Abstract
Genistein (GEN), a type of soy isoflavones, is similar to estrogen structurally and functionally. The effects of dietary gen on the reproductive performance and bone status of breeder hens were investigated. A total pf 720 laying broiler breeder (LBB) hens were randomly allocated into 3 groups with supplemental dietary GEN doses (0, 40, 400 mg/kg). Each treatment has 8 replicates of 30 birds. The results indicated that supplemental GEN significantly improved the egg production and eggshell strength of LBB hens. Dietary GEN was deposited into the egg yolk, which decreased malonaldehyde in the follicle and egg yolk. The levels of vitellogenin (VTG), progesterone, and follicle-stimulating hormone in the serum of GEN-treated groups were elevated compared with the control group. Furthermore, GEN treatment downregulated the mRNA expression of insulin-like growth factor binding protein in the fallopian tube, whereas 40 mg/kg GEN treatment upregulated estrogen receptor α expression. Both the mRNA expression of VTG-II in the liver and mRNA expression of amphiregulin in the fallopian tube were upregulated after 40 and 400 mg/kg GEN treatment. In the 400 mg/kg GEN-treated group, the levels of calcitonin and alkaline phosphatase in the serum were increased compared with the control group, which was consistent with the increased levels of calcium and phosphorus in the tibia. Supplemental GEN (400 mg/kg) improved the tibia strength of LBB hens, whereas 40 mg/kg GEN had better effects on laying performance. In summary, dietary GEN could improve the egg production and quality, as well as the bone status of LBB hens during the late egg-laying period.
key words: genistein, reproductive hormone, bone, egg quality
INTRODUCTION
Soybean isoflavones (ISF) are the non-steroidal estrogenic compounds derived from legumes and other forage grass (Cao et al., 2015). Genistein (4, 5, 7-trihydroxy-isoflavone, GEN), a type of phytoestrogens, accounts for about two thirds of ISF in soy-derived products (Price and Fenwick, 1985; Patel et al., 2016). The structure of GEN is similar to estrogen (E2). GEN can exert hypolipidemic, antineoplastic, and antioxidant effects in animal models (Wei et al., 1995; Park et al., 2007; Patel et al, 2016). Isoflavones can also reportedly promote the growth and reproductive performance, along with the improved quality of livestock prodution (Sahin et al., 2007; Steinshamn, 2010; Shin et al., 2012; Retana-Márquez et al., 2016). However, some research studies reported that phytoestrogens made adverse effects on the reproductive function of ruminants and avian species (Ljungkvist, 1967; Adams, 1995). Results from available studies reveal that dose-dependent effects of GEN vary with sex, age, and hormonal status (Slikker et al., 2001; Naaz et al., 2003; Penza et al., 2006; Ruhlen et al., 2008; Castellano et al., 2011).
Laying broiler breeder (LBB) hens are mainly bred by the weight traits. However, the laying-peak period of LBB hens is short. Furthermore, the egg production and quality decrease fast during the late egg-laying period. Egg production and bone mineralization are 2 related physiological processes, which are regulated by the neuroendocrine system. The effects of GEN on the reproductive performance and bone status of LBB hens remain unknown. Vitellogenin (VTG), the precursor of the livetin, not only supplies energy for embryonic development, but also serves as the carrier for nonpolar molecules. E2 is the pivotal factor involved in vitellogenesis (Ni et al., 2012). E2 can also increase the levels of growth hormone (GH) and insulin-like growth factor (IGF-1) in the plasma, which promote the growth performance of animals (Slootweg et al., 1997). Isoflavone was reported to enhance the secreting rhythms of progesterone (P4), GH, and tetraiodothyronine (T4) of Zhedong White geese through the hypothalamic–pituitary–gonadal axis (Zhao et al., 2013). Therefore, we speculated that GEN might improve the reproductive performance of LBB hens through hormone regulation.
Calcium (Ca) and phosphorus (P) play important roles in the formation of eggshells and bones. It is suggested that estrogen-like compounds can promote the intestinal absorption of Ca (Arjmandi et al., 2005). There is a direct relationship between the supplemental levels of ISF and the content of Ca in ovariectomized mice (Fonseca and Ward, 2004). Some studies indicate that ISF-rich food can exhibit bone-preserving effects, which alleviate osteoporos of postmenopausal women (Arjmandi et al., 1996). Thus, this study was aimed to evaluate the effects of supplemental GEN on the reproductive performance and bone status of LBB hens and elucidate its possible molecular mechanism.
MATERIALS AND METHODS
Birds and Experimental Design
The experimental animal protocol for this study was approved by the Animal Care and Use Committee of China Agricultural University (CAU/NO.160515–2). A total of 720 55-wk-old LBB hens (Ross 308) were weighed and randomly divided into 3 groups (A, B, and C). Each group involves 8 replicates of 30 birds each. Birds were raised in wired 2-level battery cages on their respective diets of an environmentally controlled house. The light regimen was 16-h light: 8-h dark. The house temperature was controlled between 18 and 25°C. As is shown in Table 1, the CSCM diet was supplemented for hens in the A group, which meets the nutritional requirements of LBB hens according to National Research Council (1994). The B and C groups were fed the CSCM diets with GEN at levels of 40 and 400 mg/kg, respectively. Supplemental GEN was the artificially synthetic product from Kaimeng Co. Chemical Plant (Xi An, China) with a purity of 99.8%. The total levels of GEN in the diets were determined by liquid chromatographic (HPLC) analysis (A = 2.97 mg/kg, B = 43.21 mg/kg and C = 402.61 mg/kg). Before the start of the experiment, all hens were fed with BMCM diet for 2 wk to deplete GEN in the body. All birds were allowed ad libitum access to water throughout the experiment. During the 8-wk formal experiment, each hen was averagely allotted 155 g of feed each day.
