Abstract
Phytase is of importance to the poultry industry because of its ability to hydrolyze phytate and release phosphorus (P) for use by poultry. However, the effect of age on phytase efficacy is not fully understood. A total of 864 day-old broiler chicks were used to investigate the effect of age and feeding length on phytase efficacy using growth performance, mineral utilization, and tibia ash as response criteria of evaluation. The experiment was arranged as a 3 × 2 × 2 factorial in a randomized complete block design with 3 diets including; a positive control (PC; 0.4% non-phytate P (nPP)), a negative control (NC; 0.2% nPP) and a NC diet supplemented with phytase at 2,000 FYT/kg; 2 ages (i.e., days 14 and 22); and 2 feeding lengths (i.e., 2 and 5 D) with 8 replicates each. Birds fed the NC had decreased (P < 0.01) body weight gain and feed efficiency compared with birds fed the PC regardless of age or feeding length. Similarly, birds fed the phytase-supplemented diet had improved (P < 0.01) performance as compared to birds fed the NC regardless of age. There were no significant differences in P utilization between birds fed for 2 to 14 D or 22 D and birds fed for 5 D to both ages. However, phytase was more efficacious at day 14 than day 22 when mineral utilization was considered because the super dose of phytase elicited greater response in birds fed the phytase supplemented diet for 2 D until day 14. In contrast, percentage tibia ash improved (P < 0.01) in birds fed phytase supplemented diet for 5 D at both ages as compared with birds fed for 2 D. In conclusion, testing phytase products, even at high doses, for 2 D during the second week in the life cycle of broiler chicks, can be recommended from the results of this study.
Key words: age, broilers, feeding length, phosphorus, phytase efficacy
INTRODUCTION
Broiler chickens are a major source of animal protein to the human population hence, research is continually carried out in diverse ways to improve their productivity while being cost-effective and maintaining a safe environment. Waste from the broiler industry has been identified as one of the major sources of environmental pollution due to the high concentration of minerals including phosphorus (Panda et al., 2007). When this waste is used as manure, phosphorus (P) accumulates in the soil and if P is leached or runoff into water bodies, it may harm aquatic life and the environment in a process called eutrophication (Panda et al., 2007). Phytate is commonly found in several cereal and oilseed grains used in animal diets. Phytate binds to nutrients such as calcium (Ca), magnesium, amino acids, and other essential nutrients forming insoluble complexes (Cowieson and Bedford, 2009). These complexes are not easily hydrolyzed by broilers due to their poor solubility in the small intestine, resulting in the unavailability of nutrients to the birds and its loss to the environment (Selle et al., 2009). The use of exogenous phytase in broiler diets has been encouraged because of its ability to hydrolyze the phytate complex, and release P and other nutrients for use by the birds (Ravindran et al., 1995). Increasing the dosage of phytase supplementation in birds has resulted in further improvement in growth performance and nutrient utilization of birds compared with the industry recommended dose of 500 to 1,000 FYT/kg diet (Rutherfurd et al., 2012). Studies have reported that high doses of phytase may lead to a more rapid and/or complete breakdown of phytate in diets thus reducing its anti-nutritional effect and eliciting a better response in birds (Shirley and Edwards, 2003). The age of birds has been known to influence their capacity to utilize nutrients especially during the early phase of rapid development (Batal and Parsons, 2002). Previous work from our lab reported that age of birds and feeding length has an impact on phytase efficacy (Babatunde et al., 2019). A reduction in the efficacy of phytase was observed with responses of birds fed a low P diet as they grew older than 2 wk. Birds fed phytase supplemented low P diets at day 14 post-hatching had increased growth performance, P and Ca utilization as compared with birds fed the same diets at days 8 and 22 post-hatching. Similarly, birds fed for 2 and 5 D had higher responses than birds fed for 16 D. However, it was not clear if there was any difference in the impact on phytase efficacy when birds were fed phytase supplemented low P diets for short periods (i.e., 2 or 5 D) at day 14 post-hatching, characterized by rapid development of organs and tissues, or at day 22 post-hatching, which is the beginning of the grower phase and the rapid accumulation of muscle in broilers. Thus, the objective of this study was to investigate the impact of these feeding lengths and age periods on phytase efficacy.
MATERIALS AND METHODS
Birds and Management
All protocols of animal experiments were reviewed and approved by the Purdue University Animal Care and Use Committee. A total of 864 one-day-old male broiler chicks (Cobb 500, Siloam Springs, AR) were obtained from a commercial hatchery. Each bird was weighed individually, tagged, and allotted to cages. Birds were raised in heated brooder battery cages (model SB 4 T; Alternative Design Manufacturing, Siloam Springs, AR) in an environmentally controlled room. All birds had unrestricted access to water and were fed a commercial starter diet in mash form formulated to contain 3,225 kcal/kg ME, 225 g/kg CP, 9 g/kg Ca, and 4.0 g/kg non-phytate P (nPP), that met or exceeded the requirements of broiler chicks (NRC, 1994) until the beginning of the experimental periods. The commercial starter diet was similar in formulation to the positive control (PC) experimental diet.
Experimental Design and Procedure
This study was a randomized complete block design with a 3 × 2 × 2 factorial arrangement of treatments comprising; 3 experimental diets; a PC diet (0.40% nPP), negative control (NC) diet (0.20% nPP), and a NC diet supplemented with phytase at 2,000 FYT/kg; 2 feeding lengths (2 or 5 D), and 2 ages (days 14 or 22). Feeding length groups included a 2 and 5 D feeding length terminating at day 14 (i.e., days 12 to 14 and days 9 to 14) and at day 22 post-hatch (i.e., days 20 to 22 and 17 to 22). Each treatment had 8 replicates with 10 birds or 8 birds per cage for groups that were fed the experimental diets until days 14 or 22, respectively. The initial BW served as the blocking factor for the 12 treatments to ensure similar average BW across the diets. A commercial starter diet, similar to the PC diet, was fed to 2 groups until days 9 and 12 respectively and then fed the experimental diets until day 14 while the other 2 groups were fed the commercial diet until days 17 and 20 respectively and then fed the experimental diets until day 22.
