Abstract
The present study aimed at investigating the impact of the current growth-related abnormalities (White-Striping—WS, Wooden Breast—WB, and Spaghetti Meat—SM) affecting broilers Pectoralis major muscles on the main quality traits, the oxidative stability of both the lipid and protein fraction as well as the water mobility assessed in fresh and frozen/thawed meat. In addition, Nuclear Magnetic Resonance spectroscopy (1 H-NMR) was applied to quantify free amino acids, histidine-containing dipeptides and metabolites involved in energy-generating pathways. Overall, the occurrence of WS, WB, and SM abnormalities remarkably affected the quality traits (pH, color, and water holding capacity) and oxidative stability of the meat, with the WB condition leading to the most detrimental effects. However, overall, freezing and subsequent thawing only partially worsened the aforementioned traits. Significant variations in free amino acids and histidine-containing dipeptides were found between abnormal muscles and their unaffected counterpart by 1 H-NMR spectroscopy and, aside from the occurrence of muscular defects, their content was remarkably reduced in frozen/thawed meat. The findings obtained by analyzing the metabolites through 1 H-NMR spectroscopy allowed to advance the knowledge concerning the impact of freezing and subsequent thawing on meat quality traits and provided useful information concerning the underlying mechanisms responsible for the development of WS, WB, and SM abnormalities in broilers.
Key words: muscular abnormality, freezing, oxidative stability, NMR, free amino acids
INTRODUCTION
During the past decade the poultry industry faced the occurrence of growth-related muscular abnormalities that mainly affect the pectoral muscles of those commercial hybrids exhibiting fast growth rate and high breast yield (Kuttappan et al., 2016; Petracci et al., 2019). In detail, white striping (WS), wooden breast (WB), and spaghetti meat (SM) affected muscles exhibit the appearance of white striations of variable thickness parallel to the myofiber direction, macroscopically pale, and out-bulging areas with focally or diffused hardened consistency and the tendency towards the separation of the fiber bundles composing the muscle tissue itself, respectively (Soglia et al., 2019). Because of their compromised visual appearance as well as remarkably altered technological and functional properties, abnormal meats are normally downgraded and diverted for further processing (Kuttappan et al., 2016; Petracci et al., 2019). Indeed, previous studies demonstrated that, in spite of their remarkably higher ultimate pH, abnormal muscles exhibited impaired water-holding and water-binding capacities as a consequence of an overall reduced protein content and functionality (Mudalal et al., 2014; Bowker and Zhuang, 2016; Soglia et al., 2016b; Baldi et al., 2018). Furthermore, resulting in increased moisture, fat, and collagen contents, the occurrence of these defects significantly alters the proximate composition and, thus, the nutritional value of the meat (Tasoniero et al., 2016; Soglia et al., 2016a; Baldi et al., 2019).
In this context, in order to fulfill the requirements of the poultry industry, the further processing of these abnormal meats does not necessarily occur at the same time of slaughter, but it frequently happens that the meat needs to be frozen and stored to be processed at a later time (Kuttappan et al., 2016). The freezing process involves a reduction in the amount of available water and through the formation of ice crystals taking place at sub-zero temperatures results in its separation in pure form (Jeremiah, 2004). It is known that freezing of fresh meat entails a significant variation in its quality traits. Indeed, depending on their size and location, the formation of ice crystals profoundly alters the ultrastructure of the meat and eventually leads to pH and solute concentration modifications (Van Den Berg, 1966; Muela et al., 2010; Leygonie et al., 2012; Wei et al., 2016; Seong et al., 2017). In addition, impairing the integrity of the muscle fibers, ice crystals might lead to the release of both mitochondrial and lysosomal enzymes within the sarcoplasm as well as to the subsequent loss of a high proportion of exudate after thawing (Martino et al., 1998; Leygonie et al., 2012). Nonetheless, oxidative reactions can occur during the frozen storage of meat having proteins and lipids as main target. In addition, previous studies demonstrated that protein and lipid oxidation are undoubtedly interlinked (Estévez, 2011). In detail, primary lipid oxidation (peroxidation) might be initiated during frozen storage of the meat leading to radical secondary lipid oxidation upon thawing (Leygonie et al., 2012). Then, reacting with protein derivatives, malonaldehyde leads to the formation of carbonyl compounds (Xiong, 2000). As a consequence, a remarkable increase in Thiobarbituric Acid Reactive Substances (TBARS) and carbonylation level was previously found in frozen/thawed meat (Soyer et al., 2010; Utrera et al., 2014). In this context, although the effect of freezing on the quality traits and the impact of muscular abnormalities on the main technological and functional properties of chicken meat have been previously investigated, no studies were carried out to evaluate the effect of freezing and subsequent thawing of P. major affected by muscular defects in broilers. In addition, information concerning the effect of abnormality and freezing on main metabolites (free amino acids, histidine-containing dipeptides, and metabolites involved in energy-generating pathways) in broiler P. major muscles is lacking. For this purpose, Nuclear Magnetic Resonance (NMR) spectroscopy represents a versatile technique since acquiring a single spectrum allows to gather quantitative and qualitative information concerning the metabolic profile of the muscle tissue (Bertocchi and Paci, 2008). Although its application in food science is expanding, only few studies were performed in order to quantify free amino acids and metabolites in meat (Mati et al., 2015; Kodani et al., 2017) and examine the metabolic profile in chicken tissue (Le Roy et al., 2016; Sundekilde et al., 2017).
In this context, the present study aimed at evaluating the main quality traits, oxidative stability of lipids and proteins and water mobility in chicken pectoral muscles affected by WS, WB, and SM abnormalities in both fresh and frozen/thawed meat. In addition, free amino acids, histidine-containing dipeptides, and metabolites involved in energy-generating pathways were quantified through 1 H-NMR spectroscopy.
