Abstract
Scoring is a common method to evaluate eggshell translucency, and it mainly depends on the area and the density of translucent spots in eggshells. However, the lack of common scoring criteria and the difficulty of quantitatively measuring spots in eggshells impede effective comparisons between research papers and greatly hinder the progress of research on translucent eggshell. To make measurement of translucent eggshells more objective, we optimized the scoring method and compared it with 2 new methods: grayscale recognition and the colorimeter method. Briefly, a total of 354 eggs from 600, 395-day-old dwarf brown laying hens were collected and classified into 4 score groups according to their degree of translucency. This subjective process was repeated 5 times. Then, we captured the profile side of each egg using a camera and measured spot characteristics using grayscale recognition, which involved measuring the quantity of spots (QS), diameter of each spot (DS), average area of each spot (AAES), sum of spot areas (SUSA), sum of shell area (SUSHA), and ratio of SUSA to SUSHA (RSS) on the eggshell. Furthermore, the L, A, and B values of each egg at the sharp, middle, and blunt ends were separately measured using a colorimeter. As a result, average values of 31.31, 29.78, 29.81, and 9.08% of all eggs were divided into score levels 1, 2, 3, and 4 (from opaque to translucent), which correspond with RSS values of 1.34, 3.23, 6.21, and 11.89%, respectively. By grayscale recognition, QS, DS, AAES, SUSA, SUSHA, and RSS all increased along with increased translucency scores (P < 0.05). Using scoring, an egg with a specific RSS value was more easily assigned to a specific score level (50%) or adjacent score levels (50%). The L, A, and B values of eggshells in score level 1 were significantly different from those of eggshells in levels 3 or 4; however, there were no significant differences between any adjacent score levels. In summary, the present study explored objective reference metrics to measure eggshell translucency.
key words: translucent eggshell, hen, scoring method, grayscale recognition, colorimeter
INTRODUCTION
Eggshell translucency is a ubiquitous problem for egg producers. Although it does not affect internal egg quality in 3 wk of storage (Wang et al., 2017), it greatly affects eggshell appearance and reduces the sale value of commodity eggs. In addition, it has been reported that translucent eggshell can be easily penetrated by Salmonella, which increase safety hazards of food eggs (Chousalkar et al., 2010). Since the phenomenon of eggshell translucency was first reported (Holst et al., 1932), little research has been conducted, largely because methods of measuring eggshell translucency were not quantitated (Almquist and Burmester, 1934; Tyler and Standen, 1969; Talbot and Tyler, 1974). Translucent eggs are usually identified by candling the eggs from the sharp end to the blunt end and are then assigned different subjective scores according to the severity of translucency (Holst et al., 1932). Moisture spots in eggshells are known to become translucent, while other areas of the shell remain opaque during candling. Translucent spots in eggshells are results of the transfer of moisture from the egg's contents through the shell membrane and its accumulation in the eggshell (Tyler and Geake, 1964; Solomon, 1991). Considering researches of physical structure and chemical composition in eggshell and shell membrane, variations of eggshell membrane may be the most important reason for translucent eggshell formation (Nie, 2013; Zhang, 2016; Wang, 2017). In addition, shell translucent characteristics such as the size of spots, clarity of spot contours, and time of appearance can be affected by the breed (Baker and Curtiss, 1957), age of hens (Solomon, 1991), food (Jiang, 2015), and by environmental factors such as temperature and humidity (Denison, 1967; Tyler and Standen, 1969). Eggshell translucency is not a permanent trait; in our previous study, we found that the trait became obvious and stable between day 5 and week 2 after the eggs were laid (Wang, 2017). Previous scoring by candling methods used 2, 3, or 4 scoring levels (Holst et al., 1932; Ray and Roberts, 2013; Wang et al., 2017). The existing scoring methods that use candling to determine the severity of shell translucency are simple and efficient, but they lack unified objective criteria or a way to quantify the density and size of spots in eggshells, which prevents researchers from comparing translucency scores from different sources.
