Abstract
Dietary prebiotics are thought to be potentially important alternatives to antibiotic growth promoters in poultry production because of their beneficial performance and health effects. The administration of dietary prebiotics has been demonstrated to improve animal health, growth performance, and microbial food safety in poultry production. In this study, we evaluated the effects of Saccharomyces- derived prebiotic refined functional carbohydrates (RFC) with yeast culture on growth performance and gastrointestinal and environmental microbiota when administered in-feed and through drinking water to broiler chickens. Broilers were administered 2 doses of prebiotic in-feed through 42 d of production and prebiotic-treated water in the final 72 h. Administration of prebiotic RFC improved ADG and decreased cecal Campylobacter counts, while the high dose also increased final BW. Additionally, significant main effects of prebiotic RFC dose were observed with the high dose improving ADG and ADFI over the finisher phase and final BW. Although the effects were not significant, the prevalence of Campylobacter in the cecum after feed withdrawal was 17% lower when broilers were administered the high prebiotic dose, and recovery of Campylobacter from litter was up to 50% lower when broilers were administered prebiotic RFC. Our results suggest that co-administration of RFC with yeast culture as a prebiotic can be used to improve growth performance and reduce human foodborne pathogens in poultry.
key words: dietary prebiotics; refined functional carbohydrates; Saccharomyces cerevisiae, Campylobacter
INTRODUCTION
Antibiotics have been used widely in poultry production because of their ability to increase weight gain (Moore et al., 1946), reduce the gastrointestinal (GI) colonization of pathogens (Lev et al., 1957; Stutz et al., 1983), and improve feed efficiency (Emborg et al., 2002). However, the use of antibiotic growth promoters (AGP) has declined due to increased concerns regarding the development of antibiotic-resistant bacteria with consequences to human and animal health (Silbergeld et al., 2008) and growing consumer demand for antibiotic-free (ABF) food production (Hume, 2011). In response, AGP use has been banned by the European Union (Cogliani et al., 2011) and limited in the United States by the Veterinary Feed Directive (Department of Health and Human Services, 2015). Therefore, the development of alternatives to AGP is of significant interest to the poultry industry. Because growth promotion by antibiotics is attributed to their effects on GI microorganisms (Visek, 1978; Gaskins et al., 2002), the GI microbiota is an important target for the development of alternatives to AGP.
Defined by expert consensus from the International Scientific Association for Probiotics and Prebiotics, a prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit (Gibson et al., 2017)”, and, when administered orally, prebiotics are referred to specifically as dietary prebiotics (Bindels et al., 2015). The administration of dietary prebiotics has been shown to enhance digestive functionality of the poultry GI tract (Nahashon et al., 1994) and positively affect animal performance by increasing BW (Torres-Rodriguez et al., 2007) and improving feed efficiency (Salianeh et al., 2011). Additionally, the administration of prebiotics has been shown to promote populations of Lactic Acid Bacteria (LAB) and other beneficial microorganisms in the GI tract that are thought to compete with pathogenic bacteria for mucosal binding sites (Patterson and Burkholder, 2003; Askelson and Duong, 2015; Broderick and Duong, 2016). The administration of prebiotics has been shown to reduce pathogens of poultry, such as Clostridium perfringens (Yang et al., 2008; Allaart et al., 2013). Further, the administration of prebiotics has been shown to reduce human foodborne pathogenic bacteria, including Salmonella (Xu et al., 2003; Chung and Day, 2004) and Campylobacter (Fernandez et al., 2000; Baurhoo et al., 2009), thus improving the microbial food safety of poultry products.
Indigestible carbohydrates are often administered as dietary prebiotics because they pass through the proximal portion of the GI tract with minimal digestion and reach the distal portion intact with the ability to interact with intestinal microbiota (Grizard and Barthomeuf, 1999; Vandeplas et al., 2010). Refined functional carbohydrates (RFC), including mannan-oligosaccharides (MOS), β-glucan, and D-mannose which account for 20 to 30% of the cell dry mass, derived from the cell wall of Saccharomyces cerevisiae, are a readily available source of prebiotics for human and animal use (Dallies et al., 1998). In previous studies, the administration of RFC as dietary prebiotics has been demonstrated to increased BW of broilers (Walker et al., 2018) and decrease the colonization of foodborne human bacterial pathogens in broiler chickens (Walker et al., 2018), broiler breeder hens and their progeny(Walker et al., 2017), and turkeys during transport stress (Huff et al., 2013).
Although the ability of prebiotics to increase performance and reduce foodborne pathogens has been widely reported, their overall effectiveness when administered to poultry is mixed. The beneficial effects of their administration are often inappropriately attributed broadly across all prebiotic products as a general class of functional feed additives. However, the ability to confer specific benefits is dependent upon the individual constituent components of a prebiotic product (Askelson and Duong, 2015). Thus, research investigating the functionality of specific prebiotic products is required. In this study, we evaluated the effects of a dietary prebiotic product composed of RFC with yeast culture on growth performance and GI and environmental microbiota when administered in-feed and through water to broiler chickens as a potential alternative to AGP.
MATERIALS AND METHODS
Experimental Animals and Husbandry
Male broiler chicks (Cobb) were obtained from a commercial hatchery on day of hatch, vaccinated for Eimeria (Advent, Huvepharma Inc, Peachtree City, GA), weighed, wing banded, and assigned randomly to pens to ensure statistically similar starting pen weights. Experimental animals were raised in 3.35 m2 floor pens on built-up litter; provided age appropriate heat and ventilation; and given access to potable water and experimental rations ad libitum. Broilers were placed at an initial stocking density of 0.075 m2 per broiler; temperature was monitored, recorded daily, and adjusted in response to bird comfort; and the lighting program followed the standard operating procedure for broilers raised at the Texas A&M University Poultry Science Research Center (Flores et al., 2019) according to the breeder's recommendations (Cobb-Vantress, 2018). All experimental procedures were performed as approved by the Texas A&M University Institutional Animal Care and Use Committee.