Experimental Diets
Ingredient composition and nutrient and energy concentration of the experimental diets are shown in Table 1. The PC and NC diets were in mash form and similar in ingredient composition but the levels of monocalcium phosphate and limestone were adjusted to give varying levels of nPP. Phytase (RONOZYME HiPhos, DSM Nutritional Products, Kaiseraugst, Switzerland) was combined with ground corn to form a premix containing 50 FTY/g. The quantity of enzyme required to liberate 1 μmol of inorganic phosphate/min from 5.0 mM sodium phytate at pH 5.5 and 37°C is known as 1 FYT (Engelen et al., 1994). The phytase premix was then supplied at 40 g/kg to the NC diet to contain 2,000 FYT/kg. Ileal digestibility and total tract retention (TTR) of nutrients in diets were determined by index method as described by Adeola and Walk (2013) using chromic oxide, incorporated into the diets, as an indigestible marker.
Table 1.
Ingredients and nutrient composition of experimental diets for broiler chickens
| Item | Positive control | Negative control | Phytase 2,000 FYT/kg |
|---|---|---|---|
| Ingredients, g/kg | |||
| Corn | 520.6 | 531.1 | 491.1 |
| Soybean meal, 480 g/kg CP | 358.0 | 356.0 | 356.0 |
| Soybean oil | 53.0 | 50.0 | 50.0 |
| Monocalcium phosphate | 13.3 | 3.8 | 3.8 |
| Limestone | 15.3 | 19.3 | 19.3 |
| Salt | 4.0 | 4.0 | 4.0 |
| Vitamin−mineral premix1 | 3.0 | 3.0 | 3.0 |
| DL-Methionine | 3.8 | 3.8 | 3.8 |
| L-Lysine·HCl | 2.9 | 2.9 | 2.9 |
| L-Threonine | 1.1 | 1.1 | 1.1 |
| Chromic oxide premix2 | 25.0 | 25.0 | 25.0 |
| Phytase premix3 | 0 | 0 | 40 |
| Total | 1000.0 | 1000.0 | 1000.0 |
| Calculated nutrients and energy, g/kg | |||
| CP | 225.3 | 225.3 | 225.3 |
| ME, kcal/kg | 3222.5 | 3228.8 | 3228.8 |
| Ca | 9.0 | 9.0 | 9.0 |
| P | 6.5 | 4.5 | 4.5 |
| Non-phytate P | 4.0 | 2.0 | 2.0 |
| Analyzed nutrients, g/kg | |||
| DM | 890 | 884 | 887 |
| Ca | 13 | 12.5 | 12.6 |
| Total P | 6.4 | 4.8 | 4.8 |
| Phytate P | 2.7 | 2.8 | 2.6 |
| Phytase activity, FYT/kg | <100 | <100 | 2210 |
Supplied the following quantities per kg of diet: vitamin A, 5,484 IU; vitamin D3, 2,643 ICU; vitamin E, 11 IU; menadione sodium bisulfite,4.38 mg; riboflavin, 5.49 mg; D-pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 μg; biotin, 55.2 μg; thiamine mononitrate, 2.2 mg; folic acid, 990 μg; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg; Mn, 66.06 mg; Cu, 4.44 mg; Fe, 44.1 mg; Zn, 44.1 mg; Se, 300 μg.
Prepared as 1 g chromic oxide added to 4 g corn.
Prepared with ground corn to contain 50 FYT per g corn.
Sample Collection and Chemical Analyses
Initial BW of birds was recorded at the start of each feeding length, while feed consumption and final BW were collected at the end of each period. Excreta were collected twice daily from each cage on the final day of the experimental periods (i.e., days 14 and 22). Excreta were oven dried at 55°C for 5 D, ground, and stored for further analyses. On days 14 and 22 post-hatching, birds in 2 groups respectively were euthanized by CO2 asphyxiation and blood samples were collected by cardiac puncture from the median BW bird in each cage into heparinized tubes. Plasma was obtained by centrifuging blood samples at 3,000 x g for 15 min at 4°C (Jiang et al., 2013) and stored at −80°C until further analyses. Ileal digesta was collected from the distal two-thirds of the ileum of all birds, flushed into plastic containers, pooled per cage, and stored at −20°C until freeze dried. Dried samples were ground with a ZM 100 grinder (Retsch ZM 100, GmbH, Haan, Germany) and passed through a 0.5 mm screen. The left tibia bone was collected from 4 birds with weights closest to the median weight per cage. Bone ash was determined from collected bones in a process previously described by Ogunwole et al. (2017). Dry matter (DM) was determined by placing samples in a drying oven at 105°C for 24 h (The Precision Scientific Co., Chicago, IL; method 934.01; AOAC International, 2000). Chromium (Cr) concentration was determined in the diet, ileal digesta, and excreta samples following a wet-ash digestion as previously described by Fenton and Fenton (1979). Phosphorus concentration was determined from digested samples by spectrophotometry, with absorbance read at 450 nm (Spectronic 21D; Milton Roy Co., Rochester, NY). Calcium concentrations in samples were determined by flame atomic absorption spectroscopy using a Varian Spectr. AA 220FS (Varian Australia Pty Ltd., Victoria, Australia; Iyayi et al., 2013). Phytase activity was estimated using methods described by Engelen et al. (1994). Blood plasma was analyzed for myo -inositol concentration by spectrophotometry using an ADVIA 1650 chemistry system (Bayer diagnostic, Puteaux, France).
Calculation and Statistical Analysis
Apparent ileal digestibility (AID) and TTR of P and Ca in the diets was determined by the index method using the following equation (Dilger and Adeola, 2006):
where CRI is the concentration of Cr in the diets, CRO is the concentration of Cr in the excreta or ileal digesta, NO is the nutrient concentration in the ileal digesta or excreta, and NI is the nutrient concentration in the diet. All values were expressed as grams per kilogram of DM.
Data were analyzed using the GLM procedure of SAS as a 3 × 2 × 2 factorial arrangement of treatments consisting 3 diets for 2 feeding periods (2 or 5 D) at 2 ages (days 14 or 22 post-hatching), with cage as the experimental unit. Statistical significance was set at P ≤ 0.05. Contrast of PC vs. NC and NC vs. NC + 2,000 phytase units/kg diets were used to examine the effect of responses of different levels of nPP and phytase, respectively. The effect of age regardless of feeding length was examined using contrast within birds fed for the same length at different ages i.e., birds fed for 2 D at days 14 or 22 (i.e., days 12 to 14 vs. 20 to 22 post-hatching) and birds fed for 5 D at days 14 or 22 (i.e., days 9 to 14 vs. 17 to 22 post-hatching). The effect of feeding length regardless of diets was examined by comparing responses of birds fed for 2 or 5 D at day 14 (i.e., days 12 to 14 vs. 9 to 14 post-hatching) and 22 (i.e., days 20 to 22 vs. 17 to 22 post-hatching), respectively. Phytase efficacy was calculated by subtracting the nutrient digestibility of birds fed the NC diet from the digestibility of birds fed the 2,000 FYT/kg phytase supplemented diet. Data was analyzed using the GLM procedure of SAS, orthogonal contrast were used to analyze the effect of age and feeding length on the phytase efficacy.