In the present study, we explored new methods to measure eggshell translucency. First, we standardized the scoring method by candling methods and evaluated the accuracy of this tested method. Second, we explored 2 new methods for measuring eggshell translucency: grayscale recognition and the colorimetric method. Grayscale recognition focuses on contour recognition and quantitative statistics of moisture spots in eggshells. The colorimetric method focuses on the brightness and absorbance values of moisture spots that are translucent and assigns them a degree of severity. Overall, the study aims to develop an objective method of measuring shell translucency and to provide accurate phenotypic data for future research on translucent eggs.
MATERIALS AND METHODS
Hens and Egg Selection
A total of 600, 395-day-old brown-egg dwarf layer (DWL) hens from a pure line were used in the experiment and were provided by CAU Poultry Breeding Corporation, Beijing, China. By 2017, the DWL line had been bred to its 16th generation. Hens shared identical conditions throughout the course of the experiment: an enclosed hen-house, individual cages, identical food, and a 16-h illumination period. The egg production rate was approximately 70%. To measure eggshell translucency, we first collected 415 eggs from the flock, laid in a single day, and marked them on the blunt end with specific Arabic numerals corresponding to individuals. Eggs with broken shells, sand shells (with ridges of small lumps of calcified material), soft shells, malformed shells, or weights that fell outside the 45 to 60 g range were not included in the experiment, leaving 354 eggs for shell translucency measurement. Prior to translucency measurements, we stored the eggs under constant conditions (temperature 20 to 25°C, RH 50 to 60%) for 5 D to render the outline of the spots clearer and more stable.
During the experiment, all animal procedures strictly followed the recommendations of the relevant national and local animal welfare bodies. Protocols were approved by the Animal Care and Use Committee of China Agricultural University (permit number: SYXK 2007-0023).
Eggshell Translucency Measurements
After being stored for 5 D, eggs were classified into 4 score levels, and the scoring procedures were repeated 5 times. Then, each egg was measured by grayscale recognition and colorimetric methods. Quantitative indicators of moisture spots on each egg were calculated using the grayscale recognition method, and the degree of eggshell translucency was ranked according to the ratio of the sum of the area of translucent spots to the total eggshell area (RSS). Then eggs were divided into 4 groups according to their RSS values, from small to large, and the number of eggs in each group was determined by taking the average of 5 independent scoring sessions. Colorimetric values of each group were measured using the colorimetric method. Details of the 3 methods used for eggshell translucency measurement are shown below.
Grading Method
When moisture spots in eggshells became obvious and stable, the grading process was conducted in a dark room. First, we scanned hundreds of eggs by candling and subjectively selected 4 eggs as reference samples for scoring levels 1 through 4, on the basis of spot size and density (Figure 1). The 4 reference eggs were placed vertically on an LED cold light source (HLK, Tongfa Corp., Dezhou, China) and candled from sharp end to blunt end; moisture spots in the eggshells appeared translucent, whereas other areas were opaque (Figure 2 a1). Then, we candled the remaining eggs and classified them into different score levels according to the reference samples. To reduce random error, the translucency score of each egg was evaluated by 3 operators. If a minimum of 2 operators assigned an egg to the same score, that score would be recorded for that egg, along with its identifying number. After all eggs were examined once, the order of all eggs was disrupted and the above procedures repeated another 4 times. The numbers of eggs that fell into each score level were calculated by the average count of the 5 repeated scores, and these numbers formed the grouping basis for the grayscale recognition method.
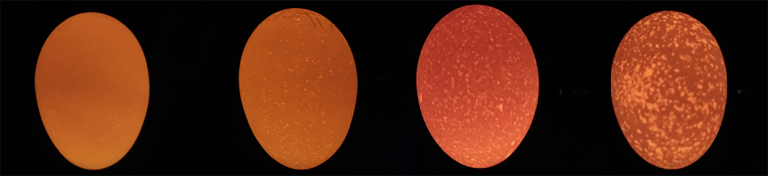
Figure 1.