Experimental Design and Diets
The effects of dietary prebiotic administration on growth performance and GI colonization of Campylobacter spp., Clostridium perfringens, and total LAB were evaluated in comparison to an AGP. Broiler chicks (n = 1720) were allocated to 6 experimental treatment groups with a total of 40 pens of 43 birds arranged, due to housing constraints, as a randomized incomplete block design and fed experimental rations with dietary prebiotic administered in-feed (Celmanax SCP, Arm and Hammer Animal and Food Production, Princeton, NJ) or through drinking water (Celmanax Liquid NC, Arm and Hammer) using the manufacturer's recommended dosages. The 6 experimental treatment groups were as follows: bacitracin methylene disalicylate (BMD)-treated (50 g t−1) feed (7 pens); untreated feed (7 pens); low-dose (50 g t−1) of prebiotic RFC in-feed (7 pens); high-dose (100 g t−1) of prebiotic RFC in-feed (7 pens); low-dose prebiotic RFC in-feed and prebiotic RFC administered via drinking water (500 ppm) beginning at 39 d post-hatch (6 pens); and high-dose prebiotic RFC in-feed and prebiotic RFC administered via drinking water (500 ppm) beginning at 39 d post-hatch (6 pens).
Broilers were fed experimental rations beginning at 0 d through 41 d post-hatch. After collecting final BW at 42 d post-hatch, feed was withdrawn for 8 h, and the study was terminated. Prebiotic-treated water was administered to the appropriate groups beginning at 39 d post-hatch (72 h prior to feed withdrawal) through study termination, while the remaining groups received untreated water over the same period. Water was provided to all treatment pens using individual hanging bucket drinkers during the water treatment period.
Experimental treatment diets (Table 1) were fed for the duration of the trial using a 3-phase feed plan: starter phase (0 to 14 d, crumble), grower phase (14 to 28 d, pellet), and finisher phase (28 to 42 d, pellet). For each phase, feed was manufactured as a single commercial-type corn/soybean meal basal diet with added phytase and 5% distiller's dried grains with solubles and divided for inclusion of dietary treatments as appropriate. Full matrix values for phytase contribution of aP, Ca, Na, digestible AA, and ME as recommended by the manufacturer were used.
Table 1.
Ingredient composition and nutrient content of the basal control diets.
| Item (%) | Starter | Grower | Finisher |
|---|---|---|---|
| Ingredients | |||
| Corn | 57.95 | 63.65 | 68.45 |
| SBM (45.6% CP) | 29.10 | 23.70 | 18.95 |
| DL-Met | 0.29 | 0.25 | 0.20 |
| Lys HCL | 0.25 | 0.23 | 0.20 |
| L-Thr | 0.09 | 0.08 | 0.07 |
| Soy Oil | 2.47 | 2.38 | 2.83 |
| Limestone | 0.87 | 0.69 | 0.66 |
| CaH4 PO4 | 0.30 | 0.00 | 0.00 |
| NaCl | 0.32 | 0.33 | 0.22 |
| NaHCO3 | 0.14 | 0.12 | 0.27 |
| Trace Minerals1 | 0.05 | 0.05 | 0.05 |
| Vitamins2 | 0.25 | 0.25 | 0.25 |
| LO- DGGS | 5.00 | 5.00 | 5.00 |
| Pork MBM | 3.00 | 3.35 | 2.99 |
| Phytase3 | 0.01 | 0.01 | 0.01 |
| Calculated nutrient | |||
| Protein | 22.00 | 19.95 | 17.82 |
| Crude Fat | 5.30 | 5.41 | 5.95 |
| Crude Fiber | 2.50 | 2.53 | 2.55 |
| Ca | 0.92 | 0.82 | 0.75 |
| aP | 0.46 | 0.41 | 0.38 |
| ME (kcal kg−1) | 3047 | 3102 | 3168 |
| dig Met | 0.59 | 0.53 | 0.46 |
| dig TSAA | 0.87 | 0.79 | 0.69 |
| dig Lys | 1.18 | 1.04 | 0.89 |
| dig Trp | 0.21 | 0.18 | 0.16 |
| dig Thr | 0.77 | 0.69 | 0.60 |
| Na | 0.046 | 0.043 | 0.039 |
| Analyzed nutrients4 | |||
| Moisture | 12.60 | 10.84 | 15.38 |
| Dry Matter | 87.40 | 89.16 | 84.62 |
| Crude Protein | 20.40 | 20.20 | 19.50 |
| Crude Fat | 5.27 | 5.07 | 2.57 |
| Fiber | 3.30 | 3.70 | 3.40 |
| Ash | 4.53 | 4.04 | 3.75 |
ME, metabolizable energy.
Trace mineral premix added at this rate yields 60.0 mg manganese, 60 mg zinc, 60 mg iron, 7 mg copper, 0.4 mg iodine, a minimum of 6.27 mg calcium, and a maximum of 8.69 mg calcium per kg of diet. The carrier is calcium carbonate and the premix contains less than 1% mineral oil.
Vitamin premix added at this rate yields 22,045 IU vitamin A, 7,716 IU vitamin D3, 91 IU vitamin E, 0.04 mg B12, 11.9 mg riboflavin, 91.8 mg niacin, 40.4 mg d-pantothenic acid, 261.1 mg choline, 2.9 mg menadione, 3.50 mg folic acid, 14.3 mg pyroxidine, 5.87 mg thiamine, 1.10 mg biotin per kg diet. The carrier is ground rice hulls.
OptiPhosPF, Huvepharma. Peachtree City, GA.
Performed by Midwest Laboratories, Inc., Omaha, NE.
Performance Data
Experimental animals and feed were weighed by pen at 0, 14, 28, and 42 d post-hatch for determination of body weight and feed consumption. Mortalities and post-mortem weight were recorded daily for the calculation of percent mortality, body weight gain, and mortality corrected FCR.
Recovery of GI Microbes
Two representative (median weight ± 5%) birds were selected from each pen, euthanized, and dissected aseptically for the collection of GI tissues at 42 d post-hatch and 8 h post-feed withdrawal. An approximately 3 cm section of the ileum proximal to the midpoint between the ileocecal junction and Meckel's diverticulum and the ceca were collected from each bird at 42 d post-hatch, while, at 8 h post-feed withdrawal, only the ceca were collected from each bird. Total LAB and Clostridium perfringens were enumerated from the ileum using cylcoheximide (100 μg mL−1, Amresco, Solon, OH) supplemented de Mann, Rogosa, and Sharpe (BD, Franklin Lakes, NJ) agar incubated in 10% CO2 at 37°C for 24 h and Tryptose Sulfite Cycloserine-Egg Yolk (BD) agar incubated at 37°C for 48 h anaerobically (Coy Laboratory Products Inc., Grass Lake, MI), respectively. Campylobacter spp. were enumerated from the cecum using Campy Cefex agar (CCA; Hardy Diagnostics, Santa Maria, CA) incubated in 10% CO2 at 37°C for 24 h. Clostridium perfringens was selectively enriched from the ileum using Fluid Thioglycollate Medium (BD) and Iron Milk Medium (HiMedia Laboratories; Mumbai, India), while Campylobacter was selectively enriched from the cecum using Bolton's Enrichment Broth (BEB; Hardy) and CCA. Specimens for which no colonies appeared on enumeration plates but were positive by selective enrichment were assigned the limit of detection for enumeration (100 cfu g−1).