MATERIALS AND METHODS
Sample Selection and Preparation
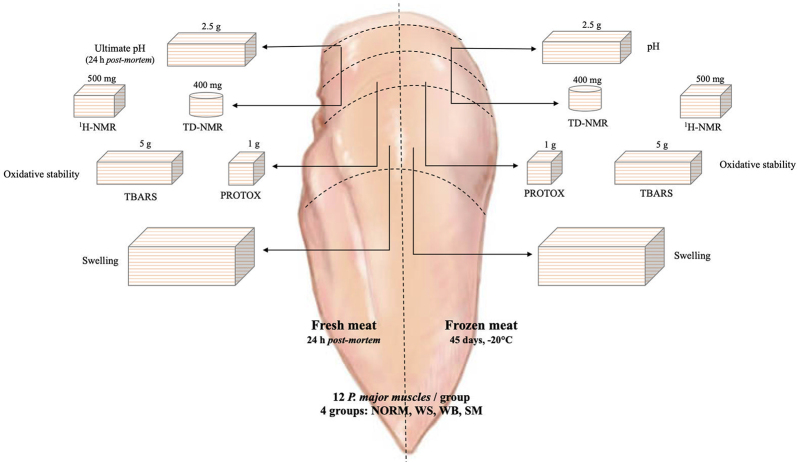
This study was conducted on a single flock of broiler chickens (females of Ross 308 strain) farmed under commercial conditions based on ad libitum access to a wheat/sorghum−soybean multiphase diet and slaughtered at 48 D of age (average weight 2.8 kg). Before slaughtering, broilers were subjected to a total feed withdrawal of 10 h along with a 3 h lairage time at the processing plant. Birds were subsequently processed under commercial conditions: electrically stunned (150 mA/bird, 400 Hz), immediately killed by severing the jugular vein and carotid artery with an automatic device and allowed bleeding for 180 s. Subsequently, birds were scalded 51 to 52°C for 215 s, plucked, eviscerated, and air-chilled passing through a cold-air flow tunnel (−6°C for 150 min) until reaching 2 to 3°C at the core. Then, a total of 48 skinless whole breasts were collected at 3 h post-mortem in the deboning area. The whole breasts were selected by 2 experienced people and classified by visual appearance and manual palpation according to the presence of muscular abnormalities into 4 experimental groups: Normal (NORM), WS, WB, and SM. Only severe cases were selected and, in order to avoid any interference, those contextually affected by other defects (i.e., hemorrhages, paleness, etc.) were not considered within this study. Then, the P. major muscle arising from the right side of the keel was weighed and subsequently longitudinally cut in half, as shown in Figure 1, in order to separate the external portion of the muscle (close to the wing), used for analyses carried out on fresh meat, from the internal part which was rapidly frozen in industrial conditions passing through a cold-air flow tunnel and stored at −20°C for 45 D. After storage, frozen samples were then subsequently thawed at 4 ± 1°C for 16 h. In detail, the half P. major thus obtained were used to assess the main quality traits (ultimate pH, color, swelling), oxidative stability (TBARS, carbonyls), water mobility and distribution (Time Domain-NMR), and evaluate the concentration of free amino acids, histidine-containing dipeptides and main metabolites (1 H-NMR) in fresh and frozen/thawed muscles affected by muscular abnormalities following the sampling procedure displayed in Figure 1. In order to minimize the variability resulting from the sampling position, sub-samples used to assess the same parameter on fresh and frozen/thawed meat were exactly excised in the adjacent position of the respective half P. major close to the median line of the muscle as shown in Figure 1.
Figure 1.
Sampling procedure adopted to assess quality traits, oxidative stability, and main metabolites (free amino acids, histidine-containing dipeptides, and metabolites involved in energy-generating pathways) in fresh and frozen/thawed broilers P. major muscles.
Quality Traits and Oxidative Stability
Color (CIE L* = Lightness, a* = Redness and b* = Yellowness) was assessed in triplicate on the bone-side surface of each muscle using a Chroma Meter CR-400 (Minolta Corp., Milan, Italy) and ultimate pH (pHu) was measured by using the 150 mM KCl and 5 mM sodium iodoacetate solution described by Jeacocke (1977). Swelling was determined according to the centrifugal method proposed by Wierbicky et al. (1957) with slight modifications. Briefly, 1.5 g of meat were homogenized (30 s at 11,000 rpm, in ice) in 4 volumes of 2% NaCl saline solution. After centrifugation (15 min, 2,000 × g at room temperature) the samples were left upside down to remove the unbound solution and subsequently weighed to quantify the percentage of solution absorbed by the meat sample.
With regard to oxidative stability, TBARS were measured according to the procedure described by Bao and Ertbjerg (2015) while carbonylation level was quantified by using the novel DNPH-based method developed by Soglia et al. (2016c).
Time Domain-NMR
Proton transverse relaxation (T2) decay curves were recorded at the operating frequency of 20 MHz with a Bruker (Milan, Italy) Minispec PC/20 spectrometer using the Carr−Purcell−Meiboon−Gill (CPMG) pulse sequence previously described by Petracci et al. (2014). The measurements were performed at a constant temperature of 25°C on a meat sample having a weight of about 400 mg and a height not exceeding the active region of the radio frequency coil. The CPMG decays were normalized by the sample weight and transformed into relaxograms through the program UPEN. Then, each relaxogram was interpreted in terms of bound, intra- and extra-myofibrillar water proton populations. To separately observe these protons populations, the relaxograms were fit to the sum of 4 exponential curves and the two with intermediate T2, describing the behavior of intra-myofibrillar protons, were combined.
1 H-NMR
The 1 H-NMR analysis was performed on 0.5 g of meat homogenized by Ultra-Turrax T25 (IKA-Werke GmbH & Co., Germany) (20 s, 11,000 rpm) in 3 mL of distilled water. Then, 1 mL of the homogenate was transferred in a tube and centrifuged (10 min, 18,000 × g at 4°C). 700 μL of the supernatant were transferred in a new tube in which 800 μL CHCl3 were added. The samples were mixed by vortex (2 min) and then centrifuged (2 min, 18,000 × g at 4°C). An aliquot (500 μL) of supernatant was transferred to a new tube and 200 μL of potassium phosphate buffer (1 M, 2 mM sodium azide; pH 7.0) in D2 O and 10 mM 3-(Trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP) were added. Following centrifugation (10 min, 18,000 × g at 4°C), 700 μL of supernatant was transferred to NMR tube for analysis. The spectra were acquired using the cpmgpr1d pulse sequence with suppression of the solvent signal and the following parameters: size of fid: 32 k, number of scans: 16, number of dummy scans: 16, spectral width: 12 ppm, acquisition time: 2.28 s, delay d1: 5 s NMR spectra were processed, adjusted and quantified with Topspin 3.1 and R software while elucidation and identification of the compounds was done with the help of the HMDB database (http://www.hmdb.ca/), Chenomx software and literature references. For quantification and calibration of the spectrum, the TSP was used (δ 0.00).
Data Processing and Analysis
Data were analyzed according to a 2 × 2 factorial design using the GLM procedure of Statistica (StatSoft Italy srl, Vigonza, Italy). In detail, the factorial ANOVA was performed to investigate the main effects of the occurrence of muscular abnormalities (NORM, WS, WB, and SM) and freezing (fresh and frozen/thawed) as well as their interaction term on the quality traits as well as on the concentration of free amino acids, histidine-containing dipeptides, and metabolites. Mean values were subsequently separated through the parametric Tukey HSD test. All statistical differences were considered significant at a level of P ≤ 0.05. In addition, in order to provide a more comprehensive representation and interpretation of the results obtained through 1 H-NMR, Principal Component Analysis (PCA) was carried out by considering those variables that, having the highest discrimination power, resulted significantly different among the groups. All statistical analyses were carried out by using Statistica software (StatSoft Italy srl, Vigonza, Italy).
RESULTS AND DISCUSSION
Quality Traits and Oxidative Stability
The findings concerning the effect of the occurrence of WS, WB, and SM abnormalities and freezing on the main quality traits and oxidative stability of meat are shown in Table 1.
Table 1.
Effect of the occurrence of muscular abnormalities and freezing treatment on the main quality traits and oxidative stability of broilers' Pectoralis major muscles (N = 96; 12 muscles/experimental group/treatment).
| Abnormality (A) |
Freezing (F) |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | NORM | WS | WB | SM | Fresh | Frozen | sem | A | F | A × F |
| pHu | 5.84b | 6.05a | 6.05a | 5.99a | 5.98 | 5.98 | 0.01 | *** | ns | ns |
| Lightness—L* | 55.9 | 56.1 | 57.6 | 57.1 | 48.0 | 47.0 | 0.3 | ns | *** | ** |
| Redness—a* | 1.64 | 1.72 | 1.73 | 1.79 | 0.14 | 0.15 | 0.06 | ns | ns | ns |
| Yellowness—b* | 7.05a,b | 6.49b | 7.63a | 7.93a | 0.02 | 0.02 | 0.13 | *** | ns | ** |
| Swelling (%) | 67.3a | 67.1a | 44.9b | 64.9a | 58.7 | 63.4 | 1.9 | *** | ns | ns |
| TBARS (mg MDA/kg of meat) | 2.19b | 3.32b | 4.05a | 3.30b | 3.91 | 2.50 | 0.19 | ** | *** | ns |
| Carbonyls (nmol/mg of protein) | 1.51 | 2.22 | 2.08 | 1.98 | 1.20 | 2.71 | 0.11 | ns | *** | ** |
= P < 0.01; *** = P < 0.001; ns = not significant.
