Abstract
This study was conducted to investigate the effects of dietary bamboo leaf extract (BLE) on growth performance, meat quality, oxidative stability, and nuclear factor erythroid 2-related factor 2 (Nrf2) related gene expression of breast meat in broilers. A total of 576 one-day-old male Arbor Acres broilers were divided into 6 groups. The control group (CTR) was fed basal diet, while BLE1, BLE2, BLE3, BLE4, and BLE5 were fed basal diet supplemented with 1.0, 2.0, 3.0, 4.0, and 5.0 g BLE per kg feed, respectively. Compared with the CTR group, BLE2 and BLE5 increased average daily feed intake from 1 to 21 D and 22 to 42 D (P < 0.05), BLE1 and BLE2 improved average daily gain (ADG) and feed to gain ratio from 22 to 42 D (P < 0.05). Throughout the trial period, the highest body weight and favorable ADG and feed to gain ratio were observed in the BLE2 group. The drip loss at 24 h and pH at 45 min postmortem of breast meat were linearly improved by BLE supplementation (P < 0.05). Shear force was significantly lower in BLE2 and BLE3 than that in CTR group. Increasing supplementation of BLE linearly improved free radical scavenging capacity and decreased malondialdehyde content of breast meat during 12 D of storage (P < 0.05). Total antioxidant capacity and glutathione peroxidase activity were linearly increased by BLE supplementation (P < 0.05). Compared with the CTR group, the mRNA expression of Nrf2 and glutathione peroxidase in BLE3, BLE4, and BLE5 groups was significantly promoted, and glutathione S-transferase gene expression was increased in BLE2, BLE4, and BLE5 (P < 0.05). The highest (P < 0.05) heme oxygennase-1 gene expression was observed in BLE5. In conclusion, broiler supplemented with BLE improved growth performance and meat quality, BLE supplementation might activate Nrf2 pathway to alleviate lipid oxidation and increase antioxidant capacity of breast meat. The dosage of 2.0 to 3.0 g/kg BLE in broiler diet was recommanded.
Key words: bamboo leaf extract, chicken, diet, meat quality, Nrf2 gene expression
INTRODUCTION
From nutritional point of view, fat content in poultry meat is relatively low (2.8 g/100 g breast) and with positive unsaturated/saturated acid ratio (Barroeta, 2007). Considering the beneficial effects of n-3 polyunsaturated fatty acids (PUFA), dietary strategies are one of common ways to enrich its content (Sirri et al., 2011). However, enrichment of n-3 PUFA in turn makes poultry meat susceptible to oxidation (Alkhalifa, 2015). At the same time, stress factors in intensive breeding environments lead to imbalance of homeostasis, accelerated oxidative stress, and lower meat quality (Kou et al., 2015). Oxidation continues postmortem and affects the shelf-life of the meat (Smet et al., 2008). Free radicals such as superoxide anion free radicals (O2-•) and hydroxyl radicals (OH•) attack unsaturated fatty acids in biological membranes, resulting in double bond rearrangement of unsaturated lipids. In turn, the membrane lipid is destroyed, and the lipid peroxide malondialdehyde is produced (Liu and Ng, 2000). Lipid oxidation is the main cause of meat quality decline and possible production of toxic compounds (Gray et al., 1996); oxidative damage to lipids may be aggravated in the immediate postmortem and, in particular, during storage (Morrissey et al., 1998). Furthermore, while high PUFA content in meat is considered healthy for human nutrition, these fatty acids are more susceptible to oxidation (Gray et al., 1996). Therefore, it is important to promote free radical scavenging capacity and antioxidant capacity of poultry meat to improve its quality and shelf life. Natural plant extracts, such as Ginkgo biloba extract (Ren et al., 2018), Marigold extract (Wang et al., 2017), and Forsythia suspensa extract (Wang et al., 2008), applied as feed additives added to broiler diets show some promise in serving this purpose.
Bamboo (Bambusoideae) is widely distributed around the world, and bamboo leaves have a long history of medicinal and culinary use in china. Bamboo leaves contain large quantities of active ingredients such as flavonoids, polyphenols, and polysaccharides (Hu et al., 2000). Bamboo leaf extract (BLE) is capable of inhibiting bacterial (Staphylococcus aureus and Escherichia coli) proliferation (Singh et al., 2010) and can be used as a food additive to combat food spoilage pathogens (Zhang et al., 2010). Bamboo leaf extract also has antioxidant and free radical scavenging properties (Guo et al., 2008; Ni et al., 2013), which have been shown to prevent low-density lipoprotein peroxidation induced by copper ions (Hu et al., 2000).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key factor in oxidative stress response of cells. Under normal conditions, intracellular Nrf2 is constantly ubiquitinated and degraded after synthesis (Lee and Johnson, 2004). However, when Nrf2 is isolated from cytoskeleton-associated protein dimer under oxidative stress, it enters the nucleus under the guidance of nuclear localization signaling and binds to antioxidant response elements, initiating the expression of downstream phase II metabolic enzymes to maintain homeostasis (Qiang et al., 2010). Elisabeth et al. (2003) explained that variety of electrophilic compounds including polyphenol and plant-derived constituents are triggers of Nrf2 signal pathway response. However, it is unclear that whether BLE could activate Nrf2 pathway to promote phase II metabolic enzymes expression, such as heme oxygennase-1 (HO-1), superoxide dismutase (SOD), catalase (CAT), which can neutralize peroxide directly, and glutathione peroxidase (GSH-Px) and glutathione S-transferase (GST), which are related to glutathione synthesis and regeneration (Kensler et al., 2007) to improve antioxidant capacity.
Currently, BLE is widely used in food, medicine, and cosmetics, but has not been applied in poultry production. Moreover, there is limited information regarding the effects of BLE on broilers, especially on oxidative stability and Nrf2 pathway of breast meat. Therefore, the objective of this study was to investigate the effects of BLE on growth performance, meat quality, oxidantive stability, and Nrf2 pathway related gene expression of breast meat in broiler chickens, and to determine an appropriate dosage.