Table 1.
Ingredients, analyzed and calculated chemical composition of the experimental diets.
| Ingredient | Content (%) | Nutrient level | |
|---|---|---|---|
| Corn | 68.99 | Avian metabolic energy (MC/Kg) | 2.83 |
| Soybean meal | 4.00 | Crude protein (%) | 16.10 |
| Corn protein | 9.15 | Calcium (%) | 3.48 |
| De-gossypol cottonseed protein | 6.00 | Tatalphosphorus (%) | 0.678 |
| Limestone | 7.76 | Available phosphorus (%) | 0.47 |
| Soybean oil | 0.50 | Methionine (%) | 0.34 |
| Dicalcium phosphate | 2.09 | Lysine (%) | 0.805 |
| NaCl | 0.35 | Met+Cys (%) | 0.626 |
| Trace mineral Premix1 | 0.30 | Threonine (%) | 0.60 |
| Choline chloride (50%) | 0.12 | Tryptophan (%) | 0.18 |
| Mycotoxin adsorbent | 0.10 | ||
| DL-Methionine | 0.0515 | ||
| Vitamin premix2 | 0.035 | ||
| Santoquin | 0.030 | ||
| Phytase | 0.016 | ||
| 4% Flavomycin | 0.015 | ||
| Lysine•HCl (78%) | 0.373 | ||
| Threonine | 0.0664 | ||
| Tryptophan | 0.0481 | ||
| Total | 100.00 | ||
Supplied the following per kg complete diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
Supplied the following (per kg complete diet): vitamin A, 12,500 IU; vitamin D3, 2500 IU; vitamin E, 30 IU; vitamin K3, 2.65 mg; thiamine, 2 mg; riboflavin, 6 mg; vitamin B12, 0.025 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; niacin, 50 mg.
Sample Collection and Procedure
At 65 wk of age, 1 chick per each replicate with body weights close to the average was selected after 10 h of feed deprivation. Blood sample was collected from the wing vein using vacuum blood collection tubes. The serum was separated out by centrifugation at 3000 × g for 15 min and stored at –20°C until it was used for the biochemical analysis. Then, the hen from each replicate was killed by jugular bloodletting. Tissue samples of the liver, follicle, and fallopian tube were immediately collected and frozen in liquid nitrogen, and kept in a freezer (−80°C) for measurements of gene expression and biochemical analysis.
Measurement of the Laying Performance and Egg Quality
The egg-laying rate, cracked egg rate, average egg weight, and feed intake were recorded daily. Ten eggs from each replicate were randomly collected at the last day of the experiment. Egg quality, including egg weight, yolk color, Haugh unit, and shell strength were measured using the digital egg tester (DET-6000, NABEL Co. Ltd, Japan) immediately. Yolk weight was measured after separating the egg white. Shell thickness was determined by the average values from the 3 locations on the egg (air cell, equator, and sharp end) by a dial pipe gauge (Mitutoyo, Japan). The egg yolk was collected and stored at –30°C for the measurement of GEN.
Detection of GEN in the Egg Yolk
The content of GEN in the egg yolk was measured using the HPLC method. Briefly, the GEN was extracted from 20 mg of the freeze-dried egg yolk sample. The sample was placed in test tube with 4 mL of 70% ethanol (containing 0.1% acetic acid) at 22°C. The sample was extracted for 8 h, with a constant agitation (shaking at 250 rpm). The extracted sample was centrifuged at 13,000 rpm for 10 min at 10°C; thereafter, 100 μL of the supernatant was transferred to an autosampler. The effluent was monitored at 260 nm. GEN monomer was used as standard (Sigma Chemical Co). The equipment for HPLC consisted of a pump (LC-20AD), a fluorescent detector (RF-10AXL), a column oven (CTO-10ASVP), an autosampler (SIL-20A), a degasser unit (DGU-20A5), and a computer system with LC Solution Software (Shimadzu). Inertsil ODS-3 C18 column (250 × 4.6 mm, 5 µm, GL Sciences Inc., Tokyo, Japan) was used as the HPLC column.
Detection of Ca, Mg, and Phosphorus in the Tibia
The fascia tissue on the surface of left tibia was stripped; then, the tibia breaking strength (TBS) was determined by 3 point bending test using the strength tester (INSTRON441l, Instron Co). All the soft tissues were stripped from the right tibia by the high-pressure treatment. The tibia was dried under 65°C for 12 h and then soaked in the mixture of alcohol and benzene 2:1 for 96 h. The dry weight was determined by the constant weight after drying at 105°C. The defatted tibia samples were ashed at 550°C in a muffle furnace for 16 h; then, the ash was dissolved in concentrated nitric acid. Ca and magnesium (Mg) were detected by the atomic absorption spectrophotometer. Phosphorus was determined by the ammonium vanadate-molybdate spectrophotometric method.
Detection of Hormones
The serum levels of progesterone (P4), follicule-stimulating hormone (FSH), luteinizing hormone (LH), calcitonin (CT), and E2 were measured using commercial double-antibody radioimmunoassay kits purchased from Shanghai Institute of Biological Products (Shanghai, China). The interassay coefficient of variation was 10%.