Mean values followed by different letters significantly differ among treatment (P < 0.05).
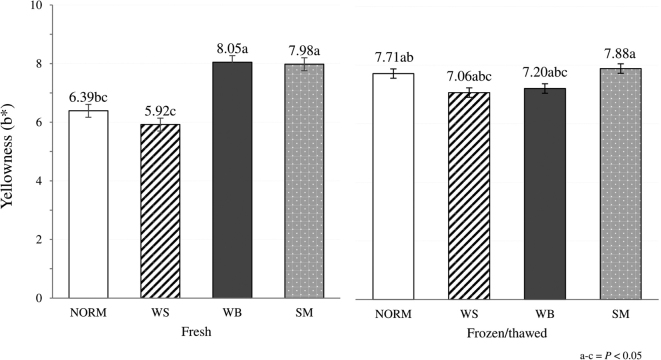
With regard to the effect of the muscular abnormality, no significant differences (P > 0.05) among the groups were found in lightness (L*), redness and carbonyls content. On the other hand, if compared with their unaffected counterpart, P. major displaying muscular abnormalities exhibited significantly (P < 0.001) higher ultimate pH. In addition, WB and SM revealed significantly higher (P < 0.001) yellowness (b*) in comparison with both NORM and WS cases. As shown in Figure 2, the significant (P < 0.001) interaction term observed for this parameter can be attributed to the withdrawal of any significant differences among the groups after freezing and subsequent thawing of the meat. Overall, the increased ultimate pH and yellowness are in general agreement with the findings reported in previous studies in which the quality traits and technological properties of muscles affected by WS, WB, and SM were evaluated (Mudalal et al., 2015; Tasoniero et al., 2016; Baldi et al., 2018). In addition, WB muscles exhibited a remarkably lower (P < 0.001) swelling, thus corroborating the findings of previous studies in which a greater WHC impairment was associated to the development of this condition if compared with WS (Mudalal et al., 2015; Soglia et al., 2016b; Baldi et al., 2019). Indeed, muscle architecture was previously found to be profoundly altered by the degenerative processes (and occasional regeneration) taking place within the tissue (Soglia et al., 2016a; Papah et al., 2017; Chen et al., 2019). With regard to swelling, WS did not exert any detrimental effect on the ability of meat to bind a saline solution thus confirming that WS did not impair the salt-induced water uptake and only marginally affected WHC of the meat (Bowker and Zhuang, 2016; Tasoniero et al., 2016). Similarly, SM affected muscles displayed swelling values comparable to that of NORM thus suggesting that the occurrence of this defect only slightly influenced the water binding ability of the meat. As for oxidative stability, WB affected muscles exhibited the highest TBARS content (4.05 vs. 2.19, 3.32, and 3.30 mg MDA/kg of meat; P < 0.01). In agreement with that, a lower oxidative stability of the lipid fraction was previously observed in WB and hypothesized to promote protein oxidation and formation of carbonyl derivatives (Soglia et al., 2016b).
Figure 2.
Effect of the occurrence of WS, WB, and SM abnormalities on yellowness (b*) assessed in both fresh and frozen/thawed P. major muscles (N = 96; 12 muscles/group/treatment).
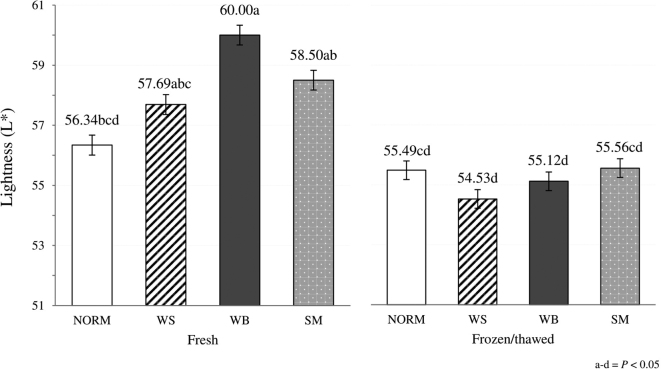
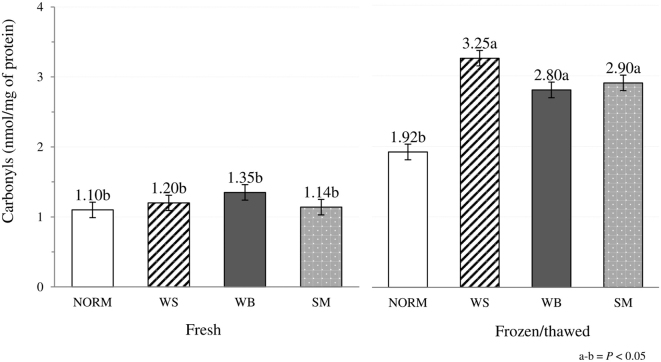
When freezing effect was considered, no significant differences (P > 0.05) between fresh and frozen/thawed meat were observed in pH, redness (a*), yellowness (b*) and swelling. In detail, even if limited information concerning the impact of freezing on pH of chicken meat is available, the absence of significant variations in pH were previously found by comparing the values assessed on fresh and frozen/thawed lamb and horse meat (Muela et al., 2010; Seong et al., 2017). Similarly, freezing and subsequent thawing of the meat did not affect the color parameters, with the only exception being an overall reduction in L* (48.0 vs. 47.0; P < 0.001). The significant (P < 0.01) interaction term observed for L* value might be ascribed to the alterations in meat structure resulting from the formation of ice crystals during freezing that might predominantly affect the structure of the abnormal muscles (Figure 3). In detail, disrupting the muscle cells, ice crystals likely aggravated the profound histological lesions previously observed in WS, WB, and SM (Soglia et al., 2019) thus resulting in a further altered muscular architecture leading to a different scattering of the incident light. On the other hand, in NORM muscles, the rapid freezing process performed under industrial conditions might have resulted in a fast development of small-sized ice crystals that, once thawed, did not affect the light scattering phenomenon. Oxidation level was remarkably affected by freezing, although contrasting results were found. TBARS assessed on frozen stored meat were found to be significantly lower if compared with the content measured on fresh meat (2.50 vs. 3.91 mg MDA/kg of meat; P < 0.001). Although the majority of the previous studies carried out on this topic evidenced an increased content of secondary lipid oxidation products after frozen storage of the meat (Soyer et al., 2010; Utrera et al., 2014; Wei et al., 2016), a significant reduction in the amount of TBARS was previously found by Utrera et al. (2014) in beef patties. This reduction might likely be attributed to the occurrence of further reactions between secondary lipid oxidation products and other meat macromolecules/compounds or to the formation of further oxidation products that were not detected as assumed in previous studies (Gray and Monahan, 1992; Hidalgo et al., 1998). Carbonylation level assessed on frozen/thawed meat was significantly higher (2.71 vs. 1.20 nmoL/mg of protein; P < 0.001) in agreement with previous studies performed on both broiler and turkey meat in which a remarkable increase in carbonyls was found after frozen storage of meat (Mercier et al., 1998; Soyer et al., 2010; Chan et al., 2011). Indeed, leading to the disruption of the cellular organelles and ultimately exposing the myofibrillar proteins to oxidative enzymes and pro-oxidant molecules, freezing results in an increased protein oxidation (Xia et al., 2009). The significant (P < 0.001) interaction term can be attributed to the notably higher protein carbonylation observed after freezing and subsequent thawing of WS, WB, and SM affected muscles, whereas no significant variations were found in NORM (Figure 4). Similarly, no significant differences in carbonyls were found by comparing P. major affected by muscular abnormalities with their NORM counterpart (Baldi et al., 2018) whereas, although comparable in term of absolute values, a significantly higher protein oxidation level was previously observed in WB and WS/WB cases (Soglia et al., 2016b).
