Abstract
This study investigated the effects of fermented soybean meal (FSBM) with or without mannan-oligosaccharide (MOS) prebiotic on growth performance, digestive functions, and hepatic IGF-1 gene expression of broiler chicken. A total of 480 day-old male broiler chickens were fed with 4 experimental diets for 6 wk. Experimental diets included corn-soybean meal diet (CON); corn-soybean meal diet + MOS prebiotic [0.2%, ActiveMOS; Biorigin, Brazile]; corn-FSBM diet [soybean meal (SBM) was totally replaced by FSBM]; and corn-FSBM + MOS prebiotic (MIX). Replacing dietary SBM with FSBM with or without MOS improved body weight gain and feed efficiency for the total grow-out period. However, the addition of MOS to the FSBM diet exhibited a greater body weight gain than other experimental diets. Villus height and villus height to crypt depth of the duodenum and jejunum were increased by feeding FSBM, MOS, and MIX diets. The ileal crude protein and energy digestibilities, as well as the activities of intestinal amylase and protease, and pancreatic protease, were improved by replacing SBM with FSBM, with or without MOS. The concentration of plasma 3-methylhistidine was reduced by FSBM and MOS, and synergistically by their combination. The MOS and FSBM diets upregulated the hepatic IGF-1 gene expression. However, there was an evident synergistic effect of FSBM supplemented with MOS in the upregulation of the hepatic IGF-1 gene expression. The outcomes of the current study indicate the FSBM and MOS had the potential to improve growth performance, hepatic IGF-1 expression, and intestinal morphology of broilers. Overall, the fermented products could be considered as functional feed that exhibits probiotic effects and the synergistic effects of prebiotics added to the fermented feeds may further improve the growth performance and gut health and functionality in broiler chicken.
key words: fermented soybean meal, mannan-oligosaccharide, probiotic, nutrient digestibility, broiler chicken
INTRODUCTION
Soybean meal (SBM) is the main protein source in livestock nutrition; it contains 40 to 50% protein and is rich in amino acids like lysine, tryptophan, threonine, isoleucine, and valine (Easter and Kim, 1999; Ravindran et al., 2014). The presence of several anti-nutritional factors (ANFs) in SBM, such as antigenic proteins, trypsin inhibitor, phytic acid, and oligosaccharides, however, reduces the absorption and digestion of nutrients and, thus, deteriorates the growth performance of birds (Li et al., 2014). Some of these ANFs like trypsin inhibitor and phytate could be eliminated by processing methods such as heating, which improves nutrient digestibility and feed conversion ratio in birds. The antigenic proteins, however, are not deactivated by heating and may result in impaired growth performance (Palacios et al., 2004). Over the past few years, microbial fermentation is considered as an effective way to degrade ANFs and enhance the nutritional quality of animal feed (Feng et al., 2007a; Jazi et al., 2017). Previous studies have demonstrated that the resulted biochemical changes by metabolic activity of microorganisms during fermentation may be leading to a decrease of ANFs and undesirable compounds and improvement of nutrient bioavailability and digestibility (Shi et al., 2017). Reports from Feng et al. (2007a) and Jazi et al. (2018a) demonstrated that fermentation can enhance the nutritional value of SBM and consequently improve growth performance. In addition, acidic pH and desirable microflora in the fermented products can influence functions of gastrointestinal (GI) tract, via an increase in beneficial bacteria and a decrease in pathogenic bacteria in a similar mode of action to probiotics. Hence, the fermented feeds can play an important role in GI health and functionality, productivity, and growth performance of birds (Jazi et al., 2017). It has been indicated that the use of fermented feeds in broiler diets improved performance traits and increased lactic acid bacteria (LAB) in the gut (Chiang et al., 2010; Ashayerizadeh et al., 2018). The improved villus height and villus height to crypt depth of the small intestinal also have been reported in broiler chickens fed with fermented products (Feng et al., 2007a; Sun et al., 2013). However, the data on the effects of feeding fermented products on nutrient digestibility, digestive enzymes activity, gut morphology and functionality in broiler chicken is scarce. Moreover, given the probiotic effects of fermented products, the hypothesis of this trial is that added prebiotic will further enhance the beneficial effects of the fermented soybean meal (FSBM) in a synergistic way. The objective of this trial was, therefore, to study the effects of FSBM compared to SBM with or without added prebiotics on growth performance, digestive enzymes activity, nutrient digestibility, gut microbial profile, intestinal morphology, and hepatic IGF-1 gene expression.
MATERIALS AND METHODS
The experiment complied with the Animal Research Ethics of our institute and the protocol was approved prior to the trial commencement.
Preparation of FSBM
The bacterial and fungus strains including Lactobacillus acidophilus (PTCC1643), Lactobacillus plantarum (PTCC1058), Bacillus subtilis (PTCC1156), and Aspergillus oryzae (PTCC5163), respectively, were sourced from the Persian Type Culture Collection Centre of the Iranian Research Organization for Science and Technology, Iran. Each kilogram of SBM as fermentation substrate was inoculated and mixed with 1 L of distilled water containing 108 CFU/mL of L. acidophilus, L. plantarum, and B. subtilis and 106 spores/mL ofA. oryzae in fermentation tanks equipped with a one-way valve to outflow of gases produced and impeded air entry at 30°C for 7 d. The FSBM samples were finally dried for 2 d at 50°C. At last, the dried samples were ground and kept at room temperature until mixed into experimental diets.
Chemical Analyses
To determine LAB and Enterobacteraiceae counts in the SBM and FSBM, 1 g of each sample was used to make 10-fold serial dilutions using buffered peptone water. Then, 0.1 mL of appropriate dilutions was spread on the plates containing modified de Man Rogosa, Sharpe (MRS) agar and eosin methylene blue agar, respectively. Plates were incubated in anaerobic (for LAB) and aerobic (for Enterobacteraiceae) conditions at 37°C for 1 d. Finally, after counting the number of colonies in each plate, the obtained number was multiplied by reversed dilution and reported as the colony forming unit (CFU) per 1 g sample. The pH value of SBM and FSBM was determined using a pH meter (Hanna Instruments, Woonsocket, USA, model HI 99163). The lactic acid concentration was determined by high-performance liquid chromatography (HPLC) according to the method of Marsili et al. (1983). The phytic acid content in SBM and FSBM was determined through the extraction of the metabolite with HCl and Na2 SO4 and the absorbance measured at 660 nm. Trypsin inhibitor activity in the fermented and unfermented samples was estimated according to the method of Smith et al. (1980), and the results are expressed as mg of trypsin inhibited per g of dry sample. The concentrations of glycinin and β-conglycinin were measured by the method of Wang et al. (2014). The amino acid compositions of SBM and FSBM samples were measured using an automated amino acid analyzer after hydrolyzing the samples with 6 M HCl at 110°C for 24 h and then sulphur-containing amino acids were oxidized using performic acid before the acid hydrolysis. Contents of dry matter (DM), ash, crude protein (CP), ether extract (EE), crude fiber were analyzed according to AOAC (2005). All analyses were performed in 3 replicates.
