Abstract
Stocking density is an important environment factor that affects the development of poultry farming, which has caused widespread concern. This study was carried out to determine the effects of stocking density on growth performance, growth regulatory factors, and endocrine hormones in broilers under appropriate environments. A total of 144 Arbor Acres male broilers (BW 1000 ± 70 g) were randomly divided into low stocking density (LSD; 6.25 birds/m2), medium stocking density (MSD; 12.50 birds/m2), and high stocking density (HSD; 18.75 birds/m2) groups, with 6 replicates in each group, and raised in 3 environmental chambers (same size) from 29-day-old to 42-day-old, respectively. The trial period lasted for 14 D with 21 ± 1°C and 60 ± 7% relative humidity, wind speed < 0.5 m/s, ammonia level<5 ppm. The results indicated that average daily food intake and average daily gain in HSD group showed significantly lower than other 2 groups (P < 0.05). Besides, the HSD group significantly reduced breast muscle yield, tibial length, tibial width, and tibial weight of broilers (P < 0.05). The HSD group increased the mRNA expression level of myostatin, and reduced the mRNA expression levels of insulin-like growth factor 1 (IGF-1) and myogenic determination factor 1 (P < 0.05). The HSD group significantly reduced the expression of parathyroid hormone-related protein in tibial growth plate (P < 0.05). The HSD group increased the serum corticosterone levels of broilers (P < 0.05), and decreased the serum IGF-1 and thyroxine (T4) levels of broiler chickens (P < 0.05) than other stocking density groups. Moreover, the serum alkaline phosphatase levels were decreased (P < 0.05) with increasing stocking density, whereas there were no significant effects on the serum 3,5,3′-triiodothyronine (T3) concentrations in 3 groups (P > 0.05). In conclusion, under appropriate environments HSD reduced the growth performance of broilers and this negative effect was likely associated with decreased growth of muscle and bone.
key words: broiler chicken, stocking density, growth performance, growth regulatory factor, endocrine hormone
INTRODUCTION
Stocking density plays an important role in poultry farming, with an increase in the number of birds per unit space potentially leading to higher returns (Estevez, 2007). However, high stocking density (HSD) can also result in increased interference and stress among broilers, with slower growth, decreased product quality, and various health problems (Sørensen et al., 2000; Thaxton et al., 2006; Tong et al., 2012; Abudabos et al., 2013). Simitzis et al. (2012) reported that increasing stocking density (from 6 to 13 birds/m2) negatively affected carcass characteristics, and the carcass weight and breast relative yield were decreased by increasing the stocking density (25, 30, 35, and 40 kg BW/m2) (Dozier et al., 2006). Stocking density not only affects breast muscle growth, but also bone growth. Some studies showed that HSD adversely affected tibia quality (relative weight, mineral composition, and biomechanical properties) (Sun et al., 2013, 2018), and leg health was shown to be severely affected by increasing stocking density (6, 15, 23, 33, 35, 41, 47, and 56 kg BW/m2) (Buijs et al., 2009). However, despite considerable focus on the impacts of stocking density on growth performance and other parameters, its effects on muscle and bone growth remain unclear. Furthermore, stocking densities in previous studies were examined under different environmental conditions with different temperatures, and little consideration of relative humidity, wind speed, and harmful gas concentrations.
Many factors affect bone and muscle growth. Previous studies identified insulin-like growth factor-1 (IGF-1) as a key regulator of muscle and bone development and metabolism in chickens (Baker et al., 1993; Ohlsson et al., 1994; Mcmurtry 1998; Van der Pol et al., 2019). The muscle-regulatory factor myogenic determination factor 1 (MyoD) is also important for muscle growth by affecting the synthesis of muscle contractile proteins (Shintaku et al., 2016), while myostatin (MSTN) is a negative regulator of muscle growth, inhibiting muscle hyperplasia and hypertrophy (Stinckens et al., 2010). Parathyroid hormone-related protein (PTHrP) is a growth factor regulating endochondral bone development, which participates in chondrocyte proliferation and further differentiation (Kronenberg, 2010). Blood corticosterone (CORT) also participates in regulating the growth of broilers, but the results of previous studies differ (Houshmand et al., 2012; Li et al., 2019).
Environmental temperature, humidity, and ammonia concentration can all affect broiler growth (Yi et al., 2016; Ma et al., 2019; Zhou et al., 2019). However, there is currently no information on the effects of stocking density on the factors regulating muscle and bone growth in broilers under appropriate environmental conditions in terms of temperature, humidity, wind speed, and gas concentrations. The aim of the present study was to investigate the effects of stocking density on growth performance, growth regulatory factors, and endocrine hormones in broilers under appropriate environments.