Detection of MDA, VTG, Ca, P, and alkaline phosphatase
The content of malondialdehyde (MDA) in the follicle and egg yolk was measured by the detection kits (Jiancheng Bioengineering Institute, Nanjing, China). The procedures were performed according to the manufacturer's instruction. The content of VTG in the serum was determined using the ELISA kits purchased from Genmay Biological Company (Shanghai, China). The levels of Ca, P, and alkaline phosphatase (ALP) in the serum were measured using a biochemical analyzer (TBA-120FR, Toshiba, Japan).
RNA Extraction and Detection, cDNA Synthesis, and Real-Time PCR Analysis
Total RNA was isolated from tissue samples using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. cDNA synthesis was performed using Prime Script RT reagent kit with cDNA eraser (TaKaRa, Dalian, Liaoning, China) according to the manufacturer's instructions. The one-step real-time RT-PCR was performed using SYBR Premix Ex TaqTM (TaKaRa, Dalian, Liaoning, China) in a real-time PCR machine (ABI7500 Applied Biosystem, Carlsbad) following the manufacturer's guidelines. The primer pairs used are shown in Table 6. β-Actin was used as the housekeeping gene. Relative mRNA expression levels of each target gene were normalized to the control using the 2−ΔΔCT method. As previously described, total RNA was treated with deoxyribonuclease, reverse transcribed, and amplified by quantitative PCR with the use of oligo nucleotide primers specific to chickens. The ratios of net intensity of target genes to β-actin were used to represent the relative level of target gene expression. The average level of 2 repeats was used for statistical analysis.
Table 6.
Primers used for quantitative real-time PCR analysis.
| Gen name | 5′ primer | Prod side |
|---|---|---|
| VTGII | F:AGCAGCAGCAGCAAGTCAAG | 120 |
| R:GTGATGCTCATGGCTGTGGT | 120 | |
| AREG | F:AAACCGAGGAGGAGGAAGAA | 121 |
| R: TTGCCATCTGAAGATGCTGT | 121 | |
| ER-a | F: TAGAGGGCATGGTGGAAATC | 122 |
| R:CACACCAGAATTGAGCAGGA | 122 | |
| IGFBP1 | F: TGTTTCCCATAAGCCAGGAC | 112 |
| R:CAAGGTCCCTGTTCTTTCCA | 112 |
F: represents forward, R: represents reward.
Statistical Analysis
The results were expressed as mean ± SD or mean ± SEM (for gene expressions), and tested by 1-way ANOVA with SPSS 11.0 for windows. A P value of ≤0.05 was considered to be statistically significant, and P values between 0.05 and 0.10 were classified as trends.
RESULTS
Laying Performance
As is shown in Figure 1, GEN supplementation (B and C) for LBB hens increased the egg laying rate during the whole experimental period compared with the control group, but with no significant difference (P = 0.089). The laying rate of the A group was significantly higher at weeks 2 and 8 compared with the control group (P < 0.05). The laying rate of the B group was significantly higher at weeks 4 and 8 compared with the control group (P < 0.05). However, there was no significant difference in the average egg weight between the 3 groups at all times studied.
Figure 1.
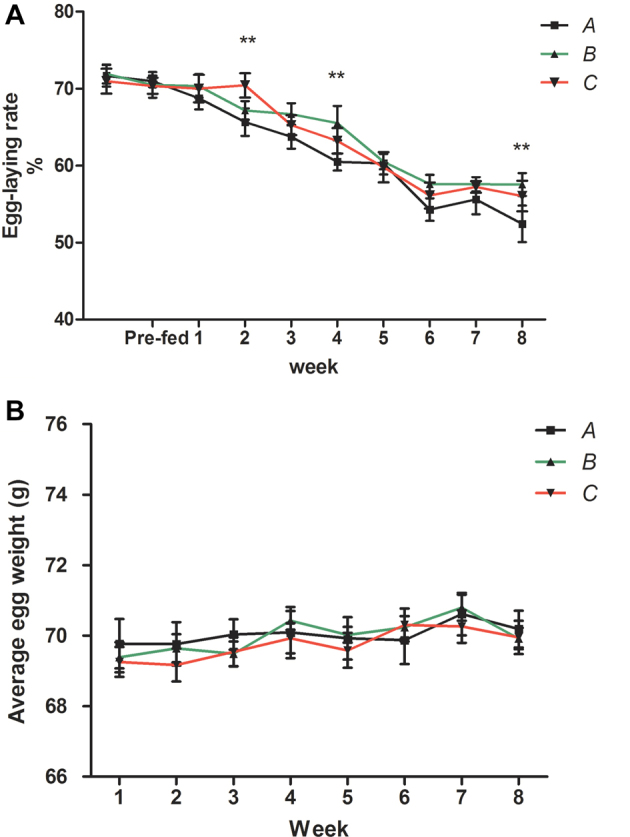
A and B panels represent the effects of dietary genistein on the egg-laying rate and average egg weight, respectively. Pre-fed represents the pre-feeding period (2 wk). Data are presented as mean value ± SD (n = 8). Mean values with (**) represent statistically significant differences, P < 0.05.
Egg Quality
Adding GEN in the diet of LBB hens had no significant effect on the albumen percentage (Table 2). Compared with the control group, 40 mg/kg and 400 mg/kg GEN treatment significantly increased the yolk color (P < 0.05). Also, the Haugh unit of the GEN-treated groups was higher than the control group (P = 0.279). 40 and 400 mg/kg dietary GEN enhanced the eggshell strength compared with the control group (P < 0.01). Similarly, there was an increased trend in the shell thickness among the 3 groups (P = 0.073).
Table 2.