Four reference samples used for scoring method, from left to right to be score 1, 2, 3, and 4.
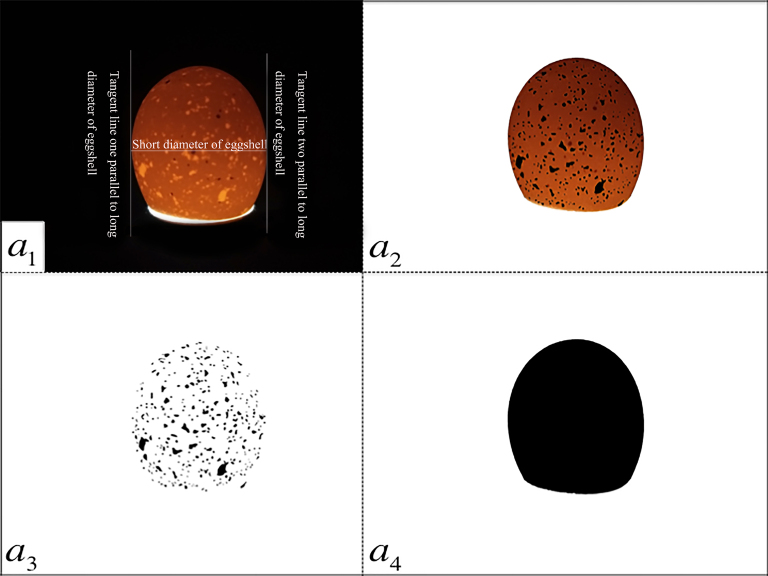
Figure 2.
Procedures of moisture spots identification on eggshell by grayscale recognition method. a1 was eggshell profile by candling from sharp end to blunt end, long diameter of eggshell is default to parallel to y-axis, the shortest distance between 2 tangent lines of eggshell parallel to y-axis is short diameter of shell, the actual length of short diameter is known and the pixels of short diameter in picture can be counted, so the ratio between actual length and graph length of eggshell is determined; a2 moisture spots on eggshell were painted black using Photoshop CS6 software; a3: spots on eggshell were extracted by Image-Pro Plus 6.0 software and opaque area of eggshell were discarded; a4: profile of both moisture spots and opaque areas on eggshell was extracted by the Image-Pro Plus software.
Grayscale Recognition Method
To measure the density and area of moisture spots in eggshells, we used a new method known as grayscale recognition. Using software combined with manual processing and automated identification, the method aims to improve the accuracy and objectivity of eggshell translucency measurements. First, the length and width of each egg were measured with an egg quality analyzer (NFN384, FNK Corp., Tokyo, Japan). Second, eggs were candled using an LED cold light source (HLK, Tongfa Corp., Dezhou, China). A random profile of eggshell was photographed in a vertical stance from a distance of 15 cm away using a digital camera (ILCE-5000 L, Sony Corp., Thailand, Japan), and the photo was saved as a 1 (Figure 2a 1). Next, a 1 was opened using Microsoft Paint software (mspaint.exe, Microsoft Corp.) in Windows 7 operating system (Microsoft Corp., Redmond, WA), and 2 tangent lines on the eggshell along the long axis of the shell were drawn to determine the short diameter of the eggshell. The shortest distance between the 2 tangent lines was the short diameter of the eggshell, and the pixels of the shell short diameter on a 1 were counted to confirm the correspondence between the actual length of the short diameter and the quantity of pixels in the picture. Next, a 1 was opened using Photoshop (Photoshop CS6, Adobe Systems Corp., San Jose, CA), and the image of the eggshell was cut out and pasted onto a blank background; each spot was selected manually and filled in black, and this file was saved as a 2 (Figure 2a 2). As the outline between translucent spots and opaque areas was blurry and not suitable for automatic recognition, this was the most important and most time-consuming step. We next opened a 2 using Image-Pro Plus 6.0 software (Media Cybernetics Corp., Silver Spring, MD) and clicked the action buttons as follows: Edit—Convert to grayscale 8–Measure—Count/size—Select, selected range from 15 to 255. These instructions changed the translucent spots to black, while the opaque area of the shell and background became white. The image was saved as a 3 (Figure 2a 3). The range above was set from 250 to 255, so that the entire eggshell became black while the background was white, and then the image was saved as a 4 (Figure 2a 4). Finally, a 3 and a 4 were opened using ImageJ software (version 1.41, National Institutes of Health) and the action buttons below were clicked: Analyse—Set Scale—Set Distance in Pixels and Known Distance—OK—Analyze—Analyze Particles—Select Options of Output Result. Then we measured quantitative indicators of translucent spots in eggshells, including quantity of spots (QS), sum area of the whole eggshell (SUSHA), sum of spot areas on the whole eggshell (SUSA), average spot area in eggshell (AAES), diameter of spots in eggshell (DS), and RSS.