Recovery of Litter Campylobacter
Immediately prior to placement and at 42 d post-hatch, litter was collected from 5 locations in each treatment pen, pooled by pen, and homogenized using Buffered Peptone Water (HiMedia) for selective enrichment of Campylobacter using BEB and CCA.
Statistical Analysis
Bacterial count and mortality data were log10 and arcsine square root transformed, respectively, for analysis. Growth performance results and bacterial counts were analyzed using ANOVA. Significantly different means were separated using Duncan's Multiple Range Test post hoc. Bacterial incidence was analyzed using Pearson's χ2 Test. Because prebiotic treatment via drinking water did not occur until the finisher phase, the relevant treatment groups, e.g., low dose prebiotic treated feed with and without water treatment, were combined for analysis during the starter and grower phases. Additionally, growth performance results and bacterial counts for treatment groups receiving the prebiotic feed supplement with or without prebiotic water treatment were analyzed using a 2 (dose) × 2 (water treatment) factorial ANOVA with main effects for in-feed dose, water treatment, and in-feed dose × water treatment, while the effects of dose and water treatment on bacterial incidence were analyzed using binomial logistic regression. Statistical significance was considered at P ≤ 0.05.
RESULTS
Growth Performance
The effects of prebiotic administration in-feed and treated water were evaluated in comparison to antibiotic-treated and untreated controls. A significant treatment effect was observed for d 42 BW (P = 0.002) and ADG over d 0 to 42 (P = 0.033) (Table 2). Body weight and ADG was greatest when broilers were fed the high prebiotic diets as compared to the low prebiotic and control diets. Although they were not significantly greater than the controls or non-water treated low prebiotic treatments, administration of the low prebiotic dose by feed with prebiotic-treated water improved BW and ADG to a level similar to the treatments administered the high prebiotic dose with or without treated water. No significant treatment effects were observed for BW on d 14 or 28 or ADG over d 0 to 14, d 14 to 28, or d 28 to 42.
Table 2.
Body weight and average daily gain of broiler chickens.
| Treatments |
BW (kg) |
ADG (g bird-day−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed1 | Water2 | d 0 | d 14 | d 28 | d 42 | Starter | Grower | Finisher | d 0–42 |
| BMD | – | 0.038 | 0.527 | 1.489 | 2.664b | 34.2 | 69.3 | 89.6 | 61.8b |
| UNT | – | 0.039 | 0.527 | 1.436 | 2.705b | 34.6 | 65.7 | 94.7 | 61.8b |
| RFC-Lo | – | 0.039 | 0.525 | 1.552 | 2.722b | 33.7 | 74.1 | 86.5 | 61.2b |
| RFC-Hi | – | 0.039 | 0.538 | 1.562 | 2.962a | 34.7 | 73.8 | 105.6 | 67.0a |
| RFC-Lo | + | 2.848a,b | 98.9 | 64.0a,b | |||||
| RFC-Hi | + | 3.040a | 110.2 | 66.6a | |||||
| P-value | 0.143 | 0.443 | 0.132 | 0.002 | 0.510 | 0.116 | 0.062 | 0.033 | |
| Pooled SEM | 0.000 | 0.003 | 0.020 | 0.034 | 0.263 | 1.339 | 2.598 | 0.687 | |
Means within a column not sharing a common superscript are significantly different (P ≤ 0.05).
In-feed treatments: BMD, 50 g t−1 bacitracin methylene disalicylate; UNT, untreated; RFC-Lo, 50 g t−1 RFC; RFC-Hi, 100 g t−1 RFC.
Drinking water treatment: RFC at 500 ppm beginning at 39 d post-hatch.
No significant treatment effect on FCR was observed for any period of the study (Table 3). However, a significant treatment effect was observed for ADFI for d 28 to 42, (P = 0.010) and d 0 to 42 (P = 0.022) (Table 3). Over both periods, ADFI was greatest when broilers were fed the high prebiotic dose and administered treated water when compared to the other treatments. Additionally, ADFI of broilers administered high prebiotic dose alone was similar to those administered the high prebiotic dose and treated water over the finisher phase and d 0 to 42.
Table 3.
Mortality corrected feed conversion ratio and average daily feed intake of broiler chickens.
| Treatments |
FCR feed:gain |
ADFI (g bird-day−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed1 | Water2 | Starter | Grower | Finisher | d 0–42 | Starter | Grower | Finisher | d 0–42 |
| BMD | – | 1.040 | 1.845 | 2.167 | 1.753 | 38.4 | 126.8 | 181.0c | 112.0b |
| UNT | – | 1.057 | 1.887 | 2.111 | 1.683 | 39.6 | 115.3 | 180.2c | 108.0b |
| RFC-Lo | – | 1.032 | 1.586 | 2.442 | 1.698 | 37.8 | 115.3 | 189.2b,c | 109.2b |
| RFC-Hi | – | 1.220 | 1.741 | 1.970 | 1.636 | 45.8 | 124.2 | 196.6a,b | 114.3a,b |
| RFC-Lo | + | 2.055 | 1.614 | 187.6b,c | 108.4b | ||||
| RFC-Hi | + | 2.011 | 1.700 | 204.4a | 121.0a | ||||
| P-value | 0.374 | 0.158 | 0.315 | 0.359 | 0.270 | 0.158 | 0.010 | 0.022 | |
| Pooled SEM | 0.046 | 0.052 | 0.064 | 0.019 | 0.000 | 1.743 | 2.189 | 2.267 | |
Means within a column not sharing a common superscript are significantly different (P ≤ 0.05).
In-feed treatments: BMD, 50 g t−1 bacitracin methylene disalicylate; UNT, untreated; RFC-Lo, 50 g t−1 RFC; RFC-Hi, 100 g t−1 RFC.
