Abstract
For healthy development, an avian embryo needs the nutritional and functional molecules maternally deposited in avian eggs. Egg white not only provides nutritional components but also exhibits functional properties, such as defenses against microbial invasion. However, the roles of the more detailed messages in embryo development remain unclear. In this study, a tandem mass tag labeling quantitation approach was used to innovatively identify the differential proteins in the egg whites of fresh eggs produced by hens with divergent high/low hatchability and in the egg whites of embryonated eggs with healthy and dead embryos. A total of 378 proteins were quantified in egg white, which is the most complete proteome identified for egg white to date, and up to 102 differential proteins were identified. GO enrichment, pathway, and hierarchical clustering analysis revealed some of the differential proteins that are the main participants in several biological processes, including blood coagulation, intermediate filament, antibacterial activity, and neurodevelopment. A list of 11 putative protein biomarkers, such as keratin (KRT19, KRT12, KRT15, and KRT6A), which is involved in cell architecture, and fibrinogen (fibrinogen alpha chain, fibrinogen beta chain, and fibrinogen gamma chain), which is related to blood coagulation, were ultimately screened. The current study screened egg white proteins that can predict low hatchability and embryonic death and deciphered the role of these proteins in embryonic development, which is meaningful for the comprehensive understanding of embryonic growth.
key words: egg white, proteomics, hatchability, embryo development, chicken
INTRODUCTION
Poultry eggs provide the material basis for embryo development, but not every poultry embryo can hatch successfully. The factors that affect chicken embryo growth include the maternal effect (age, breed, and nutritional status), egg quality (egg weight, eggshell strength, and egg size), and incubation environment (temperature, egg turning, and humidity) (Cavero et al., 2011; King'ori, 2011). In the poultry industry, hatchability is an important parameter that reflects the reproduction performance of breeding hens. Hens of the same breed and age often show divergent (high/low) hatchability, even when their eggs possess the same egg quality and are incubated in the same environment. Genetic differences in the hens should be the major determinant of the compositional differences in egg contents. The chicken embryo utilizes the nutritional and functional molecules from the egg white and yolk under the regulation of its own genes. During the first half of incubation, the egg yolk is the main sources of energy and lipoproteins for the embryo, while the egg white functions as water sources and the white proteins are assimilated later after transfer into other extra-embryonic tissues such as yolk sac and amniotic sac (Sugimoto et al., 1989; Akazawa et al., 2019; Da Silva et al., 2019). Besides, the vitelline membrane embraces the yolk and forms a natural physical separation from the egg white, thus prevents the subsequent leakage of egg yolk toward egg white (Réhault-Godbert et al., 2019). During incubation, vitelline membrane supports yolk sac growth and vascularization functioning in the nutrient transfer from the yolk to embryo. Hence, these extracellular structures play critical roles in chick embryo growth and development. For the egg white, some functional proteins are highly likely to be involved in embryo development, which was supported in our previous study in which we screened 52 conserved proteins simultaneously in the egg whites of chicken, duck, goose, turkey, quail, and pigeon; these proteins were mainly related to responses to external stimuli, blood coagulation, cellular or vascular wound healing, and host defense (Sun et al., 2017). Hence, egg white proteins may be very important in providing a stable environment and protecting the early embryonic cells from death.
Chicken egg white, as a major part of eggs, contains approximately 88.5% water, 10.5% proteins, and 1% carbohydrate, and inorganic ions (Romanoff and Romanoff, 1949). During early embryo development, the water in egg white moves into the yolk, leading to rapid dehydration and the formation of sub-embryonic fluid (Willems et al., 2014), which initiates the utilization of proteins (Carinci and Manzoli-Guidotti, 1968). The egg white proteins mainly comprise ovalbumin (54%), ovotransferrin (12%), ovomucoid (11%), lysozyme (3.5%), and ovomucin (3.4%) (Kovacs-Nolan et al., 2005). These major proteins have been universally explored in the certain fields such as food processing, medicine, and health care. Nevertheless, the roles that egg white proteins play, particularly those with low abundance, in developing embryos are unknown. With the development of proteomic analysis technology, in the last decade, increasing numbers of chicken egg white proteins have been detected. Initially, in 2006, Guérin-Dubiard et al. (2006) identified 16 proteins with 2-dimensional electrophoresis and liquid chromatography-mass spectrometry (LC-MS/MS). Up to now, a total of 284 egg white proteins have been successively detected (D'ambrosio et al., 2008; Mann, 2007; Mann and Mann, 2011). Notably, all of these studies were performed on the egg whites of fresh unfertilized eggs. To decipher the changes in egg white proteins in chicken embryo development, some researchers have performed proteomic analysis during early incubation. Ovalbumin was observed to have a wide range of degradation on the 7th D of incubation (Qiu et al., 2012; Liu et al., 2015), and some heparin-binding proteins, including lysozyme, gallinacin-11, and beta-microseminoprotein-like protein, were degraded during the first 4 D of incubation (Guyot et al., 2016a,b). Whereas the abundance of vitellogenin and zona pellucida C protein significantly increased during the first 9 D of incubation (Wang and Wu, 2014), some protein precursors, including ovoinhibitor, clusterin, and apolipoprotein-D, showed a significant increase on the 7th D of incubation (Liu et al., 2013). However, whether and how these egg white proteins affect embryo development remains unknown.
Here, we used a tandem mass tag (TMT) quantitative proteomics approach coupled with LC-MS/MS to innovatively explore the differential proteins in egg white from eggs produced by hens with divergent high/low hatchability and in egg whites from early death and healthy embryos. This allowed us to screen the crucial proteins that may act as valuable biomarkers to predict low hatchability or early embryonic death, and update our understanding of the physical mechanisms resulting in early embryonic mortality.
MATERIALS AND METHODS
Bird Management and Sample Collection
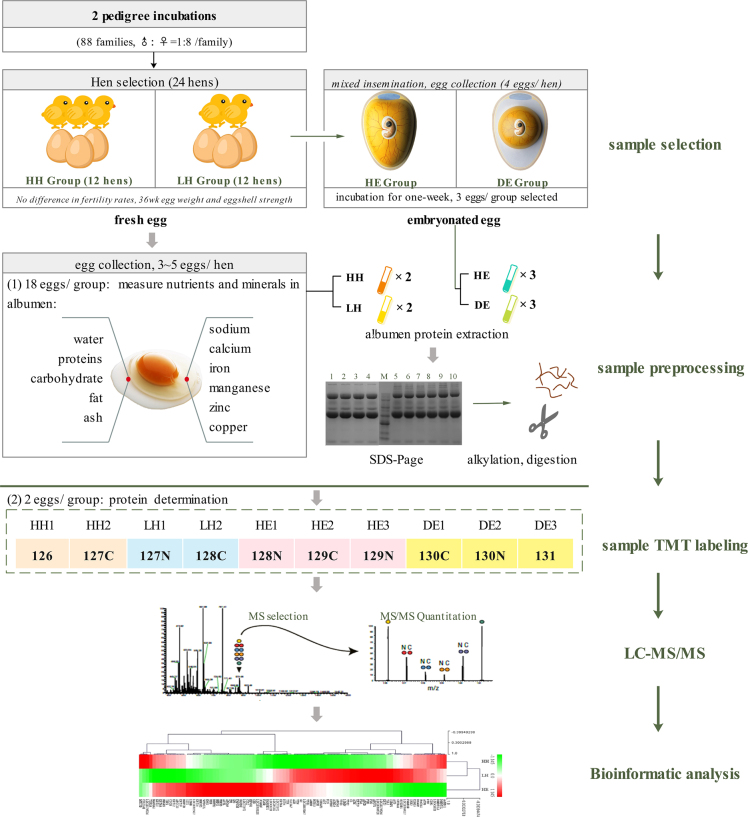
A population derived from Rhode Island Red chickens on a breeding farm of Beijing Huadu Yukou Poultry Industry Co., Ltd., was used as the experimental flock. This population consisted of 88 males and 704 females (♂: ♀ = 1:8). All birds were caged individually and subjected to a 16-h light/8-h dark cycle (16L: 8D). Egg quality traits, including egg weight and eggshell breaking strength, were measured for all hens at 36 wk of age. Then, at age 40 to 42 wk, groups of 8 hens were artificially inseminated by a single cock, and an average of 15.5 fertilized eggs per hen was finally obtained and incubated (38°C, 70% humidity). According to the records on pedigree incubation and egg quality traits, 2 groups of hens (12 for each) were selected for their divergent high/low hatchability (HH, LH; 100% vs. <65%) but equivalent egg quality. To eliminate the effect of sperm quality on hatchability, every high/low pair of hens was inseminated by the same cock. Then, fresh eggs from these 24 hens were collected for 3 successive days for the following uses: a total of 4 fresh eggs collected from hens with hatchability of 100, 100, 50.00, and 53.33% were used for differential proteomic analysis, and 36 eggs were used for nutritional component measurements. The 12 LH hens were inseminated with the mixed sperm from several cocks to allow the collection of fertilized eggs for 5 successive days, and a total of 47 eggs were obtained and incubated (38°C, 70% humidity). These eggs were candled from the 3rd D to identify the healthy and dead embryos. On the 7th D, 3 healthy eggs (HE) and 3 dead eggs (DE, alive on the 6th D) were collected for egg white protein extraction and proteomic analysis. Finally, a total of 10 egg white samples were used for TMT labeling protein identification and quantitation.