Colorimetric Method
Moisture spots in eggshells render the shell translucent, reflecting less light from these areas and transmitting more light than other opaque areas (Solomon, 1991). Thus, a colorimeter has the potential to measure the change of shell color of eggs that have different degrees of translucency. RGB, CMYK, and LAB are 3 universal models for color measurement. The LAB model has a wider gamut than RGB and CMYK models and more uniform color distribution than the RGB model; therefore, we selected LAB color model for the current experiment (León et al., 2006). After egg images were captured for grayscale recognition, the L, A, and B values of 3 parts (blunt, middle, and sharp ends) of each egg itself were measured using a portable spectrophotometer (CM-2600d, Konica Minolta, Inc., Tokyo, Japan). L represents luminosity, ranges from 0 to 100, and corresponds to color changes from black to white; A ranges from –120 to 120 and corresponds to color changes from green to red; and B ranges from –120 to 120 and corresponds to color changes from blue to yellow. For this method, eggs were divided into 4 groups according to RSS rankings by the grayscale recognition method, and color attributes of each group were compared.
Statistical Analysis
Outliers—values outside mean ± 3 SD—were excluded. Differences between groups were analyzed by the general linear model using SPSS software (version 23.0, SPSS Inc., Chicago, IL) and modeled as follows:
where μ is the overall average, ai is effect of groups, andeij is residual error. The significance of differences between groups was analyzed by Duncan multiple comparison.
Before variance analysis, percentage data for RSS (<30%) were first subjected to data conversion, using the following formula:
where X represents the observed values and Y the values after conversion.
RESULTS AND DISCUSSION
Results of statistical analyses of eggs in each score level are shown in Table 1. Eggs were classified into levels 1 (31.31% of all eggs), 2 (29.78%), 3 (19.81%), and 4 (9.08%). Fewer eggs received score level 4 than received score levels 1, 2, or 3 (P < 0.05), while there was no significant difference in incidence among the number of eggs scored at levels 1, 2, and 3. The standard deviations of the number of eggs at each score level were approximately 10% of the corresponding average value, meaning the method applied in the study is reliable for egg translucency measurement (Reed et al., 2002). Although in previous studies very little statistical evaluation was performed on scores of shell translucency, the concept of score levels was mentioned (Holst et al., 1932; Ray and Roberts, 2013). Lack of a uniform standard means that in a flock with less severe eggshell translucency, an egg of medium translucency may be classified as score 3 or 4 using the 4 score levels measurement method, while in a flock with severe eggshell translucency, an egg with the same degree of translucency may be classified as score 1 or 2 (Jiang, 2015; Wang, 2017). This indicates that criteria for identification of eggshell translucency by scoring method are not consistent across different research studies. Furthermore, even within the same study, the scoring method is subjective and it depends on the operator. The present study used 3 operators to reduce random human error. We recommend that when evaluating eggshell translucency by the scoring method, factors such as hen breed, age, temperature, and humidity (Tyler and Standen, 1969; Solomon, 1991) should be taken into consideration, and sample pictures of translucent eggs should be used as references.
Table 1.