Drinking water treatment: RFC at 500 ppm beginning at 39 d post-hatch.
A significant treatment effect was observed for mortality for the grower period, d 14 to 28, (P = 0.026) and d 0 to 42 (P = 0.016) (Table 4). Although mortality was lowest when broilers were administered BMD, BMD administration did not significantly reduce mortality when compared to untreated broilers over either period. Over the grower period, mortality of broilers fed either prebiotic dose was also not different than that of the untreated group. Similarly for d 0 to 42, mortality of broilers administered the high prebiotic dose alone or the low prebiotic dose with or without treated water was not significantly different than the BMD-treated or untreated controls. However, mortality of broilers receiving the high prebiotic dose and prebiotic-treated water was greater than the antibiotic-treated and untreated broilers. No significant treatment effects on mortality were observed over the remaining periods.
Table 4.
Mortality of broiler chickens.
| Treatments |
Mortality (%) |
||||
|---|---|---|---|---|---|
| Feed1 | Water2 | Starter | Grower | Finisher | d 0-42 |
| BMD | – | 3.99 | 0.35b | 1.10 | 5.32b |
| UNT | – | 4.65 | 1.74a,b | 0.00 | 6.31b |
| RFC-Lo | – | 6.31 | 3.25a | 0.00 | 8.97a,b |
| RFC-Hi | – | 7.75 | 3.15a | 0.45 | 8.53a,b |
| RFC-Lo | + | 0.75 | 10.30a,b | ||
| RFC-Hi | + | 0.46 | 13.18a | ||
| P-value | 0.264 | 0.026 | 0.495 | 0.016 | |
| Pooled SEM | 0.53 | 0.41 | 0.19 | 0.68 | |
Means within a column not sharing a common superscript are significantly different (P ≤ 0.05).
In-feed treatments: BMD, 50 g t−1 bacitracin methylene disalicylate; UNT, untreated; RFC-Lo, 50 g t−1 RFC; RFC-Hi, 100 g t−1 RFC.
Drinking water treatment: RFC at 500 ppm beginning at 39 d post-hatch.
Gastrointestinal Microbiota
Cecal Bacteria
A significant treatment effect was observed on counts of Campylobacter spp. in the cecum at d 42 (P = 0.012) (Figure 1 A). Administration of prebiotic reduced Campylobacter up to 1.0 log10 cfu g−1 cecal contents when compared to broilers fed BMD-treated or untreated feed, with the fewest Campylobacter being recovered from broilers administered the high prebiotic dose and treated water. Although a significant treatment effect was not observed on incidence in the cecum prior to (P = 0.253) or after (P = 0.080) feed withdrawal (Table 5), Campylobacter tended to be detected in fewer ceca from broilers administered prebiotic-treated water during the feed withdrawal period as compared to the other treatments.
Figure 1.
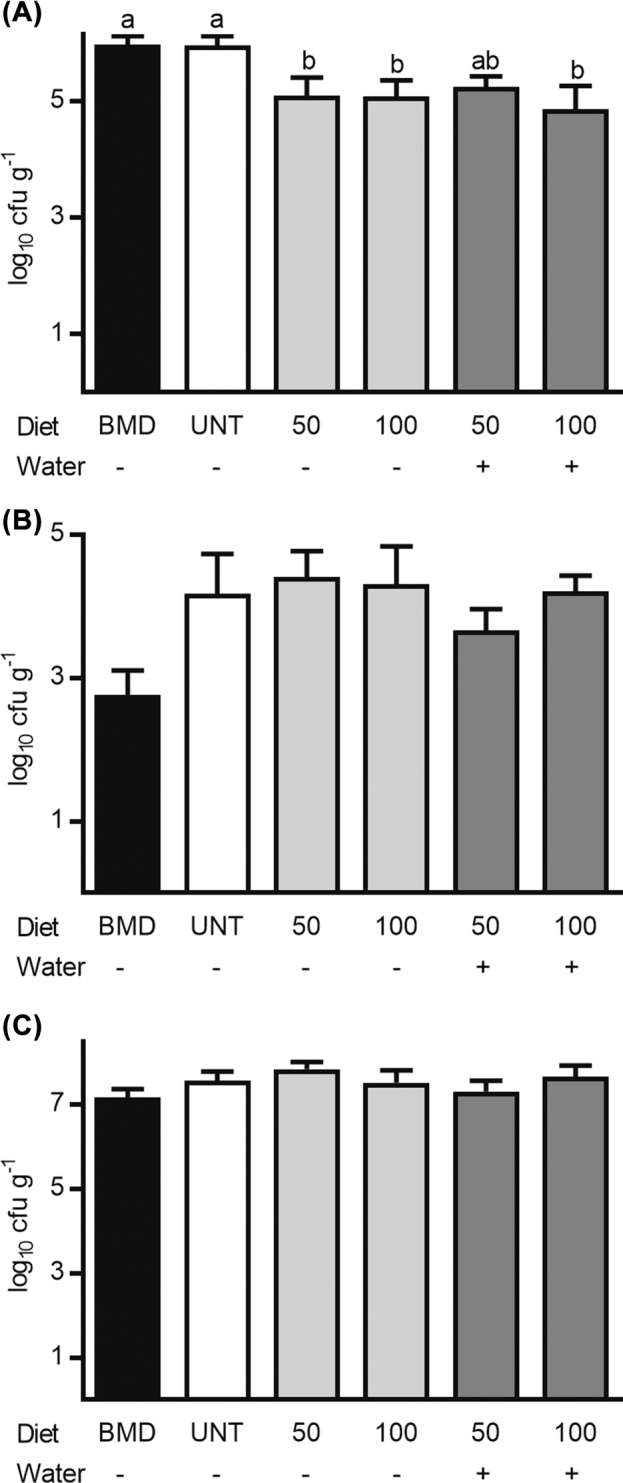
Enumeration of bacteria from broiler chickens. At 42 d post-hatch (A) Campylobacter was enumerated from the cecum of broiler chickens, and (B) C. perfringens and (C) total LAB were enumerated from the ileum. Counts are reported as the mean ± SEM log10 cfu g−1 digestive contents. Different letters above bars indicate means are significantly different (P ≤ 0.05).
Table 5.