MATERIALS AND METHODS
The experimental protocol was approved by the Animal Experimental Welfare and Ethical Inspection Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences.
Birds and Treatments
One-day-old Arbor Acres (AA) male chickens were reared in 1-tier cages with a standard corn–soybean-meal diet in accordance with NRC (1994) requirements for AA chickens. The room temperature was adjusted according to the management guide for AA broilers. A total of 144 healthy male birds with similar BWs (1,000 ± 70 g) were selected at 29 days old, divided randomly into 3 groups, and transferred to separate environmental chambers. Each environmental chamber had an intelligent control system able to detect and adjust parameters in real time to ensure that the environmental conditions were maintained at the appropriate levels, to accuracies of ±1°C, relative humidity ±7%, wind velocity <0.5 m/s, and ammonia level <5 ppm. The 3 chambers were maintained at 21 ± 1°C, 60 ± 7% relative humidity, wind velocity <0.5 m/s, ammonia level <5 ppm, and 24-h light in the trial period. All 3 chambers were the same size and each included 6 one-tier cages (0.80 m length × 0.80 m width × 0.40 m height, cage floor area approximately 0.64 m2), furnished with one-tube feeders (0.82 m length × 0.07 m width × 0.06 m depth) and 3 nipple drinkers hanging outside each cage. Each cage was a replicate, and each group included 6 replications. Birds in the 3 groups were housed in the cages at low (LSD, 6.25 birds/m2, 4 birds/cage), medium (MSD, 12.50 birds/m2, 8 birds/cage), and high stocking densities (HSD, 18.75 birds/m2, 12 birds/cage), respectively. These were equivalent to 15.63, 31.25, and 46.88 kg BW/m2 predicted at 42 D at an estimated final BW of 2.5 kg. The trial period lasted for 14 D. The broilers had ad libitum access to feed and water.
Sampling Collection and Chemical Analysis
All the broilers were observed daily, and mortality was recorded. When the birds reached 42 days old, 2 birds close to the average BW for the cage were selected at random from each cage. Blood samples were collected (5 mL; brachial vein, syringe) and the birds were then euthanized by CO2 asphyxiation.
Growth Performance Measurement
All the birds were weighed at the beginning (29 D) and end (42 D) of the experiment to calculate the average daily gain (ADG). Feed intake was recorded daily to calculate the average daily feed intake (ADFI) and feed conversion ratio (FCR).
The eviscerated carcass, both breast muscles, and the left tibia of each sampled bird were weighed individually. The breast muscle yield was expressed as the weight of both breast muscles as a percentage of the eviscerated carcass weight. The length and width of the left tibia were measured using digital Vernier calipers (AB021015202, Shanghai Tools Co., Ltd., Shanghai, China).
Serum Endocrine Hormones
Serum samples were obtained by centrifugation at 3,000 × g for 20 min at 4°C and kept at −20°C for further analysis. Serum levels of 3,5,3′-triiodothyronine (T3), thyroxine (T4), and CORT were measured by radioimmunoassay (RIA) using a gamma RIA counter (GC-2010, Anhui Ustczonkia Scientific Instruments Co., Ltd., Anhui, China). All the procedures were carried out according to the manufacturer's protocol.
Gene Expression of Regulatory Factors
Breast muscle samples (approximately 1 cm3) were collected from the same location, frozen in liquid nitrogen, and stored at −80°C for analysis. mRNA levels of IGF-1, MyoD, and MSTN in the breast muscles were examined. Total RNA was extracted from each breast muscle sample using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RT-PCR was performed using a LightCycler 96 system (LightCycler 96 system, Roche, Basel, Switzerland) based on the general RT-PCR method. The primers for the target genes were designed and confirmed (Table 1). β-Actin was used as the reference gene. The gene expression data were expressed as relative quantification and calculated using the 2-ΔΔCt method.
Table 1.
Primers used for quantitative RT-PCR.
| Primer name1 | Primer sequence2 5'-3' | Product size (bp) | GenBank accession number |
|---|---|---|---|
| β-actin | F: TGCTGTGTTCCCATCTATCG | 150 | NM_205518 |
| R: TTGGTGACAATACCGTGTTCA | |||
| IGF-1 | F: ACCTTGGCCTGTGTTTGCTTAC | 111 | NM_001004384 |
| R: AGCCTCTGTCTCCACATACGAAC | |||
| MSTN | F: TACCCGCTGACAGTGGATTTC | 153 | NM_001001461 |
| R: GCCTCTGGGATTTGCTTGG | |||
| MyoD | F:GGAGAGGATTTCCACAGACAACTC | 113 | NM_204214 |
| R: CTCCACTGTCACTCAGGTTTCCT |
β-actin, beta-actin; IGF-1, insulin-like growth factor-1; MSTN, myostatin; MyoD myogenic determination factor 1.
