Abstract
The poultry industry has recently undergone transitions into antibiotic free production, and viable antibiotic alternatives, such as probiotics, are necessary. Through in ovo probiotic inoculation, beneficial microflora development in the gastrointestinal tract may occur prior to hatch without negatively impacting chick performance. Therefore, the objective of the present study was to observe the impacts of the injection of probiotic bacteria individually or combined into fertile broiler hatching eggs on hatch and live performance characteristics. A total of 2,080 fertile broiler hatching eggs were obtained from a commercial source. On day 18 of incubation, 4 in ovo injected treatments were applied: 1.) Marek's Disease (HVT) vaccination, 2.) L. animalis (∼106 cfu/50μl), 3.) E. faecium (∼106 cfu/50μl), and 4.) L. animalis + E. faecium (∼106 cfu & ∼106 cfu/50μl each). On day of hatch, hatchability and hatch residue data were recorded. A portion of male chicks from each treatment were placed in a grow-out facility for a 21 d grow-out (18 chicks/pen × 10 pens/treatment = 720 male chicks) with a corn and soy bean meal-based diet without antibiotics or antibiotic alternatives. Performance data and gastrointestinal samples were collected on days 0, 7, 14, and 21. Results indicated no differences in all hatch parameters between treatments (P > 0.05) except for % pipped, where the L. animalis treatment had lower % pipped eggs compared to the HVT control and E. faecium treatments (P = 0.04). No differences were observed in body weight gain or mortality (P > 0.05). Probiotic treatments altered gastrointestinal tissue length, weight, and pH. This resulted in all in ovo injected probiotic treatments increasing feed conversion ratio (FCR) from days 7 to 14 as compared to the control (P = 0.01). Differences in FCR were not observed in any other week of data collection (days 0 to 7, 14 to 21, or 0 to 21; P > 0.05). Although probiotics altered live performance from days 7 to 14, these data suggest that in ovo inoculations of L. animalis and E. faecium in combination are viable probiotic administration practices that potentially improve hatch characteristics and gastrointestinal tract development.
key words: in ovo injection, chick mortality, probiotic, broiler, hatchability
INTRODUCTION
Probiotic applications in the poultry industry have grown in popularity in recent years. Due to extensive research, these studies observed improvements to broiler performance, modulation of the immune system, and reduction in pathogens within the gastrointestinal tract (Haghighi et al., 2006; Kabir, 2009; Pender et al., 2017). Commercially, probiotics have the potential to prevent gastrointestinal diseases and promote the colonization of beneficial bacteria within the gut. This colonization by probiotic products, referred to as competitive exclusion, promotes the presence of beneficial bacteria in the gastrointestinal tract thus preventing or reducing the presence of pathogenic bacteria. Probiotic applications have been proven to alter the intestinal microflora which can, in turn, elicit an immune response (Kabir, 2009; Pender et al., 2017). It has been observed that the introduction of probiotics to chicks immediately after hatch has stimulated the production of natural antibodies, stimulated immune-related gene expression and reduced pathogen presence within the gastrointestinal tract (Haghighi et al., 2006; de Oliveira et al., 2014; Slawińska et al., 2014; Ploweic et al., 2015).
There are many applied species of probiotics, including Lactobacillus animalis and Enterococcus faecium. Lactobacillus spp. are gram positive, non-spore forming cocci that secrete lactic acid into their external environment (Tannock, 1992). Previous studies have found that lactic acid production by Lactobacillus spp. regulates environmental pH to create an environment incompatible with many pathogenic bacteria species (Hutkins and Nannen, 1993). Additionally, Lactobacillus spp. are noted for high adhesion to the epithelial lining of the gastrointestinal tract (Tannock, 1992; Ehrmann et al., 2002; Barrangou et al., 2012). In broilers, large populations of Lactobacillus spp. have been identified in the duodenum, jejunum, and ileum of the small intestines (Lu et al., 2003). Enterococcus spp. are gram positive, non-spore forming cocci that produce lactic acid (Tannock, 1992). Enterococci have also been found to secrete bacteriocins capable of inhibiting microbial activity in the bacteria surrounding it (Tannock, 1992; Araujo and Ferreira, 2013; Ness et al., 2014). Enterococcins, or bacteriocins secreted by Enterococcus spp., include gelatinase, cytolysin and hyaluronidase; all of these enterococcins play an integral role in degrading cell walls (Franz et al., 2007). Enterococcus species have been previously identified in the ileum of the small intestines of broiler chickens (Lu et al., 2003).
Presently, probiotics are mostly utilized through oral consumption in the poultry industry, and this application method has observed improvements in growth performance and gastrointestinal tract morphology (Yeo and Kim, 1997; Awad et al., 2009; Jeong and Kim, 2014; Bai et al., 2017). However, there are limitations with using probiotics as feed additives. Fuller (1989) proposes that the avian species obtains its microflora from the nest it hatches in; however, a broiler chick can be exposed to pathogenic bacteria upon hatch without contacting beneficial bacteria commonly found in the nest prior to placement in a house. This modern method of animal rearing may be hindering the natural development of the chick's microflora (Fuller, 1989). In the commercial hatchery, many pathogenic bacteria inhabit contact surfaces and the air which may potentially impact chick health (Kim and Kim, 2010). Therefore, a new method of early probiotic delivery through the use of commercial in ovo technology may provide a preliminary step towards the chick establishing a healthy microflora before hatch, thereby reducing the impact environmental pathogens have on the growth potential of the chick. To effectively utilize this technology, research is necessary to determine the combinations of probiotic bacteria species required to properly develop the gastrointestinal microflora.