Quantity distribution of translucent eggs classified by the scoring method over 5 replicates.
| Indicate | Time | Score 1 | Score 2 | Score 3 | Score 4 | Total |
|---|---|---|---|---|---|---|
| Quantity of egg | 1st | 123 | 109 | 90 | 32 | 354 |
| 2nd | 123 | 100 | 95 | 27 | 345 | |
| 3rd | 99 | 107 | 106 | 32 | 344 | |
| 4th | 115 | 92 | 105 | 32 | 344 | |
| 5th | 82 | 107 | 119 | 34 | 342 | |
| Mean | 108.4 ± 17.71a | 103 ± 7.03a | 103 ± 11.2a | 31.4 ± 2.61b | 345.8 | |
| Percentage (%) | 31.31 ± 4.88a | 29.78 ± 1.93a | 29.81 ± 3.56a | 9.08 ± 0.78b | 100 |
Among 4 score levels mean without a common superscript differ (P < 0.05).
Results of translucency spots measured by the grayscale recognition method are presented in Table 2, and the frequency distribution of RSS before and after conversion is presented in Figure 3. Table 2 shows how eggs were divided into 4 groups (1, 2, 3, and 4) according to their RSS values, ranked from small to large. The RSS values of groups 1, 2, 3, and 4 are 1.34, 3.23, 6.21, and 11.89%, respectively, corresponding to the 4 translucency score levels. As shell translucency increased, QS, SUSA, AAES, and DS also increased, and there was a significant difference between any 2 of the 4 groups (P < 0.05). The overall coefficients of variation (CV) values of RSS, SUSA, and DS were 24.81, 26.58, and 18.78% (22%), and the variations were small enough to distinguish mean values of each group (Reed et al., 2002). Meanwhile, RSS group 1 showed the largest variation, indicating that the group may be divided into 2 scoring levels. Thus, a 5-level scoring system may also be suitable for the translucent shell measurement. The overall CV values of QS and AAES were relatively large, at 38.66 and 38.79%, respectively. Shells with low-density large spots or high-density small spots had the same SUSA and shared the same translucency group, which can increase variations in QS and AAES within each group. Both scoring and grayscale recognition methods focus on the areas and density of translucent spots in eggshell. In fact, grayscale recognition is an extension of the scoring method and can quantify spots in eggshells, reflecting the degree of shell translucency relatively objectively. However, there is scope for improvement of the grayscale recognition method. When eggs are photographed in a dark room, an incomplete shell profile and the inclined angles of the blunt end, the middle, and the sharp end can make SUSA, SUSHA, AAES, and DS values smaller than they should be. As RSS is SUSA/ SUSHA, the indicator RSS may be less affected by any errors.
Table 2.
Quantitative indicators of spots on eggshell corresponding with eggs of 4 score groups.
| Indicate/Group | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| N | 100 | 93 | 92 | 28 |
| Range of RSS (%) | ≤2.22 | 2.22 < P ≤ 4.38 | 4.38 < P ≤ 9.02 | >9.02 |
| RSS (%) | 1.34 ± 0.52a | 3.23 ± 0.54b | 6.21 ± 1.16c | 11.89 ± 2.98d |
| C.V. (%) | 38.8 | 16.71 | 18.67 | 25.06 |
| QS | 127.51 ± 59.01a | 248.82 ± 93.03b | 344.48 ± 116.38c | 403.74 ± 150.26d |
| C.V. (%) | 46.27 | 37.38 | 33.78 | 37.21 |
| SUSHA (cm2) | 17.52 ± 1.28 | 17.57 ± 1.4 | 17.26 ± 1.56 | 17.26 ± 1.67 |
| C.V. (%) | 7.31 | 7.96 | 9.03 | 9.67 |
| SUSA (cm2) | 0.23 ± 0.1a | 0.56 ± 0.11b | 1.07 ± 0.22c | 2.03 ± 0.46d |
| C.V. (%) | 43.47 | 19.64 | 20.56 | 22.66 |
| AAES (cm2) | 0.0021 ± 0.00092a | 0.0025 ± 0.00082b | 0.0033 ± 0.0010c | 0.0058 ± 0.0028d |
| C.V. (%) | 43.8 | 32.8 | 30.3 | 48.27 |
| DS (cm2) | 0.050 ± 0.010a | 0.056 ± 0.0093b | 0.064 ± 0.010c | 0.083 ± 0.019d |
| C.V.(%) | 20 | 16.6 | 15.62 | 22.89 |
C.V.: coefficient of variation; QS: quantity of spots on eggshell; SUSHA: sum area of the whole eggshell; SUSA: sum of spots areas on the whole eggshell; AAES: average spots area in eggshell; DS: diameters of spots on eggshell; RSS: ratio of SUSA to SUSHA.