Measurements of Nutrients and Minerals
A total of 36 eggs from the HH and LH groups (18 eggs/group) were used to measure the quantities of nutrients and minerals in the egg whites. For each group, egg whites from 6 eggs were mixed into one sample for the measurements. Then, samples of the egg whites were analyzed for water content by oven drying (934.01), protein by Kjeldahl (N × 6.25; 925.31), fat by acid hydrolysis (925.32), and ash by incineration (923.03), according to the Association of Official Analytical Chemists (AOAC) method (AOAC, 2000). The carbohydrate content was calculated by the difference method. Measurements of minerals containing sodium, calcium, iron, manganese, zinc, and copper were conducted using inductive coupled plasma atomic emission spectroscopy according to AOAC Method 984.27 (AOAC, 2000). All nutrients and minerals in each sample were measured by duplicate technical repeats.
Protein Extraction
The egg white liquid of each egg was first treated by ultrasonic centrifugation at 4°C until clarification. Then, 60 μ L of this clear liquid was removed and added to 1.14 mL of splitting buffer (8 M urea, 0.1% SDS, 1 × PI, 1 mM PMSF) in a 1.5-mL tube. The liquid was homogenized by low-temperature ultrasound on ice for at least 30 min until the liquid was clarified. Then, the solutions were centrifuged at 4°C and 12,000 × g for 10 min. The protein concentration was determined using a BCA Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL, USA). Afterward, 100 μ g of this mixture was used in the following step.
Tandem Mass Tag Labeling
After protein extraction, protein reduction, alkylation, digestion, and labeling were performed following the manufacturer's instructions for TMT Mass Tagging Kits and Reagents (Thermo Scientific Pierce, Rockford, IL, USA) as described by Sun et al. (2017). In this study, a total of 10 samples were individually labeled with TMT 10-plex reagents (Thermo Scientific Pierce, Rockford, IL, USA) using TMT-126, 127C (HH), TMT-127 N, 128C (LH), TMT-128 N, 129C, 129 N (HE), TMT-130C, 130 N, and 131 (DE). Figure 1 shows the messages of the sample grouping in detail.
Figure 1.
Workflow for proteomic analysis of chicken egg whites. A total of 24 hens were selected according to their pedigree incubation and egg quality trait records and divided into high (HH) and low hatchability (LH) groups. The quantities of major nutrients were measured in the egg whites of these 2 groups. In addition, the eggs produced by the hens of the LH group were collected for incubation again to select the healthy (HE) and dead chicken embryo (DE) groups. Finally, the egg whites from these 4 groups were separated and used for proteomic analysis. TMT, tandem mass tag; and LC-MS/MS, liquid chromatography-mass spectrometry.
Fractionation of the TMT-Labeled Peptides with an RP-C18 Chromatographic Column
The TMT-labeled peptides were fractionated by RP chromatography using a flow rate of 0.4 to 1 mL/min in an RP-C18 column (Thermo Scientific Pierce, Rockford, IL, USA). Buffer A, consisting of 2% acetonitrile (ACN) in water (pH = 10), and buffer B, consisting of 90% ACN in water (pH = 10), were used for gradient elution (Supporting Information, Table S1). Fractions were collected in a 1.5-mL tube every minute from 2 to 67 min. The samples taken from 24 to 63 min were combined according to the chromatogram, and finally, 20 fractions were collected and dried with a vacuum concentrator and then frozen at −80°C.
LC-MS/MS
After sufficient dissolution of each fraction, the peptides were loaded on a LC-MS/MS (mass spectrometer: Thermo Scientific Q Exactive, San Jose, CA, USA; HPLC system: NCS3500, Dionex, Sunnyvale, USA). The liquid phase was composed of buffer C, with 0.1% formic acid in water, and buffer D, with 0.1% formic acid in 99.9% ACN. Separation of the peptides was achieved by the gradient elution procedure at a flow rate of 300 nL/min for 65 min (Supporting Information, Table S2). The mass spectrum was obtained with a scan range of m/z 350 to 1600 and at a resolution of 17,500 for HCD spectra for the determination of the secondary mass spectrometry sequence.
Protein Identification and Quantification
LC-MS/MS raw data were analyzed using Proteome Discoverer, with the SEQUEST algorithm, to conduct dataset matching for protein identification against the downloaded protein dataset for Gallus gallus from the National Center for Biotechnology Information non-redundant protein sequence database. Peptide and protein identification were filtered with a 99% confidence level and less than 1% false discovery rate. Ultimately, to determine the differential proteins between the HH and LH group, HE and DE group, and LH and HE group, proteins with a 1.5-fold change or greater difference in their abundance were defined as showing up- and downregulation of expression, respectively.
Statistical and Bioinformatic Analysis
The differences in hatchability and egg quality traits were examined by one-way ANOVA, and the differences in nutrient quantities were examined by t -tests (SAS Institute, Cary, NC, USA). Protein classification was completed by using the DAVID 6.8 tool, according to molecular function, biological process, cellular component, and related pathways. Hierarchical clustering analysis was performed to identify protein abundance changes in the differential proteins among the HH, LH, and HE groups using the MeV tool. The workflow is summarized in Figure 1.
RESULTS
Phenotype Comparison between the High and Low Hatchability Groups
The first step of our study was to select 2 groups of hens differing significantly in hatchability but not in other traits (Table 1). The hatchability in the HH group was 100%, which was much higher than that of the LH group (59.30%, P < 0.01). The average hatchability of the whole population was 88.73%, which differed significantly from those of the HH and LH groups (P < 0.01). Other parameters that influence incubation, such as fertility, egg weight, and eggshell strength, showed no significant differences among the HH group, LH group, and the whole population.
Table 1.
Comparison of hatchability, fertility and egg quality traits between 2 groups.
| High hatchability (12 hens) | Low hatchability (12 hens) | Population (704 hens) | |
|---|---|---|---|
| Hatchability of fertile eggs (%)1 | 100A | 59.30±5.93C | 88.73±10.45B |
| Fertility (%)2 | 95.40±5.32 | 92.72±8.35 | 95.65±5.33 |
| Egg weight (g)3 | 55.77±3.25 | 58.44±4.37 | 57.48±3.81 |
| Eggshell strength (kg/cm2)3 | 3.165±0.692 | 3.234±0.610 | 3.237±0.658 |
Means values with no common superscripts within each row differ significantly (P < 0.01) when tested with one-way ANOVA. All values are shown as mean ± SD except the hatchability of 100%.
The hatchability of each hen was calculated by an average of 15.5 fertilized eggs.
The fertility of each hen was calculated by an average of 16.2 eggs.
The results were measured by 3 eggs per hen in all the 3 groups.
Nutrients may also influence chicken embryo development. Here, we measured the quantities of the main nutrients and minerals in the egg whites (Table 2). No significant differences were observed in the quantities of nutrients, including water, crude protein, fat, carbohydrate, ash, and minerals, including sodium, calcium, iron, manganese, and zinc, suggesting that nutritional composition was not the key factor in the divergent hatchability.
Table 2.
Nutrient and mineral composition in egg whites of 2 groups.
| Nutrients | High hatchability (6 eggs/sample, 3 samples) | Low hatchability (6 eggs/sample, 3 samples) | P- Value |
|---|---|---|---|
| Water(g/100 g) | 88.40±0.36 | 88.83±0.74 | 0.4121 |
| Protein(g/100 g) | 8.51±0.31 | 8.11±0.70 | 0.4127 |
| Fat (g/100 g) | 0.040±0.034 | 0.019±0.009 | 0.3484 |
| Carbohydrate(g/100 g) | 2.63±0.45 | 2.43±0.81 | 0.7287 |
| Ash(g/100 g) | 0.63±0.03 | 0.64±0.03 | 0.2054 |
| Na(g/kg) | 1.890±0.121 | 1.927±0.072 | 0.6761 |
| Ca(mg/kg) | 35.90±4.52 | 41.23±4.12 | 0.2054 |
| Cu(mg/kg) | 0.069±0.010 | 0.079±0.008 | 0.2216 |
| Fe(mg/kg)1 | <3 | <3 | – |
| Mn(mg/kg)1 | <0.3 | <0.3 | – |
| Zn(mg/kg)1 | <2 | <2 | – |
The contents of Fe, Mn, Zn in egg white was lower than threshold detection limit.