Previous studies indicate that in ovo probiotic injections may improve flock health (Cox et al., 1992; Meijerhof and Hulet, 1997; de Oliveira et al., 2014; Triplett et al., 2018); however, it is necessary to evaluate the effects that different probiotic species have on hatchability, post-hatch live performance, and chick health. Although many experiments have studied the in ovo inoculation of probiotics, these studies have small experimental units, inoculate eggs manually, and utilize methodologies inapplicable in an industry setting (de Oliveira et al., 2014; Pender et al., 2017; Teague et al., 2017). This study evaluates the in ovo injection of Enterococcus faecium and Lactobacillus animalis, individually and in combination, using commercial Inovoject® technology. Both probiotic cultures have been commonly observed as commensal bacteria within the crop, gizzard, duodenum, jejunum and ileum of birds (Ranjitkar et al., 2016). Therefore, this study observed the effects of an in ovo injected probiotic combination on performance parameters such as hatch of transfer, chick weight, gastrointestinal tissue (GIT) weights, GIT lengths, GIT pH, feed conversion and body weight gain during the first 21 d of growth.
MATERIALS AND METHODS
In vitro Analysis of Bacterial Compatibility between L. animalis and E. faecium Cultures
Before determining the compatibility of the 2 cultures, growth at 12 and 24 h of incubation at 37°C in selective media was measured for each probiotic bacteria individually. For L. animalis, the culture was grown anaerobically in De Man, Rogosa and Sharpe Broth (MRS; Millipore Sigma, St. Louis, MO) at 37°C and spread on MRS agar plates. For E. faecium, the culture was grown aerobically in Tryptic Soy Broth (TSB; Millipore Sigma, St. Louis, MO) at 37°C and spread on Bile Esculin agar (BEA; Millipore Sigma, St. Louis, MO) plates. Desirable incubation time where growth reached at least 106 cfu/mL for each culture was determined to be 12 h. In all in vitro procedures, standard plate count procedures were followed. Plates were counted when colony growth for a 10-fold dilution was within the range of 30 to 300 colonies per plate. All cultures were plated and grown in duplicate, with average cfu/mL calculated as an average of the duplicated plates.
After a bacteria growth curve was defined for each culture, the compatibility of L. animalis and E. faecium when grown in the same environment was assessed. To accomplish this, 1 mL from a 10 mL L. animalis stock culture that was incubated anaerobically in MRS broth for 24 h at 37°C and 1 mL of E. faecium from a 10 mL stock culture incubated aerobically in TSB for 24 h at 37°C were inoculated into 8 mL of fresh TSB. Two separate combination cultures containing the same amount of each inoculum were created (2 tubes containing 10 mL of TSB broth inoculated with L. animalis and E. faecium). The combined probiotic bacteria culture, containing 1 mL E. faecium culture and 1 mL L. animalis culture, was incubated for 12 h at 37°C. One tube was incubated aerobically for 12 h at 37°C and the other was incubated anaerobically for 12 h at 37°C. After the 12 h incubation period, a 10-fold serial dilution was conducted from the incubated cultures. From each tube, the combined culture was spread on BEA and MRS agar plates and incubated for 24 h at 37°C, where both agar types were grown in aerobic and anaerobic conditions. Aerobically, E. faecium achieved 106 cfu/mL on BEA agar. Anaerobically, L. animalis achieved 106 cfu/mL on MRS agar. After determining the growth curves for the individual cultures and determining the compatibility of the cultures when grown in the same environment, it was determined that both probiotic types can coexist compatibly and yield a 106 cfu/ml concentration in their respective aerobic and anaerobic environments, therefore the experiment proceeded to the in vivo trial.
Preparation of the Applied Treatments
The applied treatments are as follows: 1.) Marek's Disease (HVT) vaccination (control), 2.) L. animalis (∼106 cfu/50μl) + HVT vaccination, 3.) E. faecium (∼106 cfu/50μl) + HVT vaccination, and 4.) a combination of L. animalis and E. faecium (∼106 cfu & ∼106 cfu/50μl, respectively) + HVT vaccination. Treatments were prepared by incubating L. animalis anaerobically and E. faecium aerobically in their respective broths (MRS and TSB, respectively) for 12 h at 37°C the day prior to injection. Enough inoculated broth was prepared of each probiotic bacteria to ensure the injection of at least 106 cfu/50 µl in ovo injection.
The amount of bacteria culture needed was calculated in reference to the 800 mL sterile diluent bag (Merial Select, Inc., Gainesville, GA) used during the egg injection process. In a 800 mL diluent bag, it was calculated that an individual injection volume of 50 µ l yields 16,000 total injections. From the growth curve conducted previously, the target growth for the bacteria culture after 12 h of incubation is 106 cfu/mL. Therefore, each vaccination would have a bacteria concentration of at least 1.0 × 106 cfu/mL. Under this presumption, the total amount of bacterial culture needed for the 800 mL diluent is 1.6 × 1010 cfu/mL (16,000 injections × 1.0 × 106 cfu/50 µ L injection = 1.6 × 1010 cfu/800 mL). The total inoculated broth necessary to achieve a 106 cfu/50 µ L injection was calculated by dividing the bacteria concentration needed for injection by the determined growth curve. For L. animalis, the average growth at 12 h was 6.0 × 107 cfu/mL. Therefore, 266.7 mL of inoculated MRS broth was necessary for the L. animalis treatment (1.6 × 1010 cfu/mL/6.0 × 107 cfu/mL). For E. faecium, the average growth at 12 h was 5.7 × 108 cfu/mL. Therefore, 28.1 mL of inoculated TSB was necessary for the E. faecium treatment (1.6 × 1010 cfu/mL/5.7 × 108 cfu/mL). Two sets of each culture volume were inoculated, where one culture volume was designated for the single probiotic treatment and the other volume was designated for the combination treatment.