Among 4 groups mean without a common superscript differ (P < 0.05).
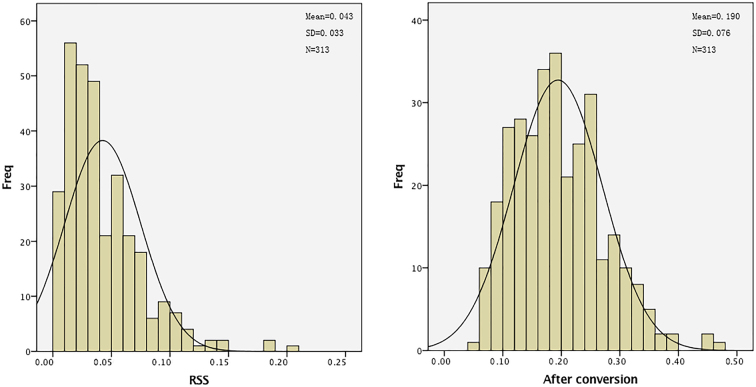
Figure 3.
Frequency distribution of RSS before and after data conversion. RSS: ratio of SUSA to SUSHA; SUSA: sum of spots areas on the whole eggshell; SUSHA: sum area of the whole eggshell; and conversion formula is as follows: Y = Arc sin (Sqrt(X)), where X are the observed values, and Y are values after conversion.
Table 3 demonstrates the reproducibility of the scoring method when the method was applied 5 times to classify shell translucency levels. Table 3 shows that for eggs with RSS values from 0 to 2.21%, an average of 70.61 and 27.14% of all eggs were scored as levels 1 and 2, respectively. In eggs with RSS values of 2.21 to 4.38%, an average of 48.47% were scored as level 2, while the remainder were almost equally divided between the adjacent score levels (1 and 3). In eggs with RSS values of 4.38 to 9.02%, 62.41% were scored as level 3 and 33.17% were scored as levels 2 and 4. In eggs with RSS above 9.02%, 99.24% were equally divided between score levels 3 and 4. By independently scoring the 313 eggs 5 times, we observed that eggs with intermediate RSS values (RSS: 2.21 to 4.38%, corresponding to score level 2, and RSS: 4.38 to 9.02%, corresponding to score level 3) were most likely (50%) to be assigned to 3 score levels: a corresponding translucency score level (50%) and 1 of the 2 adjacent score levels (50%). Eggs with small RSS values (RSS: 0 to 2.21%, corresponding to score level 1 and appearing opaque) and large RSS values (RSS: 9.02 to 100%, corresponding to score level 4 and appearing translucent) were most likely (>97%) to be assigned to 2 score levels: a corresponding and one adjacent score level. Fewer than 3% of all eggs were assigned to translucency score levels that deviated by 2 levels from the corresponding translucency score level, and no eggs were assigned to score levels deviating by 3 levels from the corresponding translucency score level. The results suggest that the scoring method has moderate reproducibility for intermediate translucent eggs and is highly accurate for the distinction between opaque (score 1) and translucent (score 4) eggshells.
Table 3.
