Abstract
This study investigated the hypothesis that dietary supplementation of leucine (Leu) above actual recommendations activates protein synthesis and inhibits protein degradation pathways on the molecular level and supports higher muscle growth in broilers. Day-old male Cobb-500 broilers (n = 180) were allotted to 3 groups and phase-fed 3 different corn-wheat-soybean meal-based basal diets during periods 1 to 10, 11 to 21, and 22 to 35 D. The control group (L0) received the basal diet which met the broiler's requirements of nutrients and amino acids for maintenance and growth. Groups L1 and L2 received basal diets supplemented with Leu to exceed recommendations by 35 and 60%, respectively, and isoleucine (Ile) and valine (Val) were supplemented to keep Leu: Ile and Leu: Val ratios fixed. Samples of liver and breast muscle and pancreas were collected on days 10, 21, and 35. The gene expression and abundance of total and phosphorylated proteins involved in the mammalian target of rapamycin pathway of protein synthesis, in the ubiquitin-proteasome pathway and autophagy-lysosomal pathway of protein degradation, in the general control nonderepressible 2/eukaryotic translation initiation factor 2A pathway involved in the inhibition of protein synthesis, and in the myostatin–Smad2/3 pathway involved in myogenesis were evaluated in the muscle, as well as expression of genes involved in the growth hormone axis. Growth performance, feed intake, the feed conversion ratio, and carcass weights did not differ between the 3 groups (P > 0.05). Plasma concentrations of Leu, Ile, and Val and of their keto acids, and the activity of the branched-chain α-keto acid dehydrogenase in the pancreas increased dose dependently with increasing dietary Leu concentrations. In the breast muscle, relative mRNA abundances of genes and phosphorylation of selected proteins involved in all investigated pathways were largely uninfluenced by dietary Leu supplementation (P > 0.05). In summary, these data indicate that excess dietary Leu concentrations do not influence protein synthesis or degradation pathways, and subsequently do not increase muscle growth in broilers at fixed ratios to Ile and Val.
Key words: leucine, mTOR, protein degradation, broiler, muscle
INTRODUCTION
The intake of nutrients accelerates protein synthesis by promoting translation initiation (Wilson et al., 2009). Amino acids are substrates for protein synthesis, but also act as signaling molecules and regulators of body protein turnover (Yoshizawa et al., 2013; Mitchell et al., 2016). After oral intake, the branched-chain amino acids (BCAA) bypass the liver, which is reflected in increased plasma concentrations of BCAA, and which allows direct action on metabolic pathways in extrahepatic tissues like the muscle (Wilson et al., 2009; Mattick et al., 2013). Among the BCAA, leucine (Leu) is unique in its capacity to stimulate protein synthesis via the mammalian target of rapamycin (mTOR) pathway as shown in skeletal muscle cells (Atherton et al., 2010). Importantly, Leu only increases protein synthesis when the availability of all other amino acids (AA) is not limiting (Wilson et al., 2010, 2011) and the sensitivity of the muscle is higher than that of the liver (Yoshizawa et al., 2013). In fasted neonatal pigs, infusion of Leu or of the Leu metabolite α-keto isocaproic acid (KICA) influenced the mTOR signaling pathway and stimulated muscle protein synthesis compared to the fasted state (Escobar et al., 2005, 2010). Likewise, parenteral administration of Leu 50% above requirements in continuously fed neonatal pigs stimulated protein synthesis (Boutry et al., 2013).
Another important determinant of muscle growth is the balance between protein synthesis and degradation. Only when protein synthesis rates exceed those of protein degradation muscle mass can be increased (Schiaffino et al., 2013). Protein synthesis and degradation pathways are also interlinked; protein kinase B/Akt positively regulates mTOR and negatively regulates the forkhead box protein O1 (FOXO1) transcription factor, which induces protein degradation (Glass, 2010). It has been observed that protein degradation may be lowered by Leu as well, and this may be mediated by downregulating either the autophagy-lysosome system (Boutry et al., 2013) or the ubiquitin-proteasome system (UPS) (Nakashima et al., 2005).
In addition, it is known that AA deprivation activates the general control nonderepressible 2 (GCN2)/eukaryotic translation initiation factor 2A (eIF2a) pathway which inhibits the mTOR pathway (Métayer et al., 2008) and stimulates autophagy (He and Klionsky, 2009). Potentially, also the myostatin–Smad2/3 pathway, which impairs protein synthesis by a downregulation of myogenic factors (Schiaffino et al., 2013), could be affected, but this has not yet been investigated in growing animals. Likewise, Leu is known to stimulate insulin secretion (Fahien and Macdonald, 2011), especially in combination with glucose (Kalogeropoulou et al., 2008) via both mTOR-dependent and independent pathways (Yang et al., 2010, 2012). It has been shown in malnourished rats that Leu supplementation increased skeletal muscle masses as well as serum insulin-like growth factor 1 (IGF1) and hepatic growth hormone receptor (GHR) levels (Gao et al., 2015). A Leu-enriched essential amino acid (EAA) preparation plus sucrose increased serum IGF1 concentration in man (Foster et al., 2012).
However, in growing farm animals, data on the effects of Leu supplementation are scarce. In 21-day-old weanling pigs, supplementing Leu to a diet with protein and AA concentrations below requirements increased phosphorylation of the mTOR downstream effectors ribosomal protein S6 kinase (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4EBP1), protein synthesis, and daily weight gains (Yin et al., 2010). A first study in chicken has shown that dietary Leu supplementation exceeding requirements in broilers aged 3, 7, and 14 D increased phosphorylation of mTOR, S6K1, and 4EBP1 in the breast muscle, but growth performance was not reported (Deng et al., 2014). Additionally, it has been shown that the effects of Leu are attenuated with age (Deng et al., 2014) and it is unclear whether Leu supplementation is effective to increase protein synthesis and growth performance during a 35-D growing period in broilers.
Therefore, we have investigated the effects of dietary Leu supplementation above requirements in comparison to a low-protein diet with Leu concentrations close to those recommended by the breeder in broilers aged 11, 22, and 36 D. Because excess Leu stimulates catabolism of all BCAA (Wiltafsky et al., 2010), and we wanted to avoid effects on growth performance due to a secondary deficiency of valine (Val) and isoleucine (Ile), we fixed the Leu: Ile and Leu: Val ratios by supplementing additionally Val and Ile to the diet. We hypothesized that dietary supplementation of Leu together with the other BCAA increases mTOR signaling and decreases proteolysis via the UPS or the autophagy-lysosomal system and thereby increases growth performance in broilers.
MATERIALS AND METHODS
Animals and Experimental Design
A total of 180-day-old male broiler chickens (Cobb 500, Cobb, Wiedemar, Germany) were allotted to 3 experimental groups for a 5-wk feeding trial. Due to the limitation in number of cages (15), the experiment was performed in 2 successive runs with 120 broilers in run 1 (8 birds per cage) and 60 broilers in run 2 (4 birds per cage). The birds were kept on cardboards in cages of 2.1 m2 equipped with nipple drinkers and automatic feeders. The broilers had free access to feed and water throughout the experiment. The mean initial body weight (41.4 ± 3.6 g; mean ± SD) was similar among experimental groups. The room temperature decreased from 27–28°C to 22–23°C during 5 wk and mean relative humidity was 63%. During the first 6 D, infrared lamps were used as additional heat sources. Light intensity was constantly at 40 lux. Close to what is recommended in the breeder guidelines, the light regime was 24 h:0 h 23 h:1 h, 22 h:2 h, 21 h:3 h, 20 h:4 h, 19 h:5 h (light: dark) at days 1, 2, 3, 4, 5, 6, and 18 h:6 h from the seventh day on.
The broilers were fed with 3 different basal diets during the starter (day 1 to 10), grower (day 11 to 21), and finisher phase (day 22 to 35). The control group (group L0) received the basal diet (Table 1) which met or exceeded the broiler's requirements for nutrients and AA for maintenance and growth according to the breeder's recommendations (Cobb-Vantress, 2015). Because the breeder guidelines do not specify Leu requirements, we ensured that dietary AA concentrations also met the recommendations specified in AMINODat 5.0 (Evonik GmbH, Hanau-Wolfgang, Germany). Leu concentrations in the basal diets reflected the natural Leu concentrations of the dietary ingredients, and were about 4% higher than those recommended in AMINODat 5.0. To the basal diets, Ile and Val were supplemented to keep Leu: Ile and Leu: Val ratios close to those recommended in AMINODat 5.0. Groups L1 and L2 received the basal diets supplemented with L-leucine (98.5%), L-isoleucine (99%), and L-valine (98%) to exceed AMINODat 5.0 recommendations by approximately 35 and 60%, respectively (Table 2). The Leu dosages had been chosen based on literature data where an activation of the mTOR pathway in 3- to 14-day-old broiler chickens had been shown with 1.73 and 2.03% Leu in the diet (10 and 30% above the breeder's recommendation) compared to a control diet which contained Leu equivalent to about 10% below breeder's recommendations (Deng et al., 2014). In addition, data from pig studies suggest an activation of the mTOR pathway when dietary Leu concentrations exceed NRC recommendations for swine (NRC, 2012) by about 40% (Yin et al., 2010) and 50% (Boutry et al., 2013).
Table 1.