MATERIALS AND METHODS
Experimental Design, Birds, and Diets
Bamboo leaf extract was obtained from Zhejiang XinHuang Biotechnology Co., Ltd. (Zhejiang, China), and its main components include flavonoids, polyphenols, and polysaccharides. A total of 576 one-day-old male Arbor Acres broiler chicks were obtained from a local commercial hatchery (Hewei Company, Anhui Province, China) and randomly allotted into 6 groups with 6 replicates containing 16 birds each. Basal diet designed for starter phase (1 to 21 D) and grower phase (22 to 42 D) (Table 1) chickens were supplied according to NRC (1994) recommendations for nutrition requirements. A control group (CTR) was fed with a basal diet, and 5 experimental groups, BLE1, BLE2, BLE3, BLE4, and BLE5, were fed the experimental diets with 1.0, 2.0, 3.0, 4.0, and 5.0 g BLE being added to basal diet per kg feed for 42 D, respectively.
Table 1.
Composition and nutrient level of basal diet.
| Item | Starter phase (1-21 D) | Grower phase (22-42 D) |
|---|---|---|
| Ingredient (%) | ||
| Corn | 57.02 | 61.36 |
| Soybean | 31.3 | 28.3 |
| Corn gluten meal | 3.7 | 1.7 |
| Soy oil | 3 | 4 |
| Dicalcium phosphate | 2 | 1.6 |
| Limestone | 1.2 | 1.3 |
| L-Lysine | 0.33 | 0.31 |
| DL-Methionine | 0.15 | 0.13 |
| Sodium chloride | 0.3 | 0.3 |
| Premix1 | 1 | 1 |
| Total | 100 | 100 |
| Nutrient levels2 | ||
| ME (MJ/kg) | 12.57 | 12.91 |
| Cp (%) | 21.42 | 19.23 |
| Lys (%) | 1.20 | 1.10 |
| Met (%) | 0.50 | 0.44 |
| Calcium (%) | 1 | 0.93 |
| Available phosphorus (%) | 0.46 | 0.39 |
Premix provided per kilogram of diet: VA 10,000 IU, VD3 3,000 IU, VE 30 IU, VK3 1.3 mg, thiamine 2.2 mg, riboflavin 8 mg, niacin 40 mg, choline chloride 600 mg, calcium pantothenate 10 mg, pyridoxine 4 mg, biotin 0.04 mg, folic acid 1 mg, VB12 0.013 mg, zinc 65 mg, iron 80 mg, copper 8 mg, manganese 110 mg, iodine 1.1 mg, selenium 0.3 mg.
Calculated value.
Housing
All birds were kept in 3-layer pens, and each replicate divided into 2 pens. Temperature was maintained at 32°C to 35°C for the first 5 D, then gradually decreased to 22°C and kept stable until the end of the experiment. During the trial period, birds had free access to feed and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (GB14925-2010, NJAU-CAST-2011-093).
Growth Performance and Sample Collection
Feed intake was recorded on daily basis, body weight (BW) and total feed consumption in each replicate were recorded at 1, 21, and 42 D to determine average daily feed intake (ADFI), average daily gain (ADG), and feed to gain ratio (F/G). At the end of the experiment, 2 birds (near the average BW of each replicate) from each replicate were selected for slaughter. A piece of meat sample was cut from left-hand breast meat just after slaughter and stored at –80°C for antioxidant enzymes and gene expression analysis. The right-hand breast meat was stripped from the body and stored at 4°C for meat quality determination and lasted 12 D for shelf life evaluation.
Meat Quality
pH: At 42 D, 2 birds from each replicate were slaughtered, and an integrity breast meat were striped from the body. After post-slaughter for 45 min, pH 45 min of breast meat was one by one measured during sampling process (Wang et al., 2013; Zhang et al., 2015a) using a portable pH meter (HI9125 portable waterproof pH/ORP meter, HANNA Instrument, Italy). Three measurements were taken and the mean value was calculated. After measured pH 45 min, breast meat was stored at 4°C for the following determination.
Meat Color
L* (lightness), a* (redness), and b* (yellowness) of the breast meat were measured using a colorimeter (Konica Minolta Sensing Inc., Osaka, Japan) 24 h postmortem (Zhang et al., 2012, 2015a). Color values at 3 different locations on the cut of meat were recorded and the means were used in further data analyses.
Drip Loss
The drip loss of breast meat was determined as described by Zhang et al. (2015a). Briefly, a 3 × 2 × 1 cm3 cut of breast meat was removed from the same location on each sample to determine drip loss. This sample was weighed and the mass was recorded as W1, and then suspended from a hook and placed in an inflatable zip-lock bag with the direction of the muscle fiber parallel to the direction of gravity and hung at 4°C for 48 h. After 24 h, the sample was removed and cleaned of moisture using filter paper, then weighed to obtain W2. This was repeated at 48 h to obtain W3. Drip loss was then calculated as a percentage, where drip loss at 24 h (%) = (W1 − W2)/W1 × 100%, and drip loss at 48 h (%) = (W1 − W3)/W1 × 100%.
Cooking Loss
Cooking loss was measured according to the method of Zhang et al. (2012). Twenty-four hour postmortem, a 4 × 3 × 1 cm3 cut of breast meat was weighed (M1) and placed in a zip-lock bag, and then cooked in a water bath at 80°C until the core temperature was maintained at 75°C for 10 min. The sample was then cooled in running water to room temperature, dried, and reweighed (M2). Cooking loss was calculated as a percentage, where cooking loss (%) = (M1 − M2)/M1 × 100%.
Shear Force
Shear force was measured according to Schmidt et al. (2013) with some modification. In brief, cooked breast meat after the above experiment was sliced into 3 equal 3 × 1 × 1 cm3 strips. Shear force of each sample was measured using a Digital Meat Tenderness Meter (C-LM3B, Northeast Agricultural University, Harbin, China) perpendicular to the muscle fiber direction. Each sample was measured 3 times and the average was used as the shear force value.
Free Radical Scavenging Capacity of Breast Meat
During 12 D of storage, at 0, 4, 8, and 12 D, 1 g breast meat was removed and homogenized with 4 mL of 0.9% sodium chloride buffer with tube embed in ice using an Ultra-Turrax homogenizer (Tekmar Co., Cincinnati, OH, USA). The OH• (Kit code number: A025-1-1) and O2-• (Kit code number: A018-1-1) scavenging activities, described as inhibition of OH• and O2-• production (Niu et al., 2016), were measured using commercial kits purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China).