Percentages of eggs with specific RSS divided in 4 different scores in 5 times scoring.
| RSS (%) | N | Score 1 (%) | Score 2 (%) | Score 3 (%) | Score 4 (%) |
|---|---|---|---|---|---|
| 0 to 2.21 | 100 | 70.61 ± 7.71a | 27.14 ± 7.3b | 2.24 ± 0.85c | 0.00 ± 0.00c |
| 2.21 to 4.38 | 93 | 27.6 ± 5.78a | 48.47 ± 4.25b | 23.47 ± 5.83a | 0.43 ± 0.59c |
| 4.38 to 9.02 | 92 | 4.39 ± 2.33a | 22.85 ± 7.74b | 62.41 ± 8.7c | 10.32 ± 2.27a |
| 9.02 to 100 | 28 | 0.00 ± 0.00a | 0.74 ± 1.65a | 49.62 ± 4.22b | 49.62 ± 4.22b |
RSS: ratio of sum of spots areas on the whole eggshell to area of the whole eggshell.
Among 4 groups mean without a common superscript differ (P < 0.05).
The shell colors of eggs with different RSS values measured by the colorimetric method are presented in Table 4. The L value of eggs in group 3 was significantly higher than that of group 1 (P < 0.05), the A value of eggs in group 4 was significantly lower than that of group 1 (P < 0.05), and the B values of groups 3 and 4 were significantly higher than that of group 1 (P < 0.05). Thus, with increasing RSS values, the L value increases while the A and B values decline. In terms of shell color phenotype, with increasing translucency, the shell becomes whiter, redder, and yellow. However, due to the variations in L, A, and B values among different groups, there was no significant difference in those values between adjacent RSS groups (P > 0.05). These findings suggest that the colorimetric method has the potential to be used to distinguish between opaque eggs (score 1) and translucent eggs (score 4), but it is not sufficiently precise to distinguish between eggs of adjacent RSS groups. In addition, as this measurement method was performed on eggs with brown shells, the feasibility of the measurement method on white-shelled eggs and blue-shelled eggs needs further verification. The present experiment aimed to distinguish shell translucency in terms of the eggshell's reflective and transmission properties caused by moisture spots. However, it is unclear whether reflective and transmission properties of translucent eggs were mainly caused by moisture spots or by variation of shell color on eggshell and it needs further investigation, which may indicate a genetic cause for translucent eggshell formation linked with genes of shell color.
Table 4.
Values of L, A, and B measured by spectrophotometer corresponding with 4 scores.
| RSS (%) | N | L | A | B |
|---|---|---|---|---|
| 0.00 to 2.21 | 100 | 65.68 ± 4.64a | 15.11 ± 2.67a | 27.43 ± 3.13a |
| 2.21 to 4.38 | 93 | 66.46 ± 4.82a,b | 14.51 ± 2.45a,b | 26.48 ± 2.91a,b |
| 4.38 to 9.02 | 92 | 67.58 ± 3.62b | 14.12 ± 3.65a,b | 25.68 ± 2.67b |
| 9.02 to 10.0 | 28 | 66.14 ± 6.66a,b | 13.79 ± 2.80b | 25.42 ± 2.97b |
RSS: ratio of sum of spots areas on the whole eggshell to area of the whole eggshell.
Among 4 grades mean without a common superscript differ (P < 0.05).
Summary
Appropriate phenotypic metrics constitute a first step in research on translucent eggshell formation, and the present study is the first to describe an existing scoring method in detail and to compare its accuracy with that of 2 other methods. Our experiment revealed the proportions of eggs that were scored at different levels for translucency and measured indicators of spot density and quantity. These results allowed us to draw the following conclusions. (1) The scoring method is efficient and suitable for translucent eggshell classification in a large flock. (2) Both grayscale recognition and the colorimetric method are more objective than the scoring method. We emphasize that the grayscale recognition method is the first method of quantitatively measuring translucent spot density and spots size in eggshells. However, digitized image processing and accurate identification of translucent spots is complex and time consuming, mainly due to the blurred boundary between opaque and translucent areas, as well as spot shape deformation at the edge of the eggshell. (3) Eggs with high RSS and low RSS values can be distinguished by the colorimetric method, but the method is not adequate for distinguishing between eggs with adjacent translucency score levels.