On the morning of the in ovo probiotic injection day, the probiotic cultures were added to the diluent bag after 12 h incubation at 37°C. To do this, all tubes of cultures were centrifuged at 4000 rpm at 4°C for 10 min to obtain a pellet. After centrifugation, all broth is removed from the formed pellet. Using a sterile needle and syringe, 1 mL diluent is drawn from the diluent bag and added to the pellet for reconstitution. The reconstituted pellet is then added to its respective diluent bag. One sterile diluent bag was designated for each treatment (4 diluent bags, total). Standard HVT vaccine (4000 doses/800 mL; Merial Select, Inc., Gainesville, GA) was added to the each diluent bag, with sterile needles and syringes changed between treatments. Inoculated diluent bags were stored at 4°C until use.
Egg Incubation
A total of 2,080 fertile Ross 708 broiler hatching eggs from a 44 wk old broiler-breeder flock were purchased from a commercial hatchery. Eggs were stored for 3 d at 21°C until setting. Eggs were assessed for cracked or misshaped shells and individually labelled by egg number, flat, and treatment where 18 flats (∼30 eggs per flat; 520 eggs total per treatment) were assigned to each treatment. To set eggs for incubation, flats were arranged in 2 NatureForm® incubator units (NatureForm Hatchery Technologies, Jacksonville, FL) where each treatment was equally represented in each unit. Incubator temperature was set at 37.5°C, and relative humidity was set at 55.0%. On day 10, all eggs were candled to identify and remove infertile, cracked, and contaminated eggs to ensure that only fertilized eggs were to be injected. On day 18, 4 treatments were applied using commercial Inovoject® equipment (Zoetis, Parsippany, NJ), eggs were transferred to hatching baskets, and set into 1 of 12 Georgia Quail Farm® hatcher units (3 GQF hatchers/treatment; Georgia Quail Farm, Savannah, GA). Each GQF unit contained 170 eggs. Incubator and hatcher disinfection was completed using a 10% Lysol solution.
Injection Procedure
On day 18 of incubation, commercial Inovoject® technology was utilized to apply treatments to the developing eggs. Embryo staging was conducted during the injection process where 1 egg was collected from 20 different flats through out the 2 NatureForm® incubator units (5 eggs/treatment, each belonging to a different flat in each treatment; 20 eggs total) for an analysis of embryo development to ensure that the injected eggs were at the appropriate stage of embryonic development. All other eggs proceeded to receive their respective in ovo probiotic treatment. However, the selected eggs for embryo staging (20 eggs total) were injected with 50 µ l Coomassie blue dye solution (Thermo Fisher Scientific, Waltham, MA) and immediately euthanized via CO2 asphyxiation. For the remaining eggs, the treatments applied are the same as those listed in “Preparation of the Applied Treatments ”. Every treatment was prepared in 800 mL of sterile diluent prior to injection (Merial Select Inc., Gainesville, GA). One flat was injected at a time, with all eggs in the flat injected at once. Immediately after injection, eggs were placed in the hatcher unit. A sanitation cycle recommended by Zoetis was completed between each treatment, and microbial samples were collected on Tryptic Soy agar (TSA; Millipore Sigma, St. Louis, MO) after each sanitation cycle to ensure that no contamination was occurring within the in ovo equipment. Eggs were set in a manner to prevent contamination between treatments: each treatment utilized 3 GQF hatchers (12 GQFs total).
Hatch Procedure
On day of hatch, hatched chicks were counted, weighed, and sexed by wing feather sexing (“Ross Broiler Management Manual”, 2009). Hatch residue analysis was conducted where unhatched eggs were counted and classified as early dead, mid dead, late dead, infertile, contaminated, or cracked according to Aviagen egg break-out guidelines (“How to… Break Out and Analyse Hatch Debris,” 2017). Male chicks from the hatch were placed in pens of 18 chicks/pen according to treatment (10 pens/treatment) in a grow-out facility at a stocking density of 0.20 m2/chick. Research pens in the house were arranged to prevent cross contamination, where no pen had contact with the other pens around it.
Grow-out and Sampling
A 21 d grow-out was carried out after hatch on used, windrowed litter from a commercial broiler house. An industry standard basal diet which met Ross 708 nutrient guidelines in crumble form was provided to birds in the starter (days 0 to 14) and grower (days 14 to 21) phases. The starter and grower diets were crumble diets consisting of corn, soy bean meal and poultry fat based on Ross 708 guidelines and did not contain antibiotics, antibiotic alternatives or anticoccidials (“Ross 708 Nutrition Specifications”, 2014). Feed and water were supplied ad libitum. Chick mortality, body weight gain, and feed consumption were obtained on days 0, 7, 14, and 21 of the grow-out. GIT samples were collected on days 0, 7, 14, and 21 of the grow-out (10 birds/treatment for each sampling day). The pH of the crop, gizzard, duodenum, jejunum, ileum, and ceca were measured with digesta contents on days 7, 14, and 21. Tissue weights with digesta for the duodenum, jejunum, ileum, ceca, bursa, and spleen were sampled. All animals in this trial were treated in compliance with the Guide for the Care and Uses of Agriculture Animals in Research and Teaching (Federation of Animal Science Societies, 2010) and the Mississippi State University Institutional Animal Care and Use Committee (IACUC Animal Welfare Assurance #A3160-01).
Statistical Analysis
All data were analyzed using SAS v.9.4 (SAS Institute, Cary, NC). Incubation data were analyzed using a completely randomized design. The experimental unit was GQF hatcher unit (3 GQFs/treatment). Live performance data were analyzed using a randomized complete block design with a split plot over time. The experimental unit was pen (10 pens/treatment). Means were separated using Fisher's Protected LSD and differences were considered significant when P ≤ 0.05 (Steel and Torrie, 1980).