Ingredient and nutrient composition of the basal diet fed during the starter (day 1 to 10), grower (day 11 to 21), and finisher (day 22 to 35) period.1
| Starter | Grower | Finisher | |
|---|---|---|---|
| Ingredient [%] | |||
| Corn | 24.4 | 29.2 | 23.2 |
| Soybean meal | 32.0 | 22.0 | 19.7 |
| Wheat | 3.98 | 9.95 | 10.95 |
| Barley | 6.87 | 0.87 | 9.95 |
| Peas | 9.95 | 9.95 | 9.95 |
| Soybean oil | 5.97 | 5.97 | 6.97 |
| Corn starch | 6.61 | 7.96 | 7.96 |
| Sunflower cake | 4.05 | 7.96 | 5.97 |
| Mineral and vitamin mix2 | 1.00 | 1.00 | 1.00 |
| Monocalciumphosphate | 1.70 | 1.48 | 1.36 |
| Limestone (calcium carbonate) | 1.55 | 1.31 | 1.02 |
| Sodium bicarbonate | 0.11 | 0.12 | 0.00 |
| Salt (NaCl) | 0.30 | 0.29 | 0.49 |
| Choline chloride 50% | 0.11 | 0.11 | 0.12 |
| L-Lysine (54.6%) | 0.60 | 1.00 | 0.75 |
| DL-Methionine (99.0%) | 0.43 | 0.35 | 0.32 |
| L-Threonine (98.5%) | 0.18 | 0.16 | 0.14 |
| L-Valine (98.0%) | 0.16 | 0.13 | 0.11 |
| L-Isoleucine (99.0%) | 0.12 | 0.12 | 0.11 |
| Nutrient composition, % as is3 | |||
| Dry matter | 89.5 (89.1) | 89.4 (89.0) | 89.5 (87.1) |
| Crude ash | 7.73 (6.66) | 6.94 (5.86) | 6.47 (5.32) |
| Crude fiber | 4.16 (4.35) | 4.25 (4.76) | 4.05 (4.55) |
| Crude fat | 7.87 (7.79) | 7.91 (7.69) | 8.79 (8.69) |
| Crude protein | 21.9 (21.9) | 19.5 (19.0) | 18.0 (17.1) |
| SID Lysine | 1.28 (1.37) | 1.30 (1.41) | 1.12 (1.16) |
| SID Methionine | 0.68 (0.68) | 0.59 (0.58) | 0.54 (0.50) |
| SID Methionine + Cysteine | 0.93 (0.93) | 0.82 (0.81) | 0.76 (0.72) |
| SID Threonine | 0.82 (0.82) | 0.71 (0.71) | 0.65 (0.62) |
| SID Tryptophan | 0.24 | 0.20 | 0.18 |
| SID Leucine | 1.41 (1.41) | 1.23 (1.24) | 1.13 (1.10) |
| SID Isoleucine | 0.88 (0.92) | 0.77 (0.79) | 0.71 (0.69) |
| SID Valine | 1.01 (1.00) | 0.88 (0.88) | 0.80 (0.75) |
| AMEn, MJ/kg | 12.63 | 12.94 | 13.23 |
Starter and grower diets contained an anticoccidiostatic drug (Maxiban, 0.375 g/kg; on top).
The mineral and vitamin mix supplied per kg diet: Ca, 3 g; Cl, 0.1 g; vitamin A, 12,000 IU; vitamin D3, 4,000 IU; vitamin E, 50 mg; vitamin K3, 3.33 mg; biotin, 250 μ g; folic acid, 1.67 mg; vitamin B1, 3.33 mg; vitamin B2, 8 mg; vitamin B6, 4.17 mg; vitamin B12, 25 μ g; nicotinamide, 69.1 mg; calcium pantothenate, 20 mg; choline chloride, 400 mg; Fe, 50 mg; Cu, 15 mg; Mn, 100 mg; Zn, 70 mg; I, 1.56 mg; Se, 0.25 mg.
Values show calculated values based on AMINODat 5.0, and values in parentheses show analyzed values. SID = standardized ileal digestible. Analyzed SID AA were calculated from analyzed AA concentrations with the help of AA digestibility values from AMINODat 5.0. The metabolizable energy content of the diet was calculated based on crude nutrient analyses of the dietary ingredients according to GfE (1999).
Table 2.
Dietary concentrations of leucine (Leu), isoleucine (Ile), and valine (Val) in the basal diet (L0) and in diets supplemented with moderate (L1) and high (L2) Leu, Ile and Val concentrations (% as is).1
| Dietary concentration, % |
|||||
|---|---|---|---|---|---|
| Phase | Treatments | Leu | Ile | Val | Ratio Leu:Ile:Val |
| Starter (1 to 10 D) | L0 | 1.41 (1.41) | 0.88 (0.92) | 1.01 (1.00) | 100:62:72 (100:65:71) |
| L1 | 1.86 (1.81) | 1.19 (1.19) | 1.38 (1.30) | 100:66:71 (100:66:72) | |
| L2 | 2.23 (2.19) | 1.43 (1.43) | 1.65 (1.58) | 100:68:70 (100:65:72) | |
| Grower (11 to 21 D) | L0 | 1.23 (1.24) | 0.77 (0.79) | 0.88 (0.88) | 100:62:71 (100:64:71) |
| L1 | 1.61 (1.61) | 1.04 (1.06) | 1.19 (1.17) | 100:67:71 (100:66:73) | |
| L2 | 1.93 (1.90) | 1.25 (1.22) | 1.42 (1.35) | 100:68:70 (100:64:71) | |
| Finisher (22 to 35 D) | L0 | 1.13 (1.10) | 0.71 (0.69) | 0.80 (0.75) | 100:62:70 (100:62:68) |
| L1 | 1.45 (1.40) | 0.96 (0.92) | 1.08 (1.00) | 100:68:72 (100:66:72) | |
| L2 | 1.73 (1.64) | 1.15 (1.07) | 1.29 (1.17) | 100:69:72 (100:65:71) | |
Values show calculated standardized ileal digestible (SID) AA concentrations based on AMINODat 5.0, and values in parentheses show analyzed SID AA concentrations. Analyzed SID AA were calculated from analyzed AA concentrations with the help of AA digestibility values from AMINODat 5.0. L-Leu (98.5%) supplementation level on top of the diet in groups L1 and L2 was 0.51 and 0.93% (starter), 0.43 and 0.78% (grower), and 0.36 and 0.68% (finisher). L-Ile (99%) supplementation in groups L1 and L2 was 0.35 and 0.62% (starter), 0.31 and 0.54% (grower), and 0.28 and 0.50% (finisher). L-Val (98%) supplementation in groups L1 and L2 was 0.41 and 0.71% (starter), 0.35 and 0.62% (grower), and 0.32 and 0.56% (finisher).
Body weight (individually) and feed consumption (per cage) were determined on days 10, 21, and 35. All experimental procedures were in accordance with the Appendix A of European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS NO. 123). The animals were humanely killed for scientific purposes in accordance with Article 4 par. 3 of the German Animal Welfare Law, which was approved by the Animal Welfare Officer of the Justus-Liebig-University, JLU No. 580_M.
Sample Collection
At slaughter (days 10, 21, and 35), weight of carcass, liver, thighs, and breast muscle have been recorded from each 18 birds (day 10 and 21) or 22 birds (day 35) per experimental group. Samples for laboratory analyses were collected exclusively during experimental run 1 from 13 birds per group and slaughter day. Plasma samples were collected and stored at –20°C for later analysis of concentrations of BCAA and their keto acids. Liver, breast muscle, and pancreas tissue were collected, snap-frozen in liquid nitrogen, and stored at –80°C for further analysis. Feed samples were stored at –20°C.
Biochemical Analyses
In lyophilized plasma, free AA concentrations were determined by Evonik via ion exchange chromatography using a Biochrom 20 amino acid analyzer, lithium column, and lithium buffers, and the protein in the samples was precipitated with aqueous sulphosalicylic acid (Sadri et al., 2017).
The plasma concentrations of the keto-acids of Leu, Ile, and Val, KICA, α-keto-β-methylvalerate (KMVA), and α-keto isovalerate (KIVA) were determined according to Li et al. (2016) via high-performance liquid chromatography (HPLC)-mass spectrometry (MS), and the method was slightly modified. In brief, plasma was thawed and centrifuged (600 U/min, 5 min, 4°C (Heraeus Fresco 21 Microcentrifuge, Thermo Fisher Scientific, Darmstadt, Germany)), and 50 μ L of centrifuged plasma or standard (α-keto isovaleric acid sodium salt, 2-keto-3-methylvaleric acid sodium salt, and α-keto isocaproic acid; Sigma-Aldrich, Darmstadt, Germany) was combined with 10 μ L of internal standard (α-keto isocaproic acid sodium salt (methyl-D3, 98%; Cambridge Isotope Laboratories, Tewksbury, MA; 10.3 μ g/mL in 50:50 methanol: water), and with 200 μ L acetonitrile, and kept on ice for 5 min to ensure proper protein precipitation. The precipitated protein was then separated by centrifugation (15,000 × g, 5 min, 4°C), and 100 μ L of the supernatant was evaporated by vacuum drying at 45°C (Concentrator plus, Eppendorf, Hamburg, Germany). The residue was resuspended in 50 μ L of water: methanol (90:10, v/v). After centrifugation (15,000 x g, 5 min, 4°C), the supernatant was transferred to an autosampler vial for analysis or stored at –20°C until analysis. An aliquot of the solution (8 μ L) was injected into the LC–MS/MS system for analysis. The HPLC system was a Hitachi LaChromUltra system (Darmstadt, Germany) consisting of the L-2160 U solvent delivery modules, an L-2200U autosampler, an L-2300 column oven, and a system controller. Separation of analytes was achieved on an Agilent Zorbax SB-C18 column (100 × 3 mm, 3.5 μ m) (Agilent, MA) fitted with a Phenomenx RP18 guard column (particle size 3 μ m, 4 × 3 mm; Phenomenex, Aschaffenburg) and temperature controlled at 45°C. The linear gradient program was set as follows with mobile phase (A) 0.05% formic acid solution and phase (B) methanol with 0.05% formic acid: 0 min, 15% B; 3 min, 30% B; 4.5 min, 30% B; 5 min, 15% B; 7 min, 15% B. The flow rate was 0.5 mL/min. Detection was achieved by coupling the HPLC to an API 3200 triple-quadrupole mass spectrometer (3200 QTrap, AB Sciex Germany GmbH, Darmstadt, Germany) using an electrospray ionization in negative mode. The mass spectrometer was correlatively optimized for declustering potential (–25), entrance potential (–7.5 for KIVA, –4.5 for KMVA, KICA, and KICA-D3), collision cell entrance potential (–16 for KIVA, –17 for KMVA, KICA, and KICA-D3), collision energy (–5), and collision cell exit potential (–1). Mass transitions were m/z 114.8–114.9 for KIVA, 129.1–129.1 for KMVA and KICA, and 132.1–132.1 for KICA-D3. Other parameters were as follows: collision gas, curtain gas, gas 1 and gas 2 (all nitrogen) medium, 25, 60, and 35 psi, respectively; dwell time 250 ms; IonSpray voltage 3,500 V; source temperature 450°C. One multiple reaction monitoring transition was recorded for each compound. For instrument control and analysis of the chromatographic data, the program Analyst 1.5.1 (AB Sciex Germany GmbH, Darmstadt, Germany) was used. The total runtime was 7 min and the elution of KIVA, KMVA, KICA, and internal standard (KICA-D3) occurred at 2.3, 4.4, and 4.8 min, respectively. The used standard curves were found to be linear in the range of 0.21 to 6.65 μ g/mL for KIVA, 0.21 to 6.7 μ g/mL for KMVA, and 0.25 to 7.8 μ g/mL for KICA with a mean correlation coefficient of 0.999 for each analyte. The detection limit was 0.05 μ g/mL for KIVA and 0.02 μ g/mL for KMVA and KICA. The precision, estimated by analyzing 5 replicates of rat plasma, was 3.9% for KIVA, 2.1% for KMVA, and 1.9% for KICA. The coefficient of variation of one sample analyzed 6 times was 3.9, 2.1, and 1.9% for KIVA, KMVA, and KICA and recovery was between 95 and 105%.