Of the 3 methods, we recommend the grayscale recognition method for quantification of eggshell translucency. In certain cases, such as case/control groups or research on large flocks, a combination of scoring by candling and grayscale recognition is appropriate for measurement.
ACKNOWLEDGMENTS
The work was supported by the Earmarked Fund for Hebei Lays and Broiler Innovation Team of Modern Agro-industry Technology Research System (HBCT2018150201), Research Funding of Hebei Agricultural University (ZD201722), and the Chinese Agricultural Research System (CARS-41).
REFERENCES
- Almquist H., Burmester B. Characteristics of an abnormal type of egg shell. Poult. Sci. 1934;13:116–122. [Google Scholar]
- Baker R., Curtiss R. Individual hen differences in egg shell mottling and the relationship of shell mottling to clutch size, internal quality and weight loss. Poult. Sci. 1957;36:904–908. [Google Scholar]
- Chousalkar K.K., Flynn P., Sutherland M., Roberts J.R., Cheetham B.F. Recovery of Salmonella and Escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int. J. Food Microbiol. 2010;142:207–213. doi: 10.1016/j.ijfoodmicro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Denison J.W. The effect of mechanical disturbance of the egg cuticle on shell mottling. Poult. Sci. 1967;46:771–772. [Google Scholar]
- Holst W.F., Almquist H.J., Lorenz F.W. A study of shell texture of the hen's egg. Poult. Sci. 1932;11:144–149. [Google Scholar]
- Jiang M. Shandong Agricultural University; Taian: 2015. Effect of calcium metabolism on eggshell quality in caged laying hens. PhD Diss. (In Chinese) [Google Scholar]
- León K., Mery D., Pedreschi F., León J. Color measurement in L*a*b* units from RGB digital images. Food Res. Int. 2006;39:1084–1091. [Google Scholar]
- Nie W. China Agricultural University; Beijing: 2013. Effects of dietary phosphorus levels on laying performance, egg shell quality and Ca and P absorption in laying herns with drawf gene. PhD Diss. (In Chinese) [Google Scholar]
- Ray A., Roberts J.R. Proceedings of the 24th Annual Australian Poultry Science Symposium, Sydney, New South Wales. 2013. The structural basis of egg shell translucency and its role in food safety of table eggs; p. 162. [Google Scholar]
- Reed G.F., Lynn F., Meade B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002;9:1235–1239. doi: 10.1128/CDLI.9.6.1235-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S.E. Egg and Eggshell Quality. Wolfe Publishing Limited; Aylesbury, UK: 1991. Translucency; pp. 111–121. [Google Scholar]
- Talbot C., Tyler C. A study of the fundamental cause of natural translucent areas in egg shells. Br. Poult. Sci. 1974;15:197–204. [Google Scholar]
- Tyler C., Geake F. The effect of water on egg shell strength including a study of the translucent areas of the shell. Br. Poult. Sci. 1964;5:277–284. [Google Scholar]
- Tyler C., Standen N. The artificial production of translucent streaks on egg shells and various factors influencing their development. Br. Poult. Sci. 1969;10:359–369. [Google Scholar]
- Wang D. China Agricultural University; Beijing: 2017. Mechanism exploration for translucent egg formation. PhD Diss. (In Chinese) [Google Scholar]
- Wang D.H., Li Y.J., Liu L., Liu J.S., Bao M., Yang N., Zhuo-Cheng H., Ning Z.H. Traits of eggshells and shell membranes of translucent eggs. Poult. Sci. 2017;96:351–358. doi: 10.3382/ps/pew328. [DOI] [PubMed] [Google Scholar]
- Zhang M. Sichuan Agricultural University; Chengdu: 2016. Research on the eggshell dark spots formation reasons and the effect of dark spots on the egg storage properties. PhD Diss. (In Chinese) [Google Scholar]