Effect of dietary GEN on the egg quality of LBB hens (n = 8).
| Group | Percentage of albumen (%) | Yolk color | Haugh unit | Shell thickness (mm) | Shell strength (kg/cm2) |
|---|---|---|---|---|---|
| A | 33.50 ± 1.30 | 9.67 ± 0.29b | 62.4 ± 4.1 | 0.326 ± 0.013 | 3.21 ± 0.37b |
| B | 33.62 ± 1.03 | 10.09 ± 0.20a | 67.2 ± 5.5 | 0.340 ± 0.012 | 3.66 ± 0.39a |
| C | 33.12 ± 0.29 | 9.99 ± 0.18a | 66.0 ± 2.4 | 0.338 ± 0.009 | 3.79 ± 0.30a |
| P-value | 0.68 | <0.01 | 0.279 | 0.073 | 0.004 |
Data are presented as mean value ± SD. Mean values without the same mark (a, b) represent statistically significant differences (P < 0.05). Each replicate has 10 eggs.
The Levels of VTG and MDA as Well as GEN Deposition
As shown in Table 3, 40 mg/kg GEN supplementation for LBB hens increased the level of VTG in the serum of LBB hens (P < 0.05). The levels of GEN in the yolk of both B and C groups were significantly increased compared with the control group (P < 0.001). The contents of MDA in the follicle (P < 0.01) and egg yolk (P = 0.091) of B and C groups were significantly lower than the control group (P < 0.01).
Table 3.
Effects of dietary GEN on VTG and MDA as well as GEN deposition (n = 8).
| Serum VTG (pg/mL) | Follicle MDA (nmol/mL) | Yolk |
||
|---|---|---|---|---|
| MDA (nmol/mL) | GEN (ug/g) | |||
| A | 61.98 ± 10.9b | 15.95 ± 4.27a | 437 ± 53 | 0.87 ± 0.20c |
| B | 91.36 ± 19.5a | 11.59 ± 4.17b | 357 ± 67 | 6.85 ± 1.47b |
| C | 75.08 ± 17.9ab | 10.14 ± 2.19b | 399 ± 59 | 14.26 ± 1.41a |
| P-value | 0.003 | 0.031 | 0.096 | <0.001 |
Data are presented as mean value ± SD. Mean values without the same mark (a, b, c) represent statistically significant differences (P < 0.05). Vitellogenin (VTG), malondialdehyde (MDA), genistein (GEN).
The Levels of Hormones in the Serum
The level of P4 in the serum of LBB hens in B and C groups was significantly increased compared with the control group (Table 4, P < 0.01). The level of FSH in the serum of B and C groups was higher than the control group (P = 0.070). However, there was no significant difference in the serum level of LH between the 3 groups.
Table 4.
Effects of GEN on reproductive hormones of LBB hens (n = 8).
| Group | P4 | LH | FSH |
|---|---|---|---|
| A | 0.27 ± 0.17b | 4.97 ± 1.48 | 2.57 ± 0.48 |
| B | 0.57 ± 0.16a | 7.10 ± 1.58 | 3.17 ± 0.63 |
| C | 0.59 ± 0.27a | 5.62 ± 1.28 | 3.07 ± 0.50 |
| P-value | 0.002 | 0.360 | 0.070 |
Data are presented as mean value ± SD. Mean values without the same mark (a, b) represent statistically significant differences (P < 0.05). Luteinizing hormone, follicule-stimulating hormone (LH, FSH, mIU/mL); progesterone (P4, ng/mL).
Serum Metabolites and Bone Indices
As shown in Table 5, 400 mg/kg GEN treatment significantly increased TBS of LBB hens compared with the control group (P < 0.05). Further detection revealed that the levels of Ca in the tibias of B and C groups were dose-dependent increased after GEN treatment (P < 0.001). The level of P in tibia of C group was higher than that in A and B groups (P = 0.053). There was no significant difference in the tibia Mg levels among the 3 groups. Furthermore, the levels of ALP (P < 0.05), P (P = 0.082), and CT (P < 0.05) in the serum of C group were increased compared with A and B groups.
Table 5.
Effects of GEN on serum metabolites and bone indexes of LBB hens (n = 8).
| Serum |
Tibia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | ALP | Ca | P | CT | TBS | Ca | Mg | P |
| A | 230 ± 33b | 4.31 ± 0.14b | 1.86 ± 0.28 | 35.96 ± 2.21b | 478 ± 45b | 20.0 ± 1.2c | 2.67 ± 0.33 | 84.9 ± 5.8b |
| B | 250 ± 37b | 4.75 ± 0.23a | 1.88 ± 0.30 | 35.90 ± 2.01b | 533 ± 58ab | 28.8 ± 1.0b | 2.43 ± 0.55 | 82.6 ± 8.8b |
| C | 352 ± 79a | 4.79 ± 0.35a | 2.20 ± 0.28 | 83.79 ± 7.67a | 676 ± 94a | 31.9 ± 2.7a | 2.56 ± 0.36 | 100.5 ± 19.0a |
| P-value | 0.041 | 0.009 | 0.082 | 0.005 | 0.001 | <0.001 | 0.678 | 0.053 |
Data are presented as mean value ± SD. Mean values without the same mark (a, b) represent statistically significant differences (P < 0.05). Alkaline phosphatase (ALP, U/L), calcitonin (CT, pg/mL), calcium in serum (Ca, mg/dL), phosphorus in the serum (P, mg/dL), tibia breaking strength (TBS, kg/cm2), calcium in tibia (Ca, %ash), magnesium (Mg, % ash), phosphorus in the bone (P, % ash).