The basal or actual activity of the branched-chain α-keto acid dehydrogenase (BCKDH) has been determined photometrically in pancreas tissue based on Nakai et al. (2000) with modifications according to Wiltafsky et al. (2010). Pancreas tissue has been chosen because i) pretests with chicken liver tissue have shown that BCKDH activity was too low to be quantified, ii) it has been shown that the BCKDH activity in pancreas tissue was twice of that in liver, and iii) the increase due to BCAA supplementation in pancreas tissue paralleled that in the liver in pigs (Wessels et al., 2016). For preparation of the tissue extract, 0.15 g of pancreas tissue, 2 stainless steel beads (5 mm, Cat. No. 69,989; Qiagen, Hilden, Germany), and 1 mL of ice-cold extraction buffer were combined into a 2-mL cup. The extraction buffer was prepared 1 D before first use and was stored at 4°C up to 5 D. Different from Nakai et al. (2000), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid in the extraction and suspension buffers was replaced by potassium phosphate (Ono et al., 2001). The samples were homogenized with a Tissue Lyzer (Qiagen) for 8 min to ensure homogenization and at 17.5 Hz to avoid foam development which occurred at higher frequency. The samples were centrifuged for 5 min at 20,000 x g at 4°C, 800 μ L of the supernatant were transferred to a new cup and 400 μ L of polyethylene glycol (27%) were added, the samples were mixed, incubated one ice for 20 min, and centrifuged at 12,000 x g for 10 min at 4°C. The supernatant was decanted, 500 μ L of ice-cold suspension buffer was added, and the pellet was dissolved by flushing with a pipet carefully to avoid air inclusion and foam development. The tissue extract was placed on ice until BCKDH activity measurement at the same day. For that, 0.15 mL of tissue extract (mix before pipetting), 0.33 mL distilled water (30°C), and 0.5 mL of assay buffer (30°C) were combined into a 1-mL polystyrol cuvette (Ref. 67.742, Sarstedt, Nümbrecht, Germany), and equilibrated within a Cary 50 Bio UV Visible spectrophotometer (Varian, Darmstadt, Germany) for 15 to 30 s, before 20 μ L of substrate (50 mM α-keto isovalerate) was added. The samples were carefully mixed, and the absorption increase was measured at 340 nm for 20 min. Different from Nakai et al. (2000), pig heart dihydrolipoamide dehydrogenase was omitted in the assay buffer. Each sample was prepared in duplicate and for each sample, a substrate blind was prepared which contained the substrate but not the tissue extract. All samples which were statistically compared have been prepared and measured on the same day, i.e., samples from the starter, grower, and finisher phase, respectively, were each analyzed on one single day. The BCKDH activity was given as nmol NADH produced per min per g tissue (nmol/min/g), and was calculated with the help of the enzyme activity formula U/L = [ΔE/min x Vg x V]/[ɛ x v x d], where U = Units, ΔE/min = ΔE/min sample – ΔE/min blind, V = dilution of tissue extract, Vg = total volume in the cuvette, ɛ = molar extinction coefficient of NADH at 340 nm (0.00622 L/μ mol x cm), v = sample volume, d = cuvette depth (1 cm). The first 2 min of measurement was excluded from the calculation of ΔE/min.
Total RNA Isolation and mRNA Quantification
RNA isolation, cDNA synthesis, and quantitative PCR (qPCR) were performed according to Zeitz et al. (2016). In brief, total RNA was isolated from 25 to 30 mg of liver and muscle tissue using Trizol reagent (Thermo Fisher Scientific/Invitrogen, Schwerte, Germany) according to the manufacturer's protocol. The RNA concentration and purity were estimated from the optical density at 260 and 280 nm, respectively, using an Infinite 200 M microplate reader and a NanoQuant Plate (both from Tecan, Männedorf, Switzerland) and RNA was stored at –80°C. The cDNA was synthesized from 2.4 μg of total RNA using 100 pmol dT18 primer (Eurofins MWG Operon, Ebersberg, Germany), 1.25 μL 10 mmol/L dNTP mix (GeneCraft, Lüdinghausen, Germany), 5 μL buffer (Fermentas, St. Leon-Rot, Deutschland), and 60 units M-MuLV Reverse Transcriptase (MBI Fermentas, St. Leon-Rot, Germany) at 42°C for 60 min, and a final inactivating step at 70°C for 10 min in Biometra ThermalCycler (Whatman BiometraW, Göttingen, Germany). The cDNA was diluted 1:2 with DNase/RNase-free water and stored at −20°C.
The qPCR was carried out on a Rotorgene 2000 system (Corbett Research, Mortlake, Australia) using 2 μL cDNA combined with 8 μL of a mixture composed of 5 μL KAPA SYBR FAST qPCR Universal Mastermix (Peqlab, Erlangen, Germany), 0.2 μL each of 10 μM forward and reverse primers, and 2.6 μL DNase/RNase-free water in 0.1 mL tubes (Ltf Labortechnik, Wasserburg, Germany). Gene-specific primer pairs were designed using PRIMER3 and BLAST and obtained from Eurofins MWG Operon (Ebersberg, Germany) (Table 3). The amplification of a single product of the expected size was approved using 2% agarose gel electrophoresis stained with GelRed nucleic acid gel stain (Biotium Inc., Hayward, CA). The Ct values of target and reference genes were obtained using Rotorgene Software 5.0 (Corbett Research). All Ct values were transformed into relative quantification data using the 2−ΔCt equation (Livak and Schmittgen 2001), but the efficiencies of the reference and target genes were determined and used for the calculations instead of using an efficiency of 2. The highest relative quantities for each gene were set to 1. These expression values of target genes were normalized using the GeNorm normalization factor (Vandesompele et al., 2002). Using the Microsoft Excel-based application GeNorm, the GeNorm normalization factor was calculated as the geometric mean of expression data (relative quantities) of the 3 most stable [liver: beta-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), succinate dehydrogenase complex flavoprotein subunit A (SDHA); muscle: ACTB, ribosomal protein L32 (RPL32), SDHA ] out of 4 tested potential reference genes (ACTB, GAPDH, RPL32, SDHA). M and V values were below the threshold level of 1.5 and 0.15, respectively (Vandesompele et al., 2002). In liver, M values were ≤0.56 for all selected reference genes, V2/V3 values were 0.16, 0.15, and 0.13, and V3/V4 values were 0.09, 0.13, and 0.13 for the starter, grower, and finisher data sets, respectively. In muscle, M values were ≤0.56 for all selected reference genes, V2/V3 values were 0.23, 0.18, and 0.21, and V3/V4 values were 0.11, 0.11, and 0.14 for the starter, grower, and finisher data sets, respectively. The normalized expression values data set was corrected for outliers. Means and SE were calculated from normalized expression data for samples of the same experimental group. The mean of the control group was set to 1, and the means and SE of the treatment groups were scaled proportionally.
Table 3.
Characteristics of the primer pairs.1
| Gene | Forward primer (from 5′ to 3′) Reverse primer (from 5′ to 3′) | PCR product size, bp | Annealing temperature,°C | PCR efficiency | NCBI GenBank |
|---|---|---|---|---|---|
| Reference genes | |||||
| ATGAAGCCCAGAGCAAAAGA | |||||
| ACTB | GGGGTGTTGAAGGTCTCAAA | 223 | 60 | 1.93 | NM_205518.1 |
| ACTGTCAAGGCTGAGAACGG | |||||
| GAPDH | AGCTGAGGGAGCTGAGATGA | 204 | 60 | 1.93 | NM_204305.1 |
| ATGGGAGCAACAAGAAGACG | |||||
| RPL32 | TTGGAAGACACGTTGTGAGC | 139 | 58 | 1.84 | NM_001252255.1 |
| ATTCCCGTTTTGCCTACGGT | |||||
| SDHA | GGGAGTTTGCTCCAAGACGA | 172 | 60 | 1.83 | XM_419054.3 |
| Target genes | |||||
| TTGATGCCCTGTTAGGTATGGAA | |||||
| ATF4 | GGTATGAGTGGAGGTTCTTTGTTGT | 139 | 60 | 2.06 | NM_204880.2 |
| GGCACCGACCGATTTAGT | |||||
| ATG5 | GCTGATGGGTTTGCTTTT | 167 | 60 | 2.04 | NM_001006409.1 |
| CCGCTATGAGACAAGGGACGAG | |||||
| ATG9A | CCGCAGGCAGATGATGAGGA | 115 | 62 | 2.02 | AM085507.1 |
| CGACTGGAGCAGGAAGAAG | |||||
| BECN1 | TCTGAGCATAACGCATCTGG | 115 | 60 | 2.15 | NM_001006332.1 |
| GCGAATGTAGGTGAAGAAGAGC | |||||
| 4EBP1 | GGCTGGTGGGAATCCTCAAA | 108 | 61.5 | 1.91 | XM_424384.4 |
| TGCCAGCTACAAGGCCGCGCAG | |||||
| FBXO32 | TGCTTGGCCAACGGAGGGGA | 296 | 64 | 1.81 | NM_001030956.1 |
| CATAACCAGCCAACACCTGC | |||||
| FOXO1 | AATTCCCACCCTTCCGTAGC | 198 | 60 | 1.92 | NM_204328.1 |
| TATGGATCTTCGGCATCTGCT | |||||
| GHR | CCAGTCTTCATCACTCCTTTTCA | 208 | 61.5 | 1.85 | NM_001001293.1 |
| CTTGAAGGTGAAGATGCACAC | |||||
| IGF1 | GCAGCAGCAGAACTGGTTA | 88 | 60 | 1.87 | NM_0,010,04384.2 |
| GGACACAGAGGAGCTTGACC | |||||
| IGF1R | TGTCAGTGGGTTGGAGGGTA | 83 | 60 | 1.85 | NM_205032.1 |
| CCCAACTGTGACAAGCATGG | |||||
| IGFBP2 | TGCTCATGGGCTGTGTAGAAG | 170 | 61.5 | 1.82 | NM_205359.1 |
| ACTCCCCAAAGTGGAGATCC | |||||
| Myf5 | CGCCATCACATCGGAGCA | 154 | 60 | 1.86 | NM_001030363.1 |
| AGGAAACCTGAGTGACAGTGGA | |||||
| Myo D | GCTTGGCTGAACGGAGCAA | 164 | 60 | 1.89 | NM_204214.2 |
| CGGGGTGGGATGGTGATG | |||||
| Myo G | TGGAGAGGAGTGGGAAAGGA | 112 | 60 | 1.92 | D90157.1 |
| AGTGAGAGTGATGCGGAGAG | |||||
| mTOR | GAAACCTTGGACAGCGGG | 120 | 58 | 1.88 | XM_417614.4 |
| CTGCGAGGGTAAAGGCATCC | |||||
| SQSTM1 | CAGGGGAAGGGTGGAACAC | 132 | 60 | 2.12 | XM_001233248.4 |
| GGTGGAGTTTGGGGGCATTA | |||||
| S6K1 | GAAGAACGGGTGAGCCTAA | 230 | 60 | 1.83 | NM_001030721.1 |
| GAACCTGCTGGTGGGAGAACA | |||||
| TRIM63 | GTGCTCCCCCTTCTTGAGTG | 76 | 61.5 | 1.84 | XM_424369.4 |
ACTB, beta-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RPL32, ribosomal protein L32; SDHA, succinate dehydrogenase complex flavoprotein subunit A; ATF4, activating transcription factor 4; ATG5, autophagy related 5; ATG9A, autophagy related 9A; FBXO32, F-box only protein 32 (Atrogin-1); BECN1, beclin 1 (ATG6); 4EBP-1, eukaryotic translation initiation factor 4E-binding protein 1; FOXO1, forkhead box protein O1; GHR, growth hormone receptor; IGF-1, insulin-like growth factor 1; IGF-1R, insulin-like growth factor 1 receptor; IGFBP-2, insulin-like growth factor-binding protein 2; Myf5, myogenic factor 5; Myg, myogenein; MyoD, class I myosin; mTOR, mammalian target of rapamycin; S6K1, ribosomal p70 S6 Kinase; SQSTM1, sequestosome 1 (p62); TRIM63, tripartite motif containing 63 (MURF1, muscle RING-finger protein-1).