Lipid Oxidation Evaluation
Lipid oxidation of breast meat was evaluated based on malondialdehyde (MDA, Kit code number: A003-1-1) content at 0, 4, 8, and 12 D of storage (Wan et al., 2016), using a commercial kit purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China).
Antioxidant Capacity Evaluation of Breast Meat
One gram of breast meat from a sample preserved at –80°C was homogenized by Ultra-Turrax homogenizer (Tekmar Co., Cincinnati, OH, USA) with 4 mL of 0.9% sodium chloride buffer with tube embed in ice and centrifuged at 4,000 rpm at 4°C for 10 min. The supernatant was used to measure SOD (Kit code number: A001-1-1), GSH-Px (Kit code number: A005-1-1), CAT (Kit code number: A007-2-1), glutathione (GSH, Kit code number: A006-1-2), and total antioxidant capacity (T-AOC, Kit code number: A015-1-2) activities using different commercial kits purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China) according to manufacturers' instructions.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Trizol Reagent (Vazyme, NanJing, China, Kit code number: R401-01) was used to extract total RNA from breast meat, which then treated by deoxyribonuclease I to remove the contaminant DNA. RNA was quantified based on the absorption of light by a Nanodrop ND-2000c spectrophotometer (Thermo Scientific, Camden, NJ) at 260 nm (A260) and 280 nm. From each sample, 1μg of RNA was used to synthesize cDNA in a 20 μL reaction mixture using the Primer-Script reagent kit (TaKaRa, Dalian, China, Kit code number: 2690A) following manufacturers' instructions. The real-time quantitative polymerase chain reaction was carried out using the SYBR Premix Ex Taq II kit (TaKaRa, Dalian, China) in an ABI 7300 fluorescence quantitative polymerase chain reaction instrument (Applied Biosystems, Foster City, CA). The 20μL reaction system included 10 μL of SYBR Premix Ex Taq buffer, 0.4μL each of forward and reverse primers and dye, 2μL of cDNA template, and 6.8 μL of distilled water. The real-time polymerase chain reaction cycling conditions were as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 30 s. The relative mRNA expression was determined using β-actin as an internal reference gene. The significance and correlation of quantitative results were analyzed using 2−△△Ct as per Livak and Schmittgen (2001). Primer sequences are shown in Table 2.
Table 2.
Primer sequences used for real-time PCR.
| Gene name1 | Primers sequence (5´-3´) | Gene bank number | |
|---|---|---|---|
| β-actin | Forward | TGCTGTGTTCCCATCTATCG | NM_205518.1 |
| Reverse | TTGGTGACAATACCGTGTTCA | ||
| Nrf2 | Forward | CGCTTTCTTCAGGGGTAGCA | NM_205117.1 |
| Reverse | AGTTCGGTGCAGAAGAGGTG | ||
| HO-1 | Forward | ACGAGTTCAAGCTGGTCACG | NM_205344.1 |
| Reverse | GGATGCTTCTTGCCAACGAC | ||
| GST | Forward | AGAGTCGAAGCCTGATGCAC | NM_001001777.1 |
| Reverse | CACTCCGCTTATCAGCAAACA | ||
| SOD | Forward | CCGGCTTGTCTGATGGAGAT | NM_205064.1 |
| Reverse | TGCATCTTTTGGTCCACCGT | ||
| CAT | Forward | GGTTCGGTGGGGTTGTCTTT | NM_001031215.2 |
| Reverse | CACCAGTGGTCAAGGCATCT | ||
| GSH-Px | Forward | GACCAACCCGCAGTACATCA | NM_001277853.2 |
| Reverse | GAGGTGCGGGCTTTCCTTTA |
Nrf2: nuclear factor erythroid 2-related factor 2; HO-1: heme oxygennase-1; GST: glutathione S-transferase; SOD: superoxide dismutase; CAT: catalase; GSH-Px: glutathione peroxidase.
Statistical Analysis
All data were preliminarily processed using Excel 2016 and analyzed using one-way analysis of variance in SPSS statistical software (Ver. 20.0 for Windows, SPSS, Inc., Chicago, IL). The data were analyzed as a completely randomized design with a replicate as an experimental unit. Duncan's multiple range test was performed to determine differences between treatments. The effects of BLE supplementation at various levels were evaluated using an orthogonal polynomial contrast test for linear and quadratic effects. Differences were regarded as significant atP < 0.05.
RESULTS
Growth Performance
In the starter phase, compared to the CTR group (Table 3), ADFI was significantly higher in the BLE2 and BLE5 groups (P < 0.05), and ABW in the BLE5 group increased significantly (P < 0.05). Meanwhile, there was no difference in F/G under any BLE dosage. In the grower phase, compared with the CTR group, ADFI and ADG in the BLE2 and BLE5 groups were significantly higher than in the CTR group (P < 0.05). However, the BLE1 and BLE2 groups showed a significant decrease in F/G (P < 0.05). Average daily gain and F/G showed quadratic improvement with increasing BLE dosage, and there was a linear and quadratic positive influence on ABW when the BLE levels increased. During the whole rearing period, ADG and F/G improved significantly in the BLE2 group over the CTR group (P < 0.05).
Table 3.
Effect of dietary BLE on growth performance of broilers.