mRNA expression of genes
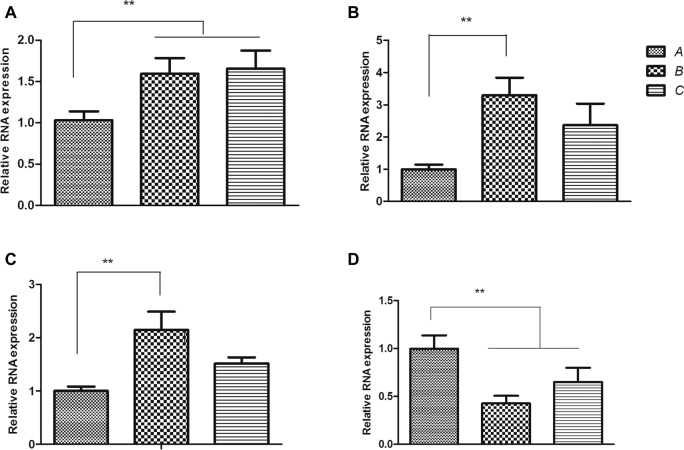
As illustrated in Figure 2 A, the mRNA expressions of VTGII in the livers of B and C groups were significantly upregulated compared with the control group (P < 0.05). The mRNA expressions of ERα (P < 0.05) and AREG (P < 0.05) in the livers of B group were up-regulated compared with the control group. Meanwhile, the mRNA expressions of IGFBP1 in the fallopian tubes of B and C groups were significantly downregulated compared with the control group (P < 0.05).
Figure 2.
A, B, C, and D panels represent the effect of GEN supplementation on the relative mRNA expression of vitellogenin (VTGII) in the liver, and amphiregulin (AREG), estrogrn receptor α (ERα), and insulin-like growth factor binding protein 1 (IGFBP1) in the shell gland of the fallopian tube, respectively. Data are presented as mean value ± SEM, n = 6. Means with (**) are significantly different, P < 0.05.
DISCUSSION
During the late egg-laying period, the egg production and egg quality of hens decrease fast (Bustany and Elwinger, 1987; Ketelaere et al., 2002). It is reported that dietary ISF improves the laying performance of ducks at the post-peak laying period, but exerts negative effects during the pre-peak laying period (Zhao et al., 2005). The efficacy of ISF, acting as either E2 agonist or antagonist, depends on the dosage, duration, and endogenous estrogenic level (Mathieson and Kitts, 1980; Cassidy, 2003). In the current study, adding GEN into the diet of LBB hens increased the egg production during the late egg-laying period. During the first 2 wk, 400 mg/kg GEN treatment showed more beneficial effects on egg production than the low-dose GEN treatment, whereas the beneficial effects of 40 mg/kg GEN became obvious during the late period of the experiment. It has been reported that high-dose GEN inhibits the activity of tyrosine protein kinase, while it exerts estrogen-like effects at the low levels (Mäkelä et al., 1995; Stahl et al., 1998). In the present study,40 mg/kg GEN supplementation for LBB hens had more obvious effects on improving the laying performance compared with the high-dose treatment during the late egg-laying period.
The egg-laying performance depends on the process of follicle formation and ovulation, which is under the regulation of reproductive hormones. In the present experiment, dietary GEN significantly increased the serum level of P4. It is reported that the laying performance of chickens is in parallel with the plasma level of P4 (Leszczynski et al., 1982). P4 could promote the maturity and ovulation of follicle through FSH and LH (Sjaastad et al., 2010). FSH is the essential hormone to follicular growth, development, dominance, maturation, and ovulation. Accordingly, GEN supplementation for LBB hens increased the levels of FSH in the serum significantly. As we all know, VTG is regulated by the endogenous E2 through the estrogen receptor (ER) pathway. VTG can directly reflect the E2 level in animal body (Tingaud-Sequeira et al., 2004). It is indicated that GEN can activate the inhibited ER due to the insufficient endogenous E2 (Hsieh et al., 1998). In the present study, GEN treatment upregulated the mRNA expression of VTG-II in the liver and the level of VTG in the serum, corresponding to the improved laying performance. Amphiregulin (AREG), one of the epidermal growth factor family, plays an important role in the blastocyst implantation (Achache and Revel, 2006). It has been suggested that E2 can upregulate the expression of AREG via estrogen receptor α (ERα) (Ciarloni et al., 2007). The overexpression of AREG significantly increases the litter size of Meishan Pigs (Wilkie et al., 1999). However, the reproductive system of poultry differs from the mammals. The report about the gene expression of AREG in the fallopian tube of hens is rare. 40 mg/kg GEN treatment upregulated the mRNA expression of AREG in the fallopian tube, which might be in favor for the egg production. Therefore, GEN could improve the egg laying performance of LBB hens by regulating the reproductive hormones (FSH and VTG) and AREG expression.
Dietary Ca is absorbed in the small intestine; it then deposits at the shell gland to form the eggshell. It is reported that daidzein, a type of ISF, can increase the eggshell thickness of laying hens (Ni et al., 2007). GEN has been reported to increase the level of Ca in the shell gland (Gao and Yamaguchi, 1998). The current study indicated that adding GEN into the diet of LBB hens enhanced the eggshell strength, and increased the serum level of Ca. GEN supplementation at the level of 400 mg/kg had the better effect on improving the egg quality. Similarly, dietary GEN at 800 mg/kg can increase the egg weight, Haugh unit, and shell thickness of quails (Akdemir and Sahin, 2009). In addition, ISF can reportedly decrease the intracellular Ca concentration in osteoclasts (Zhao et al., 2016). It is reported that E2 can upregulate the expression of ATP-dependent Ca pump in plasma membrane of human uterus (Yang et al., 2011). GEN might have the similar effect on increasing Ca in the serum. However, we found that supplemental GEN decr eased the P level in the serum of LBB hens. Thus, dietary GEN can improve the eggshell quality through enhancing Ca metabolism.