RESULTS
Nutrient analyses and phytase activity in diets (Table 1) were within acceptable ranges when sampling and analysis variations were considered. Although, analyzed Ca concentration in the diets were higher than the formulated concentration. This may be a result of limestone being used as a filler in mineral and vitamin premixes or as a flow agent in soybean meal. Phytase activity in diets were analyzed at below 100 units/kg for the PC and NC diets and 2,210 units/kg for the NC diet supplemented with phytase at 2,000 FYT/kg.
Growth performance data are presented in Table 2. Growth performance responses of birds fed the NC diet were lower (P < 0.01) than birds fed the PC diet. The effect of diets on BW gain (BWG) were lower in birds fed for a short period of 2 D as compared with birds fed the experimental diets for 5 D regardless of the age of the birds thus, resulting in a diet x feeding length interaction (P < 0.01). Birds fed the experimental diets for 2 or 5 D to day 22 had an increased BWG as compared with birds fed for the same period to day 14, resulting in an age x feeding length interaction (P < 0.01). The BWG of birds fed the NC diet for 2 D were lower (P < 0.01) than birds fed the PC diet for the same length at days 14 and 22 (by 18 and 11%, respectively). Similarly, BWG of birds fed the NC diet for 5 D until days 14 and 22 were lower (P < 0.01) than birds fed the PC for the same period at both ages (by 14 and 17%, respectively). The BWG of birds fed the NC supplemented with phytase at 2,000 FYT/kg was increased (P < 0.01) across all the ages and feeding lengths when compared with birds fed the NC diet. Age effect was evaluated by comparing responses of birds fed for the same length at different ages (i.e., 2 D feeding length at days 14 and 22 or 5 D feeding length at days 14 and 22), while feeding length effect was evaluated by comparing responses of birds fed for different lengths at the same age (i.e., 2 and 5 D feeding length at day 14 or at day 22). Results of age effect on BWG when comparing birds fed the phytase supplemented NC diets and the NC diets revealed that birds fed for 2 or 5 D at day 14 had lower improvements in BWG as compared with birds fed for the same period at day 22, respectively. Birds fed for a longer period at both ages had a greater improvement in BWG with phytase supplementation as compared with birds fed for a shorter period when feeding length effect was considered. Similarly, birds fed the NC diet across all age and feeding length had reduced (Table 2; P < 0.01) feed intake and feed efficiency when compared to birds fed the PC diet. Further, birds fed the phytase supplemented diets had improved feed intake and efficiency when compared with birds fed the NC diet. Birds at day 14 had decreased (P < 0.01) feed intake but increased (P < 0.01) feed efficiency compared with birds at day 22 with increased (P < 0.01) feed intake and decreased (P < 0.01) feed efficiency.
Table 2.
Effect of phytase, age, and feeding length on growth performance and tibia ash percentage in broiler chickens
| Age, D | Feeding length, day | Diet1 | Final BW (g) | BW gain, g/bird | Feed intake, g/bird | G:F, g/kg | Tibia ash, % | Number of replicates |
|---|---|---|---|---|---|---|---|---|
| 14 | 2 (days 12 to 14) | PC | 442 | 103 | 133 | 770 | 47.7 | 8 |
| 14 | 2 (days 12 to 14) | NC | 424 | 84 | 122 | 689 | 45.2 | 8 |
| 14 | 2 (days 12 to 14) | 2,000 | 437 | 97 | 128 | 762 | 46.7 | 8 |
| 14 | 5 (days 9 to 14) | PC | 456 | 233 | 285 | 816 | 46.2 | 8 |
| 14 | 5 (days 9 to 14) | NC | 423 | 200 | 268 | 741 | 41.3 | 8 |
| 14 | 5 (days 9 to 14) | 2,000 | 446 | 223 | 276 | 808 | 46.0 | 8 |
| 22 | 2 (days 20 to 22) | PC | 993 | 143 | 217 | 659 | 50.9 | 8 |
| 22 | 2 (days 20 to 22) | NC | 976 | 127 | 211 | 599 | 48.7 | 8 |
| 22 | 2 (days 20 to 22) | 2,000 | 991 | 142 | 210 | 676 | 50.0 | 8 |
| 22 | 5 (days 17 to 22) | PC | 1,013 | 376 | 529 | 711 | 50.9 | 8 |
| 22 | 5 (days 17 to 22) | NC | 957 | 313 | 491 | 639 | 46.2 | 8 |
| 22 | 5 (days 17 to 22) | 2,000 | 996 | 358 | 501 | 715 | 49.5 | 8 |
| PC | 726 | 214 | 291 | 739 | 49 | 32 | ||
| NC | 695 | 180 | 273 | 667 | 45 | 32 | ||
| 2,000 | 718 | 205 | 279 | 740 | 48 | 32 | ||
| 14 | 438 | 157 | 202 | 764 | 46 | 48 | ||
| 22 | 988 | 243 | 360 | 666 | 49 | 48 | ||
| 2 | 710 | 116 | 170 | 692 | 48 | 48 | ||
| 5 | 715 | 284 | 392 | 738 | 47 | 48 | ||
| 2 | PC | 718 | 123 | 175 | 714 | 49 | 16 | |
| 2 | NC | 700 | 105 | 167 | 644 | 47 | 16 | |
| 2 | 2,000 | 714 | 119 | 169 | 719 | 48 | 16 | |
| 5 | PC | 734 | 304 | 407 | 764 | 49 | 16 | |
| 5 | NC | 690 | 256 | 380 | 690 | 44 | 16 | |
| 5 | 2,000 | 721 | 291 | 389 | 761 | 48 | 16 | |
| 14 | 2 | 434 | 95 | 128 | 740 | 47 | 24 | |
| 14 | 5 | 442 | 219 | 276 | 789 | 44 | 24 | |
| 22 | 2 | 986 | 137 | 213 | 644 | 50 | 24 | |
| 22 | 5 | 989 | 349 | 507 | 688 | 49 | 24 | |
| P values | ||||||||
| Diet | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Age | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Feeding length | 0.29 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Diet × Age | 0.57 | 0.25 | 0.33 | 0.77 | 0.72 | |||
| Diet × Feeding length | 0.06 | <0.01 | 0.05 | 0.96 | 0.02 | |||
| Age × Feeding Length | 0.56 | <0.01 | <0.01 | 0.83 | 0.22 | |||
| Diet × Age × Feeding length | 0.56 | 0.22 | 0.21 | 0.94 | 0.80 | |||
| PC vs. NC | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| NC vs. 2,000 | <0.01 | <0.01 | 0.14 | <0.01 | <0.01 | |||
| Days 12 to 14 vs. 9 to 14 | 0.25 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Days 20 to 22 vs. 17 to 22 | 0.73 | <0.01 | <0.01 | <0.01 | 0.09 | |||
| Days 12 to 14 vs. 20 to 22 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Days 9 to 14 vs. 17 to 22 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
PC = positive control; NC = negative control; 2,000 = NC + 2,000 phytase units/kg; G:F = gain to feed ratio.