Animals and Experimental Design
A total of four hundred and eighty 1-day-old male broiler chickens (Ross 308) with uniform body weight were obtained from a commercial hatchery (Navid Morgh Guilan Company, Iran) and randomly allocated to 1 of 4 experimental diets in 6 replicates (pens) each with 20 birds in a completely randomized design. Experimental diets included corn-SBM diet (CON); corn-SBM diet + mannan-oligosaccharide (MOS) prebiotic [0.2%, ActiveMOS; Biorigin, Brazile]; corn-FSBM diet [SBM was totally replaced by FSBM]; and corn-FSBM + MOS prebiotic (MIX). Experimental diets, in mash form, were formulated to meet nutritional requirements based on Ross guidelines (Ross Broiler Management Handbook, Aviagen International, 2014) for starter (1 to 10 d), grower (11 to 24 d), and finisher (25 to 42 d) phases. For the period of days 35 to 42, diets were mixed with 0.3% of titanium dioxide as an external marker for determination of apparent ileal digestibility of nutrients. Table 1 presents the composition of experimental diets and calculated nutrient level. All birds had ad libitum access to water and experimental diets and light regimen was set at 22L: 2D throughout the study period. The temperature was 34°C for the first 3 d of the study and then gradually reduced by 3°C weekly reaching 22°C.
Table 1.
Ingredients and nutrients composition of the experimental diets.
| Experimental diets |
||||||
|---|---|---|---|---|---|---|
| Starter (1 to 10 d) |
Grower (11 to 24 d) |
Finisher (25 to 42 d) |
||||
| Diet composition (%) | Control | FSBM | Control | FSBM | Control | FSBM |
| Corn | 51.07 | 54.40 | 50.76 | 54.30 | 55.80 | 58.97 |
| Soybean meal | 37.19 | – | 39.99 | – | 34.57 | – |
| Fermented soybean meal | 0 | 34.50 | – | 37.11 | – | 32.08 |
| Corn gluten meal | 4 | 4 | – | – | – | – |
| Vegetable oil | 3.23 | 2.64 | 5.40 | 4.77 | 5.96 | 5.41 |
| Limestone | 0.81 | 0.83 | 0.68 | 0.70 | 0.63 | 0.65 |
| Di-calcium phosphate | 2.14 | 2.14 | 1.91 | 1.92 | 1.71 | 1.71 |
| Salt | 0.36 | 0.36 | 0.37 | 0.37 | 0.37 | 0.37 |
| Vitamin premix1 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Mineral premix2 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| DL-methionine | 0.28 | 0.29 | 0.25 | 0.26 | 0.23 | 0.24 |
| L-lysine | 0.34 | 0.28 | 0.13 | 0.07 | 0.23 | 0.07 |
| Threonine | 0.08 | 0.07 | 0.01 | – | – | – |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated composition | ||||||
| Metabolizable energy (Kcal/kg) | 3,000 | 3,000 | 3,100 | 3,100 | 3,200 | 3,200 |
| Crude protein (%) | 23 | 23 | 21.5 | 21.5 | 19.5 | 19.5 |
| SID lysine (%) | 1.28 | 1.28 | 1.15 | 1.15 | 1.03 | 1.03 |
| SID methionine (%) | 0.63 | 0.63 | 0.55 | 0.55 | 0.52 | 0.52 |
| SID methionine + cysteine (%) | 0.95 | 0.95 | 0.87 | 0.87 | 0.80 | 0.80 |
| SID threonine (%) | 0.86 | 0.86 | 0.77 | 0.77 | 0.68 | 0.68 |
| Calcium (%) | 0.96 | 0.96 | 0.87 | 0.87 | 0.79 | 0.79 |
| Available phosphorous (%) | 0.48 | 0.48 | 0.435 | 0.435 | 0.395 | 0.395 |
| Sodium (%) | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
Supplied per kg of diet: 1.8 mg all-trans-retinyl acetate; 0.02 mg cholecalciferol; 8.3 mg alphatocopheryl acetate; 2.2 mg menadione; 2 mg pyridoxine HCl; 8 mg cyanocobalamin; 10 mg nicotine amid; 0.3 mg folic acid; 20 mg D-biotin; 160 mg choline chloride.
Supplied per kg of diet: 32 mg Mn (MnSO4_H2O); 16 mg Fe (FeSO4_7H2O); 24 mg Zn (ZnO); 2 mg Cu (CuSO4_5H2O); 800 µ g I (KI); 200 µ g Co (CoSO4); 60 µ g Se (NaSeO3).
Growth Performance and Sample Collection
Body weights (BW) of the birds and their feed intakes (FI) were recorded weekly, and BW gain, FI, and feed conversion ratio (FCR) were calculated for starter, grower, and finisher phases, as well as the total grow-out period. The mortality rate was recorded daily.
At the end of the experiment, 2 birds with BW similar to the mean BW of the pen replicate were selected and euthanized by cervical dislocation and the GI tract was cleaved to sample the digesta contents of crop, small intestine (from the segments of distal end of duodenum to the ileocecal junction), and pancreas to study pH, gut microbial composition, intestinal histomorphology, nutrient digestibility, and digestive enzymes activity. The liver samples were immediately transferred to liquid nitrogen after recording their weight and kept in a −80°C freezer until RNA extraction analysis. The concentration of plasma 3-methylhistidine was determined by HPLC according to the method described by Hayashi et al. (1987).
Digesta pH, and Microbial Profile Analyses
The crop, ileum, and cecal pH values were measured using a digital pH meter (Hanna Instruments, Woonsocket, USA, model HI 99163) after mixing 1 g of digesta of each GI tract segment with 2 mL of distilled water.
To determine microbial composition, 1 g of the crop, ileal, and cecal digesta was diluted with 9 mL of 1% peptone water and homogenized for 1 min to make dilution series. Bacteria count was determined by plating 0.1 mL portions of appropriately diluted culture on MRS-agar plates for LAB; violet red bile agar for coliform bacteria; plate count agar for the total anaerobic bacteria and tryptose sulphite cycloserine agar for Clostridium perfringens after incubating plates for 24 to 48 h at 37°C under anaerobic conditions. All bacteria were counted and expressed as total CFU/g digesta, and results are reported as Log10-transformed data.
Intestinal Histomorphology
To study intestinal histomorphology, a 1-cm segment of the midpoint of the duodenum, jejunum, and ileum from each bird was removed and fixed in 10% buffered formaldehyde for 48 h. Each of these intestinal segments was embedded in paraffin, a 5-μm section of each sample was cut using microtome, placed on a glass slide, and stained with hematoxylin and eosin for examination under a light microscope. Morphological parameters were determined using the ImageJ software package (http://rsb.info.nih.gov/ij/). Villus height was measured from the tip of the villus to the villus crypt junction and crypt depth was measured from the base upwards to the region of transition between the crypt and villus. A total of 10 villi per section and 2 sections per tissue sample were analyzed.
Nutrient Digestibility
To measure nutrients digestibility, the ileal digesta was collected from the posterior half between Meckel's diverticulum and 2 cm prior to the ileo-ceco-colonic junction. The digesta samples of birds were collected and stored at −20°C and finally freeze-dried to determine digestibility of DM, CP, EE, and organic matter (OM) according to the method of AOAC (2005). Gross energy (GE) of samples was analyzed using an adiabatic bomb calorimeter (Gallenkamp autobomb, Leicestershire, UK). The nutrients' apparent digestibility values are calculated as follows:
where AID is the apparent ileal digestibility of a nutrient in the diet; Marker diet is the concentration of an indigestible marker in the diet; Nutrient digesta is the nutrient concentration in the ileal digesta; Marker digesta is the indigestible-marker concentration in the ileal digesta; and Nutrient diet is the nutrient concentration in the diet.
Digestive Enzyme Activity
The samples of pancreas and jejunum digesta were individually diluted 4 and 10 times with phosphate-saline buffer and were then centrifuged at 3,000 × g for 15 min and 18,000 × g for 20 min at 4°C, respectively. The supernatant was collected into several Eppendorf tubes and stored at −70°C to assess different digestive enzymes' activity. The Amylase activity was assayed by the method of Somogyi (1960). One enzyme unit was defined as the amount of amylase forming reducing sugars equivalent to 1 mg of glucose per mg of intestinal digesta protein or pancreas during 30 min of incubation at 38°C. Corn starch was used as the substrate in this assay.