F, forward; R, reverse.
PTHrP Expression and Serum Parameters levels
Tibial growth plate samples were collected, frozen in liquid nitrogen, and stored at −80°C until analysis. PTHrP expression in the tibial growth plate was measured by western blot. Briefly, proteins were separated, transferred to nitrocellulose membranes in Tris-glycine buffer at 90 V for 90 min, and then blocked in Tween Tris buffer solution containing 5% nonfat dry milk for 2 h at room temperature. The membranes were incubated in primary antibody solution containing rabbit anti-PTHrP (LS-C295770; diluted: 1:200; Lifespan Bioscience Inc., Seattle, WA) at 4°C for 18 h. After washing 3 times for 10 min each with PBS-T solution, the membranes were incubated with secondary antibody (goat anti-rabbit IgG horseradish peroxidase, diluted 1:10,000) for 1 h at room temperature, and detected using enhanced chemiluminescence western blotting reagents (Thermo Fisher Scientific, Inc., Waltham, MA). The expression of the measured protein was presented as the ratio of the level in the sample relative to the level of β-actin. All the antibodies used had been validated previously for use with chicken samples.
Serum IGF-1 levels were measured by RIA using a gamma RIA counter (GC-2010, Anhui Ustczonkia Scientific Instruments Co., Ltd.). Serum alkaline phosphatase (AKP) levels were detected using an automatic biochemical analyzer (Hitachi 7600, Hitachi Ltd, Tokyo, Japan). All procedures were carried out according to the manufacturer's instructions.
Statistical Analysis
The experimental design was a single factor design, a 1-way analysis of variance (ANOVA) model and Duncan's multiple comparison were used to analyze the data by SAS 9.2 (SAS Institute Inc., Cary, NC). The data were reported means ± SD. Statistical significance was set at the level of P ≤ 0.05.
RESULTS
Growth Performance
During the trial period, all the birds show no signs of clinical diseases, and no mortality. As shown in Table 2, ADFI and ADG in HSD group were significantly lower than that in LSD group and MSD group (P < 0.05), whereas FCR showed no significant difference in 3 stocking density groups (P > 0.05).
Table 2.
Effects of stocking density on ADFI, ADG, and FCR of broilers.1
| Items2 | LSD | MSD | HSD | P-value |
|---|---|---|---|---|
| ADFI (g) | 156.02a±0.33 | 154.60a±0.17 | 147.75b±1.82 | <0.01 |
| ADG (g) | 81.45a±1.37 | 83.05a±1.18 | 78.26b±1.52 | 0.01 |
| FCR (g:g) | 1.92±0.03 | 1.86±0.03 | 1.89±0.03 | 0.20 |
LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2).
Means within a column with different superscripts are different at P < 0.05.
All means reported as means ± SD (n = 12).
ADFI = average daily gain; ADG = average feed intake; FCR = ADFI/ADG.
The tibial parameters and breast muscle yield of broilers are reported in Table 3. Compared with LSD group and MSD group, the HSD group significantly reduced breast muscle yield (P ≤ 0.05), tibial length, and tibial weight of broilers (P < 0.05), and there was no significant difference in LSD group and MSD group. Tibial width was significantly reduced for HSD group compared to MSD group (P < 0.05).
Table 3.
Effects of stocking density on tibial parameters and breast muscle yield of broilers.1
| Items | LSD | MSD | HSD | P-value |
|---|---|---|---|---|
| Length (mm) | 108.37a±0.83 | 109.00a±0.95 | 103.40b±0.56 | <0.01 |
| Width (mm) | 13.93a,b±1.11 | 15.61a±1.38 | 12.86b±0.39 | 0.01 |
| Weight (g) | 22.75a±0.79 | 23.83a±3.30 | 19.28b±1.08 | 0.02 |
| Breast muscle yield (%) | 29.91a±2.47 | 30.60a±2.89 | 25.41b±0.60 | 0.05 |
LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2).
Means within a column with different superscripts are different at P ≤ 0.05.
All means reported as means ± SD (n = 12).
Regulatory Factors Gene Expression
The mRNA expression level of MSTN in the HSD group was significantly higher than in LSD group, and it was also higher for MSD than for LSD (P < 0.05; Table 4). The mRNA expression levels of IGF-1 and MyoD in HSD group were significantly lower than in MSD and LSD groups (P < 0.05), and the IGF-1 expression level was higher for LSD than for MSD.
Table 4.