| Diet treatment3 |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTR | BLE1 | BLE2 | BLE3 | BLE4 | BLE5 | SEM1 | Linear2 | Quadratic2 |
| 1-21 D | |||||||||
| ADFI (g) | 44.02c | 45.18a–c | 45.91a | 44.57a–c | 44.35b,c | 45.53a,b | 0.20 | 0.320 | 0.366 |
| ADG (g) | 31.03 | 31.79 | 32.91 | 31.72 | 31.28 | 33.09 | 0.29 | 0.188 | 0.872 |
| F/G | 1.42 | 1.42 | 1.39 | 1.40 | 1.42 | 1.37 | 0.01 | 0.348 | 0.781 |
| ABW (g) | 690.9b | 706.8a,b | 731.8a,b | 706.1a,b | 697.0a,b | 736.8a | 5.96 | 0.142 | 0.894 |
| 22-42 D | |||||||||
| ADFI (g) | 124.53b | 130.26a,b | 133.82a | 128.71a,b | 128.22a,b | 133.53a | 1.12 | 0.132 | 0.449 |
| ADG (g) | 62.77b | 68.72a | 71.46a | 67.37a,b | 66.98a,b | 69.05a | 0.78 | 0.126 | 0.046 |
| F/G | 1.98a | 1.89b | 1.87b | 1.92a,b | 1.91a,b | 1.93a,b | 0.01 | 0.573 | 0.023 |
| ABW (g) | 2015d | 2190a–c | 2277a | 2125b,c | 2112c,d | 2227a,b | 19.27 | 0.024 | 0.032 |
| 1-42 D | |||||||||
| ADFI (g) | 87.13 | 90.16 | 92.57 | 89.30 | 88.66 | 91.35 | 0.77 | 0.400 | 0.424 |
| ADG (g) | 50.21b | 53.19a,b | 55.50a | 52.30a,b | 52.20a,b | 53.66a,b | 0.55 | 0.302 | 0.145 |
| F/G | 1.74a | 1.70a,b | 1.67b | 1.71a,b | 1.70a,b | 1.71a,b | 0.01 | 0.451 | 0.058 |
Means within the same row with no common superscript differ significantly (P < 0.05). ADFI: Average daily feed intake; ADG: Average daily gain; F/G: Feed to gain ratio; ABW: Average body weight.
Standard error of the means.
Orthogonal polynomials were used to evaluate linear and quadratic responses to the levels of BLE treatment.
CTR: basal diet BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively.
Meat Quality
Compared with the CTR group, groups BLE3 and BLE4 showed significantly lower drip loss at 24 h and higher pH at 45 min (P < 0.05; Table 4). Bamboo leaf extract supplementation linearly decreased drip loss at 24 h. Bamboo leaf extract supplementation quadratically decreased shear force, with the lowest shear force measured in the BLE3 group. Drip loss at 48 h and cooking loss showed no significant difference among groups. In addition, L*, a*, and b* meat color values did not differ in supplemented groups compared to the CTR group.
Table 4.
Effect of dietary BLE on meat quality of broilers.
| Diet treatment3 |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTR | BLE1 | BLE2 | BLE3 | BLE4 | BLE5 | SEM1 | Linear2 | Quadratic2 |
| 24 h drip loss (%) | 3.83a | 3.63a,b | 3.46a,b | 2.94b | 2.80b | 3.42a,b | 0.12 | 0.035 | 0.102 |
| 48 h drip loss (%) | 4.59 | 4.55 | 4.21 | 3.57 | 3.60 | 4.15 | 0.14 | 0.051 | 0.161 |
| Cooking loss (%) | 14.25 | 12.80 | 12.54 | 14.04 | 13.96 | 14.37 | 0.32 | 0.405 | 0.175 |
| pH 45 min | 6.53b | 6.59a,b | 6.63a,b | 6.66a | 6.69a | 6.53b | 0.02 | 0.366 | 0.003 |
| L* | 47.40b,c | 47.52b,c | 46.31c | 49.14a,b | 50.27a | 49.43a,b | 0.37 | 0.004 | 0.559 |
| a* | 2.87a | 2.30a,b | 2.47a,b | 1.93b | 2.06b | 2.30a,b | 0.10 | 0.049 | 0.091 |
| b* | 8.75a–c | 8.41b,c | 8.98a–c | 8.16c | 10.14a,b | 10.32a | 0.24 | 0.013 | 0.119 |
| Shearing force (N) | 28.55a | 21.22b,c | 19.51b,c | 18.67c | 23.54a–c | 24.76a,b | 0.81 | 0.400 | <0.001 |
L*: Lightness; a*: Redness; b*: Yellowness.
Means within the same row with no common superscript differ significantly (P < 0.05).
Standard error of the means.
Orthogonal polynomials were used to evaluate linear and quadratic responses to the levels of BLE treatment.
CTR: basal diet BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively.
Free Radical Scavenging Capacity
The free radical scavenging capacity of breast meat decreased over 12 D of storage, regardless of BLE dosage (Table 5). Compared with the CTR group, all supplemented groups except for BLE3 showed significantly higher O2-• scavenging capacity immediately postmortem and after 4 D of storage (P < 0.05). With the exception of 8 D of storage, a linear relationship was observed between BLE level and O2-• scavenging capacity during storage. There was a linear increase in OH• scavenging capacity on days 0 and 4. In addition, the OH• scavenging capacity of BLE groups were significantly higher than the CTR group, with the exceptions of the BLE3 group at day 0, and BLE1 and BLE3 groups at days 4 (P < 0.05). BLE5 showed the highest free radical scavenging capacity among all groups during the storage period.
Table 5.
Effect of dietary BLE on free radical scavenging capacity of breast meat during 12 D of storage of broilers.
| Diet treatment3 |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTR | BLE1 | BLE2 | BLE3 | BLE4 | BLE5 | SEM1 | Linear2 | Quadratic2 |
| O2-• (U/g prot) | |||||||||
| 0 D | 125.00c | 138.23a,b | 136.64a,b | 131.73b,c | 138.55a,b | 141.28a | 1.25 | 0.001 | 0.047 |
| 4 D | 118.14c | 127.86a,b | 130.05a,b | 123.73b,c | 132.04a | 133.08a | 1.21 | <0.001 | 0.406 |
| 8 D | 114.64b | 119.63a,b | 123.19a | 121.04a,b | 122.84a | 119.42a,b | 1.01 | 0.123 | 0.030 |
| 12 D | 110.31b | 113.50a,b | 113.37a,b | 114.78a,b | 116.98a,b | 118.09a | 0.93 | 0.009 | 0.957 |
| OH• (U/mg prot) | |||||||||
| 0 D | 68.39b | 72.31a | 72.72a | 71.87a,b | 73.11a | 75.48a | 0.57 | 0.001 | 0.694 |
| 4 D | 65.11c | 66.89b,c | 68.69b | 64.96c | 69.42b | 73.91a | 0.62 | <0.001 | 0.022 |
| 8 D | 63.24 | 64.31 | 66.58 | 63.34 | 65.16 | 66.80 | 0.55 | 0.133 | 0.931 |
| 12 D | 61.09a,b | 61.75a,b | 61.09a,b | 59.59b | 61.59a,b | 63.46a | 0.43 | 0.260 | 0.086 |
O2-•: Superoxide anion free radicals; OH•: Hydroxyl radicals.
Means within the same row with no common superscript differ significantly (P < 0.05).