Lipase activity was measured according to the method described by Tietz and Fiereck (1966). Lipase activity unit was equal to the volume (mL) of 0.05 M NaOH required to neutralize the fatty acid released during 6 h incubation with 3 mL of lipase substrate at 38°C per mg of intestinal digesta protein or pancreas. Protease activity was determined using Lynn and Clevette-Redford's method (1984), where the protease activity unit was equal to the mg of azocasein breakdown during 2 h of incubation at 38°C per mg of intestinal digesta protein or pancreas. The Lowry et al. (1951) method was used to measure the protein concentration using ovine serum albumin as standard.
RNA Extraction and Preparation
Total RNA was isolated from each liver tissue by RNX-plus Kit (SinaClon, Iran). The samples were frozen in liquid nitrogen and were crushed and homogenized for RNA extraction (Bottje et al., 2009; Kong et al., 2011). After RNA purification and removal of DNA remnants by DNasel (kit-SinnaGen), the RNA quantity was measured by NanoDrop spectrophotometer ND-1000 (Absorbance 260 and 280 nm) and RNA integrity was validated by agarose-gel electrophoresis.
cDNA Synthesis
Reverse transcription (RT) of mRNAs was performed using RevertAid kit (Fermentas/Life Science/Isogene). A RT-PCR was carried out in a 20 µ L reaction volume according to the manufacturer' s instructions. The mixture contained total RNA, oligo dT, DEPC water, 5 × reaction buffer, RiboLock RNase Inhibitor, M-MuLV Reverse transcriptase. The RT reaction was incubated at 65°C for 5 min, 42°C for 60 min, and heat inactivation at 70°C for 5 min. The synthesized cDNA was stored at −20°C for further analysis.
Real-Time Reverse Transcription-PCR
A real-time PCR (Corbett, USA) was carried out to assess the expression pattern of target genes. Primers for IGF1 (forward primer, 5′-AGCAGTAGACGCTTACACCAC-3′; reverse primer, 5′-AGGTGGCTTTATTGGAGCACA-3′) and β -actin (forward primer, 5′-CATTGTCCACCGCAAATGCT-3′; reverse primer, 5′-TAATCCTGAGTCAAGCGCCA-3′) as the housekeeping and target gene, respectively, were designed by AllelID7.0 software. Each quantitative PCR (qPCR) reaction was performed with primers for either the target or the housekeeping gene: 10 µ L SYBR Green PCR Master Mix (SYBR biopars, GUASNR, Iran), 6 pM of each primer, 5% DMSO, and 10 ng of cDNA. Amplification conditions were comprised of an initial denaturation step of 3 min at 90°C, followed by a 2-step program (10 s of denaturation at 90°C and 30 s of annealing-elongation at 60°C), which was repeated 40 times. Then, samples were visualized on an agarose gel (1.5%) to confirm the replication of each specified gene. Finally, the cycle of threshold (Ct) for the IGF1 gene was subtracted to the corresponding Ct of the β -actin gene and the obtained data was analyzed by 2−dCt method.
Statistical Analyses
Data related to the effects of microbial fermentation on chemical composition of SBM and FSBM were analyzed using a T -test. Data related to the feeding trial of broiler chickens were checked for normality and then analyzed in a completely randomized design using the GLM procedures of SAS software (SAS Institute, 2003). When a significant effect of treatments was detected, means were compared using Tukey's HSD test atP < 0.05 level of probability.
RESULTS
FSBM Characteristics
Chemical composition, ANFs, microbial characteristics, and pH of SBM and FSBM are presented in Table 2. The results indicated that the fermentation process significantly increased the contents of CP (P = 0.003), ash (P = 0.03), and some amino acids including lysine (P = 0.001), phenylalanine (P = 0.008), glutamine (P = 0.001), and tyrosine (P = 0.03). The fermentation process considerably reduced pH from 5.96 to 3.71 in FSBM (P = 0.002). The lower pH in FSBM was associated with a 2.5-fold higher LAB count (3.87 vs. 9.22 Log10 CFU/g; P < 0.001) and a 6-fold higher lactic acid concentration (28.63 vs. 180.15 mmol/kg;P < 0.001). The Enterobacteriaceae count (4.52 vs. 1.38 Log10 CFU/g; P = 0.002), phytic acid content (0.72 vs. 0.20 g/100 g; P < 0.001), trypsin inhibitor content (2.61 vs. 0.85 mg/g; P = 0.001), β-conglycinin (40.92 vs. 17.01 mg/g; P < 0.001), and glycinin (63.27 vs. 25.27 mg/g; P < 0.005) were effectively reduced by the fermentation process in the FSBM.
Table 2.
Analyzed composition of SBM and FSBM (% of dry matter basis).
| Feed |
||||
|---|---|---|---|---|
| Item | SBM | FSBM | SEM | P-value |
| Dry matter | 92.32 | 90.69 | 1.064 | 0.339 |
| Crude protein | 43.55b | 46.37a | 0.321 | 0.003 |
| Ether extract | 1.25 | 1.18 | 0.023 | 0.116 |
| Crude fiber | 6.62 | 6.27 | 0.162 | 0.203 |
| Ash | 5.80b | 6.14b | 0.071 | 0.029 |
| Indispensable amino acids | ||||
| Arginine | 2.87 | 2.95 | 0.120 | 0.662 |
| Histidine | 1.04 | 1.08 | 0.019 | 0.179 |
| Isoleucine | 1.63 | 1.57 | 0.054 | 0.461 |
| Leucine | 3.34 | 3.32 | 0.133 | 0.907 |
| Lysine | 2.52b | 2.84a | 0.027 | 0.001 |
| Methionine | 0.60 | 0.64 | 0.026 | 0.307 |
| Phenylalanine | 1.95b | 2.08a | 0.019 | 0.008 |
| Threonine | 1.81 | 1.95 | 0.085 | 0.301 |
| Valine | 1.77 | 1.86 | 0.032 | 0.123 |
| Dispensable amino acids | ||||
| Alanine | 1.52 | 1.73 | 0.075 | 0.125 |
| Asparagine | 4.60 | 5.22 | 0.197 | 0.088 |
| Cysteine | 1.28 | 1.31 | 0.022 | 0.448 |
| Glutamine | 7.01b | 8.36a | 0.067 | 0.001 |
| Glycine | 1.63 | 1.84 | 0.060 | 0.072 |
| Proline | 2.45 | 2.58 | 0.051 | 0.149 |
| Serine | 2.25 | 2.27 | 0.028 | 0.639 |
| Tyrosine | 1.64b | 1.79a | 0.031 | 0.032 |
| Tryptophan | 0.58 | 0.67 | 0.014 | 0.167 |
| pH | 5.69a | 3.71b | 0.111 | 0.002 |
| Lactic acid (mmol/kg) | 28.63b | 180.15a | 0.967 | <0.001 |
| Lactic acid bacteria (Log10 CFU/g) | 3.95b | 9.22a | 0.220 | <0.001 |
| Enterobacteriaceae (Log10 CFU/g) | 4.52a | 1.38b | 0.324 | 0.002 |
| Phytic acid (g/100g) | 0.72a | 0.20b | 0.021 | <0.001 |
| Trypsin inhibitor (mg/g) | 2.61a | 0.85b | 0.160 | 0.001 |
| β-conglycinin (mg/g) | 40.92a | 17.01b | 1.058 | <0.001 |
| Glycinin (mg/g) | 63.27a | 25.56b | 2.762 | 0.005 |
SBM = soybean meal; FSBM = fermented soybean meal. Means with different superscripts in each row are statistically different (P < 0.05).