Protein Immunoblot Analysis
For western blot analyses, approximately 300 mg of frozen muscle tissue was homogenized in RIPA buffer (50 mM Tris, 150 mM NaCl, 10% glycerol, 0.1% SDS, 10% Triton X-100, 1 mM EDTA, 0.5% deoxycholate, pH 7.5) which included a protease inhibitor cocktail (1:200; Sigma-Aldrich, Steinheim, Germany) and a phosphatase inhibitor solution (1 mM sodium orthovanadate, 5 mM NaF; Sigma-Aldrich) at 30 Hz for 3 min in the Tissue Lyzer (Qiagen) and immediately put on ice afterwards. The samples were then centrifuged (16,200 × g, 4°C, 15 min), and the supernatant was stored at –20°C. Protein concentrations in the homogenates were determined by the bicinchoninic acid protein assay kit (UP40840A, Interchim, Montluçon, France) with bovine serum albumen (BSA) as standard. From each homogenate, 20, 30, 40, or 70 μ g protein for later detection of Akt and FOXO1, rpS6 and eIF2α, mTOR, or S6K1, respectively, was separated on SDS-PAGE using a 6% stacking and 10% running SDS-gel, and electrotransferred to a nitrocellulose membrane (Pall, Pensacola, FL) using a blotting buffer with 20% methanol for 1.5 h or, for mTOR detection, with 5% methanol for 3 h. In each SDS-PAGE, a 10 to 250 kDA molecular weight marker (Pageruler prestained protein ladder; Thermo Scientific, Dreieich, Germany) was separated in order to evaluate the size of detected proteins. Loading of equal amounts of protein in each line was verified by Ponceau S (Carl Roth, Karlsruhe, Germany) staining, and the membranes were washed 3 × 5 min in 1 x Tris-buffered saline with Tween20 (TBST). Before choosing the protein load per lane, it had been verified that loading higher amounts of protein increased fluorescence intensity of bands and that the amount of protein selected for loading was within the linear range. Blocking was done in 5% skimmed milk powder in TBST for 1 h at room temperature or, for mTOR detection, in 5% BSA at 4°C overnight. Afterwards, membranes were washed 3 × 5 min in 1 x TBST. The membranes were incubated with primary antibodies against total and phosphorylated Akt (Cat. 4685 and 4051, CST, Danvers, MA), FOXO1 (Cat. 9454 and 9461, CST), eIF2α (Cat. 9722 and 9721, CST), mTOR (Cat. 2972 and 2971, CST), S6 ribosomal protein (rpS6) (Cat. 2217 and 2211, CST), and S6K1 (Cat. 9202 and 9234, CST), at a dilution of 1:1000 in 5% skimmed milk powder in 1 x TBST for phosphorylated Akt and in 5% BSA in 1 x TBST for the other antibodies at 4°C overnight. Additionally, the total protein amount of the membranes has been quantified using the Ponceau S stained membranes, and has been used for total protein normalization (Aldridge et al., 2008; Eaton et al., 2013). The membranes were washed 3 times for 10 min in 1 x TBST, and then incubated at room temperature (25°C) with a horseradish peroxidase conjugated secondary polyclonal rabbit anti-mouse IgG antibody for phospho-Akt (ab6728; Abcam) for 1.5 h at a dilution of 1:40,000 in 1 x TBST, and with a polyclonal goat anti-rabbit IgG antibody (A0545; Sigma) for the other primary antibodies at room temperature for 1.5 h at a dilution of 1:10,000 in 1 x TBST. Washing was 3 × 10 min in 1 x TBST. Afterwards, blots were developed by ECL Select Western Blotting Detection Reagent (GE Healthcare, Munich, Germany) for 5 min at 25°C, and the intensities of the specific bands were detected with a Bio-Imaging system (G: BOX, Syngene, Cambridge, UK) after an exposure time of 0.25 to 1 min or 1 to 3 min for mTOR detection, and quantified by Syngene Gene Tools software (nonlinear dynamics).
Calculations and Statistical Analysis
The data set has been tested for normal distribution of the residuals. Cage was used as the experimental unit for performance parameters. Bird was the experimental unit for carcass evaluation, and for biochemical and molecular data evaluation. The performance data were subjected to 2-way analysis of variance (ANOVA) considering diet, experimental run, and their interaction. Probability of error P < 0.05 was considered significant. Because the samples for laboratory analyses were only collected in one experimental run, the statistical analysis in these cases considered only experimental treatment as fixed factor. The Tukey test was used as post hoc test. Probability of error P < 0.05 was considered significant.
RESULTS
Growth Performance and Carcass Characteristics
During the whole 35-D growth period, the performance of broilers (body weight gains, feed intake, feed: gain ratio) did not significantly differ between the 3 groups (P > 0.05, Table 4). During the starter period (day 1 to 10), the feed: gain ratio was significantly lower in group L1 than in group L0 (P < 0.05); during the grower period (day 11 to 21), weight gain of the broilers was significantly higher in group L1 than group L0 (P < 0.05, Table 4).
Table 4.
Performance and carcass characteristics of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine (Leu) at fixed ratios of leucine: isoleucine and leucine: valine during the 35 D growth period.1
|
P value |
|||||||
|---|---|---|---|---|---|---|---|
| L0 | L1 | L2 | SEM | group | run | group* run | |
| Performance | |||||||
| Whole period (day 1 to 35) | |||||||
| Initial body weight, g | 41.2 | 41.7 | 41.3 | 0.27 | 0.42 | <0.001 | 0.45 |
| Final body weight, g | 2,394 | 2,447 | 2,340 | 40.1 | 0.18 | 0.24 | 0.54 |
| Body weight gain, g | 2,352 | 2,405 | 2,299 | 40.3 | 0.18 | 0.24 | 0.54 |
| Feed intake, g | 3,379 | 3,344 | 3,268 | 48.6 | 0.25 | 0.82 | 0.33 |
| Feed: gain ratio, g/g | 1.42 | 1.41 | 1.40 | 0.009 | 0.73 | 0.14 | 0.92 |
| Day 1 to 10 | |||||||
| Body weight gain, g | 241 | 243 | 234 | 4.0 | 0.20 | 0.06 | 0.60 |
| Feed intake, g | 274 | 266 | 260 | 5.1 | 0.20 | 0.04 | 0.67 |
| Feed: gain ratio, g/g | 1.14a | 1.09b | 1.10a,b | 0.011 | 0.036 | 0.09 | 0.30 |
| Day 11 to 21 | |||||||
| Body weight gain, g | 677b | 725a | 696a,b | 12.0 | 0.031 | 0.23 | 0.60 |
| Feed intake, g | 891 | 926 | 889 | 18.8 | 0.37 | 0.99 | 0.87 |
| Feed: gain ratio, g/g | 1.31 | 1.29 | 1.27 | 0.016 | 0.33 | 0.73 | 1.00 |
| Day 22 to 35 | |||||||
| Body weight gain, g | 1,415 | 1,421 | 1,364 | 32.3 | 0.40 | 0.32 | 0.38 |
| Feed intake, g | 2,215 | 2,151 | 2,119 | 40.5 | 0.19 | 0.98 | 0.13 |
| Feed: gain ratio, g/g | 1.53 | 1.54 | 1.53 | 0.016 | 0.80 | 0.43 | 0.52 |
| Carcass characteristics | |||||||
| Day 10 | |||||||
| Eviscerated carcass weight, g | 171 | 171 | 170 | 5.5 | 0.99 | 0.59 | 0.79 |
| Dressing percentage, % | 60.8 | 60.0 | 61.5 | 1.08 | 0.36 | 0.62 | 0.23 |
| Thighs, % of live weight2 | 17.8 | 17.5 | 17.6 | 0.26 | 0.89 | 0.35 | 0.92 |
| Breast muscle, % of live weight | 11.9 | 11.4 | 11.5 | 0.31 | 0.32 | 0.98 | 0.45 |
| Day 21 | |||||||
| Eviscerated carcass weight, g | 653 | 701 | 642 | 21.6 | 0.24 | 0.44 | 0.27 |
| Dressing percentage, % | 69.0 | 69.6 | 67.3 | 0.65 | 0.45 | 0.62 | 0.29 |
| Thighs, % of live weight2 | 19.2 | 19.4 | 19.0 | 0.22 | 0.69 | 0.009 | 0.05 |
| Breast muscle, % of live weight | 18.2a | 18.3a | 16.5b | 0.37 | 0.005 | 0.16 | 0.31 |
| Day 35 | |||||||
| Eviscerated carcass weight, g | 1,724 | 1,744 | 1,688 | 37.8 | 0.50 | 0.79 | 0.43 |
| Dressing percentage, % | 72.2 | 72.5 | 71.7 | 0.39 | 0.26 | 0.14 | 0.35 |
| Thighs, % of live weight2 | 19.6 | 19.0 | 19.6 | 0.24 | 0.11 | 0.52 | 0.85 |
| Breast muscle, % of live weight | 22.3a | 22.6a | 21.2b | 0.36 | 0.024 | 0.37 | 0.30 |
The data set was analyzed by 2-way ANOVA with Leu concentration, experimental run, and their interaction as fixed factors. The Tukey test was used as post-hoc test. The experimental unit was the cage for performance data (n = 10) and was the individual animal for carcass characteristics (n = 13).