IGFBP1 and IGFs bind with the same receptor, which control the energy distribution between the growth and basal metabolism (Scott et al., 2002; Leu et al., 2003). Our study suggested that dietary GEN downregulated the mRNA expression of IGFBP1 in the fallopian tube. Thus, GEN could weaken the inhibition of IGFBP1 towards the IGFs signaling pathway, allotting more energy to the fallopian tube. The shell gland cell is the target of E2. Phytoestrogens prefer to bind with ERα in the fallopian tube (Cárdenas and Pope, 2005; Wang et al., 2014). In the current study, the mRNA expression of ERα in the shell gland was increased in the GEN-treated groups, which could promote Ca secretion (Ljungkvist, 1967). The result was not consistent with the report about the effect of daidzein on ERα (Ni et al, 2007), which might be due to the endogenous E2 levels.
GEN deposition increased the egg yolk color, which was accorded with the result detected by HPLC and Saitoh's report (Saitoh et al., 2001). GEN has the strongest antioxidant capability among the 4 types of ISF (Jungbauer and Medjakovic, 2014). The phenolic hydroxyl in the structure of ISF can react with free radicals in vivo or vitro (Jiang et al., 2007a, b). In our experiment, GEN treatment decreased the MDA levels in the follicle and egg yolk, which improved lipid peroxidation and increased Haugh unit. Therefore, the increased GEN in the egg yolk can increase the shelf-life of eggs.
As we all know, the caged LLB hens during the late egg-laying period suffer from bone metabolic diseases (Sahin et al., 2003), which causes serious economic losses to poultry industry. GEN can reportedly inhibit the secretion of acidic materials from osteoclasts (Barnes and Blair, 1996). In the present study, we found that supplemental GEN increased the levels of Ca and P in the tibia, as well as enhanced the tibia strength. 400 mg/kg supplemental GEN had the better effects on improving the tibia strength. ALP can assist osteoblasts hydrolyzing organophosphorus into inorganic phosphorus. Interestingly, GEN could reportedly increase the activity of ALP in osteoblasts (Anderson et al., 1999). In the present work, we also found that GEN treatment increased the serum level of ALP, which was consistent with the change of P in the bone. The positive effect of estrogenic compounds on calcium absorption in human intestine has been suggested (Arjmandi et al., 2005). Supplemental GEN at the level of 400 mg/kg decreased CT in the serum, which might be another reason for the enhanced bone strength. Therefore, dietary GEN enhanced the absorption and deposition of Ca and P through CT and ALP, which improved the bone strength of LBB hens during the late laying period.
In conclusion, dietary GEN upregulated the secretion of reproductive hormones (P4 and FSH) and the mRNA expressions of VTG-II in the liver, as well as the expressions of genes (AREG and IGFBP1) in the fallopian tube, which improved the laying performance of LBB hens. Supplemental GEN increased the serum levels of ALP and CT, as well as downregulated the mRNA expression of IGFBP1 in the eggshell gland, which improved Ca metabolism and eggshell strength. 40 mg/kg supplemental GEN had more significant effects on improving laying performance, whereas 400 mg/kg GEN had better effects on enhancing bone strength.
ACKNOWLEDGMENTS
This work was financially supported by China Agriculture Research System (CARS-42–13).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
The appropriate scientific section for this paper is Nutrition and Metabolism.
REFERENCES
- Achache H., Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- Adams N.R. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- Akdemir F., Sahin K. Genistein supplementation to the quail: effects on egg production and egg yolk genistein, daidzein, and lipid peroxidation levels. Poult. Sci. 2009;88:2125–2131. doi: 10.3382/ps.2009-00004. [DOI] [PubMed] [Google Scholar]
- Anderson J.B., Anthony M., Messina M., Garne S.C. Effects of phyto-oestrogens on tissues. Nutr. Res. Rev. 1999;12:75–116. doi: 10.1079/095442299108728875. [DOI] [PubMed] [Google Scholar]
- Arjmandi B.H., Alekel L., Hollis B.W., Amin D., Stacewiczsapuntzakis M., Guo P., Kukreja S.C. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J. Nutr. 1996;126:161–167. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- Arjmandi B.H., Lucas E.A., Khalil D.A., Devareddy L., Smith B.J., Mcdonald J., Arquitt A.B., Payton M.E., Mason C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr. J. 2005;4:176–188. doi: 10.1186/1475-2891-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Blair H.C. Genistein for use in inhibiting osteroclasts. US. 1996;55:211–221. [Google Scholar]
- Bustany Z.A., Elwinger K. Shell and interior quality and chemical composition of eggs from hens of different strains and ages fed different dietary lysine levels. Acta Agr. Scand. 1987;37:175–187. [Google Scholar]