Growth Performance
The effects of replacing SBM with FSBM with or without MOS on the growth performance of broiler chicken are reported in Table 3. The FI of the birds was not affected by any of the experimental diets for the total grow-out period. BW gain, however, was improved by replacing SBM with FSBM during d 11 to 24 (P < 0.001), d 25 to 42 (P < 0.001), and the total grow-out period (d 1 to 42; P = 0.001). MOS addition to the CON diet improved birds BW gain during day 11 to 24 (P < 0.001), but not for the rest of the experimental period. Birds had the largest BW gain when fed the MIX diet (P < 0.001), which indicated a synergy between FSBM and the added MOS as a prebiotic. Feed conversion ratio was the lowest when the birds were fed FSBM, with or without MOS for d 11 to 24 (P = 0.040), d 25 to 42 (P = 0.002), and the total grow-out period (d 1 to 42; P = 0.003). The dressing percentage was not affected by the experimental diets; however, abdominal fat (%) was reduced when birds were fed FSBM with or without MOS (P = 0.001).
Table 3.
Effects of experimental diets on growth performance in broiler chickens.1
| Treatments2 |
||||||
|---|---|---|---|---|---|---|
| Item | CON | MOS | FSBM | MIX | SEM | P-value |
| Body weight gain, g | ||||||
| d 1 to 10 | 225.71 | 228.35 | 231.60 | 230.84 | 4.574 | 0.797 |
| d 11 to 24 | 878.50c | 897.22b | 908.01b | 933.20a | 5.447 | <0.001 |
| d 25 to 42 | 1551.45c | 1574.64b,c | 1597.25b | 1625.58a | 8.029 | <0.001 |
| d 1 to 42 | 2655.67c | 2700.22b,c | 2736.87b | 2789.62a | 16.671 | 0.001 |
| Feed intake, g | ||||||
| d 1 to 10 | 275.85 | 283.61 | 283.50 | 279.45 | 5.935 | 0.760 |
| d 11 to 24 | 1388.23 | 1394.58 | 1400.25 | 1419.10 | 11.907 | 0.318 |
| d 25 to 42 | 2907.68 | 2915.84 | 2899.07 | 2917.55 | 20.136 | 0.910 |
| d 1 to 42 | 4571.76 | 4594.03 | 4582.82 | 4616.10 | 25.153 | 0.643 |
| Feed conversion ratio | ||||||
| d 1 to 10 | 1.22 | 1.24 | 1.22 | 1.21 | 0.010 | 0.235 |
| d 11 to 24 | 1.57a | 1.55a,b | 1.54a,b | 1.52b | 0.013 | 0.040 |
| d 25 to 42 | 1.87a | 1.85a | 1.82b | 1.79b | 0.011 | 0.002 |
| d 1 to 42 | 1.72a | 1.70a,b | 1.67b,c | 1.65c | 0.009 | 0.003 |
| Mortality (%; d 1 to 42) | 2.50 | 1.67 | 2.50 | 0.83 | 0.715 | 0.942 |
| Dressing percentage | 71.47 | 71.95 | 72.04 | 72.60 | 0.390 | 0.507 |
| Abdominal fat (%) | 1.56a | 1.48a | 1.19b | 1.22b | 0.065 | 0.001 |
Means with different superscripts in each row are statistically different (P < 0.05).
Data represent means of 6 replicates per treatment.
CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM.
Replacing SBM with FSBM reduced the concentration of plasma 3-methylhistidine, which was similar to the MOS diet. The MIX diet, however, further reduced the plasma 3-methylhistidine concentration in a synergistic way, indicating the symbiotic effects (Figure 1;P = 0.001).
Figure 1.
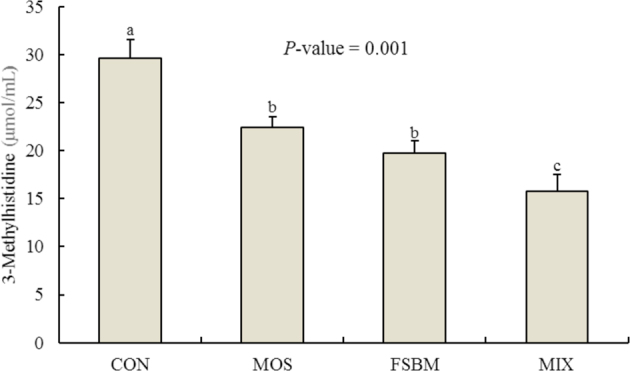
Effects of experimental diets on concentration of plasma 3-methylhistidine in broiler chickens. Bars with no common letter differ statistically (P < 0.05). CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM.
Microbiota Composition, pH, and Intestinal Morphology
The effects of experimental diets on microbiota composition (Log10 CFU/g) and the pH of the crop, ileum, and cecal of broiler chicks are presented in Table 4. The MOS diet did not change crop pH and LAB count compared to the CON diet, however, replacing SBM with FSBM with and without the addition of MOS has reduced crop pH (P = 0.001) and increased the LAB count (P = 0.001). The pH in ileum was reduced by the addition of MOS to the CON diet (P < 0.001) that was similar to the FSBM diet. Birds on the MIX diet had the lowest ileum pH compared to the CON and MOS diets but did not differ from the FSBM diet. The ileum LAB count was higher in birds fed the FSBM with and without MOS (P = 0.002) compared to the CON diet but did not differ from the MOS diet. Birds had the highest cecal LAB count when fed the MIX diet. The MOS, FSBM, and MIX diets reduced both C. perfringens and coliform in the ileum and cecum compared to the CON diet (P = 0.005). Total anaerobic bacteria count was not altered in the crop and ileum by the experimental diets.
Table 4.
Effect of experimental diets on microbiota composition (Log10 CFU/g) and the pH of the crop, ileum, and cecal in broiler chickens.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Item | CON | MOS | FSBM | MIX | SEM | P-value |
| Crop | ||||||
| pH | 4.53a | 4.49a | 4.28b | 4.20b | 0.065 | 0.001 |
| Lactic acid bacteria | 6.87b | 6.95b | 7.35a | 7.48a | 0.117 | 0.001 |
| Total anaerobic bacteria | 6.65 | 6.50 | 6.59 | 6.36 | 0.103 | 0.231 |
| Ileum | ||||||
| pH | 6.72a | 6.54b | 6.42b,c | 6.39c | 0.046 | <0.001 |
| Lactic acid bacteria | 7.58b | 7.76a,b | 7.96a | 7.91a | 0.071 | 0.002 |
| Clostridium perfringens | 4.26a | 4.02b | 3.89b | 3.95b | 0.067 | 0.002 |
| Coliform | 5.70a | 5.34b | 5.26b | 5.14b | 0.101 | 0.005 |
| Total anaerobic bacteria | 7.15 | 7.02 | 7.14 | 6.93 | 0.130 | 0.594 |
| Cecal | ||||||
| pH | 6.01a | 5.62b | 5.56b | 5.47b | 0.068 | <0.001 |
| Lactic acid bacteria | 8.13c | 8.54b | 8.70a,b | 9.02a | 0.100 | <0.001 |
| Clostridium perfringens | 4.52a | 4.26b | 4.12b | 4.19b | 0.084 | 0.011 |
| Coliform | 5.97a | 5.51b | 5.40b | 5.32b | 0.118 | 0.001 |
| Total anaerobic bacteria | 7.68a | 7.40b | 7.33b | 7.49a,b | 0.087 | 0.040 |
CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM
Means with different superscripts in each row are statistically different (P < 0.05).