RESULTS
Verification of Injection Procedures
Embryo staging conducted after the injection procedure on day 18 of incubation determined that the injection procedure was accurate. Embryos were at day 18 of development with 3 lobes in the yolk sac and the intestines were enclosed in the body cavity. Also, the coomassie blue dye applied for embryo staging was on the feathers of the embryo, which confirmed that the injection was in the amnion. Probiotic concentrations for each treatment were as follows: no bacterial growth in the diluent treatment, 3.1 × 109 cfu/50 μl of L. animalis and 0 cfu/50 μl of E. faecium in the L. animalis treatment, 0 cfu/50μl of L. animalis and 5.4 × 106 cfu/50μl of E. faecium in the E. faecium treatment, and 3.0 × 107 cfu/50 μl of L. animalis and 4.0 × 106 cfu/50 μl of E. faecium for the combination treatment.
Hatch Parameters
No difference in hatch of transfer was observed between the L. animalis, the combination, the HVT control, and E. faecium treatments (P = 0.65; Table 1). During the hatch residue analysis, no significant differences were found in percent late dead, cracked, contaminated or cull eggs (P ≥ 0.05; Table 1). There was a significant difference in percent pipped eggs between treatments (P = 0.039; Table 1) where eggs in the HVT control, E. faecium, and the combination treatments were not different from each other; however, they were significantly greater than the eggs of the L. animalis treatment (Table 1). There were no significant differences in average chick weight between the applied treatments (P = 0.39; Table 1).
Table 1.
Effect of in ovo injected L. animalis, E. faecium, or their combination on hatch parameters.
| Treatments | HVT Control | L. animalis | E. faecium | Combination | SEM | P value |
|---|---|---|---|---|---|---|
| % hatch of transfer | 93.37 | 94.62 | 93.24 | 93.52 | 1.09 | 0.6498 |
| % late dead eggs | 4.43 | 4.93 | 4.05 | 3.10 | 1.34 | 0.2309 |
| % pipped eggs | 1.98a | 0b | 1.58a | 1.47a | 1.50 | 0.0389 |
| % cracked eggs | 0.22 | 0 | 0.45 | 0 | 0.37 | 0.2175 |
| % contaminated eggs | 0 | 0 | 0.45 | 0.58 | 0.52 | 0.4635 |
| % cull eggs | 0 | 0 | 0 | 0 | . | . |
| Avg Chick Weight (g) | 46.2 | 45.9 | 46.2 | 45.4 | 3.60 | 0.3900 |
Different superscripts (a — b) indicate significant differences in the means of treatments, where differences are considered significant at P ≤ 0.05. N = 3, where each replicate in the treatment was a GQF hatcher unit (∼170 eggs/GQF; 520 total eggs/treatment).
Live Performance Parameters
For all phases of the 21 d grow-out, there were no significant differences in mortality or live weight gain between all treatments (P > 0.05; Table 2). There was a difference in feed conversion ratio (FCR) only in the day 7 to 14 phase, where the chicks in the E. faecium and the combination treatments yielded greater FCR than the chicks in the HVT control treatment. However, no treatments were significantly different from the L. animalis treatment (P = 0.01; Table 2). Unlike the days 7 to 14 phase, no significant differences in FCR were observed among treatments on days 0 to 7, 14 to 21, or 0 to 21 phases (P > 0.05; Table 2)
Table 2.
Effect of in ovo injected L. animalis, E. faecium, or their combination on live performance parameters for days 0 to 21.
| Phase | Parameter measured | HVT Control | L. animalis | E. faecium | Combination | SEM | P value |
|---|---|---|---|---|---|---|---|
| Days 0 to 7 | Live Weight Gain/Bird (kg) | 0.09 | 0.10 | 0.09 | 0.09 | 0.03 | 0.8283 |
| Feed Conversion Ratio | 1.30 | 1.28 | 1.31 | 1.30 | 0.03 | 0.9639 | |
| Mortality | 0.56 | 0.62 | 1.23 | 2.22 | 0.91 | 0.5030 | |
| Days 7 to 14 | Live Weight Gain/Bird (kg) | 0.23 | 0.22 | 0.22 | 0.21 | 0.01 | 0.2114 |
| Feed Conversion Ratio | 1.29b | 1.31a,b | 1.34a | 1.35a | 0.01 | 0.0096 | |
| Mortality | 0.00 | 0.65 | 0.00 | 0.00 | 0.31 | 0.3913 | |
| Days 14 to 21 | Live Weight Gain/Bird (kg) | 0.39 | 0.38 | 0.40 | 0.39 | 0.01 | 0.5091 |
| Feed Conversion Ratio | 1.42 | 1.47 | 1.42 | 1.47 | 0.03 | 0.4692 | |
| Mortality | 0.63 | 0.67 | 0.67 | 0.71 | 0.64 | 0.8086 | |
| Day 0 to 21 | Live Weight Gain/Bird (kg) | 0.72 | 0.70 | 0.71 | 0.69 | 0.02 | 0.6485 |
| Feed Conversion Ratio | 1.37 | 1.39 | 1.40 | 1.43 | 0.02 | 0.1558 | |
| Mortality | 1.11 | 1.23 | 1.85 | 2.78 | 1.32 | 0.7655 |
Different superscripts (a — b) indicate significance between treatments within the performance parameter, where differences are considered significant at P ≤ 0.05. N = 10, where each replicate in the treatment is a pen (18 birds/pen; 180 total birds/treatment).