Standard error of the means.
Orthogonal polynomials were used to evaluate linear and quadratic responses to the levels of BLE treatment.
CTR: basal diet; BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively.
Malondialdehyde Content
Malondialdehyde concentration increased from 0 to 12 D of storage, regardless of BLE dosage (Table 6). At day 0, MDA concentrations were significantly lower in all experimental groups except BLE1 than in the CTR group (P < 0.05), and a linear decrease occurred as dietary BLE increased. BLE2 meat samples on day 4 and BLE5 samples on day 8 showed the lowest MDA concentrations measured. At day 12, dietary BLE significantly decreased MDA concentrations in all groups except BLE1 in comparison to the CTR group (P < 0.05). Meanwhile, the BLE3 group showed the lowest MDA concentrations, and with BLE supplementation increased, the MDA concentration in breast meat was linearly and quadratically decreased.
Table 6.
Effect of dietary BLE on MDA concentrations of breast meat in broilers during 12 D of storage.
| Diet treatment3 |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTR | BLE1 | BLE2 | BLE3 | BLE4 | BLE5 | SEM1 | Linear2 | Quadratic2 |
| MDA (nmol/mg prot) | |||||||||
| 0 D | 0.431a | 0.374a,b | 0.353b | 0.358b | 0.314b,c | 0.277c | 0.011 | <0.001 | 0.962 |
| 4 D | 0.561a | 0.426b | 0.398b | 0.479a,b | 0.467a,b | 0.449b | 0.014 | 0.180 | 0.029 |
| 8 D | 0.714a | 0.647a,b | 0.617a,b | 0.684a,b | 0.645a,b | 0.531b | 0.022 | 0.057 | 0.575 |
| 12 D | 1.068a | 0.973a,b | 0.771b,c | 0.655c | 0.782b,c | 0.719c | 0.035 | <0.001 | 0.031 |
MDA: malonaldehyde.
Means within the same row with no common superscript differ significantly (P < 0.05).
Standard error of the means.
Orthogonal polynomials were used to evaluate linear and quadratic responses to the levels of BLE treatment.
CTR: basal diet; BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively.
Breast Meat Antioxidant Capacity
Total antioxidant capacity activity in BLE3, BLE4, and BLE5 were significantly higher than the CTR (P < 0.05; Table 7). In addition, there was a linear increase in T-AOC activity as BLE dose increased. Compared with the CTR group, GSH-Px activity in the BLE5 group also significantly increased (P < 0.05), and a linear increase in GSH-Px activity was observed as BLE dosage increased. No significant differences were observed among groups in terms of CAT activity or GSH content.
Table 7.
Effect of dietary BLE on antioxidant enzyme activities of breast meat of broilers.
| Diet treatment3 |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTR | BLE1 | BLE2 | BLE3 | BLE4 | BLE5 | SEM1 | Linear2 | Quadratic2 |
| T-AOC (U/mg prot) | 0.095c | 0.117b,c | 0.174a–c | 0.182a,b | 0.212a | 0.193a,b | 0.012 | 0.001 | 0.212 |
| CAT (U/mg prot) | 0.334 | 0.514 | 0.379 | 0.378 | 0.347 | 0.381 | 0.024 | 0.568 | 0.555 |
| SOD (U/mg prot) | 20.669b | 21.939a | 21.061a,b | 21.462a,b | 20.830a,b | 20.505b | 0.163 | 0.251 | 0.052 |
| GSH (mg/g prot) | 4.808 | 5.042 | 5.577 | 5.173 | 5.087 | 5.182 | 0.112 | 0.489 | 0.211 |
| GSH-Px (U/mg prot) | 37.397b | 38.906a,b | 40.287a,b | 41.261a,b | 39.877a,b | 42.457a | 0.557 | 0.011 | 0.641 |
T-AOC: Total antioxidant capacity; CAT: Catalase; SOD: Superoxide dismutase; GSH: glutathione; GSH-Px: Glutathione S-transferase.
Means within the same row with no common superscript differ significantly (P < 0.05).
Standard error of the means.
Orthogonal polynomials were used to evaluate linear and quadratic responses to the levels of BLE treatment.
CTR: basal diet; BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively.
Gene Expression
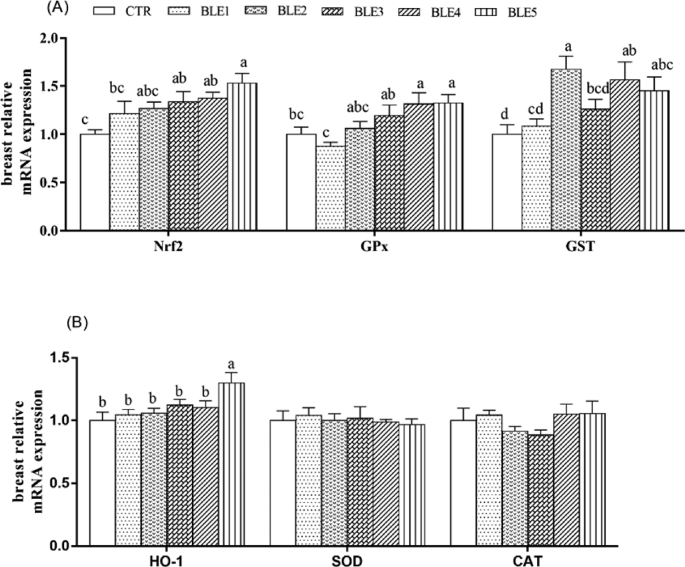
mRNA expression of Nrf2, GSH-Px, and GST increased under BLE treatment, except for GSH-Px in BLE1 (Figure 1 A). In addition, the BLE3, BLE4, and BLE5 groups showed significantly higher Nrf2 mRNA expression compared to the CTR group (P < 0.05). Expression of GSH-Px and GST were also significantly increased in the BLE4 and BLE5 groups (P < 0.05), and the greatest GST expression was observed in the BLE2 group. Furthermore, the BLE5 group showed the highest HO-1 mRNA expression among all groups (P < 0.05; Figure 1 B). Superoxide dismutase and CAT mRNA expression were not significantly different among groups.
Figure 1.