The intestinal characteristics of broiler chicken when fed with different experimental diets are shown in Table 5. All the experimental diets, including the MOS, FSBM, and MIX diets, increased villus height in duodenum (P < 0.002) and jejunum (P < 0.001) compared to the CON diet. The crypt depth was reduced by the MIX diet compared to the CON diet (P < 0.03). The villus height to crypt depth ratio in the duodenum (6.71 vs. 6.46 µ m in the MOS and CON, respectively) and jejunum (5.43 vs. 5.05 in the MOS and CON, respectively) was increased by the addition of MOS to the SBM diet (P < 0.001) compared to the CON diet. However, the MIX diet further increased the villus height to crypt depth in the duodenum (7.16 vs. 6.46 µ m in MIX and CON, respectively) and jejunum (5.72 vs. 5.05 µ m in MIX and CON, respectively) compared to the CON diet (P < 0.001), indicating the synergistic effects.
Table 5.
Effects of experimental diets on small intestinal morphology (μm) of broiler chickens.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| CON | MOS | FSBM | MIX | SEM | P-value | |
| Villus height | ||||||
| Duodenum | 1068.25b | 1092.20a,b | 1109.34a | 1115.73a | 8.870 | 0.002 |
| Jejunum | 679.05b | 705.60a | 721.95a | 727.41a | 8.511 | 0.001 |
| Ileum | 430.82 | 436.20 | 445.70 | 451.25 | 8.055 | 0.285 |
| Crypt depth | ||||||
| Duodenum | 165.83a | 163.15a,b | 160.01a,b | 155.85b | 2.391 | 0.031 |
| Jejunum | 134.70 | 130.56 | 132.64 | 127.20 | 2.767 | 0.274 |
| Ileum | 101.53 | 96.52 | 99.15 | 98.61 | 2.238 | 0.474 |
| Villus height/crypt depth | ||||||
| Duodenum | 6.46c | 6.71b | 6.94a,b | 7.16a | 0.082 | <0.001 |
| Jejunum | 5.05c | 5.43b | 5.47a,b | 5.72a | 0.096 | 0.002 |
| Ileum | 4.26 | 4.54 | 4.50 | 4.59 | 0.105 | 0.148 |
CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM.
Means with different superscripts in each row are statistically different (P < 0.05).
Ileal Nutrient Digestibility, Digestive Enzymes Activities, and Hepatic IGF-1 Expression
The ileal digestibility of DM, CP, EE, GE, and OM of different experimental diets is shown in Table 6. The CP ileal digestibility was not altered when the MOS was added to the CON diet. However, the FSBM improved the CP ileal digestibility compared to the CON and the MOS diets (P = 0.02) that was not different from the MIX diet. The ileal digestibility of GE was improved by replacing SBM with FSBM with or without MOS (P = 0.001).
Table 6.
Effects of experimental diets on apparent ileal digestibility of nutrients in broiler chickens.
| Treatments |
||||||
|---|---|---|---|---|---|---|
| CON | MOS | FSBM | MIX | SEM | P-value | |
| Dry matter | 72.25 | 72.65 | 72.09 | 72.44 | 0.431 | 0.814 |
| Crude protein | 76.40b | 76.31b | 78.95a | 78.14a,b | 0.701 | 0.023 |
| Ether extract | 83.68 | 84.15 | 86.07 | 86.73 | 1.070 | 0.144 |
| Gross energy | 79.22b | 80.45b | 85.30a | 84.97a | 1.251 | 0.001 |
| Organic matter | 71.73 | 70.68 | 71.46 | 71.80 | 0.689 | 0.645 |
CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM.
Means with different superscripts in each row are statistically different (P < 0.05).
The effects of experimental diets on intestinal and pancreatic digestive enzyme activities are presented in Table 7. The activity of intestinal amylase (P = 0.004) and protease (P = 0.020), as well as the activity of the pancreatic protease (P < 0.001), was improved by replacing SBM with FSBM with and without MOS.
Table 7.
Effects of experimental diets on intestinal and pancreatic digestive enzyme activity (U/mg of digesta protein).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| CON | MOS | FSBM | MIX | SEM | P-value | |
| Intestine | ||||||
| Lipase | 18.62 | 18.56 | 18.97 | 19.15 | 0.538 | 0.871 |
| Amylase | 13.40b | 13.32b | 16.24a | 16.01a | 0.719 | 0.004 |
| Protease | 79.25c | 81.50b,c | 86.03a,b | 88.50a | 2.209 | 0.020 |
| Pancreas | ||||||
| Lipase | 46.54 | 45.85 | 47.21 | 46.62 | 1.776 | 0.960 |
| Amylase | 35.29 | 36.14 | 34.95 | 36.33 | 1.274 | 0.883 |
| Protease | 148.80b | 153.50b | 167.46a | 170.61a | 2.253 | <0.001 |
CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM
Means with different superscripts in each row are statistically different (P < 0.05).
The hepatic IGF-1 expression of birds when fed with different experimental diets is illustrated in Figure 2. The MOS diet upregulated the hepatic IGF-1 gene expression. By replacing SBM with FSBM, an upregulation in the hepatic IGF-1 gene was observed that was similar to the MOS diet. The MIX diet, however, further increased the expression of the hepatic IGF-1 in a synergistic way (P = 0.001), indicating the synergistic effects.
Figure 2.
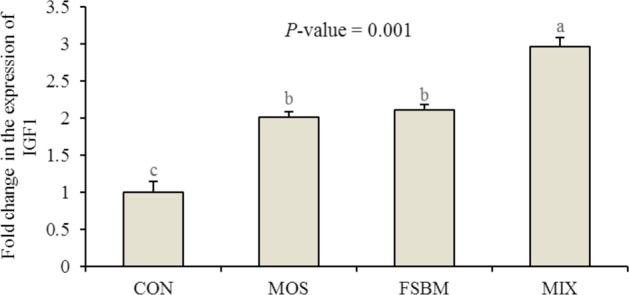
Effects of experimental diets on hepatic IGF-1 expression in broiler chickens. Bars with no common letter differ statistically (P < 0.05). CON = control; MOS = mannan-oligosaccharides; FSBM = fermented soybean meal; MIX = MOS + FSBM.
DISCUSSION
SBM is the most commonly used protein source in animal nutrition. The presence of some ANFs, such as trypsin inhibitor, antigenic proteins, and phytic acid, however, can deteriorate birds' growth performance if not removed or deactivated. Microbial fermentation has been suggested as an effective and less-expensive technique to reduce the ANFs and improve the nutritive value of plant-based proteins by providing probiotics and their metabolites in the feed. In the current study, A. oryzae removed the oxygen from the fermentation media and created anaerobic conditions for the growth and development of L. acidophilus, L. plantarum, and B. subtilis. The biological activity of B. subtilis improves the condition for the growth and proliferation of LAB (Jazi et al., 2017), and in return, the LAB activity reduces the pH of the feed by producing lactic acid and establish a protective condition against the growth and viability of pathogenic organisms such as Enterobacteriaceae. The fermentation process by the combination of fungal and bacterial species in the current study has effectively increased the LAB counts (2.5-fold increase) and lactic acid concentration (6-fold increase), which resulted in a 35% reduced pH of FSBM. Similar outcomes have been reported in previous studies, which have indicated a reduced pH and increased LAB population and lactic acid concentrations in fermented products (Shi et al., 2017; Jazi et al., 2018a). The results of the current study have demonstrated the effects of the fermentation process on improved nutritive value of FSBM with increased CP, lysine, phenylalanine, glutamine, tyrosine, as well as reduced phytic acid, trypsin inhibitor, and allergenic proteins (β-conglycinin and glycinin) contents in the FSBM. The reduction of these ANFs may be attributed to the microbial or fungal enzymes degrading these compounds. B. subtilis has been reported to produce protease, cellulase, and phytase enzymes (Sun et al., 2012) and lactobacillus to produce phytase, cellulase, xylanase, and glucanase enzymes (Taheri et al., 2009). The microbial protease and phytase enzymes might be responsible for the reduction of the allergic proteins and phytic acid contents, respectively (Li et al., 2014). The increase in the CP or amino acid contents could also originate from the microbial protein synthesis due to the increased microbial population during the fermentation (Chen et al., 2013; Ashayerizadeh et al., 2017). Moreover, the reduction of water-insoluble and non-protein compounds, such as fiber, during the extraction process may also contribute to the increased protein content in the FSBM (Sun et al., 2015).