Tissue Weight Relative to Live Body Weight
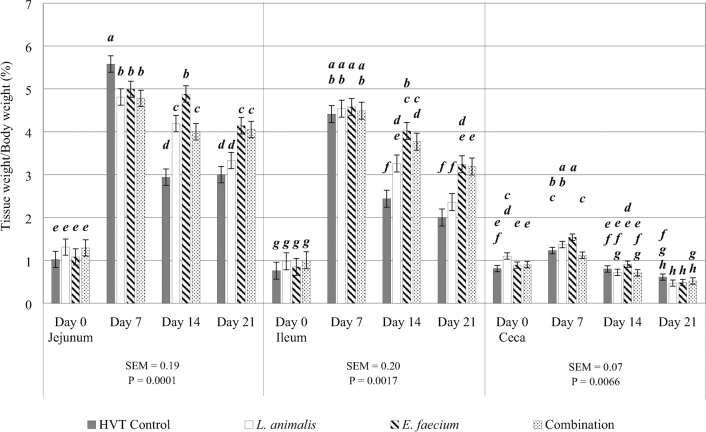
Treatment by day interactions for tissue weight relative to body weight were observed in the jejunum, ileum, and ceca (P = 0.0001, 0.002, and 0.007, respectively; Figure 1). No significant differences between treatments were observed in the jejunum on day 0 or in the ileum on days 0 and 7. However, jejunum and ileum weights were significantly greater in all probiotic treatments when compared to the control treatment on day 14. Similarly, chicks in ovo injected with E. faecium or the combination both observed greater jejunum and ileum weights on day 21. For the ceca, chicks in ovo injected with L. animalis obtained greater ceca weights relative to body weight on day 0. On day 7, chicks in the E. faecium treatment observed greater ceca weights when compared to chicks of the control and combination treatments. No difference in ceca weights among treatments was observed after day 7.
Figure 1.
Treatment by day interactions were observed in the jejunum, ileum, and ceca for tissue weight relative to live body weight. The HVT control injection is represented by the gray shaded bar. The L. animalis injected probiotic treatment is represented by the white shaded bar. The E. faecium injected probiotic treatment is represented by the striped bar. The L. animlais + E. faecium combination injected probiotic treatment is represented by the dotted bar. Tisssue weight relative to total body weight (%) is on the y-axis. Tissue sampled (jejunum, ileum, and ceca) and day of sampling (days 0, 7, 14, and 21) are on the x-axis. SEM and P values are located underneath each of their respective tissues. Differences were considered significant at P ≤ 0.05, error bars for each tissue represent the SEM for that tissue, and N = 10 (10 pens/treatment; one bird randomly sampled from each pen on each sampling day). Although all tissues with a treatment by day interaction for tissue weight relative to body weight are provided on the same figure, the analysis for the treatment by day interaction is by individual tissues. Significant differences are noted by alphabetical superscripts, where each change in a letter represents a significant difference among treatments. Jejunum superscripts are a—e, ileum superscripts are a—g, and ceca superscripts are a—h.
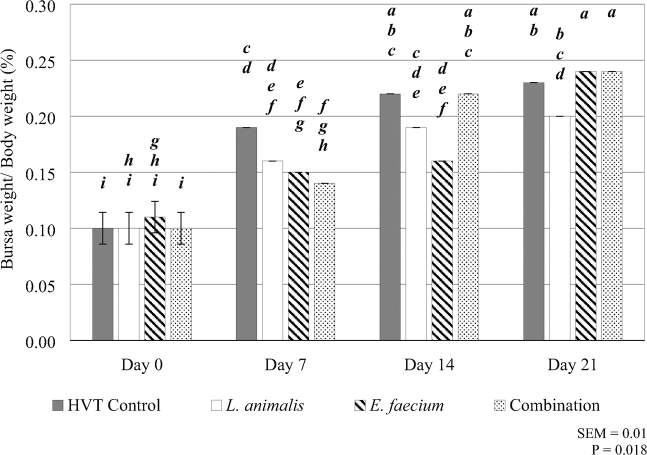
Significant treatment by day interactions were also observed in bursa weight relative to body weight where chicks in ovo injected with E. faecium or the combination both obtained smaller bursa weights relative to body weight on day 7 when compared to those injected with the HVT control. By day 21, chicks in ovo injected with E. faecium or the combination treatments both yielded significantly larger average bursa weights when compared to the in ovo injection of L. animalis (P = 0.02; Figure 2).
Figure 2.
A treatment by day interaction was observed for Bursa of Fabricius weight relative to live body weight. The HVT control injection is represented by the gray shaded bar. The L. animalis injected probiotic treatment is represented by the white shaded bar. The E. faecium injected probiotic treatment is represented by the striped bar. The L. animlais + E. faecium combination injected probiotic treatment is represented by the dotted bar. Bursa weight relative to total body weight (%) is on the y-axis, and day of grow-out (days 0, 7, 14, and 21) is on the x-axis. The SEM and P value is located in the bottom right corner of the figure. Differences were considered significant at P ≤ 0.05, error bars represent the SEM, and N = 10 (10 pens/treatment; one bird randomly sampled from each pen on each sampling day). Significant differences are noted by alphabetical superscripts, where each change in a letter represents a significant difference among treatments. The superscripts for this figure include a—i.