Effects of dietary BLE on Nrf2 and related gene expression of breast meat of broilers. Note: a, b, c, d means within the same gene of the histogram with no common superscript differ significantly (P < 0.05). CTR: basal diet; BLE1, BLE2, BLE3, BLE4, and BLE 5 group, basal diet adding 1.0, 2.0, 3.0, 4.0, and 5.0 g/kg BLE, respectively; (A) including Nrf2: nuclear factor erythroid 2-related factor 2; GSH-Px: glutathione peroxidase; GST: glutathione S-transferase; (B) including HO-1: heme oxygennase-1; SOD: superoxide dismutase; CAT: catalase.
DISCUSSION
Dietary supplementation of BLE improved growth performance in broiler chickens. It is worth mentioning that during the whole rearing period, the BLE2 group showed improved ADFI and ADG by 6.2% and 10.5%, and F/G was significantly decreased by 4.0%, as compared with the CTR group. Li et al. (2017) reported that supplementation with bamboo leaf flavonoids at 2.5 g/kg improved BW gain by 17.6% in broiler chickens. It has been suspected that flavonoids might act as growth hormones in animals due to their hydroxyl groups of aglycone being positioned in space just like estrogen (Havsteen, 2002). Our results showed that there was a linear and quadratic increase in final BW with the supplementation of BLE, and its supplemented dosage at 2.0 g/kg was the most effective, and the dose-dependent effect may be a result of increasing concentration of flavonoids in the diet. As reported in the literature, when birds suffered with challenge, such as heat stress (Dai et al., 2009; Niu et al., 2009) and immune-suppression (Yang et al., 2011), growth performance will be limited. Research shows that supplementation with 1.6 g/kg bamboo leaf flavonoids could counteract growth-suppressing effects of heat stress (Qi et al., 2014) and immune suppression (Zhang et al., 2015b) in broiler chickens. We deduced that flavonoids in BLE may play a major role in promoting growth. However, because of the intricacy nutrient composition of BLE, further study is necessary to explain the possible mechanism. In addition, broilers treated with fermented Ginkgo biloba leaves (Niu, et al., 2016), Marigold extract (Wang et al., 2017), and Broccoli extract (Mueller, et al., 2012), which contain flavonoids, polyphenols that are similar to BLE components, could enhance antioxidant capacity and feed palatability to improve growth performance in chickens. Except the excellent antioxidant property, BLE also has a specific fragrance (Zhang, 2002), which might improve feed palatability, it is pardonable to speculate that the improvement of feed intake might partly result from increasing concentration of dietary BLE.
Water-holding capacity (WHC), tenderness, pH, and meat color are usually used to evaluate meat quality (Castellini et al., 2002). Water-holding capacity including drip loss and cooking loss which refer to the ability to maintain water and are related to nutrition, flavor, and juiciness. Tenderness is considered as the most important trait of meat for consumers, and is affected by the quantity and structure of connective tissue and myofibrils (Wang et al., 2017).
Jiang et al. (2007) reported that meat with higher WHC could alleviate lipid oxidation. Yang and Chen (1993) demonstrated that pH value reflects the rate of carcass glycolysis and associated with shelf life of meat, since oxidation seems to occur rapidly in low-pH meat. Our study shows that supplementation of BLE linearly promoted WHC, and quadratically improved pH 45 min value and shear force, and a dosage of 3.0 g/kg BLE supplementation exhibits a better effect. These results indicated that, to some extent, oxidation was slowed down due to its higher pH and WHC with BLE supplementation. As was reported in the literature that flavonoids, such as alfalfa flavonoids (Ouyang et al., 2016), and genistein (Kamboh and Zhu, 2013) could improve meat quality in broiler chickens. Although there are no reports on the improvement of meat quality by BLE, bamboo leaf flavonoids might be attributed to meat quality improvement. Previous studies have similarly indicated that diets with natural flavonoids, such as soy isoflavone (Jiang et al., 2007), quercetin (Goliomytis et al., 2014) improved meat quality which might have been caused by the increase in antioxidant capacity in broilers. Thus, it is reasonable to suggest that BLE, with flavonoids exhibiting excellent antioxidant ability, improved meat quality in breast meat possibly by attenuating oxidation. In addition, meat with a higher WHC may show a higher pH value and accelerated tenderization, yielding improved quality (Qiao et al., 2001).
Natural antioxidants can also improve WHC by modulating the redox state (Rajput et al., 2014), and increased antioxidant capacity yields a suite of other benefits to meat quality. Our present study indicated that supplementation of BLE linearly improved free radical scavenging capacity in breast meat, and has a dose-independence effect during storage. Luo et al. (2011) reported that the semi-inhibitory concentration of bamboo leaf flavonoids to O2-• was 11.7 μg/mL. Favorable free radical scavenging capacity in breast meat due to increasing BLE inclusion might attribute from increase of dietary flavonoids concentration. In addition, supplementation with BLE linearly lowered MDA concentrations over 12 D of storage, a dosage of 2.0 g/kg exhibits better lipid oxidation inhibition effect. Higher free radical scavenging capacity and lower MDA concentrations occurred at BLE supplementation greater than 1.0 g/kg, with these results indicating that BLE improves the redox state of breast meat. It is clearly demonstrated that flavonoids possess the ability to scavenging free radicals and chelate transition metal ions to prevent lipid oxidation (Ross and Kasum, 2003). Gong et al. (2015) proved that BLE has a strong scavenging effect on free radicals in vitro studies, and other studies have demonstrated that dietary of supplementation with plant extracts rich in flavonoids or polyphenols can increase free radical scavenging capacity and reduce lipid oxidation in broilers (Wan et al., 2016; Kamboh et al., 2017). Such extracts may enhance hydrogen-donating ability to improve free radical scavenging capacity (Zhang et al., 2015a). Furthermore, polyphenols also play a role in inhibiting the formation of thiobarbituric acid-reactive substances (Dejong and Lanari, 2009). Hence, it is reasonable to speculate that the favorable scavenging free radical capacity of BLE might be attributable to the presence of flavonoids and polyphenols in the extract. In our study, diets supplemented with BLE exceeding 2.0 g/kg led to significantly increased activity of T-AOC in breast meat compared to CTR group. In addition, an increase in SOD and GSH-Px level was observed. Previous studies similarly have shown that BLE can significantly increase SOD, GSH-Px, and CAT contents in hyperlipidemic rats, and can improve total antioxidant capacity (Liu et al., 2014; Zhang et al., 2014). According to the present results, it is suggested that BLE promotes antioxidant capacity of breast meat by improving free radical scavenging capacity and inhibiting oxidative processes.