In the current study, replacing SBM with FSBM, with or without the addition of MOS improved BW gain and FCR for the total grow-out period (d 1 to 42). However, the FI during the grow-out period was not affected by FSBM, with or without the addition of MOS. Therefore, given that no differences in FI between the experimental diets were observed, increased BW gain indicates a higher nutrient digestibility and utilization in FSBM-fed birds. Similarly, Sun et al. (2013) and Jazi et al. (2017) reported improved growth performance when replacing SBM with fermented product in broiler diets. These authors suggested that the improvement of the nutritional value of feedstuffs and reduction in ANFs after fermentation as well as improved intestinal health, may have a beneficial impact on growth performance. In the present study, the enhanced growth performance of birds fed FSBM diets was associated with improved intestinal morphology, microbial community, digestive enzymes' activity, and increased expression of hepatic IGF-1, which is considered as the anabolic growth factor.
The positive effects of prebiotic on birds BW gain in this study are in agreement with previous studies that reported an improved growth performance of broiler chicken when fed with dietary MOS (Chee et al., 2010; Jazi et al., 2018b). Based on the literature, the improved growth performance of birds when prebiotics are included in the diet, may originate from encouraging the growth of beneficial bacteria, the pathogens-binding capability of MOS, and enhanced GI tract functionality (Faber et al., 2011; Pourabedin et al., 2014). The addition of MOS to the FSBM in the current study further improved the BW gain of the birds for the total grow-out period, which was an indication of a synergistic relationship between prebiotics and fermented feeds. The synergy between FSBM and MOS was evident in a few traits in the current study that are presented accordingly. However, we could not find any reported results of supplementing prebiotics to fermented feed products and it seems that the current study is the first to explore the synergy between fermented feed and a prebiotic, MOS in this study.
Replacing SBM with FSBM with or without MOS reduced abdominal fat content, which may originate from the increased LAB count when birds are fed the FSBM. Two mechanisms have been suggested for LAB to reduce abdominal fat. First, the LAB is reported to break down the bile salts and increase their excretion in the faces. This increases the need for bile salt production in the liver. Second, it has been shown that LAB down-regulate the activity of acetyl CoA carboxylase, which is a rate-limiting enzyme in the hepatic fatty acid production (Kalavathy et al., 2003). The reduced fatty acid output from the liver will restrict the triglyceride esterification and fat deposition in the body (Shirani et al., 2019).
In the present study, plasma 3-methylhistidine concentration was reduced by FSBM and MOS and a further reduction by their combination. The plasma or urinary 3-methylhistidine is a biomarker of muscle protein degradation. Due to the difficulties of urine sampling in chicken, we used plasma 3-methylhistidine concentration measurements to study the effects of FSBM and MOS on muscle protein degradation (Nagasawa et al., 1998). These outcomes indicate that feeding FSBM and MOS may lead to suppressing the skeletal muscle protein degradation. Similar results have been reported where the gene expression of the enzymes involved in the skeletal muscle protein breakdown were down-regulated by using probiotics in the broiler chicken diet (Saleh et al., 2013).
The GI tract of poultry is populated with microorganisms, of which the microbial composition and activity significantly affect the bird's metabolism, immune response, gut functionality, and growth performance (Pan and Yu, 2013). Replacing SBM with FSBM in the present study reduced pH, increased LAB counts in crop, ileum, and cecal and reduced ileal and cecal C. perfringens and coliform of broiler chicken. These observations are in line with previous studies that have reported an increase in the LAB counts in the intestine, a reduced pH, and improved intestinal morphology by the inclusion of fermented feeds in the diet (Sun et al., 2013; Jazi et al., 2017; Ashayerizadeh et al., 2018). The low pH and high LAB counts in fermented feeds may reduce the pH in the upper part of the GI tract, particularly crop, and thereby improves the gut microbial balance by providing an environment that promotes the beneficial bacterial growth and establishment, such as LAB, and eliminates pathogens (Shabani et al., 2019). The aforementioned effects of fermented feed on gut microbiota are very similar to the ones of probiotics in broiler chicken (Chiang et al., 2010). Our results showed that dietary MOS reduced pH, increased LAB population, and reduced C. perfringens and coliform in the ileum and cecal of broiler chicken. These outcomes were in line with the results of Chee et al. (2010) who found that supplementation with MOS in broiler diets increased population of lactobacilli and LAB and reduced C. perfringens and coliform in the ileum compared to the control group. The underlying mechanisms of MOS in improving gut microbial profile may include stimulation of the growth of beneficial bacteria, increase in short-chain fatty acids (SCFA) concentration as an end-product of fermentation, binding ability of mannose group of the MOS to the enteric pathogens, and finally reduction in the attachment of pathogenic bacteria into the enterocytes (Xu et al., 2003; Jazi et al., 2018b).
The villus height and villus height to crypt depth are important indicators of intestinal digestion and absorption capacity so that an increase in villus height to crypt depth ratio, indicating an improvement in intestinal nutrient digestion and absorption (Shirani et al., 2019). In the current study, the FSBM diet with or without MOS improved gut morphology that was reflected in a greater villus height, reduced crypt depth, and increased villus height to crypt depth. These results are in agreement with previous studies, which demonstrated that the inclusion of fermented cottonseed meal improved intestinal morphology of broilers (Sun et al., 2013). Similarly, Chiang et al. (2010) demonstrated an increase in the villus height and villus height to crypt depth ratio in jejunum and ileum of chickens fed fermented rapeseed meal. The mode of action of fermented feeds in improved intestinal morphology varies among different studies. Nonetheless, the general proposed mechanisms of action are through improving intestinal microflora balance in favor of beneficial microbes (Jazi et al., 2018a) and reducing ANFs during fermentation, such as antigenic proteins (Feng et al., 2007a). In addition, the metabolic activity of microorganisms during fermentation can increase enzymes and other compounds such as peptides, amino acids, and organic acids, which may enhance gut function (Jazi et al., 2019). The improved intestinal morphology in birds fed the FSBM and MIX diet can lead to enhanced nutrient digestibility and improved growth performance.
The improvement of intestinal mucosal structure is thought to be as a consequence of the effects of MOS on the gut microbial composition and activity and microbial metabolites. These results are in line with previous studies, which have reported that the dietary supplementation with MOS increased villus height in the small intestinal of broiler chickens (Chee et al., 2010; Pourabedin et al., 2014).