Recovery of Campylobacter from cecum and litter.
| Treatments |
Cecum (%)1 |
Litter (%)2 |
|||
|---|---|---|---|---|---|
| Feed3 | Water4 | Pre | Post | d 0 | d 42 |
| BMD | – | 92.9 | 100.0 | 100.0 | 71.4 |
| UNT | – | 100.0 | 100.0 | 100.0 | 100.0 |
| RFC-Lo | – | 85.7 | 100.0 | 100.0 | 71.4 |
| RFC-Hi | – | 75.0 | 83.3 | 100.0 | 50.0 |
| RFC-Lo | + | 85.7 | 100.0 | 100.0 | 42.9 |
| RFC-Hi | + | 100.0 | 83.3 | 100.0 | 66.7 |
| P-value | 0.253 | 0.080 | 0.283 | ||
Campylobacter positive ceca pre- and post-feed withdrawal.
Campylobacter positive pens a 0 and 42 d post-hatch.
In-feed treatment: BMD, 50 g t−1 bacitracin methylene disalicylate; UNT, untreated; RFC-Lo, 50 g t−1; RFC-Hi, 100 g t−1.
Drinking water treatment: RFC at 500 ppm.
Ileal Bacteria
Although a significant treatment effect was not observed on counts of C. perfringens (P = 0.057) or total LAB (P = 0.331) in the ileum of broilers at d 42 (Figure 1 B-C), fewer C. perfringens tended to be recovered from broilers fed the BMD-treated diet and the low prebiotic-treated diet with prebiotic water administration compared to broilers fed the untreated control or other prebiotic diets.
Litter Campylobacter
A significant treatment effect was not observed on Campylobacter prevalence in the litter at d 0 or 42 (Table 5). Campylobacter was detected in all pens prior to placement of the study. Although a significant effect was not observed on day 42 (P = 0.283), Campylobacter was detected in litter from fewer pens in which broilers were administered prebiotic or BMD-treated feed than for the untreated control.
Main Effects Analyses
The main effects of prebiotic dose in-feed and administration of prebiotic-treated water on growth performance (Table 6) and GI microbiota (not shown) were also evaluated. No significant dose × water interactions were observed for any growth performance measure. A significant main effect of prebiotic dose was observed on d 42 BW (P = 0.002), d 28 to 42 ADG (P = 0.004), and d 28 to 42 ADFI (P = 0.012), with the high dose increasing each performance measure. Although the effect was not significant (P = 0.059), FCR of broilers administered the high dose tended to be lower when compared to the low dose. However, a significant main effect of the administration of prebiotic-treated water over the final 72 h of production was not observed for any of the growth performance measures.
Table 6.
Main effects of feed and water additives on growth performance of broiler chickens.
| Finisher (28–42 d) |
||||
|---|---|---|---|---|
| BW |
ADG |
ADFI |
FCR |
|
| Main effects | 42 d (kg) | (g bird-day−1) | (g bird-day−1) | (feed:gain) |
| Dose1 | ||||
| RFC-Lo | 2.785 | 92.7 | 188.4 | 2.248 |
| RFC-Hi | 3.001 | 107.9 | 200.5 | 1.991 |
| Water2 | ||||
| Untreated | 2.842 | 96.0 | 192.9 | 2.206 |
| Treated | 2.944 | 104.6 | 196.0 | 2.033 |
| P-value | ||||
| Feed | 0.022 | 0.004 | 0.012 | 0.059 |
| Water | 0.123 | 0.082 | 0.486 | 0.193 |
| Feed × Water | 0.707 | 0.408 | 0.292 | 0.113 |
| Pooled SEM | 0.038 | 2.823 | 2.483 | 0.072 |
In-feed RFC dose: RFC-Lo, 50 g t−1; RFC-Hi, 100 g t−1.
Drinking water treatment: Treated, RFC at 500 ppm.
No significant main effects or interactions on counts of Campylobacter, total LAB, or C. perfringens were observed (not shown). Additionally, no significant association of dose or water treatment was observed on the incidence of Campylobacter in the cecum or litter.
DISCUSSION
Sub-therapeutic antibiotics have been administered widely in livestock production because of their ability to increase growth and manage infections by bacterial pathogens. However, limitations on their use in animal production have increased need for the development of potential alternatives to AGP. Growth promotion by antibiotics is attributed to their effect on the GI microbiota (Dibner and Richards, 2005). Administration of dietary prebiotics has been demonstrated to promote populations of beneficial bacteria and decrease populations of pathogens in the GI tract in poultry (Patterson and Burkholder, 2003), and prebiotics have been suggested as potential alternatives to AGP because of their ability to improve growth performance similarly to antibiotics (Huyghebaert et al., 2011). Although their benefits are often inappropriately attributed broadly across all prebiotics as a class of functional additives, the ability to confer specific benefits is dependent upon the individual constituents of a prebiotic product (Askelson and Duong, 2015). Refined functional carbohydrates derived from the cell wall of Saccharomyces cerevisiae, including mannan oligosaccharides, β-glucan, and D-mannose, are widely used as prebiotics, and although some improvement to animal growth has been reported (Walker et al., 2018), most research related to their effects in poultry have focused on pathogen reduction (Huff et al., 2013; Walker et al., 2017). In this study, we evaluated the effect of a prebiotic, composed of RFC with yeast culture, on growth performance and GI and environmental microbiota when administered in-feed and through water to broiler chickens as a potential alternative to AGP.
Overall, we observed results similar to those that have reported prebiotic administration can improve broiler growth performance parameters (Torres-Rodriguez et al., 2007; Awad et al., 2009; Mookiah et al., 2014). In our study, administration of the high prebiotic RFC dose, with or without prebiotic-treated water, increased final BW and cumulative ADG (Table 2). In a previous study, RFC administration was reported to increase BW at 28 d and 42 d of female broilers (Walker et al., 2018), while a separate study reported BWG of male broilers tended to be greater when RFC were applied as a synbiotic in combination with a direct-fed Bacillus subtilis culture (Gómez et al., 2012). In our study, finisher phase and cumulative ADFI was greater when broilers were administered the high dose of prebiotic with prebiotic-treated water, whereas no significant treatment effect was observed for FCR during any phase of the study (Table 3). These data suggest that the improvements in BW and ADG observed in this study were the result of increased feed intake. However, improved FCR has been reported previously when broilers were administered RFC (Gómez et al., 2012) and other dietary prebiotics (Hooge, 2004; Li et al., 2008; Salianeh et al., 2011). Evaluation of the effect of the dose of prebiotic RFC administered in-feed determined that final BW and ADG and ADFI over the finisher period was greater and FCR tended to be lower when broilers were administered the high dose when compared to the low dose (Table 6). However, administration of prebiotic RFC via drinking water over the final 3 d of production was not observed to have a significant effect on growth performance. Further research will be required to determine the most effective dosage and timing of RFC administration in-feed or by drinking water.