- Cárdenas H., Pope W.F. Estrogen receptors in the uterus and ovarian follicles of gilts treated with dihydrotestosterone. Domest. Anim. Endocrinol. 2005;29:523–533. doi: 10.1016/j.domaniend.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Cao J., Echelberger R., Min L., Sluzas E., Mccaffrey K., Buckley B., Patisaul H.B. Soy but not bisphenol A (BPA) or the phytoestrogen genistin alters developmental weight gain and food intake in pregnant rats and their offspring. Reprod. Toxicol. 2015;58:282–294. doi: 10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A. Potential risks and benefits of phytoestrogen-rich diets. Int. J. Vitam. Nutr. Res. 2003;73:120–126. doi: 10.1024/0300-9831.73.2.120. [DOI] [PubMed] [Google Scholar]
- Castellano J.M., Bentsen A.H., Sánchezgarrido M.A., Ruizpino F., Romero M., Garciagaliano D., Aguilar E., Pinilla L., Diéguez C., Mikkelsen J.D. Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152:3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Ciarloni L., Mallepell S., Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc. Natl. Acad. Sci. USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca D., Ward W.E. Daidzein together with high calcium preserve bone mass and biomechanical strength at multiple sites in ovariectomized mice. Bone. 2004;35:489–497. doi: 10.1016/j.bone.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Gao Y.H., Yamaguchi M. Zinc enhancement of genistein's anabolic effect on bone components in elderly female rats. Gen. Pharmacol. 1998;31:199–202. doi: 10.1016/s0306-3623(98)00022-6. [DOI] [PubMed] [Google Scholar]
- Hsieh C.Y., Santell R.C., Haslam S.Z., Helferich W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- Jiang Z.Y., Jiang S.Q., Lin Y.C., Xi P.B., Yu D.Q., Wu T.X. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poult. Sci. 2007;86:1356–1362. doi: 10.1093/ps/86.7.1356. [DOI] [PubMed] [Google Scholar]
- Jiang S., Jiang Z., Wu T., Ma X., Zheng C., Zou S. Protective effects of a synthetic soybean isoflavone against oxidative damage in chick skeletal muscle cells. Food Chem. 2007;105:1086–1090. [Google Scholar]
- Jungbauer A., Medjakovic S. Phytoestrogens and the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2014;139:277–289. doi: 10.1016/j.jsbmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Ketelaere B.D., Govaerts T., Coucke P., Dewil E., Visscher J., Decuypere E., Baerdemaeker J.D. Measuring the eggshell strength of 6 different genetic strains of laying hens: Techniques and comparisons. Br. Poult. Sci. 2002;43:238–244. doi: 10.1080/00071660120121454. [DOI] [PubMed] [Google Scholar]
- Leszczynski D.E., Toda T., Kummerow F.A. Influence of dietary sex hormones on chick lipid metabolism. Horm. Metab. Res. 1982;14:183–189. doi: 10.1055/s-2007-1018964. [DOI] [PubMed] [Google Scholar]
- Leu J.I., Crissey M.A., Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J. Clin. Invest. 2003;111:129–139. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungkvist H.I. Light and electron microscopical study of the effect of oestrogen on the chicken oviduct. Acta Endocrinol. 1967;56:391–402. doi: 10.1530/acta.0.0560391. [DOI] [PubMed] [Google Scholar]
- Mäkelä S.I., Pylkkänen L.H., Santti R.S., Adlercreutz H. Dietary soybean may be antiestrogenic in male mice. J. Nutr. 1995;125:437–445. doi: 10.1093/jn/125.3.437. [DOI] [PubMed] [Google Scholar]
- Mathieson R.A., Kitts W.D. Binding of phyto-oestrogen and oestradiol-17 beta by cytoplasmic receptors in the pituitary gland and hypothalamus of the ewe. J. Endocrinol. 1980;85:317–325. doi: 10.1677/joe.0.0850317. [DOI] [PubMed] [Google Scholar]
- Naaz A., Yellayi S., Zakroczymski M.A., Bunick D., Doerge D.R., Lubahn D.B., Helferich W.G., Cooke P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- Ni Y.D., Wu J., Tong H.Y., Huang Y.B. Effect of dietary daidzein supplementation on egg laying rate was associated with the change of hepatic VTG-II mRNA expression and higher antioxidant activities during the post-peak egg laying period of broiler breeders. Anim. Feed Sci. Technol. 2012;177:116–123. [Google Scholar]
- Ni Y., Zhu Q., Zhou Z., Grossmann R., Chen J., Zhao R. Effect of dietary daidzein on egg production, shell quality, and gene expression of ER-α, GH-R, and IGF-IR in shell glands of laying hens. J. Agric. Food Chem. 2007;55:6997–7001. doi: 10.1021/jf071085r. [DOI] [PubMed] [Google Scholar]
- Park J.S., Woo M.S., Kim D.H., Hyun J.W., Kim W.K., Lee J.C., Kim H.S. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J. Pharmacol. Exp. Ther. 2007;320:1237–1245. doi: 10.1124/jpet.106.114322. [DOI] [PubMed] [Google Scholar]
- Patel S., Peretz J., Pan Y.X., Helferich W.G., Flaws J.A. Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol. Appl. Pharmacol. 2016;293:53–62. doi: 10.1016/j.taap.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penza M., Montani C., Romani A., Vignolini P., Pampaloni B., Tanini A., Brandi M.L., Alonsomagdalena P., Nadal A., Ottobrini L. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Early Hum. Dev. 2006;147:5740–5751. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- Price K.R., Fenwick G.R. Naturally occurring oestrogens in foods. Food Addit. Contam. 1985;2:73–106. doi: 10.1080/02652038509373531. [DOI] [PubMed] [Google Scholar]