Quantification of Egg White Proteins
Four groups of eggs—HH and LH fresh eggs, HE and DE on the 7th D of incubation—were used to screen the proteomic profiles of the egg whites. A total of 378 proteins (1,041 peptides, 374 genes) were quantified in the egg whites (Table 3 and Table S3), which was the largest number of chicken egg white proteins quantified to date. Furthermore, 15 and 39 proteins were overrepresented in the HH and LH groups, respectively (Table S4). For the incubated eggs, 13 and 24 proteins were observed to be upregulated in the HE and DE groups, respectively (Table S5). In addition, a total of 35 proteins in LH fresh egg white significantly increased after 7 D of incubation (relative to HE egg white); simultaneously, 28 proteins decreased (Table S6). Table 4 lists the top 20 differential proteins in the HH vs. LH and HE vs. DE groups. The differentially presented proteins between every pair of groups showed overlap (Figure 2), such as 20 proteins that overlapped between fresh and embryonated eggs (HH and LH vs. HE and DE). In addition, 9 proteins were observed in all 3 comparisons, and a total of 102 proteins were present in at least one comparison.
Table 3.
Description of quantified proteins (peptides) in chicken egg whites.
| Fresh eggs1 |
Incubated eggs (7 D)1 |
||||
|---|---|---|---|---|---|
| HH | LH | HE | DE | ||
| Identified proteins | 395 | ||||
| Quantified proteins | 378 | ||||
| Quantified peptides | 1041 | ||||
| HH vs. LH | HE vs. DE | LH vs. HE | |||
| Upregulated2 | 15 | 13 | 28 | ||
| Downregulated2 | 39 | 24 | 35 | ||
| Total number | 54 | 37 | 63 | ||
HH, high hatchability group; LH, low hatchability group; HE, healthy embryo group; and DE, dead embryo group.
Upregulated: fold change (FC) ≥ 1.5; downregulated: FC ≤ 2/3.
Table 4.
Description of the top 20 differentially presented proteins in each comparison.
| Accession | Description | Gene symbol | Unique peptides | Fold change |
|---|---|---|---|---|
| HH vs. LH1 | ||||
| NP_989,992.1 | Ovomucin precursor | MUC5B | 3 | +5.66 |
| XP_0,03642131.2 | Collagen alpha-1(XXIII) chain | COL23A1 | 1 | +3.04 |
| NP_990187.1 | Neuronal growth regulator 1 precursor | NEGR1 | 2 | +2.25 |
| XP_01515,5141.1 | Keratin, type I cytoskeletal 47 kDa isoform X2 | KRT12 | 2 | +2.12 |
| NP_001001312.2 | Keratin, type I cytoskeletal 15 | KRT15 | 1 | +2.05 |
| XP_001233972.1 | Keratin, type I cytoskeletal 19 | KRT19 | 1 | +2.04 |
| XP_015155851.1 | Keratin 6A isoform X2 | KRT6A | 2 | +1.80 |
| NP_989508.1 | L-lactate dehydrogenase B chain | LDHB | 1 | +1.80 |
| XP_015149381.1 | Pancreatic secretory trypsin inhibitor-like | LOC101749216 | 1 | +1.75 |
| XP_419762.5 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IA | MAN1A1 | 3 | +1.60 |
| XP_015148167.1 | Alpha-2-macroglobulin | A2M | 1 | −8.20 |
| NP_001161155.1 | Fibrinogen beta chain precursor | FGB | 2 | −5.50 |
| NP_001001195.1 | Keratin, type II cytoskeletal 5 | KRT5 | 2 | −4.57 |
| NP_990687.2 | Fibrinogen alpha chain isoform 2 precursor | FGA | 5 | −4.05 |
| XP_003642540.2 | Whey acidic protein-like isoform X2 | LOC771972 | 1 | −3.99 |
| NP_990320.2 | Fibrinogen gamma chain precursor | FGG | 2 | −3.46 |
| XP_004935550.1 | Angiotensinogen | AGT | 1 | −3.08 |
| XP_015129390.1 | Myosin-7-like, partial | LOC101749246 | 1 | −2.94 |
| XP_015148426.1 | Filamin-B isoform X4 | FLNB | 1 | −2.49 |
| NP_990814.2 | Apovitellenin-1 precursor | APOV1 | 5 | −2.45 |
| HE vs. DE2 | ||||
| NP_990269.1 | Apolipoprotein A-IV precursor | APOA4 | 1 | +12.07 |
| XP_001235178.3 | Uncharacterized protein LOC771972 isoform X3 | LOC771972 | 1 | +9.95 |
| XP_015148426.1 | Filamin-B isoform X4 | FLNB | 1 | +5.41 |
| XP_003642540.2 | Whey acidic protein-like isoform X2 | LOC771972 | 1 | +3.59 |
| NP_989865.1 | Ovocalyxin-32 precursor | RARRES1 | 7 | +3.35 |
| XP_004935550.1 | Angiotensinogen | AGT | 1 | +2.46 |
| NP_990651.1 | Avidin precursor | AVD | 11 | +2.24 |
| XP_003641562.1 | Beta-microseminoprotein-like | LOC100858647 | 2 | +1.77 |
| NP_001004399.1 | Lymphocyte antigen 86 precursor | LY86 | 8 | +1.75 |
| XP_015128741.1 | Succinate dehydrogenase assembly factor 1, mitochondrial | SDHAF1 | 1 | +1.64 |
| NP_990814.2 | Apovitellenin-1 precursor | APOV1 | 5 | −6.89 |
| NP_001038098.1 | Apolipoprotein B precursor | APOB | 122 | −5.09 |
| NP_001026447.1 | Vitellogenin-2 precursor | VTG2 | 69 | −4.46 |
| NP_001004390.1 | Hemoglobin subunit rho | HBBR | 1 | −4.24 |
| XP_015129008.1 | Vitelline membrane outer layer protein 1 homolog | LOC107049720 | 1 | −3.55 |
| NP_001004408.1 | Vitellogenin-1 precursor | VTG1 | 40 | −3.52 |
| XP_015146355.1 | Vitellogenin-3 | VTG3 | 25 | −3.00 |
| NP_001034255.1 | Surfactant protein A1 precursor | SFTPA1 | 1 | −2.51 |
| NP_001001313.2 | Keratin 6A | KRT6A | 1 | −2.23 |
| NP_990340.2 | Keratin, type I cytoskeletal 19 | KRT19 | 1 | −2.12 |
HH, high hatchability group; LH, low hatchability group.
HE, healthy embryo group; DE, dead embryo group.
Figure 2.
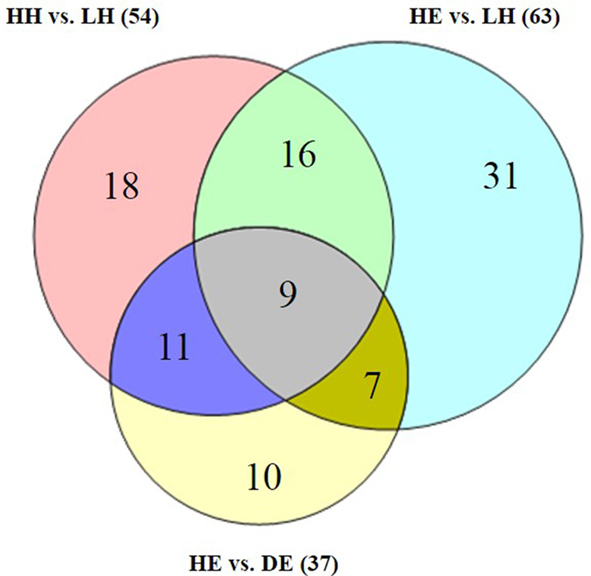
Venn diagrams for the shared and specific differential proteins in 3 comparisons. HH, high hatchability group; LH, low hatchability group; HE, healthy embryo group; and DE, dead embryo group.