Figure 3.
Effect of the occurrence of WS, WB, and SM abnormalities on lightness (L*) assessed in both fresh and frozen/thawed P. major muscles (N = 96; 12 muscles/group/treatment).
Figure 4.
Effect of the occurrence of WS, WB, and SM abnormalities on carbonyls content assessed in both fresh and frozen/thawed P. major muscles (N = 96; 12 muscles/group/treatment).
Time Domain-NMR
As expected, a complete re-organization in the distribution of the water fractions within the muscular compartments due to muscular abnormalities and freezing effect was found by evaluating the NMR relaxation properties (Table 2).
Table 2.
Effect of the occurrence of muscular abnormalities and freezing treatment on NMR relaxation properties of broilers' Pectoralis major muscles (N = 96; 12 muscles/experimental group/treatment).
| Abnormality (A) |
Freezing (F) |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | NORM | WS | WB | SM | Fresh | Frozen | sem | A | F | A × F |
| Relative intensity (%) | ||||||||||
| Bound water | 3.2a | 2.7b | 2.7b | 2.7b | 2.8 | 2.7 | 0.1 | *** | ns | ns |
| Intramyofibrillar water | 81.9a | 72.4b | 63.2c | 72.5b | 71.7 | 73.4 | 1.0 | *** | ns | ns |
| Extramyofibrillar water | 15.0b | 24.9b | 34.1a | 24.8b | 25.4 | 23.9 | 1.0 | *** | ns | ns |
| T2 relaxation time (ms) | ||||||||||
| Bound water | 1.75b | 2.08a,b | 2.34a | 2.20a,b | 2.12 | 2.06 | 0.06 | ** | ns | ns |
| Intramyofibrillar water | 39.00c | 41.92a,b | 43.43a | 41.19b | 42.26 | 40.34 | 0.33 | *** | ** | ns |
| Extramyofibrillar water | 113.28b | 114.27b | 128.40a | 112.04b | 114.56 | 119.92 | 1.97 | ** | ns | ns |
= P < 0.01; *** = P < 0.001; ns = not significant.
Mean values followed by different letters significantly differ among treatment (P < 0.05).
With regard to the effect of the muscular abnormality, if compared with NORM, a significant reduction (P < 0.001) in the relative intensities of the proton populations ascribed to bound and intramyofibrillar water was observed in WS, WB, and SM muscles that concurrently displayed an increased (P < 0.001) proportion of the extramyofibrillar water fraction. In addition, all the water fractions identified within the abnormal muscles were less tightly bound, as evidenced by the longer T2 relaxation times, in comparison with the same parameters assesses on NORM. These findings agree with those reported in a couple of previous investigations in which water mobility and distribution were assessed on SM affected muscles (Baldi et al., 2018, 2019). Similarly, longer T2 relaxation times were observed in WB affected muscles (Soglia et al., 2016b; Tasoniero et al., 2017; Xing et al., 2017). In addition, in a previous study, T2 relaxation time for the proton population ascribed to the intramyofibrillar water fraction was found to be highly correlated (r = +0.84) to sarcomere length (Bertram et al., 2002). In this context, sarcomere stretching observed in WB (Tijare et al., 2016; Velleman et al., 2018) might affect the water-protein interactions and likely contribute to explain the longer T2 relaxation times observed.
When the main effect of freezing was considered, remarkably longer (P < 0.001) T2 relaxation times were observed in frozen/thawed meat for the proton population ascribed to the intramyofibrillar water, evidencing that this fraction was weakly bound to the myofibrillar structure. No significant differences among the experimental groups were found concerning T2 relaxation times recorded for bound and extramyofibrillar water. These aspects might be explained by considering that, although abnormal muscles had a lower amount of proteins (that thus resulted in a lower relative intensity of bound water) (Mudalal et al., 2014; Bowker and Zhuang, 2016; Soglia et al., 2016b), those fibers having normal appearance (not showing any sign of degeneration) might interact with the bound water fraction with the same binding strength. In addition, similarly, the extramyofibrillar water not released as a consequence of freezing and subsequent thawing of the meat might be hold within the extracellular compartment by the same capillary forces that resulted in analogous T2 values. These results are in agreement with those obtained by Bowker and Zhuang (2017) who did not observe any significant difference in cooking loss between fresh and frozen chicken meat. In addition, in a recent study, a remarkable increase in drip loss and changes in the water distribution were observed through magnetic resonance imaging in frozen and thawed meat (Frelka et al., 2018).
1 H-NMR
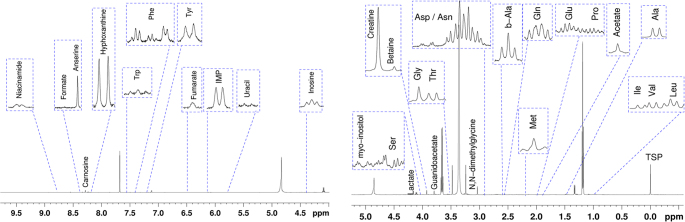
The findings concerning the impact of breast meat abnormalities and freezing on the concentration of free amino acids, histidine-containing dipeptides, and of the main metabolites of meat are shown in Table 3 whereas the identification of the compounds in the spectrum is shown in Figure 5.
Table 3.
Effect of the occurrence of muscular abnormalities and freezing treatment on the concentration (mg/100 g of meat) of free amino acids, histidine-containing dipeptides, and main metabolites in broilers' Pectoralis major muscles (N = 96; 12 muscles/experimental group/treatment).