The improved growth performance observed in prebiotic-treated poultry has been attributed to the effects on digestion, digestive function, and the GI microbiota reported when prebiotics are administered (Askelson and Duong, 2015). Indeed, increased ileal nutrient digestibility, nitrogen retention, villus height (Gómez et al., 2012), and colonization by Bifidobacterium spp. and Lactobacillus spp. (Yang et al., 2009) and reduced Salmonella prevalence (Walker et al., 2017; Walker et al., 2018) have been observed when poultry were administered RFC and other dietary prebiotics. Although the ability of prebiotics to improve GI health and reduce pathogen colonization through their modification of the GI microbiota has been reported widely, the mechanisms responsible are not well understood.
The selective utilization of dietary prebiotics has been suggested to promote populations of beneficial bacteria. Many LAB and other GI tract-associated bacteria secrete extracellular hydrolases which degrade prebiotic oligosaccharides including fructooligosaccharides (FOS) and MOS (Goh and Klaenhammer, 2015). The mono- and disaccharide products of this hydrolysis are available to be utilized by all microorganisms in the GI tract which possess the appropriate phosphotransferase system transporters (Altermann et al., 2005; Azcarate-Peril et al., 2008). However, some bacteria including Lactobacillus acidophilus NCFM (Altermann et al., 2005; Barrangou et al., 2006) produce FOS-specific ATP-binding cassette transporters which enable them to import the prebiotic oligosaccharide for hydrolysis by intracellular β-fructosidases (Barrangou et al., 2003). Import and intracellular hydrolysis may provide a selective advantage through the non-altruistic utilization of FOS and other prebiotic oligosaccharides. Whether any poultry GI tract-associated microorganisms are capable of similar non-altruistic utilization of MOS or other prebiotic oligosaccharides has not been determined.
In our study, we observed reduced cecal colonization by Campylobacter spp. in RFC-treated broilers prior to feed withdrawal, with a reduction of greater than 1 log10 cfu g−1 of cecal contents in broilers receiving the high dose in-feed and treated water as compared to the untreated control. However, administration of the prebiotic treatment via drinking water was not observed to further reduce counts of Campylobacter in the cecum prior to feed being withdrawn. A quantitative risk assessment estimated that a 1 log10 decrease in the number of Campylobacter on a contaminated carcass would result in an up to 80% reduction in the cases of human foodborne illness (Rosenquist et al., 2003).
Although not a prebiotic functionality per se because it does not involve selective utilization, agglutination of bacteria by RFC has been suggested to inhibit adhesion of pathogens to the GI mucosa resulting in their passage through the GI tract without the opportunity to colonize (Oyofo et al., 1989; Spring et al., 2000; Walker et al., 2017). Mannose binding of FimH-like adhesins on type 1 fimbriae of E. coli and Salmonella has been demonstrated to block their adhesion to the GI mucosa (Oyofo et al., 1989; Spring et al., 2000). Although Campylobacter spp. are not known to possess similar adhesins, mannose-binding lectins have been observed in Campylobacter jejuni (Day et al., 2009).
Clostridium perfringens and LAB have been suggested to be potentially important markers of GI health and mediators of performance in poultry. Indeed, Askelson et al. (2018) reported greater counts of total LAB to be correlated with reduced FCR and increased counts of C. perfringens to be correlated with increased FCR. Prebiotics have been demonstrated previously to reduce C. perfringens counts in broilers (Biggs et al., 2007) and promote populations of beneficial bacteria including the LAB (Gibson and Roberfroid, 1995; Teitelbaum and Walker, 2002; Patterson and Burkholder, 2003). No significant differences in counts of C. perfringens or total LAB were observed in this study. However, fewer C. perfringens tended to be recovered from broilers that were given low dose prebiotic-treated feed and prebiotic-treated water.
Campylobacter has been widely considered to be a commensal inhabitant of the GI tract of poultry and is able to contaminate poultry products during processing (Achen et al., 1998; Herman et al., 2003). Built-up litter consumed by broilers has been suggested to be a primary vector for the transfer of Campylobacter between birds within the same flock and from one flock to the next (Montrose et al., 1985; Sahin et al., 2015). Additionally, consumption of litter by broiler chickens has been demonstrated to increase during feed withdrawal prior to processing (Corrier et al., 1999), suggesting feed withdrawal may be a potentially important critical control point at which a intervention may be applied to reduce the incidence of human foodborne pathogens in poultry. Thus, the effects of RFC administration in-feed and through drinking water on Campylobacter prevalence in the ceca before and after an 8 h feed withdrawal and in the litter were investigated in the current study (Table 5). In our study, a significant treatment effect was not observed on Campylobacter prevalence pre- or post-feed withdrawal. However, it is interesting to note that the prevalence of Campylobacter after the feed withdrawal period did tend to be lower when broilers were administered prebiotic-treated water. These data suggest administration of prebiotic RFC in drinking water may potentially be useful for reducing the risks to foodborne illness associated with increased consumption of litter during feed withdrawal. Likewise, although the effect was not statistically significant, Campylobacter was detected in the litter from fewer pens housing RFC-treated or BMD-treated broilers than when compared to untreated control. Administration of RFC with yeast culture has been demonstrated to reduce prevalence of Salmonella in the cecum (Walker et al., 2017) and litter (Walker et al., 2018). However, the effects of RFC and yeast culture on Campylobacter prevalence have not been evaluated previously, and experiments conducted using experimentally infected animals will be required to understand the effectiveness and application of RFC for reducing Campylobacter in poultry and as a potential intervention to mitigate the increased risk of GI contamination by foodborne pathogens during feed withdrawal.
In addition to promoting growth, BMD has been administered to poultry in order to reduce mortality (Brennan et al., 2003). In this study, mortality of BMD-treated broilers was not significantly lower than the untreated broilers, and, overall, mortality of RFC-treated broilers was not observed to be significantly different from the BMD-treated or untreated control. RFC administration has not been previously reported to affect mortality of broiler chickens (Gómez et al., 2012; Walker et al., 2017; Walker et al., 2018).