Birds fed the P deficient NC diet had reduced (P < 0.01) tibia ash when compared to birds fed the PC diet across all the feeding length and age groups (Table 2). However, this decline in tibia ash was more apparent in birds fed for 5 D at days 14 and 22 (4.9 and 4.7%, respectively) than in birds fed for 2 D at the same ages (2.5 and 1.5%, respectively) resulting in a diet x feeding length interaction (P < 0.01). With phytase supplementation, birds had improved (P < 0.01) tibia ash when compared with birds fed the NC diet. Birds fed the phytase diet for 2 D at day 14 had a 1.5 percentage point improvement in tibia ash as compared with birds fed the NC diet for the same period. Similarly, birds fed the phytase diet for 5 D until day 14 had 4.7 percentage point improvement in tibia ash when compared to birds fed the NC diet. Birds fed the phytase supplemented diet for 2 and 5 D until day 22 had a 1.3 and 3.3 percentage point improvement in tibia ash as compared to birds fed the NC diet for the same period. Thus, the phytase induced improvements in tibia ash were more apparent at day 14 than at day 22 when both feeding lengths were considered.
Nutrient digestibility and retention responses of birds to diets are presented in Table 3. The AID of P in birds fed the NC diet were lower (P < 0.01) when compared with birds fed the PC diet across all age and feeding length groups. In birds fed the NC for 2 D until day 14, a 10% decrease in AID of P was observed when compared with birds fed the PC, while in birds fed the NC for 5 D until day 14, a 7.7% decrease in P digestibility was observed. Similarly, birds fed the NC diet for 2 D until day 22 had a 6% decrease in P digestibility as compared with birds fed the PC diet while birds fed the NC diet for 5 D until day 22 had a 9.5% decrease in P digestibility. The effect of age on the impact of the low P diet on birds was observed when comparing the P digestibility of birds fed the PC and the NC at days 14 and 22. Birds fed for 2 D until day 14 had an increased effect of the NC diet as compared with birds fed for 2 D until day 22. However, birds fed for 5 D until day 22 had an increased effect of the NC than birds fed for 5 D until day 14. The feeding length effect on the impact of the NC on birds was evaluated by comparing the P digestibility of birds fed for the same length until days 14 and 22. Thus, comparing birds fed the NC for 2 and 5 D until days 14 or 22 revealed that birds fed for 2 D at day 14 had an increased impact on P digestibility than birds fed for 5 D while on day 22, the opposite was observed. Birds fed the phytase supplemented diets had improved (P < 0.01) P digestibility above birds fed the PC or NC diets. Birds fed the phytase diet for 2 D at days 14 and 22 had a 24 and 19% improvement in P digestibility over birds fed the NC diets for that same period. Meanwhile, birds fed the phytase diet for 5 D at days 14 and 22 had approximately a 19% improvement in P digestibility over birds fed the NC at the same period. Birds fed the NC diets had decreased (P < 0.01) P retention, ileal digestibility, and TTR of Ca when compared with birds fed the PC diets across all the periods. Phytase supplementation improved the AID of Ca and TTR of P and Ca in birds across all age and feeding length groups when compared with birds fed the NC diets. There was an age and feeding length effect on AID of Ca as birds at day 14 had increased (P < 0.01) Ca digestibility as compared with birds at day 22 regardless of feeding length while birds fed for 5 D had increased (P < 0.05) Ca digestibility as compared with birds fed for 2 D regardless of age. There was no age or feeding length effect on the TTR of Ca. There was no age or feeding length effect on P digestibility however, there was a feeding length effect on TTR of P with birds fed for 2 D having a lower (P < 0.01) P retention than birds fed for 5 D regardless of age. Phytase efficacy was determined by subtracting responses of birds fed the NC diet from birds fed the phytase supplemented NC diet and results are presented in Table 4. There was no significant effect of age or feeding length on phytase efficacy when P and Ca digestibility and retention were considered. However, in birds fed diet supplemented with phytase for 2 D, the magnitude of improvement in P digestibility was higher in birds fed until day 14 than in birds fed until day 22. A similar trend was observed in birds fed for 5 D until days 14 and 22. Birds fed for 2 D at both ages had an increased effect of phytase on P digestibility than birds fed for 5 D at both ages. There was no impact of age but there was a feeding length effect on phytase efficacy in tibia ash (Table 4) with birds fed the phytase supplemented NC diet for 5 D having an increased (P < 0.01) improvement of phytase on tibia ash as compared with birds fed for 2 D regardless of age. However, phytase was more efficacious on tibia ash in birds fed for 5 D until day 14 considering an increased (P < 0.05) improvement in tibia ash when compared with birds fed for 2 D until day 14. Plasma myo-inositol levels in birds fed the phytase supplemented diets until day 14 were higher (P < 0.05) than birds fed the NC diet for the same period. However, birds fed the phytase supplemented diets for 5 D had a higher concentration of inositol as compared with birds fed for 2 D (Figure 1 A). Similarly, birds fed the phytase diets for 2 or 5 D until day 22 had a higher inositol concentration as compared with birds fed the NC diet for the same period until day 22 (Figure 1 B).