| Abnormality (A) |
Freezing (F) |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | NORM | WS | WB | SM | Fresh | Frozen | sem | A | F | A × F |
| Free amino acids (mg/100 g of meat) | ||||||||||
| Threonine | 27.51b | 40.97a | 42.69a | 38.21a | 31.45 | 43.24 | 1.08 | *** | *** | ns |
| Serine | 14.40b | 19.92a | 20.38a | 20.65a | 21.42 | 16.25 | 0.46 | *** | *** | ns |
| Asparagine | 9.26a | 6.38b | 4.72c | 5.63b,c | 6.81 | 6.18 | 0.26 | *** | ns | ns |
| Glutamine | 19.83c | 29.89b | 36.72a | 30.91b | 33.06 | 25.61 | 0.87 | *** | ** | ns |
| Proline | 9.37b | 12.69a | 13.37a | 12.43a | 12.87 | 11.06 | 0.27 | *** | *** | ns |
| Glycine | 32.83b | 49.46a | 43.25a | 45.57a | 45.00 | 40.56 | 1.05 | *** | *** | ns |
| Alanine | 47.91b | 69.30a | 70.09a | 65.26a | 65.58 | 60.70 | 1.18 | *** | *** | ns |
| Valine | 20.91b | 28.50a | 27.36a | 28.05a | 24.57 | 27.84 | 0.52 | *** | ns | ns |
| Methionine | 15.27b | 19.64a | 20.14a | 19.67a | 18.61 | 18.75 | 0.31 | *** | *** | ns |
| Isoleucine | 13.59b | 18.10a | 17.13a | 17.61a | 15.05 | 18.17 | 0.36 | *** | ns | ns |
| Leucine | 22.35b | 28.22a | 27.36a | 27.74a | 23.79 | 29.05 | 0.51 | *** | *** | ns |
| Tyrosine | 47.80b | 57.16a | 54.29a | 57.28a | 51.52 | 56.74 | 0.75 | *** | *** | ns |
| Aspartate | 12.19b | 19.71a | 19.96a | 19.98a | 20.01 | 15.91 | 0.48 | *** | *** | ns |
| Beta-alanine | 26.29a | 22.66a,b | 17.83b | 17.15b | 24.11 | 17.86 | 0.90 | *** | *** | ns |
| Glutamate | 73.55c | 98.68b | 111.33a | 100.13b | 96.92 | 94.92 | 2.00 | *** | ns | ns |
| Phenylalanine | 18.73b | 23.21a | 22.20a | 23.70a | 19.99 | 23.93 | 0.39 | *** | *** | ns |
| Tryptophan | 5.67b | 8.61a | 8.67a | 8.47a | 6.81 | 8.89 | 0.25 | *** | *** | *** |
| Histidine-containing dipeptides (mg/100 g of meat) | ||||||||||
| Anserine | 477.27a | 326.12b | 299.92b | 341.47b | 394.79 | 327.59 | 11.16 | *** | *** | ns |
| Carnosine | 153.20a | 111.22b | 82.45b | 82.37b | 120.51 | 91.11 | 5.08 | *** | *** | ns |
| Metabolites—energetic metabolism (mg/100 g of meat) | ||||||||||
| IMP | 106.55a | 50.34b | 26.39c | 33.52b,c | 63.86 | 44.55 | 3.94 | *** | *** | ns |
| Creatine | 415.86a | 334.69b | 315.83b | 332.10b | 391.09 | 308.15 | 7.57 | *** | *** | ns |
| Lactate | 731.65a | 535.59b | 515.83b | 545.22b | 643.28 | 520.86 | 14.43 | *** | *** | ns |
| Formate | 0.22 | 0.23 | 0.26 | 0.22 | 0.30 | 0.16 | 0.01 | ns | *** | ns |
| Fumarate | 1.09 | 1.04 | 1.08 | 0.97 | 1.03 | 1.05 | 0.02 | ns | ns | ns |
| Guanidoacetate | 280.01a | 207.42b | 200.29b | 220.93b | 252.80 | 201.53 | 5.70 | *** | *** | ns |
| Hypoxanthine | 36.41b | 55.70a | 65.68a | 54.63a | 63.31 | 42.89 | 1.84 | *** | *** | ns |
| Metabolites–other compounds (mg/100 g of meat) | ||||||||||
| Niacinamide | 8.07a | 7.06a,b | 6.58b | 6.89b | 8.17 | 6.13 | 0.16 | *** | *** | ns |
| Uracil | 0.86b | 2.37a | 2.95a | 2.22a | 2.68 | 1.51 | 0.13 | *** | *** | ns |
| Arabinose | 10.12a | 4.73b | 6.04b | 5.96b | 7.44 | 5.99 | 0.39 | *** | * | ns |
| Inosine | 62.12a | 45.16b | 41.00b | 50.76b | 51.75 | 47.77 | 1.56 | *** | ns | ns |
| Myo-inositol | 19.95b | 21.23a,b | 24.78a | 22.42a,b | 23.79 | 20.39 | 0.60 | ** | *** | ns |
| Betaine | 28.77a | 21.53b,c | 19.41c | 23.22b | 22.90 | 23.57 | 0.61 | *** | ns | ns |
| N,N-dimethylglycine | 8.03a | 6.94a,b | 5.25c | 6.53b | 7.60 | 5.78 | 0.20 | *** | *** | ns |
| Acetate | 2.78b | 3.82a | 3.94a | 3.73a | 3.94 | 3.20 | 0.09 | *** | *** | ns |
= P < 0.05; ** = P < 0.01; *** = P < 0.001; ns = not significant.
Mean values followed by different letters significantly differ among treatment (P < 0.05).
Figure 5.
Representative 600 MHz 1 H-NMR spectrum displaying the assignment of the compounds. The spectra were acquired using the cpmgpr1d pulse sequence.
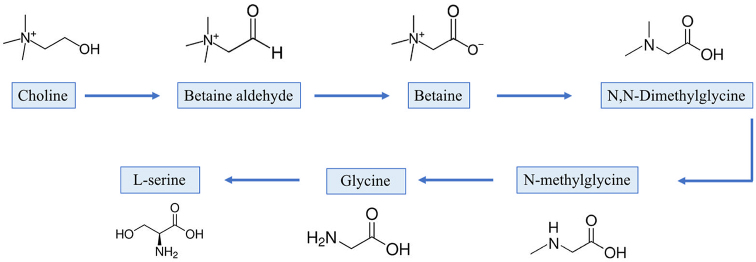
When considering the effect of the muscular abnormality, a remarkable (P < 0.001) increase in the concentration of almost all the free amino acids was found within the abnormal muscles, with the only exception being the amount of beta-alanine which was lower in WB and SM cases. This finding might be attributed to the degenerative processes taking place within these muscles (Soglia et al., 2019) that, leading to the breakdown of muscular proteins, might result in the release of peptides and amino acids (Kodani et al., 2017). Thus, in agreement with Boerboom et al. (2018), the significantly higher (P < 0.001) content of free amino acids resulting from protein catabolism observed in abnormal muscles corroborates the hypothesis of an insufficient vascularization of the muscle tissue and consequent impairment of waste product displacement (Papah et al., 2017; Sihvo et al., 2018; Chen et al., 2019). In addition, since occasional regeneration of the muscle fibers was previously observed (Soglia et al., 2016a; Papah et al., 2017; Baldi et al., 2018; Chen et al., 2019), such increase in free amino acids content might represent an attempt of the muscle tissue to overcome necrosis and repair the damaged fibers. Besides, the remarkably higher glycine concentration observed in WS, WB, and SM affected cases might be the consequence of the increased collagen synthesis (ultimately leading to fibrosis) taking place within these muscles, as glycine is included in each third position of the primary structure of the collagen molecule (Purslow, 2005). Higher contents of branched amino acids valine, leucine, isoleucine, and proline observed in WS, WB, and SM affected muscles were previously found in WB and identified as indicators of extracellular matrix remodeling (Abasht et al., 2016). In addition, if compared with their NORM counterpart, WS, WB, and SM affected muscles exhibited significantly higher contents of glutamine and glutamate (19.83 vs. 29.89, 36.72, and 30.91 mg/100 g of meat;P < 0.001; 73.55 vs. 98.68, 111.33, and 100.13 mg/100 g of meat; P < 0.001, respectively). Intriguingly, similar results were found in mdx mice and dystrophic muscles (Griffin et al., 2001; Martins-Bach et al., 2012) and hypothesized to be related to the intense regenerative processes associated to these conditions (Martins-Bach et al., 2012). Indeed, providing amino groups, glutamine and glutamate have an important role in the synthesis of several molecules, including peptides (Nelson and Lehninger, 2005). The significant (P < 0.001) interaction term observed for tryptophan can be attributed to the increased concentration of this amino acid after freezing and subsequent thawing of WS, WB, and SM affected muscles, whereas no significant variations were observed in NORM (data not shown). This result might be related to the degenerative processes taking place within these muscles that, being aggravated and exacerbated by the formation of ice crystals during freezing, likely result in the release of free tryptophan. With regard to the histidine-containing dipeptides, significantly lower (P < 0.001) levels of anserine and carnosine were found by comparing abnormal muscles with their NORM counterpart. Overall, these findings are in agreement with previous studies performed on WB chickens and mdx mice (Martins-Bach et al., 2012; Sundekilde et al., 2017) and might be explained by considering the anti-oxidative