., Birol K. Ultrasound in the meat industry: general applications and decontamination efficiency. Int. J. Food Microbiol. 2015;198:59–69. doi: 10.1016/j.ijfoodmicro.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Hafez H.M., Stadler A., Kosters J. Surveillance of Salmonella in turkey Survs and processing plants. Dtsch. Tierarztl. Wochenschr. 1997;104:33–35. [PubMed] [Google Scholar]
- Hannah J.F., Cason J.A., Richardson J.R., Cox N.A., Hinton A., Jr., Buhr A.R.J., Smith D.P. Effect of stomaching on numbers of bacteria recovered from chicken skin. Poult. Sci. 2011;90:491–493. doi: 10.3382/ps.2009-00477. [DOI] [PubMed] [Google Scholar]
- Henao O.L., Scallan E., Mahon B., Hoekstra R.M. Methods for monitoring trends in the incidence of foodborne diseases: Foodborne Diseases Active Surveillance Network 1996–2008. Foodborne Pathog. Dis. 2010;7:1421–1426. doi: 10.1089/fpd.2010.0629. [DOI] [PubMed] [Google Scholar]
- Hinton A., Cason J.A. Bacterial flora of processed broiler chicken skin after successive washings in mixtures of potassium hydroxide and lauric acid. J. Food Prot. 2008;71:1707–1713. doi: 10.4315/0362-028x-71.8.1707. [DOI] [PubMed] [Google Scholar]
- Ingram L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1989;9:305–319. doi: 10.3109/07388558909036741. [DOI] [PubMed] [Google Scholar]
- Jang J.H., Jang J.S., Lee S.Y., Kim H.S., Kang S.M., Park J.H. Growth inhibition effects of ethanol and sodium chloride on bacillus cereus. Korean J. Food Sci. Technol. 2003;35:998–1002. [Google Scholar]
- James C., Vincent C., de Andrade Lima T.I., James S. J. The primary chilling of poultry carcasses-a review. Int. J. Refrig. 2006;29:847–862. [Google Scholar]
- Kalathenos P., Russell N.J. In: Food preservatives. Russell N.J., Gould G.W., editors. Springer; Boston, MA: 2003. Ethanol as a food preservative; pp. 196–217. [Google Scholar]
- Karaoglu M., Aksu M.I., Esenbuga N., Kaya M., Macit M., Durdag H. Effect of dietary probiotic on the pH and color characteristics of carcasses, breast fillets and drumsticks of broilers. J. Animal Sci. 2004;78:253–259. [Google Scholar]
- Kim B.Y., Lee J.Y., Ha S.D. Growth characteristics and development of a predictive model for Bacillus cereus in fresh wet noodles with added ethanol and thiamine. J. Food Prot. 2011;74:658–664. doi: 10.4315/0362-028X.JFP-10-131. [DOI] [PubMed] [Google Scholar]
- Ko J.K., Ma Y.H., Song K.B. Effect of chlorine dioxide treatment on microbial growth and qualities of chicken breast. J. Food Sci. Nutr. 2005;10:122–129. [Google Scholar]
- Kwak T.Y., Kim N.H., Rhee M.S. Response surface methodology-based optimization of decontamination conditions for Escherichia coli O157:H7 and Salmonella Typhimurium on fresh-cut celery using hermoultrasound and calcium propionate. Int. J. Food Microbiol. 2011;150:128–135. doi: 10.1016/j.ijfoodmicro.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Lachenmeier D.W. Safety evaluation of topical application of ethanol on skin and inside the oral cavity. J. Occup. Med. Tocicol. 2008;3:26–30. doi: 10.1186/1745-6673-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.Y., Park S.Y., Kang I.S., Ha S.D. The evaluation of combined chemical and physical treatments on the reduction of resident microorganisms and Salmonella Typhimurium attached to chicken skin. Poult. Sci. 2014;93:208–215. doi: 10.3382/ps.2013-03536. [DOI] [PubMed] [Google Scholar]
- Leighton T.G. Acad. Press; San Diego, CA: 1994. The Acoustic Bubble. [Google Scholar]
- LeLièvre V., Besnard A., Schlusselhuber M., Desmasures N., Dalmasso M. Phages for biocontrol in foods: What opportunities for Salmonella sp. Control along the dairy food chain? Food Microbiol. 2019;78:89–98. doi: 10.1016/j.fm.2018.10.009. [DOI] [PubMed] [Google Scholar]
- McClements D.J. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci. Technol. 1995;3:S253–S260. [Google Scholar]
- McKee S. American Meat Science Association; 2012. Salmonella control in poultry processing. 65th Annual reciprocal meat conference.http://goo.gl/61Bdk [Google Scholar]
- Noriega E., Shama G., Laca A., Diaz M, Kong M.G. Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiol. 2011;28:1293–1300. doi: 10.1016/j.fm.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Notermans S., Kampelmacher E.H. Heat destruction of some bacterial strains attached to broiler skin. Brit. Poultry Sci. 1975;15:351–361. doi: 10.1080/00071667508416199. [DOI] [PubMed] [Google Scholar]