Table 3.
Effect of phytase, age, and feeding length on nutrient digestibility and retention responses of broiler chickens
| Age, D | Feeding length, day | Diet2 | AID1 DM, % | AID P, % | AID Ca, % | TTR1 DM, % | TTR P, % | TTR Ca, % | Number of replicates |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 2 (days 12 to 14) | PC | 72.0 | 56.0 | 58.2 | 66.5 | 50.1 | 44.7 | 8 |
| 14 | 2 (days 12 to 14) | NC | 70.0 | 45.7 | 46.0 | 65.8 | 35.1 | 27.0 | 8 |
| 14 | 2 (days 12 to 14) | 2,000 | 71.3 | 69.6 | 62.9 | 69.3 | 58.2 | 42.6 | 8 |
| 14 | 5 (days 9 to 14) | PC | 70.2 | 55.0 | 59.6 | 70.0 | 54.9 | 45.0 | 8 |
| 14 | 5 (days 9 to 14) | NC | 69.2 | 47.3 | 52.3 | 69.5 | 46.4 | 24.4 | 8 |
| 14 | 5 (days 9 to 14) | 2,000 | 71.2 | 65.2 | 62.7 | 70.3 | 63.0 | 36.9 | 8 |
| 22 | 2 (days 20 to 22) | PC | 70.7 | 54.4 | 50.4 | 69.6 | 48.9 | 46.2 | 8 |
| 22 | 2 (days 20 to 22) | NC | 69.7 | 48.5 | 44.7 | 69.6 | 37.5 | 28.6 | 8 |
| 22 | 2 (days 20 to 22) | 2,000 | 72.0 | 67.3 | 54.5 | 71.3 | 58.3 | 45.0 | 8 |
| 22 | 5 (days 17 to 22) | PC | 71.1 | 58.3 | 57.5 | 71.4 | 51.8 | 45.5 | 8 |
| 22 | 5 (days 17 to 22) | NC | 70.2 | 48.8 | 49.0 | 71.3 | 43.7 | 29.0 | 8 |
| 22 | 5 (days 17 to 22) | 2,000 | 72.8 | 66.4 | 56.1 | 73.2 | 63.5 | 40.2 | 8 |
| PC | 71.0 | 55.9 | 56.4 | 69.40 | 51.4 | 45.4 | 32 | ||
| NC | 69.8 | 47.5 | 48.0 | 69.1 | 40.6 | 27.2 | 32 | ||
| 2,000 | 71.8 | 67.1 | 59.0 | 71.0 | 60.8 | 41.1 | 32 | ||
| 14 | 70.6 | 56.4 | 57.0 | 68.6 | 51.3 | 36.7 | 48 | ||
| 22 | 71.1 | 57.3 | 52.0 | 71.1 | 50.6 | 39.1 | 48 | ||
| 2 | 71.0 | 57.0 | 52.8 | 68.7 | 48.0 | 39.0 | 48 | ||
| 5 | 70.8 | 56.8 | 56.2 | 71.0 | 53.9 | 36.8 | 48 | ||
| 14 | 2 | 71.1 | 57.1 | 55.7 | 67.2 | 47.8 | 38.1 | 24 | |
| 14 | 5 | 70.1 | 55.8 | 58.2 | 70.0 | 54.8 | 35.4 | 24 | |
| 22 | 2 | 70.8 | 56.7 | 49.9 | 70.2 | 48.2 | 40.0 | 24 | |
| 22 | 5 | 71.3 | 57.8 | 54.2 | 72.0 | 53.0 | 38.2 | 24 | |
| P values | |||||||||
| Diet | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Age | 0.25 | 0.51 | <0.01 | <0.01 | 0.47 | 0.17 | |||
| Feeding Length | 0.66 | 0.95 | 0.04 | <0.01 | <0.01 | 0.20 | |||
| Diet × Age | 0.39 | 0.68 | 0.43 | 0.90 | 0.51 | 0.86 | |||
| Diet × Feeding length | 0.60 | 0.34 | 0.47 | 0.54 | 0.07 | 0.45 | |||
| Age × Feeding length | 0.06 | 0.34 | 0.57 | 0.37 | 0.23 | 0.77 | |||
| Diet × Age × Feeding length | 0.81 | 0.66 | 0.62 | 0.46 | 0.47 | 0.89 | |||
| PC vs. NC | 0.01 | <0.01 | <0.01 | 0.63 | <0.01 | <0.01 | |||
| NC vs. 2,000 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Days 12 to 14 vs. 9 to 14 | 0.11 | 0.47 | 0.27 | <0.01 | <0.01 | 0.27 | |||
| Days 20 to 22 vs. 17 to 22 | 0.31 | 0.52 | 0.06 | 0.02 | <0.01 | 0.48 | |||
| Days 12 to 14 vs. 20 to 22 | 0.61 | 0.83 | 0.01 | <0.01 | 0.73 | 0.44 | |||
| Days 9 to 14 vs. 17 to 22 | 0.04 | 0.26 | 0.08 | <0.01 | 0.18 | 0.24 |
AID = apparent ileal digestibility; TTR = total tract retention; DM = dry matter; P = phosphorus; Ca = calcium.
PC = positive control; NC = negative control; 2,000 = NC + 2,000 phytase units/kg.
Table 4.