Gene expression of Nrf2-related mRNAs also indicated favorable changes under BLE supplementation. It has been verified that phytogenic extracts activate the Nrf2 signal pathway to induce GSH synthesis in broilers (Aruna et al., 2008; Zhang et al., 2018). The results of the present study showed that supplementation with BLE enhances gene expression of Nrf2, GSH-Px, and GST in breast meat. While SOD and CAT gene expressions were shown without significant difference, which was consistent with the corresponding antioxidant enzymes activities. In addition, Scapagnini et al. (2002) illumiated that natural compounds such as polyphenols may be potent inducers of HO-1 in cells. HO-1 is important for reducing the total production of reactive oxygen species by decomposing heme, releasing carbon monoxide, and biliverdin to counteract cellular dysfunction (Lee et al., 2018). In the present study, HO-1 gene expression was upregulated with increasing BLE supplementation. Since the MDA concentration during 12 D of storage, GSH-Px enzyme activity and Nrf2 pathway related gene expression were improved; it is reasonable to believe that BLE strengthens oxidative stability by improving free radical scavenging capacity and activating GSH-related enzyme gene expression in the Nrf2 signaling pathway. However, as the relationship between antioxidant enzymes and the Nrf2 signaling pathway involving BLE is not fully understood, further research should be carried out to elucidate this aspect.
In summary, broiler chickens supplemented with BLE at a dosage of 2.0 g/kg presented a priority of growth performance. Bamboo leaf extract supplementation linearly improved antioxidant capacity of breast meat. Water-holding capacity and tenderness of breast meat were linearly or quadratically improved by BLE supplementation, and a dosage of 3.0 g/kg BLE showed preferably effects. During 12 D of storage, lipid oxidation was linearly decreased by BLE inclusion, and 2.0 g/kg of which present optimal effects. Taken together, it is suggested that BLE as a potential feed additive with an appropriate dosage from 2.0 to 3.0 g/kg was recommended in poultry production.
ACKNOWLEDGMENTS
This research was financially supported by The National Natural Science Foundation of China (31601973).
REFERENCES
- Alkhalifa H. Production of added-value poultry meat: enrichment with n-3 polyunsaturated fatty acids. World Poult. Sci. J. 2015;71:319–326. [Google Scholar]
- Aruna K., Saravanan R., Samuel C., Se-Ran Y., Megson I.L., Irfan R. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol-Lung Cell. Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Barroeta A.C. Nutritive value of poultry meat: relationship between vitamin E and PUFA. Worlds Poult. Sci. J. 2007;63:277–284. [Google Scholar]
- Castellini C., Mugnai C., Dalbosco A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002;60:219–225. doi: 10.1016/s0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Dai S.F., Wang L.K., Wen A.Y., Wang L.X., Jin G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009;50:333–340. doi: 10.1080/00071660902806947. [DOI] [PubMed] [Google Scholar]
- Dejong S., Lanari M.C. Extracts of olive polyphenols improve lipid stability in cooked beef and pork: contribution of individual phenolics to the antioxidant activity of the extract. Food Chem. 2009;116:892–897. [Google Scholar]
- Elisabeth B., Martha H., Pengfei G., Erin K., Green C.J., Roberta F., Jawed A., Roberto M. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliomytis M., Tsoureki D., Simitzis P.E., Charismiadou M.A., Hagertheodorides A.L., Deligeorgis S.G. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 2014;93:1957–1962. doi: 10.3382/ps.2013-03585. [DOI] [PubMed] [Google Scholar]
- Gong J., Xia D., Huang J., Ge Q., Mao J., Liu S., Zhang Y. Functional cmponents of bamboo shaving and bamboo leaf extracts and their antioxidant activities in vitro. J. Med. Food. 2015;18:453–459. doi: 10.1089/jmf.2014.3189. [DOI] [PubMed] [Google Scholar]
- Gray J.I., Gomaa E.A., Buckley D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996;43:111–123. doi: 10.1016/0309-1740(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Guo X.F., Yue Y.D., Feng T., Jin W. Detection of antioxidative capacity of bamboo leaf extract by scavenging superoxide anion free radical. Spectrosc. Spect. Anal. 2008;28:1823–1826. [PubMed] [Google Scholar]
- Havsteen B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Therap. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Hu C., Zhang Y., Kitts D.D. Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. henonis leaf extract in vitro. J. Agric. Food Chem. 2000;48:3170–3176. doi: 10.1021/jf0001637. [DOI] [PubMed] [Google Scholar]
- Jiang Z.Y., Jiang S.Q., Lin Y.C., Xi P.B., Yu D.Q., Wu T.X. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poult. Sci. 2007;86:1356–1362. doi: 10.1093/ps/86.7.1356. [DOI] [PubMed] [Google Scholar]
- Kamboh A.A., Memon A.M., Mughal M.J., Memon J., Bakhetgul M. Dietary effects of soy and citrus flavonoid on antioxidation and microbial quality of meat in broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:235–240. doi: 10.1111/jpn.12683. [DOI] [PubMed] [Google Scholar]
- Kamboh A.A., Zhu W.Y. Individual and combined effects of genistein and hesperidin supplementation on meat quality in meat-type broiler chickens. J. Sci. Food Agric. 2013;93:3362–3367. doi: 10.1002/jsfa.6185. [DOI] [PubMed] [Google Scholar]
- Kensler T., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kou T., Hu Z.P., Dong L., He J.T., Bai K.W., Wang T. Effect of N,N-Dimethylglycine sodium on slaughter performance, meat quality indices and antioxidant performance of broilers. Food Sci. 2015;36:179–184. [Google Scholar]
- Lee J.M., Johnson J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee M.T., Lin W.C., Wang S.Y., Lin L.J., Yu B., Lee T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2 and NF-κB dominated pathways in broiler chickens. Poult. Sci. 2018;97:2419–2434. doi: 10.3382/ps/pey076. [DOI] [PubMed] [Google Scholar]