Values with superscripts with no common letter differ at P <P < 0.05.
Thighs including skin and bones.
Carcass weights, dressing percentage, and thigh weights in relation to the live weight at days 10, 21, and 35 did not significantly differ between the 3 groups of broilers (P > 0.05, Table 4). However, the relative breast muscle weight at day 21 was significantly lower in group L2 than in groups L0 and L1 (P < 0.05, Table 4); the relative breast muscle weight at day 35 was significantly lower in group L2 than in group L1 (P < 0.05, Table 4).
Effects of Dietary Leu Supplementation on BCAA Metabolism at Fixed Leu: Ile and Leu: Val Ratios
Principally, higher dietary concentrations of BCAA in broilers fed BCAA-supplemented diets were reflected in higher plasma concentrations of BCAA and their keto acids (Figures Figure 1, Figure 2). At day 10, Val plasma concentrations were significantly higher in groups L1 and L2 than in group L0 (P < 0.05, Figure 1). At day 21, Leu, Ile, and Val plasma concentrations were significantly higher in group L2 than in groups L0 and L1 (P < 0.05, Figure 1). At day 35, Leu and Val plasma concentrations were significantly higher in group L2 than in L0 (P < 0.05, Figure 1). The plasma concentrations of KICA, KIVA, and KMVA at day 10 were significantly higher in groups L2 and L1 than in group L0 (P < 0.05, Figure 2). The plasma concentrations of KICA, KIVA, and KMVA at day 21 were significantly higher in group L2 than in groups L0 and L1 (P < 0.05, Figure 2). Plasma concentrations of KICA and KIVA at day 35 concentrations were significantly higher in group L2 than in group L0 (P < 0.05); the concentration of KMVA at day 35 did not significantly differ between the 3 groups of broilers (Figure 2). The activity of the BCKDH in the pancreas increased by Leu supplementation in a dose-dependent manner, and it was higher in group L2 than in group L0 at days 21 and 35 (P < 0.05, Figure 3).
Figure 1.
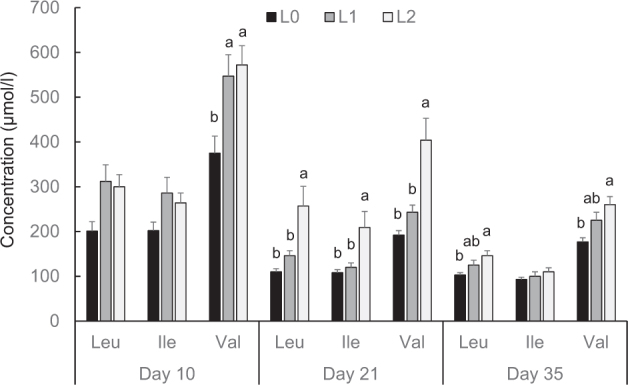
Concentrations of leucine (Leu), isoleucine (Ile), and valine (Val) in the plasma of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at days 10, 21, and 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor and the Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Columns with no common letter differ significantly at P < 0.05 within broiler age.
Figure 2.
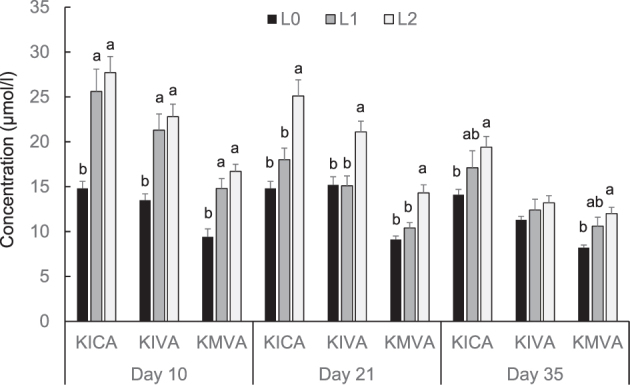
Concentrations of α-keto isocaproate (KICA, keto acid of leucine), keto β-methylvalerate (KMVA, keto acid of isoleucine), and α-keto isovalerate (KIVA, α-keto acid of valine) in the plasma of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at days 10, 21, and 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor and the Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Columns with no common letter differ significantly at P < 0.05 within broiler age.
Figure 3.
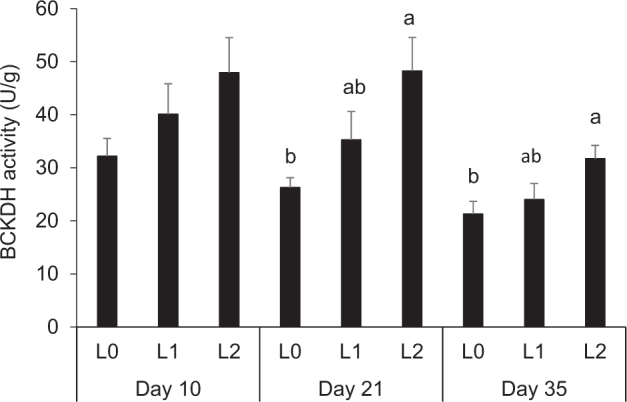
Activity of branched-chain α-keto acid dehydrogenase (BCKDH) in pancreas of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at days 10, 21, and 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor and the Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Columns with no common letter differ significantly at P < 0.05 within broiler age.
Effects of Dietary Leu Supplementation on the Expression of Genes Involved in the Somatotropic Axis in Liver and Muscle at Fixed Leu: Ile and Leu: Val Ratios
As indicators of the somatotropic axis in the liver, relative mRNA abundances of GHR, IGF1, insulin-like growth factor binding protein 2 (IGFBP2), and insulin-like growth factor receptor ( IGFR) were determined. Overall, the expression of these genes was only slightly affected by BCAA supplementation, and the few effects observed were not consistent within the 3 different ages (days 10, 21, 35) considered. At day 10, the mRNA abundances of IGF1, IGFBP2, and IGFR in the liver were not significantly different between the 3 groups (P > 0.05, data not shown); the relative mRNA abundance of GHR was significantly higher (P < 0.05) in group L1 than in group L2 (L0: 1.00 ± 0.04; L1: 1.16 ± 0.08; L2: 0.92 ± 0.07, means ± SE). At day 21, the mRNA abundances of GHR, IGFBP2, and IGFR in the liver were not significantly different between the 3 groups (P > 0.05, data not shown); the relative mRNA abundance of IGF1 was significantly lower (P < 0.05) in group L2 than in group L0 (L0: 1.00 ± 0.10; L1: 0.80 ± 0.07; L2: 0.63 ± 0.08, means ± SE). At day 35, relative mRNA abundances of GHR and IGF1 in the liver were significantly higher in group L1 than in group L0 (P < 0.05, Figure 4), and the relative mRNA abundance of IGF1R was significantly lower in group L2 than in group L1 (P < 0.05, Figure 4). Relative mRNA concentration of IGFBP2 in the liver did not significantly differ between the 3 groups at day 35 (P > 0.05, Figure 4).
Figure 4.
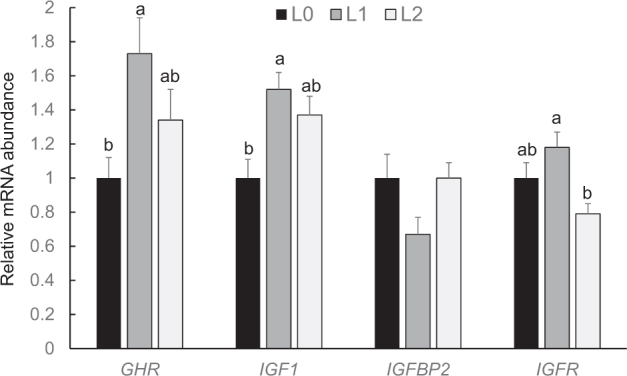
Relative mRNA abundances (fold of L0) of genes involved in the GH axis in the liver of broilers fed control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at day 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor. The Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Values with superscripts with no common letter differ at P < 0.05.
In the muscle, mRNA abundances of the IFGR and IGFBP2 at day 10 were significantly higher (P < 0.05) in group L2 than in group L0 (IFGR: L0: 1.00 ± 0.05; L1: 1.04 ± 0.06; L2: 1.26 ± 0.09; IGFBP2: L0: 1.00 ± 0.13; L1: 1.02 ± 0.09; L2: 1.53 ± 0.19; means ± SE). However, the mRNA abundances of GHR and IGF1 in the muscle at day 10 and those of GHR, IGF1, IFGBP2, and IGFR at days 21 and 35 did not significantly differ between the 3 groups (P > 0.05, data not shown).
Effects of Dietary Leu Supplementation on Genes Involved in Molecular Pathways of Protein Synthesis and Protein Degradation in the Muscle of Broilers at Fixed Leu: Ile and Leu: Val Ratios
In general, the investigation of molecular pathways of protein synthesis and degradation in the muscles of broilers revealed that those pathways remained largely unaffected by Leu supplementation. At day 10, relative mRNA abundances of the genes involved in the mTOR pathway (mTOR, 4EBP1, S6K1) and in the GCN2 pathway [activating transcription factor 4 ( ATF4), sequestosome 1 ( SQSTM1) ] were not significantly different between the 3 groups (P > 0.05, data not shown). Relative mRNA abundances of class I myosin ( MyoD) and myogenin ( MyoG), 2 genes involved in myogenesis, were also not significantly different between the 3 groups of broilers at day 10 (P > 0.05, data not shown). In contrast, the relative mRNA abundance of myogenic factor 5 ( Myf5) at day 10 was significantly higher (P < 0.05) in group L2 than in groups L0 and L1 (L0: 1.00 ± 0.10; L1: 1.18 ± 0.15; L2: 1.86 ± 0.12; means ± SE). At day 21, relative mRNA abundances of all the genes involved in the mTOR pathway, the GCN2 pathway, and myogenesis were not significantly different between the 3 groups of broilers (P > 0.05, data not shown). At day 35, relative mRNA abundances of the genes involved in the mTOR pathway and the GCN2 pathway as well as mRNA abundances of Myf5 and MyoD were not significantly different between the 3 groups of broilers (P > 0.05, Figure 5); the mRNA abundance of MyoD was significantly lower in group L2 than in group L0 (P < 0.05, Figure 5).
Figure 5.