Enrichment Analysis of Differential Proteins
GO enrichment and pathway analysis were conducted using the DAVID 6.8 tool with a Bonferroni P -value < 0.05 (Table 5) to evaluate the potential functions of these differential proteins. The upregulated proteins in the LH group were first clustered in GO terms related to blood coagulation process, including 4 terms: blood coagulation and fibrin clot formation (GO: 0072378), fibrinolysis (GO: 0042730), positive regulation of heterotypic cell adhesion (GO: 0034116), and plasminogen activation (GO: 0031639). This finding is consistent with the enriched reactome pathway of platelet degranulation (r-gaa-114608), which indicates blood coagulation and cellular or vascular injury. Additionally, the upregulated LH proteins were enriched for chylomicron (GO: 0042627), very low-density lipoprotein particle (GO: 0034361), nutrient reservoir activity (GO: 0045735), and lipid transporter activity (GO: 0005319). However, for the proteins that were upregulated in the HH group, only 1 term, intermediate filament (GO: 0045111), was enriched; this term encompassed 3 types of keratins mainly expressed in epithelial tissue and participating in cytoskeleton formation.
Table 5.
GO terms and pathways of differential proteins in chicken egg whites.
| Description1 | GO terms2 | Count | Bonferroni | Gene symbol |
|---|---|---|---|---|
| Upregulated in HH compared to LH | ||||
| Intermediate filament | C:0045111 | 3 | 6.00E-03 | KRT12, KRT14, KRT15 |
| Downregulated in HH compared to LH | ||||
| Blood coagulation | P:0072378 | 4 | 1.40E-04 | APOH, FGA, FGB, FGG |
| P:0042730 | 5 | 1.60E-05 | APOH, FGA, FGB, FGG, PLG | |
| P:0034116 | 3 | 1.80E-02 | FGA, FGB, FGG | |
| P:0031639 | 4 | 1.70E-03 | APOH, FGA, FGB, FGG | |
| Nutrient reservoir activity | F:0045735 | 4 | 1.00E-05 | APOV1, VTG1, VTG2, VTG3 |
| Lipid transporter activity | F:0005319 | 5 | 3.90E-03 | APOA1, APOB, VTG1, VTG2, VTG3 |
| Extracellular exosome | C:0070062 | 17 | 9.50E-06 | ALB, AMBP, AGT, APOA1, APOB, APOH, FGA, FGB, FGG, FLNB, GC, IGFBP7, KRT19, LGMN, RARRES1, TTR, VTN |
| Plasma lipoprotein particle | C:0042627 | 4 | 6.10E-05 | APOA1, APOB, APOH, APOV1 |
| C:0034361 | 4 | 2.10E-04 | APOA1, APOB, APOH, APOV1 | |
| Reactome pathway for downregulated proteins in HH compared to LH | ||||
| Platelet degranulation | R-gaa-114608 | 7 | 1.30E-05 | ALB, APOA1, APOH, FGA, FGB, FGG, PLG |
| Downregulated in HE compared to LH | ||||
| Defense response to bacterium | P:0042742 | 5 | 1.70E-03 | OvoDA1, AvBD11, AVD, FGA, FGB |
| Blood coagulation | P:0072378 | 3 | 9.80E-03 | FGA, FGB, FGG |
| P:0034116 | 3 | 3.00E-03 | FGA, FGB, FGG | |
| Downregulated in HE compared to DE | ||||
| Nutrient reservoir activity | F:0045735 | 4 | 6.80E-07 | APOV1, VTG1, VTG2, VTG3 |
| Lipid transporter activity | F:0005319 | 5 | 6.60E-03 | APOV1, APOB, VTG1, VTG2, VTG3 |
HH, high hatchability group; LH, low hatchability group; HE, healthy embryo group; and DE, dead embryo group.
GO term categories: biological process (P), molecular function (F), and cellular component (C). GO term enrichment and pathway analysis were performed on DAVID 6.8 tool, https://david.ncifcrf.gov/.
Proteins that significantly decreased after 7 D of incubation relative to fresh eggs (LH vs. HE) were only markedly enriched for the term defense response to bacterium (GO: 0042742), indicating the gradual decline of antimicrobial proteins in egg white during embryo growth. In addition, 3 fibrinogen proteins (fibrinogen alpha chain (FGA), fibrinogen beta chain (FGB), and fibrinogen gamma chain (FGG)) that were enriched under the term blood coagulation (GO: 0072378 and GO: 0034116) declined after incubation (HE) relative to unincubated eggs (LH). Finally, between HE and DE, 5 proteins were overrepresented in the DE group (APOV1, APOB, VTG1, VTG2, and VTG3) and were enriched in terms of nutrient reservoir activity (GO: 0045735) and lipid transporter activity (GO: 0005319).
Hierarchical Clustering Analysis of Differential Proteins
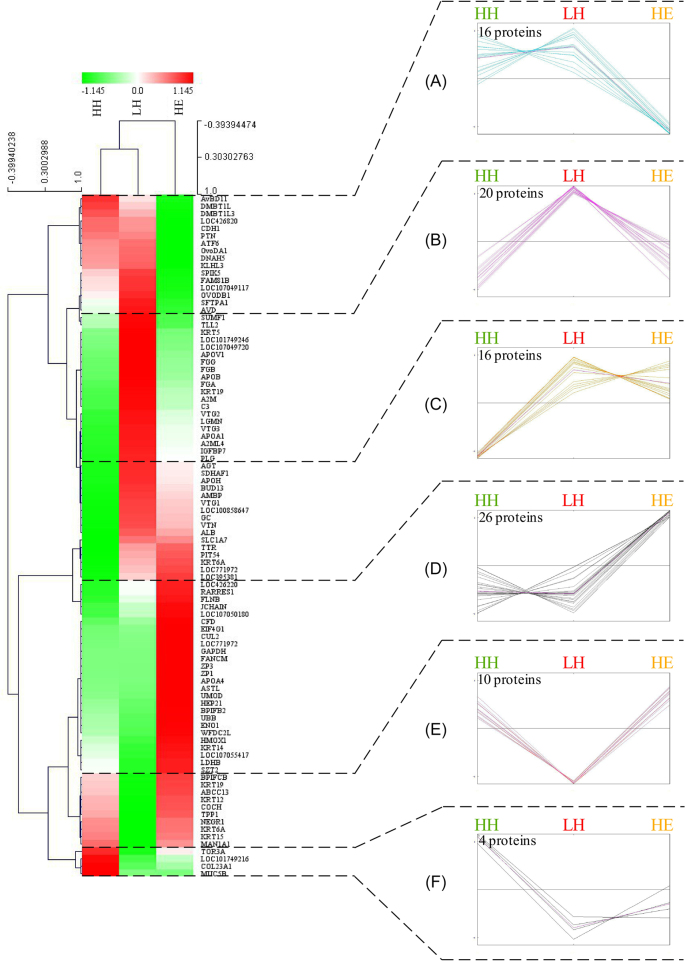
During the analysis, certain proteins such as keratins (KRT6A, KRT12, and KRT15) were observed to be overrepresented in HH relative to the LH group, but after 7 D incubation of LH eggs (HE), these proteins had significantly increased to levels equivalent to those in HH, indicating the positive roles of these proteins in embryo development. Hence, hierarchical clustering analysis was then performed on 92 differential proteins among the HH, LH, and HE groups to reveal the major patterns of protein changes. As seen in the dendrogram plot of Figure 3, 6 clusters corresponding to the different patterns of protein changes were obtained for these 3 groups. Cluster A and cluster D consisted of proteins that differed significantly before and after incubation. Specifically, cluster A showed the apparent decline of 16 proteins after 7 D of incubation, whereas cluster D contained 26 proteins that increased during early incubation. Cluster B and cluster E were more interesting. A total of 20 proteins in cluster B were overrepresented in LH relative to the HH group, but after 7 D of incubation of the LH eggs (HE), these proteins significantly decreased and were equivalent to the levels observed in HH, indicating the high abundance of these proteins could be regarded as a signal of low hatchability. Cluster E, which consisted of 10 proteins opposite to cluster B, showed the same changes as those described for keratin content in the above text. These proteins may predict the healthy development of chicken embryos. Finally, cluster C and cluster F showed large differences between the HH and LH groups, but little difference was found in protein abundance before and after incubation (LH vs. HE). The distinct variation patterns in the proteins contributed to screen typical proteins in egg white associated with hatchability and embryonic development.
Figure 3.
Hierarchical classification analysis of 92 differential proteins in the egg albumen of unfertilized eggs produced by hens differing in hatchability (HH and LH) and embryonated eggs with healthy embryos (HE). Dendrogram (left) and graphs of the 6 clusters (right) showed the variation patterns of differential proteins for the HH, LH, and HE groups. HH, high hatchability group; LH, low hatchability group; and HE, healthy embryo group.