capacity of these dipeptides that were found to effectively inhibit oxidation and act as strong scavengers against radicals (Liu et al., 2003; Fu et al., 2009). In this context, the remarkably higher TBARS as well as the degenerative processes taking place in WS, WB, and SM cases (Soglia et al., 2019), might have required an increased antioxidant protection that resulted in the depletion of these compounds, and concurrently suggests an altered redox homeostasis (Abasht et al., 2016). In addition, carnosine exerts several physiological properties (i.e., pH buffering and increasing Ca2+ sensitivity) relevant to muscular function and homeostasis (Boldyrev et al., 2013). In detail, being directly involved in the synthesis of carnosine, the remarkable (P < 0.001) decrease in beta-alanine found in abnormal muscles might contribute to further explain their lower carnosine content. The occurrence of muscular abnormalities profoundly altered the concentration of the main metabolites involved in energy-generating pathways, with the only exceptions being the amount of fumarate and formate which did not differ among the experimental groups (P > 0.05). In detail, if compared with NORM, significantly lower creatine contents were found in WS, WB, and SM affected muscles (415.86 vs. 334.69, 315.83, and 332.10 mg/100 g of meat; P < 0.001). Thus, considering its major role in the energetic metabolism, the lower creatine content observed in abnormal muscles further corroborated the hypotheses of metabolism perturbations associated to the development of these conditions (Abasht et al., 2016; Zambonelli et al., 2016; Brothers et al., 2019). In agreement with this, lower (P < 0.001) concentrations of inosine-5-monophosphate were found in WS, WB, and SM affected cases that concurrently exhibited remarkably (P < 0.001) increased levels of hypoxanthine and uracil. In agreement with Abasht et al. (2016), an increased concentration of these last 2 metabolites might be the result of a more pronounced nucleotide degradation taking place within the abnormal cases and further contribute to exacerbate the impaired redox homeostasis observed in these muscles. Overall, if compared with their NORM counterpart, WS, WB, and SM affected muscles exhibited a remarkably lower lactate concentration (731.65 vs. 535.59, 515.83, and 545.22 mg/100 g of meat, respectively; P < 0.001), in agreement with their higher ultimate pH. With regard to the other compounds identified, the remarkably lower content of N,N-dimethylglycine observed in WB affected cases was further investigated by MetaboAnalyst in order to evaluate its eventual implication in the occurrence of WS, WB, and SM abnormalities. As shown in Figure 6, N,N-dimethylglycine is formed through the removal of a methyl group from betaine. By the removal of 2 methyl groups, N,N-dimethylglycine may lead to the formation of glycine (having N-methylglycine as an intermediate product) that can be subsequently converted resulting in the synthesis of serine and methionine. As lower concentrations of betaine and N,N-dimethylglycine were observed in WB affected muscles that concomitantly exhibited significantly higher concentrations of methionine, glycine, and serine, a shift in this metabolic pathway leading to the synthesis of amino acids might be hypothesized. Indeed, a higher requirement of amino acids might be a direct consequence of the degenerative and regenerative processes observed in these muscles (Soglia et al., 2016a; Papah et al., 2017; Chen et al., 2019).
Figure 6.
Schematic representation of the metabolic pathway that through the removal of 2 methyl groups from betaine results in the formation of N,N-dimethylglycine and glycine (having N-methylglycine as an intermediate product). Then, glycine can be subsequently converted resulting in the synthesis of serine and methionine.
When considering the effect of freezing treatment, the concentration of almost all the free amino acids was significantly (P < 0.001) affected. Only the amount of asparagine, valine, isoleucine, and glutamate did not differ (P > 0.05) between fresh and frozen/thawed meat. In detail, the concentration of threonine, glutamine, methionine, tyrosine, phenylalanine, and tryptophan was found to be remarkably (P < 0.001) increased in frozen-stored meat whereas serine, proline, glycine, valine, beta-alanine, and aspartate decreased (P < 0.001). This trend might be explained by considering that at −20°C the activity of the proteolytic enzymes, although slow down, proceeds within the muscle tissue leading to the breakdown of muscular proteins and resulting in the release of peptides and amino acids (Kodani et al., 2017). On the other hand, serine, proline, glycine, valine, beta-alanine, and aspartate might be released after thawing as an indirect consequence of the ultrastructural changes resulting from the formation of ice crystals during freezing (Leygonie et al., 2012). As to the histidine-containing dipeptides, if compared with the findings obtained on fresh meat, the concentrations of anserine and carnosine in frozen/thawed meat were remarkably reduced (P < 0.001). These results might be associated to the significantly higher protein oxidation level (P < 0.001) measured on frozen/thawed meat and might be the consequence of a further depletion of anserine and carnosine as a result of their anti-oxidative capacity against the free radicals generated during frozen storage. In addition, this hypothesis is further corroborated by the significantly higher level of taurine observed in fresh WS, WB, and SM affected muscles (data not shown). Indeed, having osmoregulatory properties, taurine exerts a protective role against tissue damages induced by hypoxia (Mankovskaya et al., 2000; Martins-Bach et al., 2012) and an accumulation in response to increased levels of reactive oxygen species has been previously demonstrated (Schuller-Levis and Park, 2004). Recently, an elevated taurine concentration was also found in WS muscles (Boerboom et al., 2018). However, in 1 H-NMR spectra, the signal ascribed to taurine was not detected in frozen/thawed P. major muscles thus supporting the anti-oxidative activity of this compound in contrasting radicals generated during frozen storage. Significant (P < 0.001) reduction in the concentrations of IMP, creatine and hypoxanthine were observed since part of these metabolites might be released as a consequence of the fluid lost following thawing process, whereas no significant differences between fresh and frozen/thawed meat were found in fumarate content. In addition, according to Kodani et al. (2017), creatine can be considered as a biomarker of meat storage: the longer the storage time, the lower the creatine concentration observed is. Furthermore, a significantly lower concentration of lactate was observed after freezing and thawing. This remarkable decrease in lactate content following freezing and thawing should have resulted in an increased pH that, as previously mentioned, was not observed. Thus, this result might be explained by considering the eventual release of H+ as a consequence of protein denaturation (Leygonie et al., 2012) that thus prevented pH variations.