In this study, we investigated the effects of prebiotic RFC administration to broiler chickens on growth performance and GI and litter microbiota. We have demonstrated the administration of RFC as a dietary prebiotic improved growth performance through increased BW, ADG, and ADFI. Although the differences were not observed to be statistically significant, FCR tended to be lower with administration of the high RFC dose. Additionally, we have demonstrated that prebiotic RFC administration also reduced cecal colonization by Campylobacter spp. and may potentially reduce Campylobacter prevalence in litter, possibly improving pre-harvest microbial food safety of poultry and poultry products. Our results suggest that administration of RFC with yeast culture as a dietary prebiotic may potentially be an important component of an antibiotic-free production program and an intervention to improve pre-harvest food safety. Because of the effectiveness and reliability of antibiotics, it is unlikely that a single alternative product will match their efficacy. Thus, the continued development of entire ABF management programs, including feed additives and improved husbandry, will likely be required to truly replace AGP in poultry production.
ACKNOWLEDGMENTS
This research was conducted with support from Texas A&M AgriLife Research and Arm and Hammer Animal and Food Production. L. K. Froebel was supported by a graduate assistantship from the Texas A&M University Department of Poultry Science.
REFERENCES
- Achen M., Morishita T.Y., Ley E.C. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 1998;42:732–737. [PubMed] [Google Scholar]
- Allaart J.G., van Asten A.J., Gröne A. Predisposing factors and prevention of Clostridium perfringens-associated enteritis. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:449–464. doi: 10.1016/j.cimid.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Altermann E., Russell W.M., Azcarate-Peril M.A., Barrangou R., Buck B.L., McAuliffe O., Souther N., Dobson A., Duong T., Callanan M., Lick S., Hamrick A., Cano R., Klaenhammer T.R. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Nat. Acad. Sci. USA. 2005;102:3906–3912. doi: 10.1073/pnas.0409188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askelson T.E., Duong T. In: Probiotics and Prebiotics: Current Research and Future Trends. Venema K., do Carmo A.P., editors. Caister Academic Press; Norfolk, UK: 2015. Perspectives on differences between human and livestock animal research in probiotics and prebiotics; pp. 447–458. [Google Scholar]
- Askelson T.E., Flores C.A., Dunn-Horrocks S.L., Dersjant-Li Y., Gibbs K., Awati A., Lee J.T., Duong T. Effects of direct-fed microorganisms and enzyme blend co-administration on intestinal bacteria in broilers fed diets with or without antibiotics. Poult. Sci. 2018;97:54–63. doi: 10.3382/ps/pex270. [DOI] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Azcarate-Peril M.A., Altermann E., Goh Y.J., Tallon R., Sanozky-Dawes R.B., Pfeiler E.A., O'Flaherty S., Buck B.L., Dobson A., Duong T., Miller M.J., Barrangou R., Klaenhammer T.R. Analysis of the genome sequence ofLactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl. Environ. Microbiol. 2008;74:4610–4625. doi: 10.1128/AEM.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R., Altermann E., Hutkins R., Cano R., Klaenhammer T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Nat. Acad. Sci. USA. 2003;100 doi: 10.1073/pnas.1332765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R., Azcarate-Peril M.A., Duong T., Conners S.B., Kelly R.M., Klaenhammer T.R. Global analysis of carbohydrate utilization by Lactobacillus acidophilus using cDNA microarrays. Proc. Nat. Acad. Sci. USA. 2006;103:3816–3821. doi: 10.1073/pnas.0511287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurhoo B., Ferket P.R., Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- Biggs P., Parsons C., Fahey G.C. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 2007;86:2327–2336. doi: 10.3382/ps.2007-00427. [DOI] [PubMed] [Google Scholar]
- Bindels L.B., Delzenne N.M., Cani P.D., Walter J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015;12:303. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- Brennan J., Skinner J., Barnum D., Wilson J. The efficacy of bacitracin methylene disalicylate when fed in combination with narasin in the management of necrotic enteritis in broiler chickens. Poult. Sci. 2003;82:360–363. doi: 10.1093/ps/82.3.360. [DOI] [PubMed] [Google Scholar]
- Broderick T.J., Duong T. Mechanisms of Lactobacillus persistence and colonization in the gastrointestinal tract of poultry, a review. Int. J. Probiotics Prebiotics. 2016;11:15–28. [Google Scholar]
- Chung C., Day D. Efficacy of Leuconostoc mesenteroides (ATCC 13146) isomaltooligosaccharides as a poultry prebiotic. Poult. Sci. 2004;83:1302–1306. doi: 10.1093/ps/83.8.1302. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress . Cobb-Vantress, Inc.; Siloam Springs, AR, USA: 2018. Cobb Broiler Management Guide. [Google Scholar]
- Cogliani C., Goossens H., Greko C. Restricting antimicrobial use in food animals: lessons from Europe. Microbe. 2011;6:274. [Google Scholar]
- Corrier D.E., Byrd J.A., Hargis B.M., Hume M.E., Bailey R.H., Stanker L.H. Presence of Salmonella in the crop and ceca of broiler chickens before and after preslaughter feed withdrawal. Poult. Sci. 1999;78:45–49. doi: 10.1093/ps/78.1.45. [DOI] [PubMed] [Google Scholar]
- Dallies N., Francois J., Paquet V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast. 1998;14:1297–1306. doi: 10.1002/(SICI)1097-0061(1998100)14:14<1297::AID-YEA310>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Day C.J., Tiralongo J., Hartnell R.D., Logue C.-A., Wilson J.C., von Itzstein M., Korolik V. Differential carbohydrate recognition by Campylobacter jejuni strain 11168: influences of temperature and growth conditions. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, Food and Drug Administration Veterinary feed directive, final rule. 21 CFR Parts 514 and 518. Fed. Regis. 2015;80:31708–31735. [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Emborg H.-D., Ersbøll A.K., Heuer O.E., Wegener H.C. Proc. Beyond Antimicrobial Growth Promoters in Food Animal Production, Foulum, Denmark. 2002. Effects of termination of antimicrobial growth promoter use for broiler health and productivity; pp. 51–56. [Google Scholar]