To further dissect the potential functions of these 92 proteins, we searched gene annotations, the literature and functional domain databases and eventually classified the proteins into 4 functional groups, including blood coagulation, nutrient transport, antimicrobial and immunity, cell/tissue development, and others (Table 6). The proteins related to blood coagulation and nutrient transport were mainly found in cluster B. The proteins participating in antimicrobial activity were distributed in almost all clusters, indicating that antibacterial activity occurred in both fresh and developmental eggs but was executed by different proteins. Finally, 39 proteins could not be assigned to any of these 4 functional groups but rather had other roles.
Table 6.
Potential functions of egg white proteins of 6 clusters.
| Potential functions for embryo |
|||||
|---|---|---|---|---|---|
| Cluster1 | Blood coagulation | Nutrient transport | Antimicrobial and immunity | Cell/tissue development | Others |
| A (16 proteins) |
|
|
DMBT1L, CDH1, OvoDA1, DNAH5, KLHL3, FAM81B, OVODB1 | ||
| B (20 proteins) |
|
|
|
|
SUMF1, LOC101749246, LOC107049720, KRT19, LGMN |
| C (16 proteins) |
|
VTG1 |
|
|
SDHAF1, BUD13, AMBP, VTN, SLC1A7, KRT6A, LOC771972 |
| D (26 proteins) |
|
|
|
LOC426220, LOC107050180, CFD, CUL2, LOC771972, GAPDH, FANCM, HEP21, ENO1, HMOX1, ZP3, ZP1, UMOD, LOC107055417 | |
| E (10 proteins) |
|
|
TPP1, NEGR1, MAN1A1 | ||
| F (4 proteins) | MUC5B | TOR3A, COL23A1, LOC101749216 | |||
The clusters of A to F correspond to the 6 clusters in Figure 3, respectively. And the proteins are showed by their corresponding gene names.
Putative Protein Biomarkers for Embryo Development Status
According to GO, pathway, and clustering analysis, and in combination with literature and database searching for each protein, a total of 11 proteins were determined to be plausible biomarkers for healthy embryo growth or early death; these proteins were related to cytoskeleton formation, blood coagulation, neurodevelopment and antibacterial activity. The functional description and annotation of these proteins are shown in Table 7, and their potential roles are explored subsequently in the discussion section.
Table 7.
Description of 11 protein biomarkers involved in embryo development.
| Functional class | Protein name (Gene symbol) | Annotation |
|---|---|---|
| Intermediate filament | Keratin, type I cytoskeletal 47 kDa isoform X2 (KRT12)1 | Expressed in epithelial tissue and participate in forming cytoskeleton |
| Keratin, type I cytoskeletal 15 (KRT15) | ||
| Keratin, type I cytoskeletal 19 (KRT19) | ||
| Keratin 6A isoform X2 (KRT6A) | ||
| Neurodevelopment | L-lactate dehydrogenase B chain (LDHB) | Expressed in embryo glycogen body |
| Neuronal growth regulator 1 precursor (NEGR1) | Promotion neurite growth and development | |
| Blood coagulation | Fibrinogen alpha chain (FGA) | Crucial coagulation proteins in hemostasis and clot formation |
| Fibrinogen beta chain (FGB) | ||
| Fibrinogen gamma chain (FGG) | ||
| Antimicrobial | Alpha-2-macroglobulin (A2M) | Inhibit a broad spectrum of proteases |
| Ovomucin precursor (MUC5B) | Potential effector of innate immunity |
Bold items are proteins that are favorable to embryo development when overrepresented.
DISCUSSION
Egg white, which surrounds the yolk, maintains a secure and stable environment for the developing embryo. Previous studies have reported that egg white components are indispensable for the start of development (Willems et al., 2014). Hence, proteins in the egg white should be closely associated with embryonic development. In the current study, we used a TMT labeling quantitation approach to identify differentially presented proteins in the egg whites of fresh eggs produced by hens with high and low hatchability (HH and LH) and in the egg whites of embryonated eggs with healthy and dead embryos (HE and DE).
Several criteria were set in the high/low hatchability model that was used in the current study. First, all hens of the same age and breed were reared under the same conditions, eliminating the effects of breed, age and environment on hatchability. In addition, each pair of hens with high/low hatchability was artificially fertilized by sperm from the same male bird to correct for the impact of sperm quality. In addition, the hens were also selected based on their egg quality traits to ensure the same egg quality between 2 groups. Finally, previous studies noted that some mineral elements such as sodium, calcium, iron, manganese, zinc, and copper are vital to the developing embryo (Miles, 2000; Vieira, 2007; Uni et al., 2012). Hence, nutrients, including water, proteins, carbohydrate, fat, and the abovementioned minerals, were measured in the egg whites, and no differences were observed. These criteria allowed us to screen the crucial proteins related to embryo development.
When compared HH to LH group, it is noteworthy that some yolk proteins such as FGA, FGB, FGG, A2M, APOV1, and myosin (Mann and Mann, 2008; Farinazzo et al., 2009) were the six of top 10 proteins overexpressed in the egg white of LH group (Table 4), which reflects that these proteins could transfer from the egg yolk to the white by crossing the vitelline membrane. The vitelline membrane forms fibrous structure network and consists of three layers (Hincke et al., 2019). The inner layer is secreted by ovarian granulosa cells, the middle and outer layers are in the infundibulum of the oviduct after ovulation (Waclawek et al., 1998; Takeuchi et al., 1999). It is highly possible that the ovarian and oviduct of LH hens have some defects in the structure or some disorders and deteriorations in the function, which impairs the integrity of the vitelline membrane and results in leakage from the egg yolk to the white. Similarly, in the comparison HE/DE, most of the top 10 upregulated proteins in DE group are the most abundant egg yolk proteins, such as APOV1, APOB, VTG1, VTG2, and VTG3, as well as main yolk sac protein hemoglobin subunit rho (Yadgary et al., 2014) and vitelline membrane major protein vitelline membrane outer layer protein 1 (VMO1) homolog (LOC107049720) (Mann, 2008) (Table 4). During chick embryo development, the cell layers of mesoderm and endoderm form the definitive wall of yolk sac and blood vessels are developed on the mesoderm next to the endoderm (Baggott, 2009). During incubation, the structure of yolk sac replaces the vitelline membrane gradually during the early incubation with a decrease in the strength and rupture of vitelline membrane. The results indicated the vitelline membrane and yolk sac of DE group more tend towards structural alteration. Schäfer et al. (1998) reported that solubilization of the vitelline membrane outer proteins (VMO1 and VMO2) from the membrane may lead to deterioration of the vitelline membrane. Coincidently, the VMO1 homolog was overexpressed in the LH and DE (Table 4, Table S5), may due to VMO1 homolog from the vitelline membrane disintegrates and transfers to the egg white, in turn, which could verify vitelline membrane deterioration results in embryonic death. The abundance of these differential proteins in egg white may reflect the oviduct defects of hens during egg formation or vitelline membrane structure alteration during incubation.
These considerations along with the hierarchical clustering analysis for differential proteins in egg white of HH, LH, and HE, facilitates to screen some specific proteins closely related to embryonic growth and development. During the evolution of avian, epidermal keratinization of the avian skin formed feathers, beaks, and claws for adaptation to the environment and to satisfy the need to forage and fly. Keratin is the predominant component of these epidermal derivatives, which are shaped as early as during avian embryo development. In our study, 4 types of keratins (KRT6A, KRT12, KRT15, and KRT19) were overrepresented in the HH and HE groups, and one type of keratin (KRT14) increased after 7 D of incubation, suggesting that these keratin proteins upregulated expressions reflect chicken embryo normal growth. Moreover, keratins are expressed in most epithelial cells and belong to the intermediate filament (GO: 0045111), which is a complex dynamic network that controls cell architecture, cell adhesion, cell migration and cell differentiation (Gruenbaum and Aebi, 2014; Lowery et al., 2015). In developing murine embryos, keratin turnover and keratin rearrangement occur in the period of cell division during the formation of the first epithelial tissue (Schwarz et al., 2015). Keratins contribute to maintaining the structural and functional integrity of the epithelial tissue. Keratin-deficient mouse embryos died from severe growth retardation or restricted cytolysis in the trophoblast layer at ED9.5 (Hesse et al., 2000; Vijayaraj et al., 2009), and mosaic knock-out mice only survived for 12 D when suffering extensive epidermal damage (Bar et al., 2014). In our study, keratins showed a significantly low presence in the LH group, indicating deficient keratin could also closely relate to early chicken embryo development.