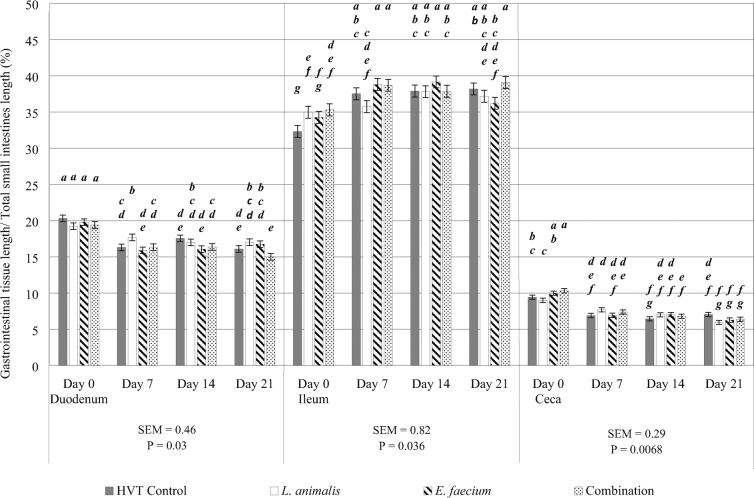
Tissue Length Relative to Small Intestines Length
There were significant treatment by day interactions for tissue length relative to total small intestines length observed in the duodenum, ileum and ceca (P = 0.03, 0.04 and 0.007, respectively; Figure 3). Although no differences were observed between treatments on days 0 or 14 for duodenum length, chicks in ovo injected with L. animalis obtained significantly longer duodenum lengths on day 7 when compared to all treatments on that day. Concurrently, the chicks in ovo injected with the combination treatment observed shorter relative duodenum lengths when compared to those injected with L. animalis or E. faecium on day 21. Unlike the duodenum, the in ovo injection E. faecium or the combination both yielded significantly longer tissues in the ileum when compared to the birds injected with the HVT control on day 0. On day 7, the relative ileum lengths in E. faecium and the combination treatments were significantly longer than L. animalis treatments. Similar to the relative duodenum length, no differences in relative ileum lengths were observed on day 14. Unlike the duodenum, the in ovo injection of the combination yielded chicks with significantly longer ileums when compared to those who received the E. faecium injection on day 21. However, ileum lengths in the E. faecium and combination treatments on day 21 were not different from L. animalis or the control relative ileum lengths on day 21. In the ceca, the birds from the combination yielded longer relative ceca tissues when compared to the HVT control and L. animalis treatments. However, no other treatment by day interactions were observed in the ceca on days 7, 14, or 21.
Figure 3.
Treatment by day interactions were observed in the duodenum, ileum, and ceca for tissue length relative to small intestines length. The HVT control injection is represented by the gray shaded bar. The L. animalis injected probiotic treatment is represented by the white shaded bar. The E. faecium injected probiotic treatment is represented by the striped bar. The L. animlais + E. faecium combination injected probiotic treatment is represented by the dotted bar. Tisssue length relative to total small intestines length (%) is on the y-axis. Tissue sampled (duodenum, ileum, and ceca) and day of sampling (days 0, 7, 14, and 21) are on the x-axis. SEM and P values are located underneath each of their respective tissues. Differences were considered significant at P ≤ 0.05, error bars for each tissue represent the SEM for that tissue, and N = 10 (10 pens/treatment; 1 bird randomly sampled from each pen on each sampling day). Although all tissues with a treatment by day interaction for tissue length relative to small intestines length are provided on the same figure, the analysis for the treatment by day interaction is by individual tissues. Significant differences are noted by alphabetical superscripts, where each change in a letter represents a significant difference among treatments. Duodenum superscripts are a—e, ileum superscripts are a—g, and ceca superscripts are a—g.
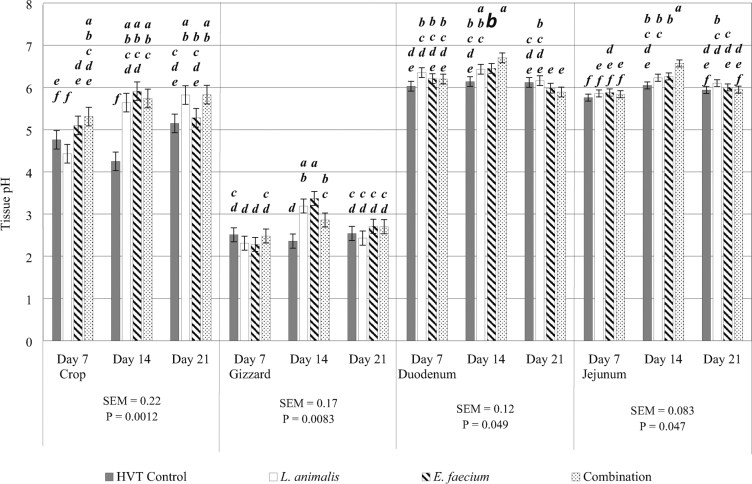
Tissue pH
Treatment by day interactions were also observed in crop, gizzard, duodenum and jejunum pH (P = 0.001, 0.008, 0.05, and 0.05, respectively; Figure 4). On day 7, no treatment differences were observed in the gizzard, duodenum or jejunum. However, it was observed that chicks in ovo injected with L. animalis obtained lower pH in the crop when compared to chicks in ovo injected with E. faecium and combination treatments. The crop and gizzard also observed decreased pH levels in the chicks in the HVT control treatment when compared to the birds of all probiotic treatments on days 14 and 21. Also, the gizzard pH on day 14 in chicks in ovo injected with the combination was not different from those of the L. animalis treatment but significantly lower than chicks in the E. faecium treatment. However, the HVT control treatment yielded a significantly lower pH in the gizzard on day 14 when compared to all probiotic treatments. The pH of the duodenum in birds who received the combination treatment on day 14 was greater than those of the HVT control treatment, but they were not different from the duodenum pH of the L. animalis or E. faecium treatments. The pH of the jejunum on day 14 was greater for birds who were in ovo injected with the combination treatment when compared to all other treatments. On day 21, no significant differences in tissue pH were observed in the gizzard, duodenum and jejunum. However, chicks in ovo injected with L. animalis or the combination had a significantly elevated pH in the crop on day 21 when compared to the control HVT injection. Crop pH in the L. animalis or combination treatments were not different from E. faecium on day 21.
Figure 4.