- Retana-Márquez S., Juárez-Rojas L., Hernández A., Romero C., López G., Miranda L., Guerrero-Aguilera A., Solano F., Hernández E., Chemineau P. Comparison of the effects of Mesquite pod and Leucaena extracts with phytoestrogens on the reproductive physiology and sexual behavior in the male rat. Physiol. Behav. 2016;164:1–10. doi: 10.1016/j.physbeh.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Ruhlen R.L., Howdeshell K.L., Mao J., Taylor J.A., Bronson F.H., Newbold R.R., Welshons W.V., vom Saal F.S. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ. Health Persp. 2008;116:322–328. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin K., Onderci M., Sahin N., Gursu M.F., Kucuk O. Dietary vitamin C and folic acid supplementation ameliorates the detrimental effects of heat stress in Japanese quail. J. Nutr. 2003;133:1882–1886. doi: 10.1093/jn/133.6.1882. [DOI] [PubMed] [Google Scholar]
- Sahin N., Onderci M., Balci T.A., Cikim G., Sahin K., Kucuk O. The effect of soy isoflavones on egg quality and bone mineralisation during the late laying period of quail. Br. Poult. Sci. 2007;48:363–369. doi: 10.1080/00071660701341971. [DOI] [PubMed] [Google Scholar]
- Saitoh S., Sato T., Harada H., Takita T. Transfer of soy isoflavone into the egg yolk of chickens. Biosci. Biotechnol. Biochem. 2001;65:2220–2225. doi: 10.1271/bbb.65.2220. [DOI] [PubMed] [Google Scholar]
- Scott B.A., Avidan M.S., Crowder C.M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296:2388–2391. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- Shin J.H., Park J.M., Kim J.M., Roh K.S., Jung W.S. The improvement of laying productivity and egg quality according to providing germinated and fermented soybean for a feed additive. Korean J. Food Sci. Anim. Resour. 2012;32:404–408. [Google Scholar]
- Sjaastad V., Sand O., Hove K., Sjaastad V., Sand O., Hove K. 2nd rev. ed. Natl. Acad. Press; Washington, DC, US.: 2010. Physiology of Domestic Animals. [Google Scholar]
- Slikker W., Jr, Scallet A.C., Doerge D.R., Ferguson S.A. Gender-based differences in rats after chronic dietary exposure to genistein. Int. J. Toxicol. 2001;20:175–179. doi: 10.1080/109158101317097764. [DOI] [PubMed] [Google Scholar]
- Slootweg M.C., Swolin D., Netelenbos J.C., Isaksson O.G., Ohlsson C. Estrogen enhances growth hormone receptor expression and growth hormone action in rat osteosarcoma cells and human osteoblast-like cells. J. Endocrinol. 1997;155:159–164. doi: 10.1677/joe.0.1550159. [DOI] [PubMed] [Google Scholar]
- Stahl S., Chun T.Y., Gray W.G. Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicol. Appl. Pharmacol. 1998;152:41–48. doi: 10.1006/taap.1998.8500. [DOI] [PubMed] [Google Scholar]
- Steinshamn H. Effect of forage legumes on feed intake, milk production and milk quality. Anim. Sci. Pap. Rep. 2010;28:195–206. [Google Scholar]
- Tingaud-Sequeira A., André M., Forgue J., Barthe C., Babin P.J. Expression patterns of three estrogen receptor genes during zebrafish (Danio rerio) development: evidence for high expression in neuromasts. Gene Expr. Patterns. 2004;4:561–568. doi: 10.1016/j.modgep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wang H., Eriksson H., Sahlin L. Estrogen receptors α and β in the female reproductive tract of the rat during the estrous cycle1. Biol. Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- Wei H., Bowen R., Cai Q., Barnes S., Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Exp. Biol. Med. 1995;208:124–130. doi: 10.3181/00379727-208-43844. [DOI] [PubMed] [Google Scholar]
- Wilkie P.J., Paszek A.A., Beattie C.W., Alexander L.J., Wheeler M.B., Schook L.B. A genomic scan of porcine reproductive traits reveals possible quantitative trait loci (QTLs) for number of corpora lutea. Mamm. Genome. 1999;10:573–578. doi: 10.1007/s003359901047. [DOI] [PubMed] [Google Scholar]
- Yang H., Choi K.C., Hyun S.H., Jeung E.B. Coexpression and estrogen-mediated regulation of TRPV6 and PMCA1 in the human endometrium during the menstrual cycle. Mol. Reprod. Dev. 2011;78:274–282. doi: 10.1002/mrd.21303. [DOI] [PubMed] [Google Scholar]
- Zhao Y.H., Liu W.F., Zeng J.Y., Liu S.C., Tan X.Y., Aljohi H., Hu S.N.A. Identification and analysis of mouse non-coding RNA using transcriptome data. Sci. China Life Sci. 2016;59:589–603. doi: 10.1007/s11427-015-4929-x. [DOI] [PubMed] [Google Scholar]
- Zhao X., Shao T., Wang Y.Q., Lu X.L., Luo J.B., Zhou W.D. The phytoestrogen daidzein may affect reproductive performance of Zhedong White geese by regulating gene mRNA levels in the HPG axis. Br. Poult. Sci. 2013;54:252–258. doi: 10.1080/00071668.2013.767439. [DOI] [PubMed] [Google Scholar]
- Zhao R.Q., Zhou Y.C., Ni Y.D., Lu L.Z., Tao Z.R., Chen W.H., Chen J. Effect of daidzein on egg-laying performance in Shaoxing duck breeders during different stages of the egg production cycle. Br. Poult. Sci. 2005;46:175–181. doi: 10.1080/00071660500064808. [DOI] [PubMed] [Google Scholar]