Neurodevelopment is an important part of embryonic growth in animals. Some proteins associated with neurodevelopment displayed upregulation in the HH and HE group relative to the LH group. For example, neuronal growth regulator 1 (NEGR1), a subgroup named IgLON of the immunoglobulin superfamily of cell adhesion molecules, functions to regulate neuronal development and synapse formation (Tan et al., 2017). It has been reported recently that NEGR1 may affect neuronal growth by promoting neurite growth and controlling the development of neurite arborization (Pischedda et al., 2014; Singh et al., 2018). Pischedda et al. (2014) reported that NEGR1 expression markedly increased during maturation of cortical neurons from mouse embryos in vitro and mice in vivo, and its downregulation resulted in a significant decrease in the length and number of neurite processes in vivo. In the current study, the elevated abundance of NEGR1 after 7 D of incubation (HE group) indicates the vital role of NEGR1 in the facilitation of embryo neurodevelopment. In addition, another 2 proteins—chicken protein TENP (BPIFB2) and L-lactate dehydrogenase B chain (LDHB)—have very close connection with embryo normal development. BPIFB2 was first identified as a nearly embryonic protein that is transiently expressed in neural precursor cells in the retina and the brain, representing 2 cell types in the vertebrate central nervous system (Yan and Wang, 1998). L-lactate dehydrogenase B chain is a subtype of lactate dehydrogenase (LDH), which is the enzyme that catalyzes the interconversion of pyruvate and lactate. Imagawa et al. (2006) reported that LDHB mRNA was the only subtype of LDH expressed in the embryo glycogen body (acting as a nutrient reservoir in chicken central nervous system) during all embryonic periods (measured after 12 D of incubation). Hence, the proteins NEGR1, BPIFB2, and LDHB in egg white were expected to transfer into the amniotic sac and support nutrients and to protect and promote nervous system development in the chicken embryo.
Some proteins in egg white relate to early embryonic death. Our results indicate that the high abundance of proteins relating to blood coagulation (GO: 0072378) or platelet degranulation (r-gaa-114608) negatively affects embryo growth. Among these proteins, the fibrinogen family proteins (FGA, FGB, and FGG), which varied from 3.46 to 5.50 times higher in the LH than the HH group in our study (Table 4), are most crucial for coagulation in hemostasis and clot formation. The coagulation system is a basic physiological defense mechanism that prevents blood loss following vascular injury (Esmon, 2004; Gentry, 2004). However, blood coagulation is also known to be a major risk factor in thrombogenesis (Cho et al., 2003). Brodsky (2002) reported that lack of fibrinogen in mice resulted in normal embryonic development, a finding that is inconsistent with our results, which showed that a low abundance of fibrinogen was found in the HH and HE groups. In humans, elevated fibrinogen plasma levels are known to be an independent predictor of mortality in patients with chronic kidney disease (Schuett et al., 2017). Elevated fibrinogen concentrations result in denser fibrin fibers and stiffer clots, which are more difficult to lyse and are associated with elevated mortality in chronic kidney disease (Martinez et al., 2013). In our study, 7 upregulated proteins in the LH group were classified into platelet degranulation, which refers to blood coagulation and cellular or vascular wound healing. One of the mechanisms for embryonic death in animals is ischemic or coagulation oncosis, which is a premortality process (Majno and Joris, 1995; Betts and King, 2001; Van Cruchten and Van Den Broeck, 2002). Platelet degranulation has been proven to increase in the acute and convalescent phases after ischemic stroke or transient ischemic attack (Mccabe et al., 2004), suggesting that early embryo death might result from ischemic necrosis caused by stresses to the fibrinogen concentration. From the above, we hypothesize that high levels of fibrinogen might closely relate to the early embryo death of chickens by accelerating the formation of denser clots.
Certain proteins with antimicrobial activity were also identified as biomarkers affecting embryo development. Antimicrobial proteins are widespread in nearly every component of the egg, including the cuticle (Rose-Martel et al., 2012; Mikšík et al., 2014), eggshell membrane (Cordeiro and Hincke, 2016), egg white, egg yolk (Réhault-Godbert et al., 2014), and vitelline membrane (Mann, 2008). In the present study, antimicrobial proteins were also present in the egg whites of almost all of the 6 clusters. Some of these overabundant proteins could forecast high hatchability or healthy embryo, such as ovomucin precursor (MUC5B) and BPIFB2, which showed higher abundance in the HH or HE group. Ovomucin, accounting for 3.4% of the total egg white proteins, is believed to be responsible for gel properties and viscosity of fresh eggs and be involved in egg white thinning (Omana et al., 2010). Ovomucin presumably binds heparin and functions as a potential effector of innate immunity that would participate in protecting eggs against pathogenic microorganisms (Guyot et al., 2016a). Some evidences show that the glycopeptides derived from ovomucin exert antibacterial activity (Watanabe et al., 1998; Kobayashi et al., 2004). In our study, its precursor, MUC5B, displayed a maximum fold change (+5.66) between the HH and LH groups. Hence, we hypothesized that MUC5B might also have antibacterial activity and be connected to the normal development of chicken embryo. Another protein, BPIFB2, also named TENP, which belongs to the bactericidal permeability-increasing protein superfamily, contains a bactericidal permeability-increasing protein domain that binds to and neutralizes lipopolysaccharides from the outer membrane of gram-negative bacteria, eventually leading to death of bacteria. Whenham et al. (2014) documented that the expression of TENP is restricted to the magnum of the oviduct and the protein TENP is a major component of egg, especially the white. This protein increased during early embryonic development, which would concur with previous study that TENP protein in egg white increases during the rapid embryonic growth period (ED7∼ED16) (Liu et al., 2015). BPIFB2, with high sequence identity (58%) to the emu protein TENP, which exhibits antibacterial activity against gram-positive bacteria (Maehashi et al., 2014), recently has been reported to be an antibacterial protein (Guyot et al., 2016a) during chicken embryo early development. Kinoshita et al. (2016) revealed that TENP was identified as the gene encoding chicken egg white ovoglobulin G2 (G2), and the sequence of the G2B band was observed to be identical to that of TENP. Moreover, variations in the G2 protein correlated to hatchability and the embryonic fatality rate. In our study, TENP was overabundant in eggs held with healthy embryos (HE), further suggesting that an overabundance of TENP in egg whites embryonic development. In contrast, other proteins, such as complement C3 precursor (C3) and alpha-2-macroglobulin (A2M), were overabundant in the LH group. A2M, which functions as a broad-spectrum protease-binding protein (Armstrong and Quigley, 1999), is synthesized only by the oviduct and accumulates in egg whites (Nagase et al., 1983). Recently, A2M has been proposed to be a maternally derived immune factor present in eggs of various species, protecting developing embryos against pathogenic attack (Wen et al., 2005; Hathaway et al., 2010; Pathirana et al., 2016). In our study, it is surprising that the abundance of A2M in the LH group was 2.74 and 8.20 times higher than that in the HE and HH groups (Table S6, Table 4), respectively. However, the mechanisms underlying this result need further research. C3, a member of the A2M family, is the central component of the innate immune system and provides important links with adaptive immunity (Carroll, 2004). Georgiou et al. (2011) documented that the C3 present in embryo culture medium plays a negative role in early embryonic development in porcine species. Hence, in our results, overabundant C3 in the LH group was considered to be unfavorable for chicken embryos.
CONCLUSION
The present study for the first time screened the crucial egg white proteins involved in embryo development with MS-based protein quantification technology. Crucial egg white proteins participating in cell architecture (KRT19, KRT12, KRT15, and KRT6A) and blood coagulation (FGA, FGB, and FGG) could play critical roles in chicken embryo development or early death. Our study provides a better understanding of the maternal genetic determinants of the variance of offspring hatchability. The final list of candidate proteins should be considered as key biomarkers in the further study of embryo development.
ACKNOWLEDGMENTS
The current study was funded in part by the Programs for Changjiang Scholars and Innovative Research in University (IRT_15R62) and the China Agriculture Research System (CARS-40).
Footnotes
Supplementary data are available at Poultry Science online.
Table S1. Gradient elution for peptide pre-separation.
Table S2. Gradient elution for liquid chromatography.
Table S3. Description of identified proteins in egg white of 4 different egg states.
Table S4. Proteins with at least 1.5-fold change abundance in HH versus LH.
Table S5. Proteins with at least 1.5-fold change abundance in HE versus DE.