Effects of stocking density on the mRNA expression of breast muscles growth-related regulatory factors of broilers.1
| Items2 | LSD | MSD | HSD | P-value |
|---|---|---|---|---|
| IGF-1 | 1.74a±0.11 | 0.93b±0.07 | 0.36c±0.04 | <0.01 |
| MSTN | 0.24b±0.01 | 0.67a±0.14 | 0.63a±0.12 | 0.01 |
| MyoD | 1.10a±0.13 | 1.14a±0.17 | 0.22b±0.03 | <0.01 |
LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2).
Means within a column with different superscripts are different at P < 0.05.
All means reported as means ± SD (n = 12).
IGF-1, insulin-like growth factor-1; MSTN, myostatin; MyoD, myogenic determination factor 1.
PTHrP Expression and Serum Parameters Levels
As shown in Figure 1, the expression of PTHrP in tibial growth plate in the HSD group was significantly lower than in the LSD and MSD groups (P < 0.05), whereas there was no significant difference in LSD and MSD groups (P > 0.05). Moreover, the serum AKP levels of broilers were decreased (P < 0.05) with increasing stocking density.
Figure 1.
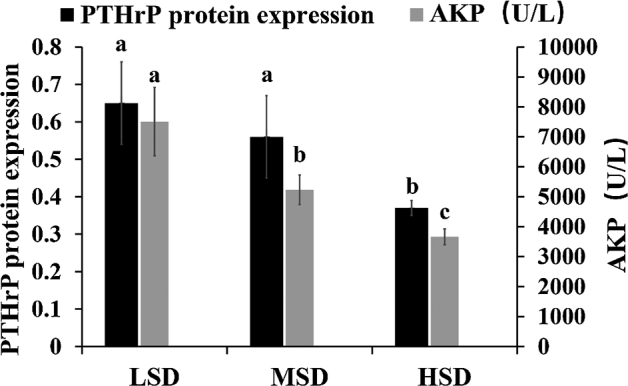
Effects of stocking density on expression of parathyroid hormone related protein (PTHrP) in tibial growth plate and serum alkaline phosphatase (AKP) levels of broilers. LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2). Each bar presents means ± SD (n = 12). Different letters are different at P < 0.05.
As shown in Figure 2, compared with LSD and MSD groups, the serum IGF-1 levels of broiler chickens in the HSD group were significantly lower (P < 0.05), and there was no significant difference in LSD and MSD groups.
Figure 2.
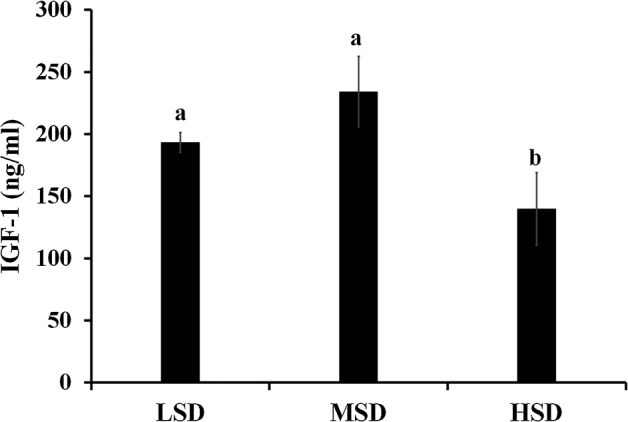
Effects of stocking density on serum insulin-like growth factor 1 (IGF-1) levels of broiler chickens. LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2). Each bar presents means ± SD (n = 12). Different letters are different at P < 0.05.
Serum Endocrine Hormones
The serum CORT levels of broilers in the HSD group were significantly higher (P < 0.05; Table 5), compared with LSD and MSD groups, and the levels of T4 in serum of broiler chickens in the HSD group were significantly lower (P < 0.05), whereas there were no significant effects on the levels of T3 in 3 groups (P > 0.05).
Table 5.
Effects of stocking density on serum endocrine hormones of broilers.1
| Items2 | LSD | MSD | HSD | P-value |
|---|---|---|---|---|
| CORT (nmol/L) | 74.40b±0.27 | 71.32b±1.06 | 83.11a±5.22 | <0.01 |
| T4 (ng/mL) | 69.41a±2.84 | 62.76a±6.90 | 49.39b±3.11 | 0.01 |
| T3 (ng/mL) | 1.38±0.28 | 1.08±0.06 | 1.23±0.24 | 0.20 |
LSD, low stocking density (6.25 birds/m2); MSD, medium stocking density (12.50 birds/m2); HSD, high stocking density (18.75 birds/m2).
Means within a column with different superscripts are different at P < 0.05.
All means reported as means ± SD (n = 12).
CORT, corticosterone; T4, thyroxine; T3, 3,5,3'-triiodothyronine.