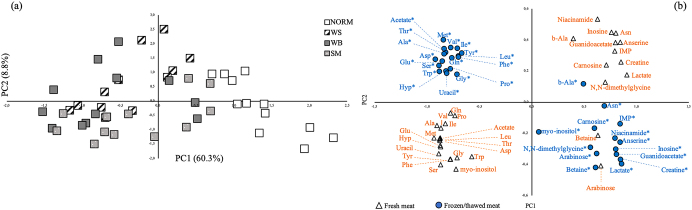
In order to provide a more comprehensive representation of the results obtained through 1 H-NMR, PCA was carried out by considering only those variables that having the highest discrimination power resulted significantly different (P < 0.001) among the groups. The first 2 components explained about 70% of the total variance, being PC1 and PC2 60.3 and 8.8%, respectively. The resulting grouping of the P. major belonging from NORM, WS, WB, and SM groups is shown in Figure 7 a. NORM muscles are grouped on the right part of the graph in which metabolites involved in energy-generating pathways and histidine-containing dipeptides (namely anserine and carnosine) are found (Figure 7 b). This demonstrates that the development of muscular abnormalities negatively correlates with the content of these compounds and, concurrently, is positively linked to the amount of free amino acids within the muscle tissue. In addition, although clustered in different regions of the graph, muscles affected by WS, WB, and SM cannot be clearly distinguished one from the others thus suggesting that these conditions are not associated with distinctive metabolites but likely involves common underlying mechanisms.
Figure 7.
Principal component analysis of the metabolites (free amino acids, histidine-containing dipeptides, and metabolites involved in energy-generating pathways) assessed through 1 H-NMR in fresh and frozen/thawed broilers' P. major muscles affected by WS, WB, and SM abnormalities. Plot of the first 2 principal components score vectors (a). Plot of the variables (free amino acids, histidine-containing dipeptides, and main metabolites) having the highest discrimination power and resulting significantly different (P < 0.001) among the groups according to the 2 principal components loading vectors (b).
Overall, the occurrence of WS, WB, and SM abnormality remarkably affected quality traits (pH, color, and water holding capacity) and oxidative stability of the meat, confirming the findings obtained in previous studies performed on WS, WB, and SM affected meat. In detail, if compared with WS and SM, the WB condition exerted the most detrimental effects. Freezing and subsequent thawing of the meat only marginally affected the quality traits, whereas protein oxidative stability was significantly impaired. In detail, significantly higher carbonyls were observed after freezing and subsequent thawing of WS, WB, and SM affected muscles, whereas no significant variations were found in NORM. However, since overall freezing and subsequent thawing of the abnormal meat did not result in a further worsening of the aforementioned traits, freezing can be an effective processing strategy to store the downgraded meats before further processing. 1 H-NMR spectroscopy highlighted significant differences in free amino acids content by comparing WS, WB, and SM affected muscles with their NORM counterpart. Thus, since free amino acids exert a relevant role in meat taste and flavor, the changes in free amino acids content observed in those muscles exhibiting WS, WB, and SM condition could likely affect the sensory traits of the resulting meat. Most of the free amino acids were found to be remarkably reduced in frozen/thawed meat. Overall, examining metabolites through 1 H-NMR spectroscopy allowed to identify several metabolic pathways providing useful information to advance the knowledge concerning the impact of freezing and subsequent thawing on meat quality traits and concerning the underlying mechanisms responsible for the development of WS, WB, and SM abnormalities in broilers.
ACKNOWLEDGMENTS
The authors acknowledge Marco Berti (Amadori Company, San Vittore di Cesena, Italy) for his technical support. We thank also Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) for the foreign doctorate scholarship.
REFERENCES
- Abasht B., Mutryn M.F., Michalek R.D., Lee V.L. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Laghi L., Tappi S., Rocculi P., Tavaniello S., Prioriello D., Mucci R., Maiorano G., Petracci M. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Res. Int. 2019;115:369–376. doi: 10.1016/j.foodres.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Bao Y., Ertbjerg P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015;110:174–179. doi: 10.1016/j.meatsci.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Bertocchi F., Paci M. Applications of high-resolution solid-state NMR spectroscopy in food science. J. Agric. Food Chem. 2008;56:9317–9327. doi: 10.1021/jf8019776. [DOI] [PubMed] [Google Scholar]
- Bertram H.C., Purslow P.P., Andersen H.J. Relationship between meat structure, water mobility and distribution: a low-field nuclear magnetic resonance study. J. Agric. Food Chem. 2002;50:824–829. doi: 10.1021/jf010738f. [DOI] [PubMed] [Google Scholar]
- Boerboom G., Kempen T.V., Navarro-Villa A., Pérez-Bonilla A. Unraveling the cause of White striping in broilers using metabolomics. Poult. Sci. 2018;97:3977–3986. doi: 10.3382/ps/pey266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Impact of white striping on functionality attributes of broiler breast meat1. Poult. Sci. 2016;95:1957–1965. doi: 10.3382/ps/pew115. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H. Freezing-thawing and sub-sampling influence the marination performance of chicken breast meat. Poult. Sci. 2017;96:3482–3488. doi: 10.3382/ps/pex117. [DOI] [PubMed] [Google Scholar]
- Brothers B.K., Zhuo Z., Papah M., Abasht B. RNA-seq analysis reveals spatial and sex differences in pectoralis major muscle of broiler chickens contributing to difference in susceptibility to wooden breast disease. Front. Physiol. 2019;10:764. doi: 10.3389/fphys.2019.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.T.Y., Omana D.A., Betti M. Effect of ultimate pH on the biochemical properties of proteins in turkey breast meat. Food Chem. 2011;127:109–117. [Google Scholar]
- Chen L.R., Suyemoto M., Sarsour A.H., Cordova H.A., Oviedo-Rondón E.O., Wineland M., Barnes H.J., Borst L.B. Temporal characterization of wooden breast myopathy (“woody breast”) severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain. Avian Pathol. 2019;48:319–328. doi: 10.1080/03079457.2019.1598541. [DOI] [PubMed] [Google Scholar]
- Estévez M. Protein carbonyls in meat systems: a review. Meat Sci. 2011;89:259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Frelka J.C., Phinney D.M., Yang X., Knopp M.V., Heldman D.R., Wick M.P., Vodovotz Y. Assessment of chicken breast meat quality after freeze/thaw abuse using magnetic resonance imaging techniques. J. Sci. Food Agric. 2018;99:844–853. doi: 10.1002/jsfa.9254. [DOI] [PubMed] [Google Scholar]
- Fu H., Katsumura Y., Lin M., Muroya Y., Hata K., Fujii K., Yokoya A., Hatano Y. Free radical scavenging and radioprotective effects of carnosine and anserine. Radiat. Phys. Chem. 2009;78:1192–1197. [Google Scholar]
- Gray J.I., Monahan F.J. Measurement of lipid oxidation in meat and meat products. Trends Food Sci. Technol. 1992;3:315–319. [Google Scholar]
- Griffin J.L., Williams H.J., Sang E., Clarke K., Rae C., Nicholson J.K. Metabolic profiling of genetic disorders: a multitissue 1 H nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue. Anal. Biochem. 2001;293:16–21. doi: 10.1006/abio.2001.5096. [DOI] [PubMed] [Google Scholar]
- Hidalgo F.J., Alaiz M., Zamora R. A spectrophotometric method for the determination of proteins damaged by oxidized lipids. Anal. Biochem. 1998;262:129–136. doi: 10.1006/abio.1998.2758. [DOI] [PubMed] [Google Scholar]