Table S6. Proteins with at least 1.5-fold change abundance in LH versus HE.
Supplementary Material
REFERENCES
- Akazawa T., Ogawa M., Hayakawa S. Migration of chicken egg-white protein ovalbumin-related protein X and its alteration in heparin-binding affinity during embryogenesis of fertilized egg. Poult. Sci. 2019 doi: 10.3382/ps/pez335. [DOI] [PubMed] [Google Scholar]
- AOAC . 17th ed. Assn. of Official Analysis Chemists; Washington, DC: 2000. Official methods of analysis. [Google Scholar]
- Armstrong P.B., Quigley J.P. α2-macroglobulin: an evolutionarily conserved arm of the innate immune system. Dev. Comp. Immunol. 1999;23:375–390. doi: 10.1016/s0145-305x(99)00018-x. [DOI] [PubMed] [Google Scholar]
- Bar J., Kumar V., Roth W., Schwarz N., Richter M., Leube R.E., Magin T.M. Skin fragility and impaired desmosomal adhesion in mice lacking all keratins. J. Invest. Dermatol. 2014;134:1012–1022. doi: 10.1038/jid.2013.416. [DOI] [PubMed] [Google Scholar]
- Baggott G.K. Development of extra-embryonic membranes and fluid compartments. Avian Biology Research. 2009;2:21–26. [Google Scholar]
- Betts D.H., King W.A. Genetic regulation of embryo death and senescence. Theriogenology. 2001;55:171–191. doi: 10.1016/s0093-691x(00)00453-2. [DOI] [PubMed] [Google Scholar]
- Brodsky S.V. Coagulation, fibrinolysis and angiogenesis: new insights from knockout mice. Nephron. Exp. Nephrol. 2002;10:299–306. doi: 10.1159/000065305. [DOI] [PubMed] [Google Scholar]
- Carinci P., Manzoli-Guidotti L. Albumen absorption during chick embryogenesis. J. Embryol. Exp. Morphol. 1968;20:107–118. [PubMed] [Google Scholar]
- Carroll M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Cavero D., Schmutz M., Icken W., Preisinger R. Improving hatchability in white egg layer strains through breeding. Lohmann Information. 2011;46:44–54. [Google Scholar]
- Cho H.J., Ham H.S., Lee D.S., Park H.J. Effects of proteins from hen egg yolk on human platelet aggregation and blood coagulation. Biol. Pharm. Bull. 2003;26:1388–1392. doi: 10.1248/bpb.26.1388. [DOI] [PubMed] [Google Scholar]
- Cordeiro C.M., Hincke M.T. Quantitative proteomics analysis of eggshell membrane proteins during chick embryonic development. J. Proteomics. 2016;130:11–25. doi: 10.1016/j.jprot.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Da Silva M., Dombre C., Brionne A., Monget P., Chessé M., De Pauw M., Mills M., Combes-Soia L., Labas V., Guyot N., Nys Y., Réhault-Godbert S. The unique features of proteins depicting the chicken amniotic fluid. Mol. Cell. Proteom. 2019;18:S174. doi: 10.1074/mcp.RA117.000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio C., Arena S., Scaloni A., Guerrier L., Boschetti E., Mendieta M.E., Citterio A., Righetti P.G. Exploring the chicken egg white proteome with combinatorial peptide ligand libraries. J. Protemone. Res. 2008;7:3461–3474. doi: 10.1021/pr800193y. [DOI] [PubMed] [Google Scholar]
- Esmon C.T. Interactions between the innate immune and blood coagulation systems. Trends. Immunol. 2004;25:536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinazzo A., Restuccia U., Bachi A., Guerrier L., Fortis F., Boschetti E., Fasoli E., Citterio A., Righetti P.G. Chicken egg yolk cytoplasmic proteome, mined via combinatorial peptide ligand libraries. J. Chromatogr. A. 2009;1216:1241–1252. doi: 10.1016/j.chroma.2008.11.051. [DOI] [PubMed] [Google Scholar]
- Gentry P.A. Comparative aspects of blood coagulation. Vet. J. 2004;168:238–251. doi: 10.1016/j.tvjl.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Georgiou A.S., Gil M.A., Alminana C., Cuello C., Vazquez J.M., Roca J., Martinez E.A., Fazeli A. Effects of complement component 3 derivatives on pig oocyte maturation, fertilization and early embryo development in vitro. Reprod. Domest. Anim. 2011;46:1017–1021. doi: 10.1111/j.1439-0531.2011.01777.x. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Aebi U. Intermediate filaments: a dynamic network that controls cell mechanics. F1000Prime. Rep. 2014;6:54. doi: 10.12703/P6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin-Dubiard C., Pasco M., Mollé D., Désert C., Croguennec T., Nau F. Proteomic analysis of hen egg white. J. Agric. Food Chem. 2006;54:3901–3910. doi: 10.1021/jf0529969. [DOI] [PubMed] [Google Scholar]
- Guyot N., Labas V., Harichaux G., Chessé M., Poirier J., Nys Y., Réhault-Godbert S. Proteomic analysis of egg white heparin-binding proteins: towards the identification of natural antibacterial molecules. Sci. Rep. 2016;6 doi: 10.1038/srep27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N., Rehault-Godbert S., Slugocki C., Harichaux G., Labas V., Helloin E., Nys Y. Characterization of egg white antibacterial properties during the first half of incubation: A comparative study between embryonated and unfertilized eggs. Poult. Sci. 2016;95:2956–2970. doi: 10.3382/ps/pew271. [DOI] [PubMed] [Google Scholar]
- Hathaway J.J.M., Adema C.M., Stout B.A., Mobarak C.D., Loker E.S. Identification of protein components of egg masses indicates parental investment in immunoprotection of offspring by Biomphalaria glabrata (gastropoda, mollusca) Dev. Comp. Immunol. 2010;34:425–435. doi: 10.1016/j.dci.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Franz T., Tamai Y., Taketo M.M., Magin T.M. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000;19:5060–5070. doi: 10.1093/emboj/19.19.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincke M.T., Da Silva M., Guyot N., Gautron J., McKee M.D., Guabiraba-Brito R., Réhault-Godbert S. Dynamics of structural barriers and innate immune components during incubation of the avian egg: critical interplay between autonomous embryonic development and maternal anticipation. J. Innate. Immun. 2019;11:111–124. doi: 10.1159/000493719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Yamamoto E., Sawada M., Okamoto M., Uehara M. Expression of lactate dehydrogenase-A and-B messenger ribonucleic acids in chick glycogen body. Poult. Sci. 2006;85:1232–1238. doi: 10.1093/ps/85.7.1232. [DOI] [PubMed] [Google Scholar]
- King'ori A.M. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci. 2011;10:483–492. [Google Scholar]
- Kinoshita K., Shimogiri T., Ibrahim H.R., Tsudzuki M., Maeda Y., Matsuda Y. Identification of TENP as the gene encoding chicken egg white ovoglobulin G2 and demonstration of its high genetic variability in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Hattori M., Hara-Kudo Y., Okubo T., Yamamoto S., Takita T., Sugita-Konishi Y. Glycopeptide derived from hen egg ovomucin has the ability to bind enterohemorrhagic Escherichia coli O157: H7. J. Agric. Food Chem. 2004;52:5740–5746. doi: 10.1021/jf0353335. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J., Phillips M., Mine Y. Advances in the value of eggs and egg components for human health. J. Agric. Food. Chem. 2005;53:8421–8431. doi: 10.1021/jf050964f. [DOI] [PubMed] [Google Scholar]
- Liu Y., Qiu N., Ma M. Comparative proteomic analysis of hen egg white proteins during early phase of embryonic development by combinatorial peptide ligand library and matrix-assisted laser desorption ionization-time of flight. Poult. Sci. 2013;92:1897–1904. doi: 10.3382/ps.2012-02986. [DOI] [PubMed] [Google Scholar]
- Liu Y., Qiu N., Ma M. Comparative proteomic analysis of egg white proteins during the rapid embryonic growth period by combinatorial peptide ligand libraries. Poult. Sci. 2015;94:2495–2505. doi: 10.3382/ps/pev176. [DOI] [PubMed] [Google Scholar]
- Lowery J., Kuczmarski E.R., Herrmann H., Goldman R.D. Intermediate filaments play a pivotal role in regulating cell architecture and function. J. Biol. Chem. 2015;290:17145–17153. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehashi K., Ueda M., Matano M., Takeuchi J., Uchino M., Kashiwagi Y., Watanabe T. Biochemical and functional characterization of transiently expressed in neural precursor (TENP) protein in emu egg white. J. Agric. Food. Chem. 2014;62:5156–5162. doi: 10.1021/jf5008117. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Mann K. The chicken egg white proteome. Proteomics. 2007;7:3558–3568. doi: 10.1002/pmic.200700397. [DOI] [PubMed] [Google Scholar]