DISCUSSION
The results of the present study revealed that HSD significantly decreased the ADFI and ADG but had no significant effect on FCR. These results were consistent with the findings of previous studies showing that HSD reduced ADFI and BW gain in chickens (Bessei, 2006; Beloor et al., 2010). Similarly, Abudabos et al. (2013) found no effect on FCR of increasing the stocking density of broilers from 37.0 to 40.0 kg/m2. Our results indicated that HSD was detrimental to growth performance in broilers. These results were consistent with Shakeri et al. (2014) who suggested that HSD was detrimental to weight gain compared with LSD. However, some studies reported no significant differences in 42-D BW, BW gain, FCR, and mortality among broilers raised at different stocking densities (30, 35, 40 kg/m2) (Rambau et al., 2016), and no detrimental effect of HSD (16 birds/m2) on growth performance or survivability (Najafi et al., 2015). Breast muscle is one of the most important parts of broilers and directly affects broiler production. Breast meat yields were decreased by increasing stocking densities to >13 birds/m2 (Dozier et al., 2006), whereas HSD had no effect on whole carcass and breast meat yields relative to BW in broilers (Dozier et al., 2005), in accord with the results of Feddes et al. (2002). In the present study, breast muscle yield was significantly reduced in the HSD group. Tibial length, width, and weight of broilers were also significantly reduced in HSD group, similar to the results of Kestin et al. (1992) who found frequent leg problems in poultry fed at high densities, and Sanotra et al. (2001) who showed increased tibial dysplasia with increasing stocking density. A recent report showed that layer breeder males kept at HSD had shorter tibias (Li et al., 2019), in agreement with the current results. Differences in feeding environments (including temperature, relative humidity, etc.), age, broiler strain, and feed kinds among studies may have contributed to inconsistent results among previous studies. However, the present study found that poor growth performance in broilers at HSD was related to specific growth regulatory factors under appropriate environments.
The reasons for changes in breast muscle yield in relation to stocking density are unclear. Breast muscle growth is regulated by many factors, including IGF-1, MyoD, and MSTN. IGF-1 provides an important involvement of growth factor systems in the growth process (Baker et al., 1993), and several studies demonstrated that skeletal muscle growth was regulated by IGF-1, which is in turn involved in many signaling pathways (Mcmurtry, 1998; Beccavin et al., 2001). IGF-1 can promote skeletal muscle hypertrophy and prevent muscle atrophy (Latres et al., 2005; Wen et al., 2014b). MyoD is one of the most important factors regulating muscle growth (Rudnicki et al., 1993). MyoD is involved in myoblast differentiation and increases the expression of contractile-apparatus genes to direct the synthesis of contractile proteins in the muscle (Yi et al., 2010; Shintaku et al., 2016). MSTN is an essential factor for muscle growth and development, and it acts as a negative regulator of muscle growth (Stinckens et al., 2010; Wen et al., 2014a). MSTN had a negative effect on muscle development before birth and muscle hypertrophy after birth in chickens (Kocamis et al., 2001). In the present study, mRNA expression levels of IGF-1 and MyoD in breast muscle were significantly lower than other groups, whereas mRNA expression level of MSTN at HSD was significantly higher. These results suggest that HSD might affect breast muscle hypertrophy and differentiation by regulating the expression of IGF-I, MyoD, and MSTN.
IGF-1 is also involved in bone growth, as confirmed by gene knockout studies (Wang et al., 2002). IGF-1 was shown to stimulate endochondral cell proliferation. Endochondral bone formation is also regulated by other autocrine/paracrine growth factors, such as PTHrP (Stevens and Williams, 1999). PTHrP regulates terminal differentiation of growth plate chondrocytes and slows the rate of differentiation of pre-hypertrophic to hypertrophic chondrocytes within the growth plate (Farquharson et al., 2001), thus facilitating the formation of complex traits and structures during bone growth and development. AKP is a specific marker of early osteoblast differentiation and is often used as a marker of osteoblast function (Collette et al., 2010). Ōzbey and Esen (2007) found that serum levels of AKP in rock partridges decreased significantly with increasing stocking density (15, 20, 25 bird/m2). In the current study, serum IGF-1 levels and PTHrP expression in the tibial growth plate were lower in HSD compared with MSD and LSD broilers. Furthermore, serum AKP levels were also lowest in the HSD group, indicating that higher stocking density inhibited tibia growth. These results suggest that HSD may reduce tibial length, width, and weight in broilers by adversely affecting tibia proliferation, differentiation, and formation.