- Jeacocke R.E. Continuous measurements of the pH of beef muscle in intact beef carcasses. Int. J. Food Sci. Technol. 1977;12:375–386. [Google Scholar]
- Jeremiah L.E. In: Encyclopedia of Meat Science. 1st edition. Devine C., Dikeman M., editors. Academic Press; 2004. Freezing and food quality; pp. 1156–1161. [Google Scholar]
- Kodani Y., Miyakawa T., Komatsu T., Tanokura M. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-01272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Le Roy C.I., Mappley L.J., La Ragione R.M., Woodward M.J., Claus S.P. NMR-based metabolic characterization of chicken tissues and biofluids: a model for avian research. Metabolomics. 2016;12:1–14. doi: 10.1007/s11306-016-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leygonie C., Britz T.J., Hoffman L.C. Impact of freezing and thawing on the quality of meat: review. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Xu G.Z., Sayre L.M. Carnosine inhibits (E)-4-hydroxy-2- nonenal-induced protein cross-linking: Structural characterization of carnosine-HNE adducts. Chem. Res. Toxicol. 2003;16:1589–1597. doi: 10.1021/tx034160a. [DOI] [PubMed] [Google Scholar]
- Mankovskaya I.N., Serebrovskaya T.V., Swanson R.J., Vavilova G.L., Kharlamova O.N. Mechanisms of taurine antihypoxic and antioxidant action. High Alt. Med. Biol. 2000;1:105–110. doi: 10.1089/15270290050074242. [DOI] [PubMed] [Google Scholar]
- Martino M.N., Otero L., Sanz P.D., Zaritzky N.E. Size and location of ice crystals in pork frozen by high-pressure-assisted freezing as compared to classical methods. Meat Sci. 1998;50:303–313. doi: 10.1016/s0309-1740(98)00038-2. [DOI] [PubMed] [Google Scholar]
- Martins-Bach A.B., Bloisea A.C., Vainzofb M., Rabbania S.R. Metabolic profile of dystrophic mdx mouse muscles analyzed with in vitro magnetic resonance spectroscopy (MRS) J. Magn. Reson. Imaging. 2012;30:1167–1176. doi: 10.1016/j.mri.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Mati M., Staruch L., Soral M. Use of NMR spectroscopy in the analysis of carnosine and free amino acids in fermented sausages during ripening. Chem. Pap. 2015;69:1319–1324. [Google Scholar]
- Mercier Y., Gatellier P., Viau M., Remington H., Renerre M. Effect of fat and vitamin E on colour stability and lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998;48:301–318. doi: 10.1016/s0309-1740(97)00113-7. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Babini E., Cavani C., Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult. Sci. 2014;93:2108–2116. doi: 10.3382/ps.2014-03911. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Muela E., Sanudo C., Campo M.M., Medel I., Beltran J.A. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2010;84:662–669. doi: 10.1016/j.meatsci.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Nelson D.L., Lehninger M.M.C. Principles of Biochemistry. Freeman W. H.; New York, NY: 2005. Amino acid oxidation and the production of urea; pp. 656–686. [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Petracci M., Laghi L., Rimini S., Rocculi P., Capozzi F., Cavani C. Chicken breast meat marinated with increasing levels of sodium bicarbonate. J. Poult. Sci. 2014;51:206–212. [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., E I., Estevez M. Wooden-Breast, White Striping, and Spaghetti Meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Purslow P.P. Intramuscular connective tissue and its role in meat quality. Meat Sci. 2005;70:435–447. doi: 10.1016/j.meatsci.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Schuller-Levis G.B., Park E. Taurine and its chloramine: modulators of immunity. Neurochem. Res. 2004;29:117–126. doi: 10.1023/b:nere.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- Seong P.N., Seo H.W., Kim J.H., Kang G.H., Cho S.H., Chae H.S., Park B.Y., Van Bal H. Assessment of frozen storage duration effect on quality characteristics of various horse muscles. Asian Australas. J. Anim. Sci. 2017;30:1756–1763. doi: 10.5713/ajas.17.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Airas N., Lindén J., Puolanne E. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Pathol. 2018;161:1–10. doi: 10.1016/j.jcpa.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Petracci M. Histology, composition and quality traits of chicken pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Soglia F., Laghi L., Canonico L., Cavani C., Petracci M. Functionality property issues in broiler breast meat related to emerging muscle abnormalities. Food Res. Int. 2016;89:1071–1076. [Google Scholar]
- Soglia F., Petracci M., Ertbjerg P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016;197:670–675. doi: 10.1016/j.foodchem.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mazzoni M., Petracci M. Current growth-related breast meat abnormalities in broilers. Avian Pathol. 2019;48:1–3. doi: 10.1080/03079457.2018.1508821. [DOI] [PubMed] [Google Scholar]
- Soyer A., Özalp B., Dalmıs Ü., Bilgin V. Effect of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120:1025–1030. [Google Scholar]
- Sundekilde U.K., Rasmussen M.K., Young J.F., Bertram H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2017;217:151–154. doi: 10.1016/j.foodchem.2016.08.104. [DOI] [PubMed] [Google Scholar]
- Tasoniero G., Bertram H.C., Young J.F., Zotte A.D., Puolanne E. Relationship between hardness and myowater properties in Wooden Breast affected chicken meat: A nuclear magnetic resonance study. LWT Food Sci. Technol. 2017;86:20–24. [Google Scholar]
- Tasoniero G., Cullere M., Cecchinato M., Puolanne E., Dalle Zotte A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by White Striping and Wooden Breast myopathies. Poult. Sci. 2016;95:2707–2714. doi: 10.3382/ps/pew215. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Utrera M., Parra V., Estévez M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014;96:812–820. doi: 10.1016/j.meatsci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Van Den Berg L. pH changes in buffers and foods during freezing and subsequent storage. Cryobiology. 1966;3:236–242. [Google Scholar]
- Velleman S., Clark D.L., Tonnings J.R. The effect of wooden breast myopathy on sarcomere structure and organization. Avian Dis. 2018;62:28–35. doi: 10.1637/11766-110217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wei R., Wang P., Han M., Chen T., Xu X., Zhou G. Effect of freezing on electrical properties and quality of thawed chicken breast meat. Asian Australas. J. Anim. Sci. 2016;30:569–575. doi: 10.5713/ajas.16.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierbicki E., Kunlie L.E., Deatherage F.E. Changes in the water holding capacity and cationic shifts during the heating and refreezing and thawing of meat as revealed by a simple centrifugal method for measuring shrinkage. Food Technol. 1957;11:60–73. [Google Scholar]
- Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009;83:239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Xing T., Zhao X., Han M., Cai L., Deng S., Zhou G., Xu X. A comparative study of functional properties of normal and wooden breast broiler chicken meat with NaCl addition. Poult. Sci. 2017;96:3473–3481. doi: 10.3382/ps/pex116. [DOI] [PubMed] [Google Scholar]
- Xiong Y.L. In: Antioxidants in Muscle Foods. Decker E.A., Faustman C., Lopez-Bote C.J., editors. Wiley; New York: 2000. Protein oxidation and implications for muscle foods quality; pp. 85–111. [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping – wooden breast myopathies. Poult. Sci. 2016;95:2771–2785. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]