- Mann K. Proteomic analysis of the chicken egg vitelline membrane. Proteomics. 2008;8:2322–2332. doi: 10.1002/pmic.200800032. [DOI] [PubMed] [Google Scholar]
- Mann K., Mann M. The chicken egg yolk plasma and granule proteomes. Proteomics. 2008;8:178–191. doi: 10.1002/pmic.200700790. [DOI] [PubMed] [Google Scholar]
- Mann K., Mann M. In-depth analysis of the chicken egg white proteome using an LTQ Orbitrap Velos. Proteome. Sci. 2011;9:7. doi: 10.1186/1477-5956-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M., Weisel J.W., Ischiropoulos H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free. Radic. Biol. Med. 2013;65:411–418. doi: 10.1016/j.freeradbiomed.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe D.J.H., Harrison P., Mackie I.J., Sidhu P.S., Purdy G., Lawrie A.S., Watt H., Brown M.M., Machin S.J. Platelet degranulation and monocyte-platelet complex formation are increased in the acute and convalescent phases after ischaemic stroke or transient ischaemic attack. Br. J. Haematol. 2004;125:777–787. doi: 10.1111/j.1365-2141.2004.04983.x. [DOI] [PubMed] [Google Scholar]
- Mikšík I., Ergang P., Pácha J. Proteomic analysis of chicken eggshell cuticle membrane layer. Anal. Bioanal. Chem. 2014;406:7633–7640. doi: 10.1007/s00216-014-8213-x. [DOI] [PubMed] [Google Scholar]
- Miles R.D. Trace minerals and avain embryo development. Ciência Animal Brasileira. 2000;2:1–10. [Google Scholar]
- Nagase H., Harris E.D., Woessner J.F., Brew K. Ovostatin: a novel proteinase inhibitor from chicken egg white. I. Purification, physicochemical properties, and tissue distribution of ovostatin. J. Biol. Chem. 1983;258:7481–7489. [PubMed] [Google Scholar]
- Omana D.A., Wang J., Wu J. Ovomucin–a glycoprotein with promising potential. Trends Food Sci. Technol. 2010;21:455–463. doi: 10.1016/j.tifs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirana A., Diao M., Huang S., Zuo L., Liang Y. Alpha 2 macroglobulin is a maternally-derived immune factor in amphioxus embryos: new evidence for defense roles of maternal immune components in invertebrate chordate. Fish Shellfish Immunol. 2016;50:21–26. doi: 10.1016/j.fsi.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Pischedda F., Szczurkowska J., Cirnaru M.D., Giesert F., Vezzoli E., Ueffing M., Sala C., Francolini M., Hauck S.M., Cancedda L., Piccoli G. A cell surface biotinylation assay to reveal membrane-associated neuronal cues: Negr1 regulates dendritic arborization. Mol. Cell. Proteomics. 2014;13:733–748. doi: 10.1074/mcp.M113.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu N., Ma M., Cai Z., Jin Y., Huang X., Huang Q., Sun S. Proteomic analysis of egg white proteins during the early phase of embryonic development. J. Proteomics. 2012;75:1895–1905. doi: 10.1016/j.jprot.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Réhault-Godbert S., Mann K., Bourin M., Brionne A., Nys Y. Effect of embryonic development on the chicken egg yolk plasma proteome after 12 days of incubation. J. Agric. Food. Chem. 2014;62:2531–2540. doi: 10.1021/jf404512x. [DOI] [PubMed] [Google Scholar]
- Réhault-Godbert S., Guyot N., Nys Y. The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients. 2019;11:684. doi: 10.3390/nu11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L., Romanoff A.J. John Wiley and Sons, Inc.; New York, NY: 1949. The avian egg. [Google Scholar]
- Rose-Martel M., Du J., Hincke M.T. Proteomic analysis provides new insight into the chicken eggshell cuticle. J. Proteomics. 2012;75:2697–2706. doi: 10.1016/j.jprot.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Drewes W., Schwägele F. Analysis of vitelline membrane proteins of fresh and stored eggs via HPLC. Z. Lebensm. Unters. Forsch. A. 1998;206:329–332. [Google Scholar]
- Schuett K., Savvaidis A., Maxeiner S., Lysaja K., Jankowski V., Schirmer S.H., Dimkovic N., Boor P., Kaesler N., Dekker F.W., Floege J., Marx N., Schlieper G. Clot structure: a potent mortality risk factor in patients on hemodialysis. J. Am. Soc. Nephrol. 2017;28:1622–1630. doi: 10.1681/ASN.2016030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., Windoffer R., Magin T.M., Leube R.E. Dissection of keratin network formation, turnover and reorganization in living murine embryos. Sci. Rep. 2015;5 doi: 10.1038/srep09007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Loreth D., Pottker B., Hefti K., Innos J., Schwald K., Hengstler H., Menzel L., Sommer C.J., Radyushkin K., Kretz O., Philips M., Haas C.A., Frauenknecht K., Lilleväli K., Heimrich B., Vasar E., Schäfer M.K.E. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease-associated Negr1 gene. Front. Mol. Neurosci. 2018;11:30. doi: 10.3389/fnmol.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Saito A., Kusakabe T., Hori K., Koga K. Flow of egg white ovalbumin into the yolk sac during embryogenesis. Biochim. Biophys. Acta. 1989;992:400–403. doi: 10.1016/0304-4165(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Sun C., Liu J., Li W., Xu G., Yang N. Divergent proteome patterns of egg albumen from domestic chicken, duck, goose, turkey, quail and pigeon. Proteomics. 2017;17 doi: 10.1002/pmic.201700145. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Nishimura K., Aoki N., Adachi T., Sato C., Kitajima K., Matsuda T. A 42-kDa glycoprotein from chicken egg-envelope, an avian homolog of the ZPC family glycoproteins in mammalian Zona pellucida. Its first identification, cDNA cloning and granulosa cell-specific expression. Eur. J. Biochem. 1999;260:736–742. doi: 10.1046/j.1432-1327.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- Tan R.P.A., Leshchyns Ka I., Sytnyk V. Glycosylphosphatidylinositol-anchored immunoglobulin superfamily cell adhesion molecules and their role in neuronal development and synapse regulation. Front. Mol. Neurosci. 2017;10:378. doi: 10.3389/fnmol.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Yadgary L., Yair R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 2012;21:175–184. [Google Scholar]
- Van Cruchten S., Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- Vieira S.L. Chicken embryo utilization of egg micronutrients. Brazil J. Poult. Sci. 2007;9:1–8. [Google Scholar]
- Vijayaraj P., Kröger C., Reuter U., Windoffer R., Leube R.E., Magin T.M. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and raptor. J. Cell. Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclawek M., Foisner R., Nimpf J., Schneider W.J. The chicken homologue of zona pellucida protein-3 is synthesized by granulosa cells. Biol. Reprod. 1998;59:1230–1239. doi: 10.1095/biolreprod59.5.1230. [DOI] [PubMed] [Google Scholar]
- Wang J., Wu J. Proteomic analysis of fertilized egg white during early incubation. EuPA Open Proteomics. 2014;2:38–59. [Google Scholar]
- Watanabe K., Tsuge Y., Shimoyamada M., Ogama N., Ebina T. Antitumor effects of pronase-treated fragments, glycopeptides, from ovomucin in hen egg white in a double grafted tumor system. J. Agric. Food Chem. 1998;46:3033–3038. [Google Scholar]
- Wen C., Zhang Z., Ma W., Xu M., Wen Z., Peng J. Genome-wide identification of female-enriched genes in zebrafish. Dev. Dyn. 2005;232:171–179. doi: 10.1002/dvdy.20210. [DOI] [PubMed] [Google Scholar]
- Whenham N., Wilson P.W., Bain M.M., Stevenson L., Dunn I.C. Comparative biology and expression of TENP, an egg protein related to the bacterial permeability-increasing family of proteins. Gene. 2014;538:99–108. doi: 10.1016/j.gene.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Willems E., Decuypere E., Buyse J., Everaert N. Importance of albumen during embryonic development in avian species, with emphasis on domestic chicken. Worlds Poult. Sci. J. 2014;70:503–518. [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R.T., Wang S.Z. Identification and characterization of tenp, a gene transiently expressed before overt cell differentiation during neurogenesis. J. Neurobiol. 1998;34:319–328. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.