Effect of age and feeding length on phytase efficacy in mineral utilization and tibia ash of broiler chickens1
| Age, D | Feeding length, day | AID2 P, % | AID Ca, % | TTR2 P, % | TTR Ca, % | Tibia Ash, % | Number of replicates |
|---|---|---|---|---|---|---|---|
| 14 | 2 (Days 12 to 14) | 23.94 | 16.87 | 23.15 | 15.57 | 1.5 | 8 |
| 14 | 5 (Days 9 to 14) | 17.95 | 10.32 | 16.68 | 12.49 | 4.8 | 8 |
| 22 | 2 (days 20 to 22) | 18.85 | 9.84 | 20.89 | 16.34 | 1.3 | 8 |
| 22 | 5 (Days 17 to 22) | 17.67 | 7.09 | 19.85 | 11.25 | 3.4 | 8 |
| 14 | 20.95 | 13.60 | 19.92 | 14.03 | 3.1 | 16 | |
| 22 | 18.26 | 8.46 | 20.37 | 13.79 | 2.3 | 16 | |
| 2 | 21.40 | 13.35 | 22.02 | 15.95 | 1.4 | 16 | |
| 5 | 17.81 | 8.71 | 18.27 | 11.87 | 4.1 | 16 | |
| P values | |||||||
| Age | 0.41 | 0.10 | 0.82 | 0.96 | 0.45 | ||
| Feeding length | 0.27 | 0.14 | 0.06 | 0.37 | 0.02 | ||
| Age × Feeding length | 0.46 | 0.54 | 0.17 | 0.83 | 0.60 | ||
| Days 12 to 14 vs. 9 to 14 | 0.19 | 0.14 | 0.02 | 0.63 | 0.04 | ||
| Days 20 to 22 vs. 17 to 22 | 0.79 | 0.53 | 0.70 | 0.43 | 0.17 | ||
| Days 12 to 14 vs. 20 to 22 | 0.27 | 0.11 | 0.41 | 0.91 | 0.87 | ||
| Days 9 to 14 vs. 17 to 22 | 0.95 | 0.46 | 0.25 | 0.85 | 0.37 |
3 Phytase 2,000 = NC + 2,000 phytase units/kg.
Data were obtained by subtracting responses of birds on NC diet from responses of birds on NC + 2,000 FYT/kg phytase supplemented diets in each of 8 blocks of cages.
AID = apparent ileal digestibility, TTR = total tract retention.
Figure 1.
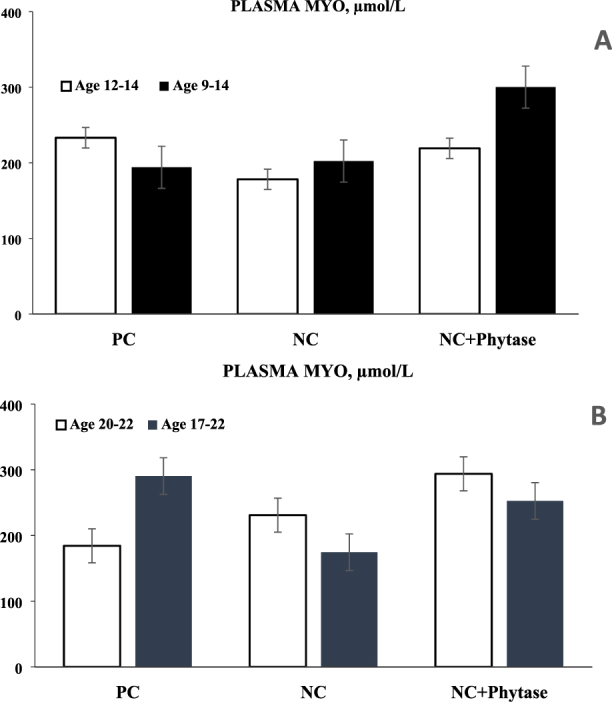
Plasma Myo -inositol (MYO) concentrations of broiler chickens fed PC (positive control), NC (negative control), and phytase (2,000 FTY/kg) supplemented NC diet. Panel A represents age day 14 when fed for 2 or 5 D from days 12 to 14 or days 9 to 14 of age. Panel B represents age 22 D when fed 2 or 5 D from days 20 to 22 or days 17 to 22. Each point represents a mean of 8 observations.
DISCUSSION
Phytase supplementation in broiler nutrition has been known to improve growth performance, nutrient and mineral utilization, bone mineralization, and the general well-being of birds, and this has been established by various studies over time (Broz et al., 1994; Dilger et al., 2004; Olukosi et al., 2013). The current study was not an exception to this trend as improvements in growth performance, mineral utilization, and bone mineralization were observed with phytase supplementation. However, the study aimed to evaluate the impact of age and feeding length on phytase efficacy. This was important because it has been established that age has an influence on the utilization of nutrients by broilers (Tarvid, 1995). Some studies have reported that birds in the first 2 wk of life have increased energy and protein digestibility and utilization as compared with older birds due to the rapid growth of organs and tissues associated with this period (Batal and Parsons, 2002; Huang et al., 2005). Other studies have reported an increase in digestibility of nutrients with increasing age (Noy and Sklan, 1995; Sell, 1996). Feeding length has been observed to have an impact on P digestibility and retention. Li et al. (2018) reported that the effect of feeding low P diets to birds for greater than 48 h could potentially confound results of P digestibility as physiological adaptations could occur in birds. Following up on a previous study in our lab, feeding P deficient diets to birds for 2 or 5 D had the greatest impact on P digestibility and phytase efficacy as compared with feeding for a longer period of 16 D (Babatunde et al., 2019). However, the study design was not suitable to determine whether feeding for a short period would have the same effect on phytase efficacy at different time point in the starter phase of chickens. Therefore, this current study evaluated the age effect on phytase efficacy by comparing responses of birds fed for 2 D at days 14 and 22 and for 5 D at days 14 and 22. It also evaluated the feeding length effect on phytase efficacy by comparing responses for birds fed for 2 and 5 D at days 14 and at 22. Phytase efficacy was determined by subtracting responses of birds fed the NC from responses of birds fed the phytase supplemented NC diet at each feeding length and age. Similarly, the effect of age and/or feeding length on P deficiency in birds were evaluated by comparing responses of birds fed the PC and NC diets at the different ages and feeding lengths.
Birds fed the P deficient diet had lower weight gain, feed intake, and feed efficiency as compared with birds fed the PC diet across all the ages and feeding lengths. This observation suggests that P was a limiting nutrient in the diet and supports observations by previous studies where broilers fed low P diets had decreased growth performance (Adeola and Walk, 2013; Dilger et al., 2004). However, the effect of the low P diet on BWG was more evident in birds fed for 2 D at day 14 than in birds fed for 5 D at this same age. This could be because an exposure to P deficiency for a short period and at a critical point in the lifecycle of the birds, characterized by the rapid growth of organs and tissues may be more detrimental than P deficiency of a longer duration. It is possible that birds may adapt to low P conditions over time (Proszkowiec-weglarz and Angel, 2013), this could explain why the impact on BWG after feeding for 5 D at day 14 was not as severe as feeding for 2 D. On the contrary, birds fed for 5 D at day 22 had a more severe effect of P deficiency on BWG than birds fed for 2 D. This could be due to birds being well developed at this phase. Thus, a short exposure to P deficiency may not be as severe as a longer exposure. This observation could be feed intake driven as the effect of the low P diet on feed intake was higher in birds fed for 5 D than in birds fed for 2 D at day 22. Phytase supplementation improved BWG, feed intake, and efficiency across all groups. However, when age effect was considered at both feeding lengths, birds at day 22 had an increased improvement with phytase supplementation than birds at day 14. This may be feed intake driven as birds at this age will consume more diet than birds at day 14, resulting in more muscle development and weight gain. When feeding length effect was considered, birds fed the phytase supplemented diet for 5 D at both ages had increased weight gain than birds fed for 2 D at both ages, this was probably due to increased feed intake over a longer period.