A healthy gut microbiota is the cornerstone of the optimum growth performance in animal. The impaired health and functioning of the GI tract affects the nutrients' digestion and absorption, and the growth performance will be retarded. Determination of nutrients digestibility is a quantitative assessment of the nutritional and physiological phenomena associated with digestion capacity and gut function (Goodarzi Boroojeni et al., 2018). In the current study, replacing SBM with FSBM, with or without MOS, improved ileal digestibility of CP and GE that was reflected in the birds' growth performance. This improvement might be related to the decreased ANFs content particularly trypsin inhibitor, phytic acid, and allergenic protein, and increased protein solubility (Feng et al., 2007b; Jazi et al., 2019). The elevated digestive enzymes activity by feeding fermented feeds in this study, also contributes to the higher nutrient digestibility, which is explained in the next section. Chiang et al. (2010) reported an improved digestibility of DM and GE, after replacing rapeseed meal with fermented rapeseed meal in the broiler chicken diet. Similar results were obtained by Aljuobori et al. (2014), where the CP digestibility was greater in fermented rapeseed meal compared to the broiler chicken fed with raw rapeseed meal.
Digestive enzymes play an important role in breaking down the feed particles and liberating nutrients for absorption, which are required for growth and general health of the birds. In previous studies, it has been shown that in addition to trypsin inhibitor, other ANFs such as antigenic proteins depress digestive enzyme activity and thereby impair the digestive capacity and absorption of nutrients (Feng et al., 2007a). On the other hand, pathogenic bacteria may prevent the secretion of digestive enzymes by damaging the villi and microvilli of the intestinal mucosa of the host (Jazi et al., 2018b). To the best of our knowledge, data reporting the underlying mechanisms by which fermented feeds may improve digestive enzymes in digestive organs is limited. Feng et al. (2007b) suggested that the elimination of ANFs such as trypsin inhibitor and soybean globulins in SBM may improve digestive enzyme activities. Sun et al. (2013) also demonstrated that replacing SBM with cottonseed meal fermented with B. subtilis in broiler diets increases protease and amylase enzyme activity compared to the control birds. The authors stated that this effect may come from the improved intestinal morphology, as well as the contribution of B. subtilis in the production of protease and amylase enzymes. Fermented feeds may also acidify the upper GI tract and provide unfavorable conditions for growth and colonization of harmful bacteria (Jazi et al., 2018a), and therefore, inhibit the negative effects of pathogens on the secretion of digestive enzymes. The results of the present study indicated that feeding birds with FSBM and MIX diets increased the activity of intestinal amylase and protease, and pancreatic protease. These observations were associated with improved intestinal morphology as increased villus height and villus height to crypt depth ration, greater ileal digestibility of key nutrients, and eventually a better growth performance.
The MOS and FSBM diets upregulated the hepatic IGF-1 gene expression similarly, in the present study. Supplementing FSBM diet with MOS, the MIX, however, further increased the hepatic IGF-1 gene expression, indicating a synergy between the FSBM and the prebiotic. The hepatic IGF-1 is one of the main growth factors in the body that stimulates muscle protein synthesis in mammals and poultry (Guobin et al., 2011). The anabolic actions of muscle protein synthesis, including amino acids uptake and incorporation into proteins, uridine, and thymidine synthesis into nucleic acid glucose uptake, cell proliferation, and suppression of protein degradation are regulated by IGF-1 (Florini et al., 1991). Yan et al. (2016) in a recent study revealed that addition of SCFA (as major end products of bacterial fermentation) to mice drinking water can increase serum IGF-1 levels. Kareem et al. (2016) reported that feeding postbiotics (metabolic end-products of LAB fermentation) and prebiotics increased hepatic IGF-1 expression when compared to the control group. Saleh et al. (2013) have also demonstrated that feeding Aspergillus awamori reduces skeletal muscle protein breakdown and stimulates growth in broilers that supports the outcomes of the current study.
The present study demonstrated that the use of FSBM in broiler diets could promote the growth performance by suppressing the skeletal muscle protein breakdown, up-regulating the hepatic IGF-1 gene expression, and improving the digestive functions, including digestive enzyme activity, nutrient digestibility, gut microbial balance, and intestinal morphology of broiler chicken. The addition of prebiotic to the diets containing FSBM further improves growth performance, demonstrating synergistic effects. These findings encourage the animal feed industry to look for cost-effective opportunities to include fermented products in the diet.
ACKNOWLEDGMENTS
This project was funded by the University of Mohaghegh Ardabili, Ardabil, Iran (Project Number #D-7914). The authors greatly acknowledge and appreciate the technical support from the staff of the University of Mohaghegh Ardabili, Ardabil, Iran.
REFERENCES
- AOAC . AOAC International; Arlington, VA, USA: 2005. Official Methods of Analysis in Association of Official Analytical Chemists. [Google Scholar]
- Aljuobori A., Zulkifli I., Farjam Soleimani A., Norhani A., Juan Boo L., Awad E.A. Effect of solid state fermentation on nutrient content and ileal amino acids digestibility of canola meal in broiler chickens. Ital. J. Anim. Sci. 2014;13:410–414. [Google Scholar]
- Ashayerizadeh A., Dastar B., Shams Shargh M., Sadeghi Mahoonak A.R., Zerehdaran S. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Vet. Microbiol. 2017;201:93–102. doi: 10.1016/j.vetmic.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Ashayerizadeh A., Dastar B., Shargh M.S., Mahoonak A.S., Zerehdaran S. Effects of feeding fermented rapeseed meal on growth performance, gastrointestinal microflora population, blood metabolites, meat quality, and lipid metabolism in broiler chickens. Livest. Sci. 2018;216:183–190. [Google Scholar]
- Aviagen International . Aviagen Ltd.; Scotland, UK: 2014. Nutrition Specifications Manual: Arbor Acers. [Google Scholar]
- Bottje W., Brand M.D., Ojano-Dirain C., Lassiter K., Toyomizu M., Wing T. Mitochondrial proton leak kinetics and relationship with feed efficiency within a single genetic line of male broilers. Poult. Sci. 2009;88:1683–1693. doi: 10.3382/ps.2009-00100. [DOI] [PubMed] [Google Scholar]
- Chee S.H., Iji P.A., Choct M., Mikkelsen L.L., Kocher A. Characterisation and response of intestinal microflora and mucins to manno-oligosaccharide and antibiotic supplementation in broiler chickens. Br. Poult. Sci. 2010;51:368–380. doi: 10.1080/00071668.2010.503477. [DOI] [PubMed] [Google Scholar]
- Chen L., Madl R.L., Vadlani P.V. Nutritional enhancement of soy meal via Aspergillus oryzae solid‐state fermentation. Cereal. Chem. 2013;90:529–534. [Google Scholar]
- Chiang G., Lu W.Q., Piao X.S., Hu J.K., Gong L.M., Thacker P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas. J. Anim. Sci. 2010;23:263–271. [Google Scholar]
- Easter B.R., Kim S.W. Opportunities for Soy Products in Animal Nutrition. Federation of Animal Science Societies; Savoy: 1999. Routes of use of soybean products; pp. 1–8. [Google Scholar]