- Fernandez F., Sharma R., Hinton M., Bedford M.R. Diet influences the colonisation of Campylobacter jejuni and distribution of mucin carbohydrates in the chick intestinal tract. Cell. Mol. Life Sci. 2000;57:1793–1801. doi: 10.1007/PL00000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C.A., Duong T., Augspurger N., Lee J.T. Efficacy of Bacillus subtilis administered as a direct-fed microorganism in comparison to an antibiotic growth promoter and in diets with low and high DDGS inclusion levels in broiler chickens. J. Appl. Poult. Res. 2019 doi: 10.3382/japr/pfz048. [DOI] [Google Scholar]
- Gaskins H., Collier C., Anderson D. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Goh Y.J., Klaenhammer T.R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Ann. Rev. Food Sci. Tech. 2015;6:137–156. doi: 10.1146/annurev-food-022814-015706. [DOI] [PubMed] [Google Scholar]
- Gómez S., Angeles M., Mojica M., Jalukar S. Combination of an enzymatically hydrolyzed yeast and yeast culture with a direct-fed microbial in the feeds of broiler chickens. Asian-Australas. J. Anim. Sci. 2012;25:665. doi: 10.5713/ajas.2011.11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizard D., Barthomeuf C. Non-digestible oligosaccharides used as prebiotic agents: mode of production and beneficial effects on animal and human health. Reprod. Nutr. Dev. 1999;39:563–588. doi: 10.1051/rnd:19990505. [DOI] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerchove D., Rollier I., De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 2003;131:1169–1180. doi: 10.1017/s0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooge D.M. Meta-analysis of broiler chicken pen trials evaluating dietary mannan oligosaccharide, 1993–2003. Int. J. Poult. Sci. 2004;3:163–174. [Google Scholar]
- Huff G.R., Huff W.E., Jalukar S., Oppy J., Rath N.C., Packialakshmi B. The effects of yeast feed supplementation on turkey performance and pathogen colonization in a transport stress/Escherichia coli challenge. Poult. Sci. 2013;92:655–662. doi: 10.3382/ps.2012-02787. [DOI] [PubMed] [Google Scholar]
- Hume M.E. Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 2011;90:2663–2669. doi: 10.3382/ps.2010-01030. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Lev M., Briggs C., Coates M.E. The gut flora of the chick: 3*. Differences in caecal flora between ‘infected’, ‘uninfected’ and penicillin-fed chicks. Br. J. Nutr. 1957;11:364–372. doi: 10.1079/bjn19570057. [DOI] [PubMed] [Google Scholar]
- Li X., Qiang L., Xu C. Effects of supplementation of fructooligosaccharide and/or Bacillus subtilis to diets on performance and on intestinal microflora in broilers. Arch. Anim. Breeding. 2008;51:64–70. [Google Scholar]
- Montrose M.S., Shane S.M., Harrington K.S. Role of litter in the transmission of Campylobacter jejuni. Avian Dis. 1985;29:392–399. [PubMed] [Google Scholar]
- Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Moore P., Evenson A., Luckey T., McCoy E., Elvehjem C., Hart E. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946;165:437–441. [PubMed] [Google Scholar]
- Nahashon S., Nakaue H., Mirosh L. Production variables and nutrient retention in Single Comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult. Sci. 1994;73:1699–1711. doi: 10.3382/ps.0731699. [DOI] [PubMed] [Google Scholar]
- Oyofo B., DeLoach J., Corrier D., Norman J., Ziprin R., Mollenhauer H. Prevention of Salmonella Typhimurium colonization of broilers with D-mannose. Poult. Sci. 1989;68:1357–1360. doi: 10.3382/ps.0681357. [DOI] [PubMed] [Google Scholar]
- Patterson J., Burkholder K. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Nielsen N.L., Sommer H.M., Nørrung B., Christensen B.B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Micrbiol. 2003;83:87–103. doi: 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Sahin O., Kassem I.I., Shen Z., Lin J., Rajashekara G., Zhang Q. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- Salianeh N., Shirzad M., Seifi S. Performance and antibody response of broiler chickens fed diets containing probiotic and prebiotic. J. Appl. Anim. Res. 2011;39:65–67. [Google Scholar]
- Silbergeld E.K., Graham J., Price L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Spring P., Wenk C., Dawson K., Newman K. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000;79:205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- Stutz M., Johnson S., Judith F. Effects of diet and bacitracin on growth, feed efficiency, and populations of Clostridium perfringens in the intestine of broiler chicks. Poult. Sci. 1983;62:1619–1625. doi: 10.3382/ps.0621619. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J.E., Walker W.A. Nutritional impact of pre-and probiotics as protective gastrointestinal organisms. Annu. Rev. Nutr. 2002;22:107–138. doi: 10.1146/annurev.nutr.22.110901.145412. [DOI] [PubMed] [Google Scholar]
- Torres-Rodriguez A., Higgins S.E., Vicente J.L.S., Wolfenden A.D., Gaona-Ramirez G., Barton J.T., Tellez G., Donoghue A.M., Hargis B.M. Effect of lactose as a prebiotic on turkey body weight under commercial conditions. J. Appl. Poult. Res. 2007;16:635–641. [Google Scholar]
- Vandeplas S., Dauphin R.D., Beckers Y., Thonart P., Thewis A. Salmonella in chicken: current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010;73:774–785. doi: 10.4315/0362-028x-73.4.774. [DOI] [PubMed] [Google Scholar]
- Visek W.J. The mode of growth promotion by antibiotics. J. Anim. Sci. 1978;46:1447–1469. [Google Scholar]
- Walker G.K., Jalukar S., Brake J. Effect of refined functional carbohydrates from enzymatically hydrolyzed yeast on the presence of Salmonella spp. in the ceca of broiler breeder females. Poult. Sci. 2017;96:2684–2690. doi: 10.3382/ps/pex054. [DOI] [PubMed] [Google Scholar]
- Walker G.K., Jalukar S., Brake J. The effect of refined functional carbohydrates from enzymatically hydrolyzed yeast on the transmission of environmental Salmonella Senftenberg among broilers and proliferation in broiler housing. Poult. Sci. 2018;97:1412–1419. doi: 10.3382/ps/pex430. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009;65:97–114. [Google Scholar]
- Yang Y., Iji P.A., Kocher A., Mikkelsen L.L., Choct M. Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br. Poult. Sci. 2008;49:550–559. doi: 10.1080/00071660802290408. [DOI] [PubMed] [Google Scholar]