Tibia ash was reduced in birds fed low P diets and increased in birds fed phytase supplemented low P diets. This was predictable as previous studies have observed this trend (Jiang et al., 2013; Onyango et al., 2004). Although no significant age effect was observed on phytase efficacy on tibia ash, a feeding length effect was observed where birds fed phytase supplemented diets for 5 D at both ages had increased improvement in tibia ash than birds fed for 2 D. This could be due to the fact that P and Ca constitute approximately 99% of bones in the body, thus, the longer the exposure of birds to P deficient diets, the greater the negative impact on tibia ash. Consequently, feeding phytase supplemented diets to birds for a longer period will ameliorate the impact of the low P diet on tibia ash given that there is more room for improvement. Birds at day 14 had a slightly higher phytase induced improvement on tibia ash than birds at day 22 regardless of the feeding length, this may be because birds at this phase are rapidly depositing hydroxyapatite on bones to increase its strength and to support their weight as they grow older (Dilger et al., 2004). Birds fed the P deficient diet had decreased P digestibility in accordance with observations from previous studies (Plumstead et al., 2008; Ravindran et al., 2006). Evaluating the age effect of P deficiency on P digestibility revealed that birds fed the NC for 2 D at day 14 had an increased reduction in AID of P when compared with birds fed for 2 D at day 22. This may be because birds at day 14 may be more sensitive to changes in dietary P than birds at day 22. Birds at day 22 had also been exposed to a commercial diet until day 20 and so a change in dietary P for 2 D may not cause a severe decrease in P digestibility. Similarly, phytase improved P digestibility more so in birds fed for 2 D at day 14 than birds at day 22, thus showing that phytase was more efficacious at day 14. Considering feeding length effect, birds fed P deficient diets for 2 D at day 14 and 22 had lower P digestibility than birds fed for 5 D at both ages. This observation agreed with the previous study from our lab (Babatunde et al., 2019) and other studies which revealed that feeding low P diet for more than 48 h could potentially confound results obtained from P digestibility. This is because adaptive changes may be stimulated in the gastro-intestinal tracts of birds to maintain P homeostasis (Li et al.,2015; Perryman et al., 2016). Calcium has been known to interact with phytate present in cereal and oilseed grains by forming complexes with phytate and preventing its hydrolysis and release to the animal (Plumstead et al., 2008). Invariably, addition of phytase to the diet breaks this complex bond and releases Ca for use by the animal. This explains why Ca digestibility and retention were improved in birds fed diet with phytase supplementation and in agreement with previous studies (Adeola and Walk, 2013; Paiva et al., 2014). A similar pattern with P utilization was observed when age and feeding length effect on phytase efficacy on Ca utilization were evaluated. Plasma myo -inositol levels were increased in birds fed phytase supplemented diets and this was similar with observations reported by Cowieson et al., (2015). This is because phytase hydrolyzes phytic acid into inositol and orthophosphate in the digestive tract (Perryman et al., 2016). Inositol which is absorbed into the blood, may be responsible for some of the benefits of phytase on growth performance, as it has recently been discovered to have insulin mimetic properties that stimulates the translocation of glucose transporters (GLUT4) to the plasma membranes of the small intestine (Cowieson et al., 2017).
In conclusion, we observed that broiler chickens may be more sensitive to P deficiency when fed for 2 D or at an earlier stage in the starter phase, invariably giving phytase an added opportunity to improve performance in birds. It is possible that phytase recovery is reduced in birds that are fed P deficient diets supplemented with phytase over a longer period. Thus, considering current practices, varying the length of phytase supplementation during the starter phase may prove more beneficial to phytase recovery with regards to P and Ca utilization. This knowledge may also prove useful during the grower or finisher phases of broiler production; however, more data might be needed to establish this notion. We can also recommend based on results from this study that phytase trials in broilers could be carried out for 2 D during the end of the second week in the lifecycle of broiler chickens for maximum sensitivity. Feeding broiler chickens for 2 D or during the second week in their lifecycle could potentially serve as a model for carrying out preliminary tests with phytase products or other feed additives. It is also possible that this model could be used to test the sensitivity of broiler chickens to deficiencies of other nutrients including amino acids, vitamins, or other minerals. However, more studies may be needed to assert this possibility.
ACKNOWLEDGMENTS
The authors appreciate DSM Nutritional Products, Kaiseraugst, Switzerland for supporting this research, Cobb-Vantress, Monticello, KY for donating the chicks and Pat Jaynes for her technical assistance.