- Nyachuba D.G. Foodborneillness: is it on the rise? Nutr. Rev. 2010;68:257–269. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell D.G., Tiwari B.K., Bourke P., Cullen P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010;21:358–367. [Google Scholar]
- Ono K., Yamamoto K. Contamination of meat with Campylobacter jejuni in Saitama. Int. J. Food Microbiol. 1999;47:211–219. doi: 10.1016/s0168-1605(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Phongphakdee K., Nitisinprasert S. Combination inhibition activity of nisin and ethanol on the growth inhibition of pathogenic gram negative bacteria and their application as disinfectant solution. J. Food Sci. 2015;80:M2241–M2246. doi: 10.1111/1750-3841.13015. [DOI] [PubMed] [Google Scholar]
- Piernas V., Guiraud J.P. Control of microbial growth on rice sprouts. Int. J. Food Sci. Technol. 1998;33:297–305. [Google Scholar]
- Piyasena P., Mohareb E., McKellar R.C. Inactivation of microbes using ultrasound: a review. Int. J. Food Microbiol. 2003;87:207–216. doi: 10.1016/s0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Rabsch W., Tschape H., Bnumler A.J. Review, non-typhoidal salmonellosis: Emerging problems. Microbes Infect. 2001;3:237–247. doi: 10.1016/s1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- Sagong H.G., Lee S.Y., Chang P.S., Heu S.G., Ryu S.R., Choi Y.J., Kang D.H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011;145:287–292. doi: 10.1016/j.ijfoodmicro.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Sagong H.G., Cheon H.L., Kim S.O., Lee S.Y., Park K.H., Chung M.S., Choi Y.J., Kang D.H. Combined effects of ultrasound and surfactants to reduce Bacillus cereus spores on lettuce and carrots. Int. J. Food Microbiol. 2013;160:367–372. doi: 10.1016/j.ijfoodmicro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Salim H.M., Lee H.R., Jo C., Lee S.K., Lee B.D. Effect of dietary zinc proteinate supplementation on growth performance, and skin and meat quality of male and female broiler chicks. Brit. Poultry Sci. 2012;5:116–124. doi: 10.1080/00071668.2012.658757. [DOI] [PubMed] [Google Scholar]
- Sams A.R., Feria R. Microbial effects of ultrasonication of broiler drumstick skin. J. Food Sci. 1991;56:247–248. [Google Scholar]
- São José J. F. B, Vanetti M.C.D. Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enteric Typhimurium on cherry tomatoes. Food Control. 2012;24:95–99. [Google Scholar]
- Scouten A.J., Beuchat L.R. Combined effect of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 2002;92:668–674. doi: 10.1046/j.1365-2672.2002.01571.x. [DOI] [PubMed] [Google Scholar]
- Seymour I.J., Burfoot D., Smith R.L., Cox L., Lockwood A. Ultrasound decontamination of minimally processed fruits and vegetables. Int. J. Food Sci. Technol. 2002;37:547–557. [Google Scholar]
- Sharma C.S., Ates A., Joseph P., Nannapanei R., Kiess A. Reduction of Salmonella in skinless chicken breast fillets by lauric arginate surface application. Poult. Sci. 2013;92:1419–1424. doi: 10.3382/ps.2012-02837. [DOI] [PubMed] [Google Scholar]
- Tack D.M., Marder E.P., Griffin P.A., Cieslak P.R., Dunn J., Hurd S., Scallan E., Lathrop S., Muse A., Ryan P., Smith K., Tobin-D'Angelo M., Vugia D.J., Holt K.G., Holpert B.J., Tauxe R., Geissler A.L. Preliminary incidence and trends of infections with pathogens transmitted commonly through food-foodborne diseases active surveillance network, 10 U.S. Sites, 2015–2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68:369–373. doi: 10.15585/mmwr.mm6816a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.J., McMeekin T.A. Contamination of broiler carcass skin during commercial processing procedures: an electron microscopic study. Appl. Environ. Microbiol. 1980;40:133–144. doi: 10.1128/aem.40.1.133-144.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jeong J.Y., Janardhanan K.K., Ryser E.T., Kang I. Microbiological quality of water immersion-chilled and air-chilled broilers. J. Food Prot. 2011;74:1531–1535. doi: 10.4315/0362-028X.JFP-11-032. [DOI] [PubMed] [Google Scholar]
- Zhang L., Singh P., Lee H.C., Kang I. Effect of hot water spray on broiler carcasses for reduction of loosely attached, intermediately attached, and tightly attached pathogenic (Salmonella and Campylobacter) and mesophilic aerobic bacteria. Poult. Sci. 2013;92:804–810. doi: 10.3382/ps.2012-02504. [DOI] [PubMed] [Google Scholar]
- Zhou B., Feng H., Luo Y. Ultrasound enhanced sanitizer efficacy in reduction of Escherichia coli O157:H7 population on spinach leaves. J. Food Sci. 2009;74:308–313. doi: 10.1111/j.1750-3841.2009.01247.x. [DOI] [PubMed] [Google Scholar]