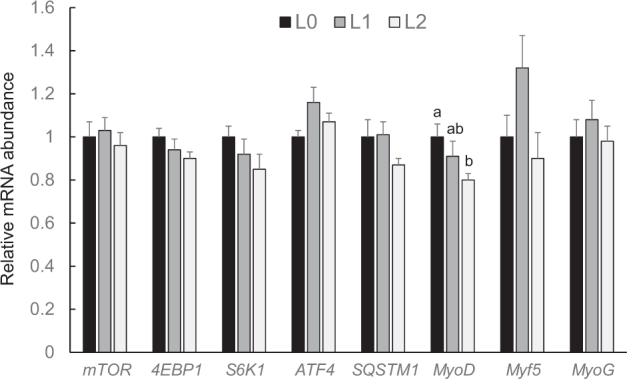
Relative mRNA abundances (fold of L0) of genes involved in the mTOR pathway (mTOR, 4EBP1, S6K1), the GCN2 pathway (ATF4, SQSTM1), and myogenesis (MyoD, Myf5, MyoG) in muscle of broilers fed control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at day 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor. The Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Values with superscripts with no common letter differ at P < 0.05.
Within the group of genes involved in the UPS pathway [FOXO1, F-box only protein 32 ( FBXO32), tripartite motif containing 63 ( TRIM63) ], and autophagy [autophagy related 5 ( ATG5), autophagy related 9A ( ATG9), beclin 1 ( BECN1) ], there were no significant differences in mRNA abundances between the 3 groups at days 10 and 21 (P > 0.05, data not shown). At day 35, relative mRNA abundances of genes involved in the UPS pathway and relative mRNA abundances of ATG9A and BECN1 were not significantly different between the 3 groups of broilers (P > 0.05, Figure 6); the mRNA abundance of ATG5 was significantly higher in group L1 than in groups L0 and L2 (P < 0.05, Figure 6).
Figure 6.
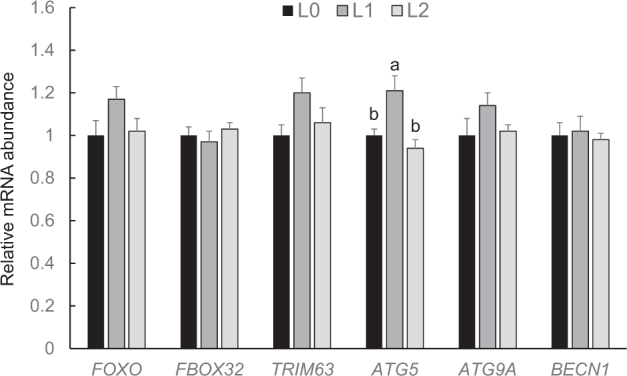
Relative mRNA abundances (fold of L0) of genes involved in the ubiquitine proteasome system (FOXO1, FBOX32, TRIM63) and autophagy (ATG5, ATG9A, BECN1) in muscle of broilers fed control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at day 35 (mean ± SE). The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor. The Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 13). a,b Values with superscripts with no common letter differ at P < 0.05.
As most of the proteins involved in the mTOR pathway of protein synthesis and of the FOXO1/Akt pathway are regulated by phosphorylation, we also determined phosphorylation levels (phosphorylated to total protein) of proteins involved in those pathways in the muscle of the broilers. At days 10, 21, and 35, relative phosphorylation levels of mTOR and the downstream targets S6K1 and rpS6 did not significantly differ between the 3 groups (P > 0.05, Figure 7 A-C). At day 35, there was however a tendency towards a reduced relative phosphorylation of mTOR in groups L1 and L2 compared to group L0 (P < 0.10, Figure 7 A). At days 10, 21, and 35, relative phosphorylation levels of Akt, FOXO1, and eIF2α also did not significantly differ between 3 groups (P > 0.05, Figure 8 A-C).
Figure 7.
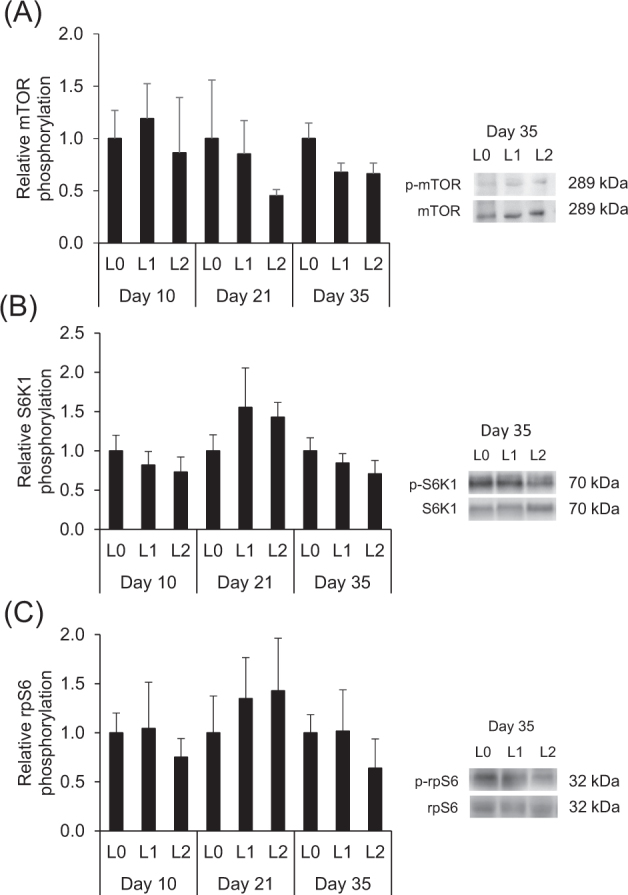
Relative phosphorylation levels (fold of L0) of mTOR (A), S6K1 (B), and rpS6 (C) in the breast muscle of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at days 10, 21, and 35 (mean ± SE). Relative phosphorylation levels were calculated as the amount of protein detected by the phosphorylated antibody divided by that detected by the antibody for detection of total protein level of the respective protein. mTOR, mammalian target of rapamycin; S6K1, ribosomal protein S6 kinase beta-1, rpS6, ribosomal protein S6. The data set was analyzed by 1-way ANOVA with leucine concentration as fixed factor and the Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 4–5). There were no significant (P < 0.05) differences between experimental groups. Representative blots for one animal of each group at day 35 are shown.
Figure 8.
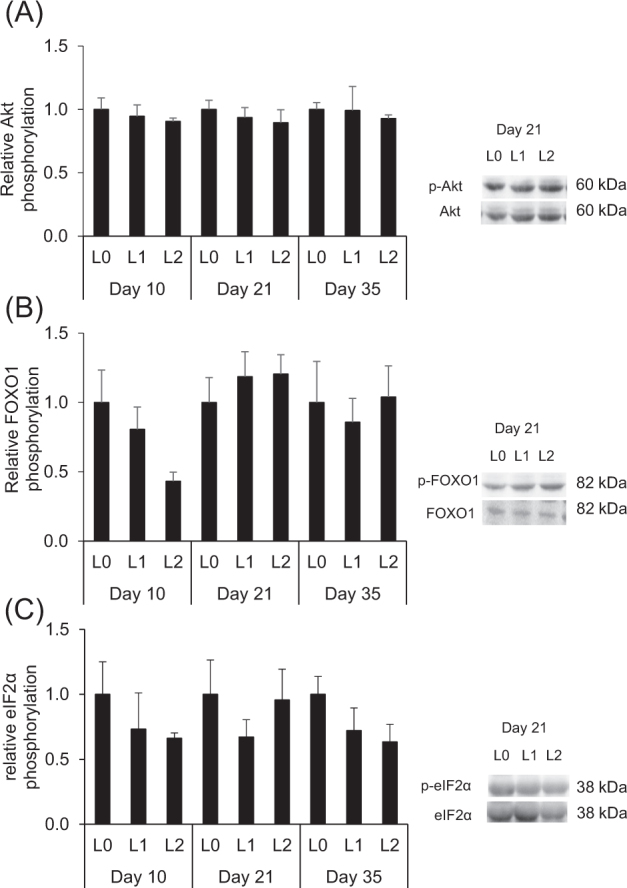
Relative phosphorylation levels (fold of L0) of Akt (A), FOXO1 (B), and eIF2α (C) in the breast muscle of broilers fed either control diets (L0) or diets supplemented with moderate (L1) or high (L2) concentrations of leucine at fixed ratios of leucine: isoleucine and leucine: valine at days 10, 21, and 35 (mean ± SE). Relative phosphorylation levels were calculated as the amount of protein detected by the phosphorylated antibody divided by that detected by the antibody for detection of total protein level of the respective protein. Akt, protein kinase B; FOXO1, forkhead box protein O1; eIF2α, eukaryotic translation initiation factor 2A. The data set was analyzed by 1-way ANOVA with BCAA concentration as fixed factor and the Tukey test was used as post hoc test. The experimental unit was the individual animal (n = 4–5). There were no significant (P < 0.05) differences between experimental groups. Representative blots for one animal of each group at day 21 are shown.
DISCUSSION
In the present study, we found that dietary Leu supplementation at fixed ratios to Ile and Val had overall minimum effects on growth performance in broilers. The only effects on growth performance observed were an increase of body weight gains in the L1 group in comparison to the L0 group in the starter period (day 1 to 10) and an increased body weight gain in L1 group in comparison to the L0 group in the grower period (day 11 to 21). Body weight gains, feed intake, and the gain: feed ratio during the whole period were not different between the 3 groups. The finding that an excess of dietary Leu (up to 60% above requirement) has no effect on growth performance agrees with a recent study in broilers in which Leu was supplied to the diets without adjusting the ratios to Ile and Val (Zeitz et al., 2019). The present study shows that high dietary Leu concentrations moderately reduce the breast muscle percentage in the broilers. Interestingly, such an effect was not observed in the recent study by Zeitz et al. (2019), in which Leu was supplemented at a similar level as in the present study, however, without supplementation of Ile and Val. The contradictory results of these 2 studies suggest that an adverse effect on breast muscle percentage could be induced by high dietary concentrations of BCAA in total but not by high Leu concentrations alone.