High CORT levels can affect bone growth and significantly reduce feed intake and BW gain in broilers (Luo et al., 2013). Serum CORT levels were significantly elevated in HSD broilers in the current study. These results were similar to those of Türkyilmaz et al. (2008), who showed that CORT levels at 42 D increased with increasing stocking density (15, 20, 25/m2), but different from those of Thaxton et al. (2006), who found no significant changes in serum CORT levels in relation to stocking density. These discrepancies may be due to differences in the housing systems used. Feddes et al. (2002) reported that broilers subjected to HSD experienced difficulties with heat and gas exchange in the microclimate. The HSD might result in increasing stress, and several researches have shown that stress increased the serum concentrations of T3 and T4 (Sahin et al., 2002; Dai et al., 2011). T3 and T4 affect almost every physiological process in the body and are important hormones supporting chicken growth (Xiao et al., 2017). However, information on the effects of stocking density on 2 hormones in birds raised under appropriate environments is limited. The current study found that serum T4 levels were reduced in broilers at HSD compared with lower densities.
To sum up, the current results indicated that there is no significant difference in growth performance between LSD and MSD, and HSD can adversely affect muscle and bone growth in broilers under appropriate environments because it might affect breast muscle hypertrophy and differentiation, tibia proliferation, differentiation, and formation, and serum endocrine hormone levels in broilers. Overall, keeping broilers at HSD thus reduces their growth performance.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2016YFD0500509). This research was also supported by Independent Research Program of State Key Laboratory of Animal Nutrition (2004DA125184G1609) and the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (ASTIP-IAS09).
REFERENCES
- Abudabos A.M., Samara E.M., Hussein E.O., Al-Ghadi A.Q.M., Al-Atiyat R.M. Impacts of stocking density on the performance and welfare of broiler chickens. Ital. J. Anim. Sci. 2013;12:66–71. [Google Scholar]
- Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Beccavin C., Chevalier B., Cogburn L.A., Simon J., Duclos M.J. Insulin-like growth factors and body growth in chickens divergently selected for high or low growth rate. J. Endocrinol. 2001;168:297–306. doi: 10.1677/joe.0.1680297. [DOI] [PubMed] [Google Scholar]
- Beloor J., Kang H.K., Kim Y.J., Subramani V.K., Jang I.S., Sohn S.H., Moon Y.S. The effect of stocking density on stress related genes and telomeric broiler chickens. Asian-Australas. J. Anim. Sci. 2010;23:437–443. [Google Scholar]
- Bessei W. Welfare of broilers: a review. World's Poult. Sci. J. 2006;62:455–465. [Google Scholar]
- Buijs S., Keeling L., Rettenbacher S., Van Poucke E., Tuyttens F.A.M. Stocking density effects on broiler welfare: identifying sensitive ranges for different indicators. Poult. Sci. 2009;88:1536–1543. doi: 10.3382/ps.2009-00007. [DOI] [PubMed] [Google Scholar]
- Collette J., Bruyère O., Kaufman J.M., Lorenc R., Felsenberg D., Spector T.D., Curiel M.D., Boonen S., Reginster J.Y. Vertebral anti-fracture efficacy of strontium ranelate according to pre-treatment bone turnover. Osteoporos. Int. 2010;21:233–241. doi: 10.1007/s00198-009-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Gao F., Zhang W., Song S., Xu X., Zhou G. Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Anim. Feed Sci. Technol. 2011;168:51–60. [Google Scholar]
- Dozier W.A., III, Thaxton J.P., Branton S.L., Morgan G.W., Miles D.M., Roush W.B., Lott B.D., Vizzier-Thaxton Y. Stocking density effects on growth performance and processing yields of heavy broilers. Poult. Sci. 2005;84:1332–1338. doi: 10.1093/ps/84.8.1332. [DOI] [PubMed] [Google Scholar]
- Dozier W.A., III, Thaxton J.P., Purswell J.L., Olanrewaju H.A., Branton S.L., Roush W.B. Stocking density effects on male broilers grown to 1.8 kilograms of body weight. Poult. Sci. 2006;85:344–351. doi: 10.1093/ps/85.2.344. [DOI] [PubMed] [Google Scholar]
- Estevez I. Density allowances for broilers: where to set the limits? Poult. Sci. 2007;86:1265–1272. doi: 10.1093/ps/86.6.1265. [DOI] [PubMed] [Google Scholar]
- Farquharson C., Seawright E., Jefferies D. Parathyroid hormone-related peptide expression in tibial dyschondroplasia. Avian Pathol. 2001;30:327–335. doi: 10.1080/03079450120066331. [DOI] [PubMed] [Google Scholar]