Treatment by day interactions were observed in the crop, gizzard, duodenum, and jejunum for tissue pH. The HVT control injection is represented by the gray shaded bar. The L. animalis injected probiotic treatment is represented by the white shaded bar. The E. faecium injected probiotic treatment is represented by the striped bar. The L. animlais + E. faecium combination injected probiotic treatment is represented by the dotted bar. Tisssue pH is on the y-axis. Tissue sampled (crop, gizzard, duodenum, and jejunum) and day of sampling (days 7, 14, and 21) are on the x-axis. SEM and P values are located underneath each of their respective tissues. Differences were considered significant atP ≤ 0.05, error bars for each tissue represent the SEM for that tissue, and N = 10 (10 pens/treatment; one bird randomly sampled from each pen on each sampling day). Although all tissues with a treatment by day interaction for tissue pH are provided on the same figure, the analysis for the treatment by day interaction is by individual tissues. Significant differences are noted by alphabetical superscripts, where each change in a letter represents a significant difference among treatments. Crop superscripts are a—f, gizzard superscripts are a—d, duodenum superscripts are a—e, and jejunum superscripts are a—f.
DISCUSSION
Hatch Parameters
This experiment utilized commercial Inovoject® equipment to inject 2 different probiotic species, L. animalis and E. faecium, individually and in combination into fertile broiler hatching eggs. Other companies have created in ovo technology similar to Inovoject®, and this study provides insight into the applicability of in ovo probiotic injections using commercially available technology. Methodology such as this provides an industry perspective on the application of a probiotic during the standard vaccination of a vaccine. Although in ovo probiotic studies have been conducted, many of these studies consist of manual injection procedures that inject greater volumes of a diluent than the volume applied with commercial in ovo injection equipment (de Oliveira et al., 2014; Madej et al., 2015; Madej and Bednarczyk, 2016; Pender et al., 2017). Even so, previous research on in ovo probiotic inoculation has found that probiotics can have positive and negative impacts on chick performance while stimulating the immune system (Sławińska et al., 2014; Madej et al., 2015; Płoweic et al., 2015; Madej and Bednarczyk, 2016; Pender et al., 2017; Triplett et al., 2018).
Concerns do exist when injecting beneficial supplements, such as probiotics, into fertile broiler hatching eggs on day 18 of incubation. It has been found that the in ovo injections of probiotics and vaccines into fertile hatching eggs may have a negative impact on hatchability, but these results are dependent on the probiotic type and injection location (Cox et al., 1992; Meijerhof and Hulet, 1997; de Oliveira et al., 2014; Triplett et al., 2018). This negative impact may exist due to the injection process: puncturing the cuticle, shell, and membranes of an egg. This may then lead to pathogenic bacteria in the external environment gaining direct access to the embryo. Sanitary conditions, such as needle sterilization, are necessary to ensure that chicken embryos are not negatively affected by the inoculation process (Johnston et al., 1997).
In the present study, no negative impacts were evident from the injection procedure. It was observed that the L. animalis treatment significantly reduced percentage pipped eggs when compared to the control treatment. This indicates that it is possible to inject L. animalis into the amnion of an embryo on day 18 of embryonic development with potential to improve hatch performance. Previous research studying the impact of probiotic in ovo injections did not observe similar results (de Oliveira et al., 2014; Pender et al., 2017; Teague et al., 2017; Triplett et al., 2018). Probiotic species and the concentration being injected (Triplett et al., 2018) as well as the volume of the injection and methods of injection (de Oliveira et al., 2014; Pender et al., 2017; Teague et al., 2017) all potentially impact hatch performance. Because beneficial bacteria species have differing primary modes of action, the injection of one bacteria species into a fertile hatching egg may induce different biological responses in the chick when compared to another probiotic bacteria species.
Some of these biological responses may be linked to bacteriocin production by some probiotic species. Bacteriocins are bacteriocidal proteins commonly secreted by lactic acid producing bacteria species such as Lactobacillus and Enterococcus (Cintas et al., 2001). Lactic acid and bacteriocin production by many probiotic species are key components of pathogen reduction in the gastrointestinal tract of their host (Guerra et al., 2007; Mountzouris et al., 2007; Aliakbarpour et al., 2012). Through their differing modes of action, probiotics have been shown to improve performance for in-feed applications where it is introduced into the gastrointestinal tract of a maturing chick (Jeong and Kim, 2014; Bai et al., 2017). Through the supplementation of probiotics in ovo, prior to the chick ever contacting pathogenic bacteria in the external environment, it may be possible to initiate early colonization of beneficial bacteria species in the gut to prevent pathogen colonization and improve chick performance upon hatch (Ballou et al., 2016). However, the same bacteria species injected in ovo may not exhibit synergistic characteristics with the embryo and impair hatch performance (Cox et al., 1992; Meijerhof and Hulet, 1997; Jeong and Kim, 2014; Bai et al., 2017; Triplett et al., 2018).
For example, previous studies have observed the possible hazards that probiotic administration may pose for humans who are immunocompromised (Oggioni et al., 1998; Hassan et al., 2018). In a meta-analysis of cancer patients who consumed probiotic supplements during their treatment, a safety analysis noted 5 out of the 25 studies yielded infections in patients which were linked to their probiotic consumption (Hassan et al., 2018). Concurrently, there are beneficial impacts that probiotic supplementation has on patients, which include diarrhea reduction and fever reduction in individuals who are in an immunocompromised state (Ceccarelli et al., 2017; Hassan et al., 2018). Due to the potential for probiotics to act pathogenically within an immunocompromised host, it is possible that chicken embryos, whose immune systems do not achieve maturations until weeks after hatch, may be negatively influenced by in ovo probiotic supplementation post-hatch (Dibner et al., 1998).