- Li Z.T., Shen Z.C., Liu S.G., Zhang D.D., Liu Y., Li K., Dou T.F., Liu L.X., Zhang X., Ge C.R. Effects of bamboo leaf flavonoids on early weight gain and some organ index of Jining Bairi chicken. Chin. Poult. 2017;39:52–54. (in Chinese) [Google Scholar]
- Liu F., Ng T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000;66:725–735. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- Liu L.L., Wu C.J., Miao L., Lou Q.M., Yu S.K. Regulation of lipid metabolism and oxidative stress by bamboo leaf flavonoid and cassia seed compound associated with ascorbyl palmitate and vitamin E succinate in hyperlipidemia rats. Food Sci. Technol. 2014;30:12–16. and 22. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y.Q., Guo H., Hu L.F., Shi L.W., Qian J.Q. Antioxidant activity of flavonoids from bamboo leaves. Food Sci. Technol. 2011;36:201–203. [Google Scholar]
- Morrissey P.A., Sheehya P.J., Galvina K., Kerry J.P., Buckley D.J. Lipid stability in meat and meat products. Meat Sci. 1998;49:S73–S86. [PubMed] [Google Scholar]
- Mueller K., Blum N.M., Kluge H., Mueller A.S. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic- and antioxidant enzymes in broiler chickens. Br. J. Nutr. 2012;108:588–602. doi: 10.1017/S0007114511005873. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ni Q., Xu G., Gao Q., Yang D., Zhang Y. Evaluation of reactive oxygen species scavenging activities and DNA damage prevention effect of Pleioblastus kongosanensis f. aureostriatus leaf extract by chemiluminescence assay. J. Photochem. Photobiol. B. 2013;128:115–121. doi: 10.1016/j.jphotobiol.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Niu Y., Wan X.L., Zhang X.H., Zhao L.G., He J.T., Zhang J.F., Zhang L.L., Wang T. Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult. Sci. 2016;96:869–877. doi: 10.3382/ps/pew313. [DOI] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Ouyang K., Xu M., Jiang Y., Wang W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2016;96:332–341. [Google Scholar]
- Qi Y., Yang Y., Ni Z., Zhang J., Wang Z., Jin Z., Xian Q., Gang S. The effects of bamboo leaf flavonoid on the growth and slaughter performance in broilers under the continued heat stress. Feed Ind. 2014;8:6–8. (in Chinese) [Google Scholar]
- Qiang Z., Jingbo P., Woods C.G., Andersen M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Rajput N., Ali S., Naeem M., Khan M.A., Wang T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on pigmentation, oxidative stability and quality of meat from broiler chickens affected by a coccidiosis challenge. Br. Poult. Sci. 2014;55:501–509. doi: 10.1080/00071668.2014.925537. [DOI] [PubMed] [Google Scholar]
- Ren X., Yang Z., Ding X., Yang C. Effects of Ginkgo biloba leaves (Ginkgo biloba) and Ginkgo biloba extract on nutrient and energy utilization of broilers. Poult. Sci. 2018;97:1342–1351. doi: 10.3382/ps/pex445. [DOI] [PubMed] [Google Scholar]
- Ross J.A., Kasum C.M. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Scapagnini G., Foresti R., Calabrese V., Stella A.M., Giuffrida C. J. Green, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Scheier R., Hopkins D.L. Preliminary investigation on the relationship of Raman spectra of sheep meat with shear force and cooking loss. Meat Sci. 2013;93:138–143. doi: 10.1016/j.meatsci.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Shukla R., Satish V., Kumar S., Gupta S., Mishra A. Antibacterial activity of leaves of bamboo. Int. J. Pharmacol. 2010;2:1–5. [Google Scholar]
- Sirri F., Minelli G., Iaffaldano N., Tallarico N., Franchini A. Oxidative stability and quality traits of n-3 PUFA enriched chicken meat. Ital. J. Anim. Sci. 2011;2:450–452. [Google Scholar]
- Smet K., Raes K., Huyghebaert G., Haak L., Arnouts S., De S.S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult. Sci. 2008;87:1682–1688. doi: 10.3382/ps.2007-00384. [DOI] [PubMed] [Google Scholar]
- Wan X.L., Song Z.H., Niu Y., Cheng K., Zhang J.F., Ahmad H., Zhang L.L., Wang T. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult. Sci. 2016;96:844–850. doi: 10.3382/ps/pew307. [DOI] [PubMed] [Google Scholar]
- Wang L., Piao X.L., Kim S.W., Piao X.S., Shen Y.B., Lee H.S. Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 2008;87:1287–1294. doi: 10.3382/ps.2008-00023. [DOI] [PubMed] [Google Scholar]
- Wang S.H., Zhang L., Li J.L., Gao F., Zhou G.H. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas. J. Anim. Sci. 2017;30:71–77. doi: 10.5713/ajas.16.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Q., Chen X., Tan H.Z., Zhang D.X., Zhang H.J., Wei S., Yan H.C. Nutrient density and slaughter age have differential effects on carcase performance, muscle and meat quality in fast and slow growing broiler genotypes. Br. Poult. Sci. 2013;54:50–61. doi: 10.1080/00071668.2012.745927. [DOI] [PubMed] [Google Scholar]
- Yang C.C., Chen T.C. Effects of refrigerated storage, pH adjustment, and marinade on color of raw and microwave cooked chicken meat. Poult. Sci. 1993;72:355–362. [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hu Z., Lu C., Bai K., Zhang L., Wang T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015;63:3880–3886. doi: 10.1021/jf505889b. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Gong J.Y., Ding Y.T., Lu B.Y., Wu X.Q., Ying Z. Antibacterial activity of water-phase extracts from bamboo shavings against food spoilage microorganisms. Afr. J. Biotechnol. 2010;9:7710–7717. [Google Scholar]
- Zhang J.Y., Lin S.Y., Zhao J., Li X.H., Wang Z.G., Li C.C., Shu G. The effects of bamboo leaf flavonoid on the immune function and growth performance in immunosuppression broilers. J. Sichuan Agric. Univ. 2015;3:314–318. (in Chinese) [Google Scholar]
- Zhang L., Zhang H.J., Qiao X., Yue H.Y., Wu S.G., Yao J.H., Qi G.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen J., Sun A., Zhao L.Y. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Food Agric. Immunol. 2014;25:386–396. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Natural functional extract of bamboo leaves flavonoid. Chin. Food Addit. 2002;3:54–58. (in Chinese) [Google Scholar]