The findings of Leu supplementation on growth performance of broilers in this study are in contrast to data reported in weaning piglets where growth was enhanced by Leu supplementation (Yin et al., 2010). It has to be considered, though, that the basal diet in Yin et al. (2010) did not meet recommendations for some AA like lysine and methionine + cysteine, and dietary Leu concentrations in the Leu-supplemented groups were not more than 25 and 40% above those recommended by NRC (2012). In addition, it has been reported that excess Leu supplementation decreases performance in chicken (Smith and Austic, 1978) and pigs (Gatnau et al., 1995), which can be explained by increased catabolism of all BCAA (Wiltafsky et al., 2010). We have also shown that BCAA supplementation increased plasma concentrations of their keto acids which indicates that the first step of BCAA catabolism, the reversible transamination catalyzed by the BCAA transaminase in muscle tissues (Harper et al., 1984; Suryawan et al., 1998), was stimulated by Leu supplementation. Likewise, as indicated by higher activity of the BCKDH in the pancreas, a tissue with high BCKDH activity (Wessels et al., 2016), the second step of BCAA catabolism has also been stimulated by BCAA supplementation. We had supplemented Val and Ile in addition to Leu to avoid negative effects on growth in our study, and despite stimulated BCAA catabolism, plasma BCAA concentrations in fact increased after BCAA supplementation which proved that a secondary deficiency of Val and Ile was avoided. In addition, it is also verified that Leu availability increased and thus that Leu could have the potential to influence protein synthesis positively and protein degradation in the muscle as hypothesized. It was therefore unexpected that we found even decreases in breast muscle percentage in group L2.
An increase in muscle mass occurs when protein synthesis rates exceed those of protein degradation (Schiaffino et al., 2013). Potentially, several molecular pathways could be altered when muscle growth is affected. Therefore, we had measured effects of Leu on 2 major pathways of protein synthesis: the IGF1-PI3K-Akt-mTOR pathway, as a positive regulator, and the myostatin–Smad2/3 pathway, as a negative regulator of protein synthesis (Schiaffino et al., 2013). Upon activation, Akt/PKB will promote anabolic processes via the mTOR pathway, and impede catabolic processes, e.g., by inhibiting proteasome- and autophagy-related protein degradation (Fan et al. 2016) which are the 2 most important pathways of skeletal muscle proteolysis (Pasiakos and Carbone, 2014). The mTOR pathway is influenced by AA (Dann and Thomas, 2006), and (re)feeding of AA accelerates the rate of protein synthesis (Vary and Lynch, 2007). Leu has been shown to stimulate protein synthesis in murine skeletal muscle cells (Atherton et al., 2010), in fasted neonatal pigs (Escobar et al., 2005, 2010), in elderly humans (Katsanos et al. 2006), and even in weaned pigs (Yin et al. 2010) and young, up to 14-day-old broilers (Deng et al., 2014). Changes in the GH/IGF1 axis which are related to growth in broilers as shown for serum IGF1 and breast muscle IGF1 expression (Xiao et al., 2017) may go along with changes in protein synthesis. For example, a Leu-rich mixture of EAA increased serum free IGF1 levels and activated the mTOR pathway in a rat model (Xia et al., 2013). Also, when rats which had been subjected to 50% food restriction for 4 wk prior to the experiment were fed Leu-supplemented diets, hepatic mRNA expression of GHR and IGF1 was increased, and the mTOR pathway activated when compared to a control group (Gao et al., 2015). In the present study, however, as mRNA abundances of genes involved in the GH/IGF1 axis were hardly and not consistently affected by Leu supplementation, and proteins involved in the mTOR pathway were not affected as well, this indicates that protein synthesis was similar between the 3 groups. The findings of this study are in agreement with a recent study in broilers in which dietary Leu was supplemented without adjustment of the ratios to Ile and Val. In that study, Leu supplementation also did not influence expression of genes involved in the GH/IGF1 and mTOR pathways as well as phosphorylation of proteins involved in the mTOR pathway (Zeitz et al., 2019).
The mTOR pathway also negatively regulates autophagy which refers to processes leading to elimination, or rather recycling, of cytoplasmic components (He and Klionsky, 2009; Boya et al., 2013). In addition, the protein kinase GCN2 is involved in autophagy and the mTOR pathway; the GCN2/eIF2α pathway is, besides the mTOR pathway, one major pathway how AA presence or absence is sensed (Battu et al., 2017). Amino acid deprivation activates the GCN2/eIF2α-pathway in order to inhibit mTOR (Métayer et al., 2008) and to stimulate autophagy (He and Klionsky, 2009). Similarly, lack of insulin or EAA generally accelerates protein degradation (Lecker et al., 2006). For example, in chick skeletal muscles, Leu inhibited the UPS as determined by gene expression analysis, an effect which was mediated through phosphoinositide 3-kinase (PI3K) and protein kinase C (PKC), whereas the mTOR pathway remained unaffected (Nakashima et al. 2005). The autophagy-lysosome system was also down-regulated when Leu was infused in formula-fed 9-day-old piglets equivalent to a dosage of 50% above requirements compared to control piglets (Boutry et al., 2013). Likewise, Leu decreased the activity of lysosomal proteases and decreased the expression of genes involved in proteasomal proteolysis when isolated rat muscles were incubated in a medium with 10 mM Leu and 5 mM glucose compared with glucose alone (Busquets et al., 2000). Notably, however, such high Leu concentrations are an order of magnitude higher than those measured in the plasma. In C2C12 myotubes, Leu starvation induced autophagy, and the data suggest that protein sequestration to the lysosome was affected (Mordier et al. 2000). We had therefore hypothesized that autophagy might be affected by Leu and considered mRNA expression of ATG5, beclin1, and ATG9 genes which are involved in phagophore biogenesis and expansion, the first step of autophagosome formation before its fusion with the lysosome which then complete the breakdown by hydrolases (Boya et al., 2013). However, neither the UPS nor the autophagy-lysosomal system was affected by supplementing Leu in concentrations above those generally recommended for growing broilers.
It should be also noted that the proteins involved in the mTOR signaling pathway are mainly regulated by post-translational mechanisms and that protein synthesis will be initiated after phosphorylation of proteins like Akt, S6K1, 4E-BP1, and rpS6 (Vary and Lynch 2007). The FOXO1 transcription factor, which induces protein degradation by increasing the expression of TRIM63 [also called Muscle RING-finger protein-1 (MURF1)] and FBXO32 (also called atrogin-1) involved in the UPS pathway, and which is required for autophagy induction, is inactivated due to phosphorylation by Akt (Glass, 2010; Zhao et al., 2010). Likewise, eIF2α, phosphorylated by GCN2, is regulated by post-translational mechanisms (He and Klionsky, 2009). However, in the present study, Leu supplementation did not influence phosphorylation of proteins involved in the mTOR pathway, UPS and autophagy, and GCN2/eIF2α signaling pathways indicating again that neither protein synthesis nor degradation was affected by Leu supplementation. Myostatin, which is involved in myogenesis and myoblast differentiation (Langley et al., 2002), activates the UPS and autophagy (Wang et al., 2015). Although it was reported that the myostatin/Smad2/3 pathway was not affected by a BCAA supplement in patients experiencing alcoholic cirrhosis as measured by myostatin phosphorylation (Tsien et al., 2015), we also investigated gene expression of the Smad2/3 target genes MyoG, MyoD, and Myf5. The finding that mRNA abundances of these genes in muscle remained unchanged confirmed that Leu supplementation did not affect this pathway.
A secondary deficiency of Ile and Val can be excluded as discussed above. Also, a scarcity of the other AA, which could be one reason that BCAA supplementation did not affect the mentioned pathways (Wilson et al. 2010; Wilson et al. 2011), can be excluded, because we fed diets which met or exceeded the recommended dietary levels. In addition, plasma concentrations of the other EAA except the BCAA were largely unaffected (data not shown) which also corroborates the view that AA uptake into tissues and their utilization for protein synthesis was not increased due to Leu supplementation which could have been expected in case of increased muscle protein synthesis.
Possible reasons why we did not find the effects of Leu on pathways of muscle protein synthesis and degradation may be related to the animal age or to the metabolic state of the animals which may affect their response to anabolic and anti-catabolic stimuli. For example, Deng et al. (2014) have shown that gene expression and relative phosphorylation of mTOR and S6K1 were increased by Leu supplementation in the muscle of broilers aged 3 or 7 D, but after 14 D, the effect was attenuated and failed to reach significance for mTOR. It is possible that we failed to detect differences because we have investigated broilers aged 11, 22, and 36 D, thus of higher age than in Deng et al. (2014). It is also worthy to note that broilers are not only in an anabolic state per se, but that the growth rate of broilers, including Cobb broilers used in this study, is extremely high and daily weight gains are, in relation to body weight, higher than in piglets, and dramatically higher than in humans. Interestingly, many studies which found that Leu supplementation stimulates muscle protein synthesis have used animals or humans in a catabolic state, like fasted piglets (Davis et al., 2008; Escobar et al., 2010), food-deprived rats (Anthony et al., 2000), or elderly humans (Katsanos et al., 2006; Rieu et al., 2006). In addition, Katsanos et al. (2006) have shown that the response in fractional rate of protein synthesis to ingestion of an EAA mix was lower in elderly than in young subjects and that an extra high proportion of Leu (41%) in the EAA mix further stimulated fractional protein synthesis rate only in the elderly indicating that Leu can reverse an attenuated response of protein synthesis. It has been suggested that Leu supplementation is only effective in cases where a decreased sensitivity of protein anabolism to Leu—like in the elderly—is probable (Balage and Dardevet, 2010) or in cases of clear catabolic states like fasting, as mentioned above. This, however, may also indicate that Leu does not stimulate protein synthesis via the mTOR pathway in young fast-growing animals like broilers which are fed according to AA requirements. Protein synthesis and inhibition of proteolysis in growing broilers may be already at its maximum under such conditions. This view is corroborated by the observation that maximal activation of the mTOR pathway seems to be reached at Leu concentrations which are at or slightly above physiological levels (Yoshizawa et al., 2013).
In conclusion, the data indicate that diets supplemented with Leu 60% above levels recommended in AMINODat 5.0 at fixed Leu: Ile and Leu: Val ratios do not or hardly influence molecular pathways of protein synthesis and degradation and do not affect performance in fast-growing broilers during a 35-D growth period.