- Feddes J.J., Emmanuel E.J., Zuidhoft M.J. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002;81:774–779. doi: 10.1093/ps/81.6.774. [DOI] [PubMed] [Google Scholar]
- Houshmand M., Azhar K., Zulkifli I., Bejo M.H., Kamyab A. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult. Sci. 2012;91:393–401. doi: 10.3382/ps.2010-01050. [DOI] [PubMed] [Google Scholar]
- Kestin S.C., Knowles T.G., Tinch A.E., Gregory N.G. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992;131:190–194. doi: 10.1136/vr.131.9.190. [DOI] [PubMed] [Google Scholar]
- Kocamis H., Mcfarland D.C., Killefer J. Temporal expression of growth factor genes during myogenesis of satellite cells derived from the biceps femoris and pectoralis major muscles of the chicken. J. Cell. Physiol. 2001;186:146–152. doi: 10.1002/1097-4652(200101)186:1<146::AID-JCP1014>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kronenberg H.M. PTHrP and skeletal development. Ann. N. Y. Acad. Sci. 2010;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- Latres E., Amini A.R., Amini A.A., Griffiths J., Martin F.J., Yi W., Lin H.C., Yancopoulos G.D., Glass D.J. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Luo J.W., Zhou Z.L., Zhang H., Ma R.S., Hou J.F. Bone response of broiler chickens (Gallus gallus domesticus) induced by corticosterone. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2013;164:410–416. doi: 10.1016/j.cbpa.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Li J., Liu W., Ma R., Li Y., Liu Y., Qi R., Zhan K. Effects of cage size on growth performance, blood biochemistry, and antibody response in layer breeder males during rearing stage. Poult. Sci. 2019 doi: 10.3382/ps/pez102. pez102. [DOI] [PubMed] [Google Scholar]
- Ma D., Liu Q., Zhang M., Feng J., Li X., Zhou Y., Wang X. iTRAQ-based quantitative proteomics analysis of the spleen reveals innate immunity and cell death pathways associated with heat stress in broilers (Gallus gallus) J. Proteomics. 2019;196:11–21. doi: 10.1016/j.jprot.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Mcmurtry J.P. Nutritional and developmental roles of insulin-like growth factors in poultry. J. Nutr. 1998;128:302S. doi: 10.1093/jn/128.2.302S. [DOI] [PubMed] [Google Scholar]
- Najafi P., Zulkifli I., Jajuli N.A., Farjam A.S., Ramiah S.K., Amir A.A., O'Reily E., Eckersall D. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int. J. Biometeorol. 2015;59:1577–1583. doi: 10.1007/s00484-015-0964-3. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academy of Sciences; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ohlsson C., Isaksson O., Lindahl A. Clonal analysis of rat tibia growth plate chondrocytes in suspension culture–differential effects of growth hormone and insulin-like growth factor I. Growth. Regul. 1994;4:1–7. [PubMed] [Google Scholar]
- Ōzbey O., Esen F. The effects of breeding systems and stocking density on some blood parameters of rock partridges (Alectoris graeca) Poult. Sci. 2007;86:420–422. doi: 10.1093/ps/86.2.420. [DOI] [PubMed] [Google Scholar]
- Rambau M.D., Mudau M.L., Makhanya S.D., Benyi K. Effects of stocking density and daily feed withdrawal periods on the performance of broiler chickens in a semi-arid environment. Trop. Anim. Health. Prod. 2016;48:1547–1554. doi: 10.1007/s11250-016-1126-2. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A., Schnegelsberg P.N.J., Stead R.H., Braun T., Arnold H.H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Onderci M., Gursu F., Cikim G. Optimal dietary concentration of chromium for alleviating the effect of heat stress on growth, carcass qualities, and some serum metabolites of broiler chickens. Biol. Trace. Elem. Res. 2002;89:53–64. doi: 10.1385/BTER:89:1:53. [DOI] [PubMed] [Google Scholar]
- Sanotra G.S., Lawson L.G., Vestergaard K.S., Thomsen M.G. Influence of stocking density on tonic immobility, lameness, and tibial dyschondroplasia in broilers. J. Appl. Anim. Welf. Sci. 2001;4:71–87. [Google Scholar]
- Shakeri M., Zulkifli I., Soleimani A.F., O'Reilly E.L., Eckersall P.D., Anna A.A., Kumari S., Abdullah F.F. Response to dietary supplementation of L-glutamine and L-glutamate in broiler chickens reared at different stocking densities under hot, humid tropical conditions. Poult Sci. 2014;93:2700–2708. doi: 10.3382/ps.2014-03910. [DOI] [PubMed] [Google Scholar]