REFERENCES
- Adeola O., Walk C.L. Linking ileal digestible phosphorus and bone mineralization in broiler chickens fed diets supplemented with phytase and highly soluble calcium. Poult. Sci. 2013;92:2109–2117. doi: 10.3382/ps.2013-03068. [DOI] [PubMed] [Google Scholar]
- AOAC International . 17th ed. AOAC Int.; Gaithersburg, MD: 2000. Official Methods of Analysis. [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Batal A.B., Parsons C.M. Effects of age on nutrient digestibility in chicks fed different diets. Poult. Sci. 2002;81:400–407. doi: 10.1093/ps/81.3.400. [DOI] [PubMed] [Google Scholar]
- Broz J., Oldale P., Perrin-Voltz A.H., Rychen G., Schulze J., Nunes C.S. Effects of supplemental phytase on performance and phosphorus utilisation in broiler chickens fed a low phosphorus diet without addition of inorganic phosphates. Br. Poult. Sci. 1994;35:273–280. doi: 10.1080/00071669408417691. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Aureli R., Guggenbuhl P., Fru-Nji F. Possible involvement of myo-inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 2015;55:710–719. [Google Scholar]
- Cowieson A.J., Bedford M.R. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? Worlds Poult. Sci. J. 2009;65:609–624. [Google Scholar]
- Cowieson A.J., Roos F.F., Ruckebusch J., Wilson J.W., Guggenbuhl P., Lu H., Ajuwon K.M., Adeola O. Time-series responses of swine plasma metabolites to ingestion of diets containing myo-inositol or phytase. Br. J. Nutr. 2017;118:897–905. doi: 10.1017/S0007114517003026. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Adeola O. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing pigs fed conventional and low-phytate soybean meals. J. Anim. Sci. 2006;84:627–634. doi: 10.2527/2006.843627x. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Onyango E.M., Sands J.S., Adeola O. Evaluation of microbial phytase in broiler diets. Poult. Sci. 2004;83:962–970. doi: 10.1093/ps/83.6.962. [DOI] [PubMed] [Google Scholar]
- Engelen A.J., van der Heeft F.C., Randsdorf P.H.G., Smit E.L.C. Simple and rapid determination of phytase activity. J. AOAC Int. 1994;77:760–764. [PubMed] [Google Scholar]
- Fenton T.W., Fenton M. An improved procedure for determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 1979;59:631–634. [Google Scholar]
- Huang K.H., Ravindran V., Li X., Bryden W.L. Influence of age on the apparent ileal amino acid digestibility of feed ingredients for broiler chickens. Br. Poult. Sci. 2005;46:236–245. doi: 10.1080/00071660500066084. [DOI] [PubMed] [Google Scholar]
- Iyayi E.A., Fru-Nji F., Adeola O. True phosphorus digestibility of black-eyed pea and peanut flour without or with phytase supplementation in broiler chickens. Poult. Sci. 2013;92:1595–1603. doi: 10.3382/ps.2012-02898. [DOI] [PubMed] [Google Scholar]
- Jiang X.R., Luo F.H., Qu M.R., Bontempo V., Wu S.G., Zhang H.J., Yue H.Y., Qi G.H. Effects of non-phytate phosphorus levels and phytase sources on growth performance, serum biochemical and tibia parameters of broiler chickens. Ital. J. Anim. Sci. 2013;12:375–380. [Google Scholar]
- Li W., Angel R., Kim S. -W., Jimenez-Moreno E., Proszkowiec-Weglarz M., Plumstead P.W. Age and adaptation to Ca and P deficiencies: 2. Impacts on amino acid digestibility and phytase efficacy in broilers. Poult. Sci. 2015;94:2917–2931. doi: 10.3382/ps/pev273. [DOI] [PubMed] [Google Scholar]
- Li W., Angel R., Kim S.W., Jiménez-Moreno E., Proszkowiec-Weglarz M., Plumstead P.W. Impacts of age and calcium on Phytase efficacy in broiler chickens. Anim. Feed Sci. Technol. 2018;238:9–17. [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ogunwole O.A., Babatunde O.O., Faboyede R.A., Adedeji B.S., Jemiseye F.O. Calcium and phosphorus retention by broiler chickens fed groundnut cake based-diet supplemented with l-lysine and dl- methionine. J. Anim. Prod. Res. 2017;29:240–248. [Google Scholar]
- Olukosi O.A., Kong C., Fru-Nji F., Ajuwon K.M., Adeola O. Assessment of a bacterial 6-phytase in the diets of broiler chickens. Poult. Sci. 2013;92:2101–2108. doi: 10.3382/ps.2012-03005. [DOI] [PubMed] [Google Scholar]
- Onyango E.M., Bedford M.R., Adeola O. The yeast production system in which escherichia coli phytase is expressed may affect growth performance, bone ash, and nutrient use in broiler chicks. Poult. Sci. 2004;83:421–427. doi: 10.1093/ps/83.3.421. [DOI] [PubMed] [Google Scholar]
- Paiva D., Walk C., Mcelroy A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 2014;93:2752–2762. doi: 10.3382/ps.2014-04148. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Rao S.V.R., Raju M.V.L.N., Gajula S.S., Bhanja S.K. Performance of broiler chickens fed low non phytate phosphorus diets supplemented with microbial phytase. J. Poult. Sci. 2007;44:258–264. [Google Scholar]
- Perryman K.R., Cattley R.C., Masey O'Neill H.V., Bedford M.R., Dozier W.A. Interactive effects of dietary adaptation period length and titration diet type on apparent ileal phosphorus digestibility and phosphorus retention in growing broilers. Poult. Sci. 2016;95:2332–2341. doi: 10.3382/ps/pew117. [DOI] [PubMed] [Google Scholar]
- Plumstead P.W., Leytem A.B., Maguire R.O., Spears J.W., Kwanyuen P., Brake J. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 2008;87:449–458. doi: 10.3382/ps.2007-00231. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013;22:609–627. [Google Scholar]
- Ravindran V., Bryden W.L., Kornegay E.T. Phytin: occurrence, bioavailability and implications in poultry nutrition. Poult. Avian Biol. Rev. 1995;6:125–143. [Google Scholar]
- Ravindran V., Morel P.C.H., Partridge G.G., Hruby M., Sands J.S. Influence of an escherichia coli-derived phytase on nutrient utilization in broiler starters fed diets containing varying concentrations of phytic acid. Poult. Sci. 2006;85:82–89. doi: 10.1093/ps/85.1.82. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S.M., Chung T.K., Thomas D.V., Zou M.L., Moughan P.J. Effect of a novel phytase on growth performance, apparent metabolizable energy, and the availability of minerals and amino acids in a low-phosphorus corn-soybean meal diet for broilers. Poult. Sci. 2012;91:1118–1127. doi: 10.3382/ps.2011-01702. [DOI] [PubMed] [Google Scholar]
- Sell J.L. Physiological limitations and potential for improvement in gastrointestinal tract function of poultry. J. Appl. Poult. Res. 1996;5:96–101. [Google Scholar]
- Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 2009;124:126–141. [Google Scholar]
- Shirley R.B., Edwards H.M., Jr Graded levels of phytase past industry standards improves broiler performance. Poult. Sci. 2003;82:671–680. doi: 10.1093/ps/82.4.671. [DOI] [PubMed] [Google Scholar]
- Tarvid I. The development protein digestion in poultry. Poult. Avian Biol. Rev. 1995;6:35–54. [Google Scholar]