ACKNOWLEDGMENTS
The authors thank Evonik Nutrition & Care GmbH for financial support.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Aldridge G.M., Podrebarac D.M., Greenough W.T., Weiler I.J. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J.C., Anthony T.G., Kimball S.R., Vary T.C., Jefferson L.S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J. Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Atherton P.J., Smith K., Etheridge T., Rankin D., Rennie M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Balage M., Dardevet D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:265–270. doi: 10.1097/MCO.0b013e328336f6b8. [DOI] [PubMed] [Google Scholar]
- Battu S., Minhas G., Mishra A., Khan N. Amino acid sensing via general control nonderepressible-2 kinase and immunological programming. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry C., El-Kadi S.W., Suryawan A., Wheatley S.M., Orellana R.A., Kimball S.R., Nguyen H.V., Davis T.A. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2013;305:E620–E631. doi: 10.1152/ajpendo.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P., Reggiori F., Codogno P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets S., Alvarez B., Llovera M., Agell N., López-Soriano F.J., Argilés J.M. Branched-chain amino acids inhibit proteolysis in rat skeletal muscle: mechanisms involved. J. Cell. Physiol. 2000;184:380–384. doi: 10.1002/1097-4652(200009)184:3<380::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress Broiler Performance & Nutrition Supplement. 2015. http://www.cobb-vantress.com
- Dann S.G., Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett. 2006;580:2821–2829. doi: 10.1016/j.febslet.2006.04.068. [DOI] [PubMed] [Google Scholar]
- Davis T.A., Suryawan A., Orellana R.A., Nguyen H.V., Fiorotto M.L. Postnatal ontogeny of skeletal muscle protein synthesis in pigs. J. Anim. Sci. 2008;86:E13–E18. doi: 10.2527/jas.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Zheng A, Liu G, Chang W, Zhang S, Cai H. Activation of mammalian target of rapamycin signaling in skeletal muscle of neonatal chicks: effects of dietary leucine and age. Poult. Sci. 2014;93:114–121. doi: 10.3382/ps.2013-03287. [DOI] [PubMed] [Google Scholar]
- Eaton S.L., Roche S.L., Llavero Hurtado M., Oldknow K.J., Farquharson C., Gillingwater T.H., Wishart T.M. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J., Frank J.W., Suryawan A., Kimball S.R., Nguyen H.V., Jefferson L.S., Davis T.A. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J. Nutr. 2005;135:1374–1381. doi: 10.1093/jn/135.6.1374. [DOI] [PubMed] [Google Scholar]
- Escobar J., Frank J.W., Suryawan A., Nguyen H.V., van Horn C.G., Hutson S.M., Davis T.A. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J. Nutr. 2010;140:1418–1424. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahien L.A., Macdonald M.J. The complex mechanism of glutamate dehydrogenase in insulin secretion. Diabetes. 2011;60:2450–2454. doi: 10.2337/db10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Kou X., Jia S., Yang X., Yang Y., Chen N. Autophagy as a potential target for sarcopenia. J. Cell. Physiol. 2016;231:1450–1459. doi: 10.1002/jcp.25260. [DOI] [PubMed] [Google Scholar]
- Foster E.B., Fisher G., Sartin J.L., Elsasser T.H., Wu G., Cowan W., Pascoe D.D. Acute regulation of IGF-I by alterations in post-exercise macronutrients. Amino Acids. 2012;42:1405–1416. doi: 10.1007/s00726-011-0837-y. [DOI] [PubMed] [Google Scholar]
- Gao X., Tian F., Wang X., Zhao J., Wan X., Zhang L., Wu C., Li N. Leucine supplementation improves acquired growth hormone resistance in rats with protein-energy malnutrition. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0125023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatnau R., Zimmerman D.R., Nissen S.L., Wannemuehler M., Ewan R.C. Effects of excess dietary leucine and leucine catabolites on growth and immune responses in weanling pigs. J. Anim. Sci. 1995;73:159–165. doi: 10.2527/1995.731159x. [DOI] [PubMed] [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie) DLG-Verlag; Frankfurt/Main, Germany: 1999. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler) [Google Scholar]
- Glass D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:225–229. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulou D., Lafave L., Schweim K., Gannon M.C., Nuttall F. Q. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57:1747–1752. doi: 10.1016/j.metabol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Langley B., Thomas M., Bishop A., Sharma M., Gilmour S., Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- Lecker S.H., Goldberg A.L., Mitch W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- Li R., Liu P., Liu P., Tian Y., Hua Y., Gao Y., He H., Chen J., Zhang Z., Huang Y. A novel liquid chromatography tandem mass spectrometry method for simultaneous determination of branched-chain amino acids and branched-chain α-keto acids in human plasma. Amino Acids. 2016;48:1523–1532. doi: 10.1007/s00726-016-2212-5. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mattick J.S.A., Kamisoglu K., Ierapetritou M.G., Androulakis I.P., Berthiaume F. Branched-chain amino acid supplementation: impact on signaling and relevance to critical illness. WIREs Syst. Biol. Med. 2013;5:449–460. doi: 10.1002/wsbm.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métayer S., Seiliez I., Collin A., Duchêne S., Mercier Y., Geraert P.-A., Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem. 2008;19:207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Mitchell W.K., Wilkinson D.J., Phillips B.E., Lund J.N., Smith K., Atherton P.J. Human skeletal muscle protein metabolism responses to amino acid nutrition. Adv. Nutr. 2016;7:828S–838S. doi: 10.3945/an.115.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordier S., Deval C., Béchet D., Tassa A., Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J. Biol. Chem. 2000;275:29900–29906. doi: 10.1074/jbc.M003633200. [DOI] [PubMed] [Google Scholar]
- Nakai N., Kobayashi R., Popov K.M., Harris R.A., Shimomura Y. Determination of branched-chain alpha-keto acid dehydrogenase activity state and branched-chain alpha-keto acid dehydrogenase kinase activity and protein in mammalian tissues. Methods Enzymol. 2000;324:48–62. doi: 10.1016/s0076-6879(00)24218-3. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Ishida A., Yamazaki M., Abe H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem. Biophys. Res. Commun. 2005;336:660–666. doi: 10.1016/j.bbrc.2005.08.138. [DOI] [PubMed] [Google Scholar]
- [NRC] National Research Council . Natl. Acad. Press; Washington, D.C.: 2012. Nutrient Requirements of Swine. [Google Scholar]
- Ono K., Hakozaki M., Suzuki T., Mori T., Hata H., Kochi H. cDNA cloning of the chicken branched-chain alpha-keto acid dehydrogenase complex. Eur. J. Biochem. 2001;268:727–736. doi: 10.1046/j.1432-1327.2001.01925.x. [DOI] [PubMed] [Google Scholar]
- Pasiakos S.M., Carbone J.W. Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise. IUBMB Life. 2014;66:478–484. doi: 10.1002/iub.1291. [DOI] [PubMed] [Google Scholar]
- Rieu I., Balage M., Sornet C., Giraudet C., Pujos E., Grizard J., Mosoni L., Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri H., von Soosten D., Meyer U., Kluess J., Dänicke S., Saremi B., Sauerwein H. Plasma amino acids and metabolic profiling of dairy cows in response to a bolus duodenal infusion of leucine. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0176647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Austic R.E. The branched-chain amino acid antagonism in chicks. J. Nutr. 1978;108:1180–1191. doi: 10.1093/jn/108.7.1180. [DOI] [PubMed] [Google Scholar]
- Suryawan A., Hawes J.W., Harris R.A., Shimomura Y., Jenkins A.E., Hutson S.M. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- Tsien C., Davuluri G., Singh D., Allawy A., ten Have G.A.M., Thapaliya S., Schulze J.M., Barnes D., McCullough A.J., Engelen M.P.K.J., Deutz N.E., Dasarathy S. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., de Preter K., Pattyn F., Poppe B., van Roy N., de Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary T.C., Lynch C.J. Nutrient signaling components controlling protein synthesis in striated muscle. J. Nutr. 2007;137:1835–1843. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]
- Wang D.-T., Yang Y.-J., Huang R.-H., Zhang Z.H., Lin X. Myostatin activates the ubiquitin-proteasome and autophagy-lysosome systems contributing to muscle wasting in chronic kidney disease. Oxid. Med. Cell. Longev. 2015;2015:1–18. doi: 10.1155/2015/684965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A.G., Kluge H., Hirche F., Kiowski A., Schutkowski A., Corrent E., Bartelt J., König B., Stangl G.I. High leucine diets stimulate cerebral branched-chain amino acid degradation and modify serotonin and ketone body concentrations in a pig model. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F.A., Suryawan A., Gazzaneo M.C., Orellana R.A., Nguyen H.V., Davis T.A. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J. Nutr. 2010;140:264–270. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F.A., Suryawan A., Orellana R.A., Gazzaneo M.C., Nguyen H.V., Davis T.A. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids. 2011;40:157–165. doi: 10.1007/s00726-010-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F.A., Suryawan A., Orellana R.A., Kimball S.R., Gazzaneo M.C., Nguyen H.V., Fiorotto M.L., Davis T.A. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J. Nutr. 2009;139:1873–1880. doi: 10.3945/jn.109.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltafsky M.K., Pfaffl M.W., Roth F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010;103:964–976. doi: 10.1017/S0007114509992212. [DOI] [PubMed] [Google Scholar]
- Xia X., Wang X., Li Q., Li N., Li J. Essential amino acid enriched high-protein enteral nutrition modulates insulin-like growth factor-1 system function in a rat model of trauma-hemorrhagic shock. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0077823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Wu C., Li K., Gui G., Zhang G., Yang H. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J. Anim. Sci. Biotechnol. 2017;8:43. doi: 10.1186/s40104-017-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Chi Y., Burkhardt B.R., Guan Y., Wolf B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010;68:270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Dolinger M., Ritaccio G., Mazurkiewicz J., Conti D., Zhu X., Huang Y. Leucine stimulates insulin secretion via down-regulation of surface expression of adrenergic α2A receptor through the mTOR (mammalian target of rapamycin) pathway: implication in new-onset diabetes in renal transplantation. J. Biol. Chem. 2012;287:24795–24806. doi: 10.1074/jbc.M112.344259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Yao K., Liu Z., Gong M., Ruan Z., Deng D., Tan B., Liu Z., Wu G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. 2010;39:1477–1486. doi: 10.1007/s00726-010-0612-5. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F., Mochizuki S., Sugahara K. Differential dose response of mTOR signaling to oral administration of leucine in skeletal muscle and liver of rats. Biosci. Biotechnol. Biochem. 2013;77:839–842. doi: 10.1271/bbb.120737. [DOI] [PubMed] [Google Scholar]
- Zeitz J.O., Käding S.C., Niewalda I.R., Machander V., de Paula Dorigam J.C., Eder K. Effects of leucine supplementation on muscle protein synthesis and degradation pathways in broilers at constant dietary concentrations of isoleucine and valine. Arch. Anim. Nutr. 2019;73:75–87. doi: 10.1080/1745039X.2019.1583519. [DOI] [PubMed] [Google Scholar]
- Zeitz J.O., Most E., Eder K. Conjugated linoleic acid influences the metabolism of tocopherol in lactating rats but has little effect on tissue tocopherol concentrations in pups. Lipids Health Dis. 2016;15:102–111. doi: 10.1186/s12944-016-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yang J., Liao W., Liu X., Zhang H., Wang S., Wang D., Feng J., Yu L., Zhu W.-G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]