- Faber T.A., Hopkins A.C., Middelbos I.S., Price N.P., Fahey G.C., Jr Galactoglucomannan oligosaccharide supplementation affects nutrient digestibility, fermentation end-product production, and large bowel microbiota of the dog. J. Anim. Sci. 2011;89:103–112. doi: 10.2527/jas.2010-3028. [DOI] [PubMed] [Google Scholar]
- Feng J., Liu X., Xu Z.R., Wang Y.Z., Liu J.X. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult. Sci. 2007;86:1149–1154. doi: 10.1093/ps/86.6.1149. [DOI] [PubMed] [Google Scholar]
- Feng J., Liu X., Xu Z.R., Lu Y.P., Liu Y.Y. Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Dig. Dis. Sci. 2007;52:1845–1850. doi: 10.1007/s10620-006-9705-0. [DOI] [PubMed] [Google Scholar]
- Florini J.R., Ewton D.Z., Magri K.A. Hormones, growth factors, and myogenic differentiation. Annu. Rev. Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Goodarzi Boroojeni F., Männer K., Rieger J., Pérez Calvo E., Zentek J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019;98:2080–2086. doi: 10.3382/ps/pey556. [DOI] [PubMed] [Google Scholar]
- Guobin C., Xiangping L., Jing L. Temporal and spatial expression of the pax-7 gene during chicken embryo and postnatal development. J. Anim. Vet. Adv. 2011;10:1785–1788. [Google Scholar]
- Hayashi K., Maeda Y., Toyomizu M., Tomita Y. High performance liquid chromatographic method for the analysis of Nt -methylhistidine in food, chicken excreta, and rat urine. J. Nutr. Sci. Vitaminol. 1987;33:151–156. doi: 10.3177/jnsv.33.151. [DOI] [PubMed] [Google Scholar]
- Jazi V., Boldaji F., Dastar B., Hashemi S.R., Ashayerizadeh A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br. Poult. Sci. 2017;58:402–408. doi: 10.1080/00071668.2017.1315051. [DOI] [PubMed] [Google Scholar]
- Jazi V., Ashayerizadeh A., Toghyani M., Shabani A., Tellez G., Toghyani M. Fermented soybean meal exhibits probiotic properties when included in Japanese quail diet in replacement of soybean meal. Poult. Sci. 2018;97:2113–2122. doi: 10.3382/ps/pey071. [DOI] [PubMed] [Google Scholar]
- Jazi V., Foroozandeh A.D., Toghyani M., Dastar B., Rezaie Koochaksaraie R., Toghyani M. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poult. Sci. 2018;97:2034–2043. doi: 10.3382/ps/pey035. [DOI] [PubMed] [Google Scholar]
- Jazi V., Mohebodini H., Ashayerizadeh A., Shabani A., Barekatain R. Fermented, soybean meal ameliorates Salmonella Typhimurium infection in young broiler chickens. Poult. Sci. 2019 doi: 10.3382/ps/pez338. in press. [DOI] [PubMed] [Google Scholar]
- Kalavathy R., Abdullah N., Jalaludin S., Ho Y.W. Effects of lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 2003;44:139–144. doi: 10.1080/0007166031000085445. [DOI] [PubMed] [Google Scholar]
- Kareem K.Y., Loh T.C., Foo H.L., Akit H., Samsudin A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016;12:163–170. doi: 10.1186/s12917-016-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B.W., Song J.J., Lee J.Y., Hargis B.M., Wing T., Lassiter K., Bottje W. Gene expression in breast muscle associated with feed efficiency in a single male broiler line using a chicken 44K oligo microarray. I. Top differentially expressed genes. Poult. Sci. 2011;90:2535–2547. doi: 10.3382/ps.2011-01435. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Lu J.J., Wu C.P., Lien T.F. Effects of probiotics and bremelain fermented soybean meal replacing fish meal on growth performance, nutrient retention and carcass traits of broilers. Livest. Sci. 2014;163:94–101. [Google Scholar]
- Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lynn K.R., Clevette-Radford N.A. Purification and characterization of hevain, a serine protease from Hevea brasiliensis. Biochem. J. 1984;23:963–964. [Google Scholar]
- Marsili R.T., Ostapenko H., Simmons R.E., Green D.E. High performance liquid chromatographic determination of organic acid. J. Food Prot. 1983;46:52–57. [Google Scholar]
- Nagasawa T., Hirano J., Yoshizawa F., Nishizawa N. Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci. Biotechnol. Biochem. 1998;62:1932–1937. doi: 10.1271/bbb.62.1932. [DOI] [PubMed] [Google Scholar]
- Palacios M.F., Easter R.A., Soltwedel K.T., Parsons C.M., Douglas M.W., Hymowitz T., Pettigrew J.E. Effect of soybean variety and processing on growth performance of young chicks and pigs1. J. Anim. Sci. 2004;82:1108–1114. doi: 10.2527/2004.8241108x. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Xu Z., Baurhoo B., Chevaux E., Zhao X. Effects of mannan oligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Can. J. Microbiol. 2014;60:255–266. doi: 10.1139/cjm-2013-0899. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Abdollahi M.R., Bootwalla S.M. Nutrient analysis, metabolizable energy, and digestible amino acids of soybean meals of different origins for broilers. Poult. Sci. 2014;93:2567–2577. doi: 10.3382/ps.2014-04068. [DOI] [PubMed] [Google Scholar]
- Saleh A.A., Hayashi K., Ohtsuka A. Synergistic effect of feeding Aspergillus awamori and Saccharomyces cerevisiae on growth performance in broiler chickens; promotion of protein metabolism and modification of fatty acid profile in the muscle. J. Poult. Sci. 2013;50:242–250. [Google Scholar]
- SAS Institute Inc . SAS Inst. Inc.; Cary, NC: 2003. SAS User's Guide. Statistics. Version 9.1. [Google Scholar]
- Shabani A., Jazi V., Ashayerizadeh A., Barekatain R. Inclusion of fish waste silage in broiler diets affects gut microflora, cecal short-chain fatty acids, digestive enzyme activity, nutrient digestibility, and excreta gas emission. Poult. Sci. 2019 doi: 10.3382/ps/pez244. in press. [DOI] [PubMed] [Google Scholar]
- Shirani V., Jazi V., Toghyani M., Ashayerizadeh A., Sharifi F., Barekatain R. Pulicaria gnaphalodes powder in broiler diets: consequences for performance, gut health, antioxidant enzyme activity, and fatty acid profile. Poult. Sci. 2019;98:2577–2587. doi: 10.3382/ps/pez010. [DOI] [PubMed] [Google Scholar]
- Shi C., Zhang Y., Lu Z., Wang Z. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017;8:50–59. doi: 10.1186/s40104-017-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Van Megen W., Twaalfhoven L., Hitchcock C. The determination of trypsin inhibitor levels in foodstuffs. J. Sci. Food Agric. 1980;31:341–350. doi: 10.1002/jsfa.2740310403. [DOI] [PubMed] [Google Scholar]
- Somogyi M. Modification of two methods for the assay of amylase. Clin. Chem. 1960;6:23–27. [PubMed] [Google Scholar]
- Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Feng J. Improvement of the nutritional quality of cottonseed meal by Bacillus subtilis and the addition of papain. Int. J. Agric. Biol. 2012;14:563–568. [Google Scholar]
- Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013;45:987–993. doi: 10.1007/s11250-012-0322-y. [DOI] [PubMed] [Google Scholar]
- Sun H., Yao X., Wang X., Wu Y., Liu Y., Tang J., Feng J. Chemical composition and in vitro antioxidant property of peptides produced from cottonseed meal by solid-state fermentation. CyTA J. Food. 2015;13:264–272. [Google Scholar]
- Taheri H.R., Moravej H., Tabandeh F., Zaghari M., Shivazad M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 2009;88:1586–1593. doi: 10.3382/ps.2009-00041. [DOI] [PubMed] [Google Scholar]
- Tietz N.W., Fiereck E.A. A specific method for serum lipase determination. Clin. Chim. Acta. 1966;13:352–358. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu X.T., Wang H.L., Li D.F., Piao X.S., Lu W.Q. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs. Livest. Sci. 2014;170:91–99. [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S., Charles J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]