- Shintaku J., Peterson J., Talbert E., Gu J.M., Ladner K., Williams D., Mousavi K., Wang R., Sartorelli V., Guttridge D. MyoD regulates skeletal muscle oxidative metabolism cooperatively with alternative NF-κB. Cell. Rep. 2016;17:514–526. doi: 10.1016/j.celrep.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen P., Su G., Kestin S.C. Effects of age and stocking density on leg weakness in broiler chickens. Poult. Sci. 2000;79:864–870. doi: 10.1093/ps/79.6.864. [DOI] [PubMed] [Google Scholar]
- Stevens D.A., Williams G.R. Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol. Cell. Endocrinol. 1999;151:195–204. doi: 10.1016/s0303-7207(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Stinckens A., Luyten T., Bijttebier J., Van d.M.K., Dieltiens D., Janssens S., De S.S., Georges M., Buys N. Characterization of the complete porcine MSTN gene and expression levels in pig breeds differing in muscularity. Anim. Genet. 2010;39:586–596. doi: 10.1111/j.1365-2052.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- Sun Z.W., Fan Q.H., Wang X.X., Guo Y.M., Wang H.J., Dong X. High stocking density alters bone-related calcium and phosphorus metabolism by changing intestinal absorption in broiler chickens. Poult. Sci. 2018;97:219–226. doi: 10.3382/ps/pex294. [DOI] [PubMed] [Google Scholar]
- Sun Z.W., Yan L., Guo Y.Y., Zhao J.P., Lin H., Guo Y.M. Increasing dietary vitamin D3 improves the walking ability and welfare status of broiler chickens reared at high stocking densities. Poult. Sci. 2013;92:3071–3079. doi: 10.3382/ps.2013-03278. [DOI] [PubMed] [Google Scholar]
- Thaxton J.P., Rd D.W., Branton S.L., Morgan G.W., Miles D.W., Roush W.B., Lott B.D., Vizzierthaxton Y. Stocking density and physiological adaptive responses of broilers. Poult. Sci. 2006;85:819–824. doi: 10.1093/ps/85.5.819. [DOI] [PubMed] [Google Scholar]
- Tong H.B., Lu J., Zou J.M., Wang Q., Shi S.R. Effects of stocking density on growth performance, carcass yield, and immune status of a local chicken breed. Poult. Sci. 2012;91:667–673. doi: 10.3382/ps.2011-01597. [DOI] [PubMed] [Google Scholar]
- Türkyilmaz M.K. The effect of stocking density on stress reaction in broiler chickens during summer. Turk. J. Vet. Anim. Sci. 2008;32:31–36. [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A., Maatjens C.M., Gussekloo S.W., Kranenbarg S., Wijnen J., Pieters R.P., Schipper H., Kemp B., van den Brand H. Light-dark rhythms during incubation of broiler chicken embryos and their effects on embryonic and post hatch leg bone development. PloS One. 2019;14 doi: 10.1371/journal.pone.0210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fosmire G.J., Gay C.V., Leach R.M., Jr. Short-term zinc deficiency inhibits chondrocyte proliferation and induces cell apoptosis in the epiphyseal growth plate of young chickens. J. Nutr. 2002;132:665–673. doi: 10.1093/jn/132.4.665. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen Y., Wu P., Wang T., Zhou Y. MSTN, mTOR and FoxO4 are involved in the enhancement of breast muscle growth by metthionine in broilers with lower hatching weight. PLOS One. 2014;9 doi: 10.1371/journal.pone.0114236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Wu P., Chen Y., Wang T., Zhou Y. Methionine improves the performance and breast muscle growth of broilers with lower hatching weight by altering the expression of genes associated with the insulin-like growth factor-I signalling pathway. Br. J. Nutr. 2014;111:201–206. doi: 10.1017/S0007114513002419. [DOI] [PubMed] [Google Scholar]
- Yi C., Yao Z., Sarkar D., Lawrence M., Sanchez G.J., Parker M.H., Macquarrie K.L., Davison J., Morgan M.T., Ruzzo W.L. Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev. Cell. 2010;18:662–674. doi: 10.1016/j.devcel.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Wu C., Li K., Gui G., Zhang G., Yang H. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J. Anim. Sci. Biotechnol. 2017;8:1–7. doi: 10.1186/s40104-017-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B., Chen L., Sa R., Zhong R., Xing H., Zhang H. High concentrations of atmospheric ammonia induce alterations of gene expression in the breast muscle of broilers (Gallus gallus) based on RNA-Seq. BMC Genomics. 2016;17:598. doi: 10.1186/s12864-016-2961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li X.M., Zhang M.H., Feng J.H. Effect of relative humidity at either acute or chronic moderate temperature on growth performance and droppings' corticosterone metabolites of broilers. J. Integr. Agr. 2019;18:152–159. [Google Scholar]