Even so, previous research that compared the in ovo application of different individual probiotic species has found that the in ovo injection of Lactobacillus spp. does not negatively affect hatchability (Triplett et al., 2018). A study by Triplett et al. (2018) observed the possible impacts that lactic acid and bacteriocin producing bacteria have on the developing embryo when injected using Inovoject® equipment. It was observed that the injection of Lactobacillus acidophilus or Bifidobacterium animalis using commercial Inovoject® equipment did not positively or negatively affect hatch of fertilized eggs when compared to the control (Triplett et al., 2018). However, a Bacillus subtilis species had a significant negative impact on hatch of fertilized eggs when compared to the control (Triplett et al., 2018). Previous research has found that Bacillus subtilis has the potential to improve broiler performance when supplemented in the feed (Jeong and Kim, 2014; Bai et al., 2017). Lactobacillus spp. and Bacillus s pp. have differing modes of action that may potentially impact the embryo differently when injected into the amnion. Lactobacillus species have high epithelial adhesion capabilities and produces lactic acid through fermentative processes, while Bacillus secretes lactate, acetoin, 2,3-butanediol, acetate, and ethanol through fermentative processes (Hutkins and Nannen, 1993; Nicholson, 2008; Lai et al., 2012; Shokryazdan et al., 2014). Although further studies are necessary to understand the interaction between these metabolic fermentation products and the broiler embryo, it has been found that 2,3-butanediol enhances natural killer cell cytotoxicity which advances spontaneous abortion in mice species (Gendron and Baines, 1988; Lai et al., 2012). Similar natural killer cells have been observed in the developing avian embryo (Jansen et al., 2010), which may have attributed to the high embryo mortality found with the injection of Bacillus subtilis by Triplett et al. (2018).
The injection of L. animalis and E. faecium, probiotics with different characteristics in vitro, into fertile broiler hatching eggs on day 18 of incubation did not demonstrate any of the negative effects observed by Triplett et al. from bacteriocin or lactic acid production (2018). This is important to note as it was found during the verification of injection procedures that the L. animalis and the combination treatments exhibited notably elevated L. animalis concentrations when compared to E. faecium concentrations. This may be due to a high growth rate observed when the L. animalis culture was grown in broth during treatment preparation. Even though L. animalis grew more rigorously in the preparation of the treatments when compared to its growth when formulating the growth curve, a bacterial concentration of 109 cfu/50 μl L. animalis injected into fertile broiler hatching eggs yielded 0% contaminated eggs in the L. animalis treatment. Also, a combined injection of L. animalis at 107 cfu/50 μl and E. faecium at 106 cfu/50 μl did not impact any hatch residue or any of the hatch parameters when compared to the HVT control treatment. This indicates that the injection of a live probiotic bacteria culture did not negatively impact hatchability, even when the injected concentration was as high as 109 cfu/50 μl L. animalis in the L. animalis injected treatment.
Other than percentage pipped eggs, the hatch parameters analyzed in the present study yielded no significant differences due to treatment. These variables, including chick weight, percent cracked, percent contaminated, or percent late dead eggs, indicate that the in ovo injection of a probiotic combination including L. animalis and E. faecium does not negatively impact the developing embryo and its ability to hatch. In an analysis by Johnston et al., 1997, it was determined that the use of in ovo technology to administer the Marek's disease vaccine does not negatively impact many hatch parameters, such as post-hatch chick mortality, when compared to post-hatch vaccination. In the present study, the L. animalis treatment had a positive impact on the developing embryo where there were significantly lower percentage pipped eggs when compared to the control. However, it is important to note that the chicks in ovo injected with E. faecium or the combination treatment were not significantly different from the HVT control treatment in hatchability of transferred eggs or percentage pipped eggs.
Live Performance and GIT Parameters
The lack of differences observed in many of the live performance parameters between the HVT control and probiotic treatments further demonstrates the efficiency and implications of using in ovo technology for probiotic supplementation. Similar results were observed in an analysis conducted by Gildersleeve et al. (1993), where in ovo vaccination of fertilized broiler hatching eggs and conventional post-hatch vaccination methods were compared in a commercial setting. With no significant differences in early post-hatch mortality in the present study, neither the in ovo technology utilized nor the probiotics introduced to the embryo negatively impacted chick mortality post-hatch.
A treatment effect for FCR was yielded during the days 7 to 14 phase in the present study, where E. faecium and the combination treatments yielded an increased FCR in comparison to those in the HVT control treatment. No other FCR treatment effects were observed in the other growth phases (days 0 to 7, 14 to 21, or 0 to 21). The performance difference on days 7 to 14 may be attributed to weight, length, and pH differences found in the GITs among the probiotic treatments when compared to the HVT control. Due to the observance of altered gastrointestinal parameters and an increased FCR during grow-out in the days 7 to 14 phase, it is possible that the in ovo injection of probiotics is capable of altering the physiological development of the chick's gastrointestinal tract during the first 21 d of life. Whether or not these alterations caused by in ovo supplementations of L. animalis and/or E. faecium are beneficial over the course of the entire grow-out is yet to be established.
Previous research in other organism models have found that the natural gut microflora is capable of modulating the expression of genes associated with gut epithelium development (Hooper et al., 2001; Lange et al., 2010). Similarly, a study by Pruszynska-Oszmalek et al. discovered that the in ovo injection of probiotics or synbiotics elevated amylase, hydrolase, and trypsin activity by the pancreas, which may potentially improve bird performance (2015). Contradictively, a study with the in-feed application of probiotics found that the supplementation decreased urease activity in broiler chicks (Yeo and Kim, 1997). Although studies are continuing to investigate the impact of probiotics on the digestive system, the interaction between the gastrointestinal microbiome and the functionality of the gastrointestinal tract is complex and not completely understood in the broiler chicken (Yeo and Kim, 1997; Lu et al., 2003; Pruszynska-Oszmalek et al., 2015; Ballou et al., 2016).
In the present study, the injected probiotic species, L. animalis and E. faecium, may be capable of diversely influencing the gastrointestinal tract of its host (Hutkins and Nannen, 1993; Franz et al., 2007; Shokryazdan et al., 2014). Other studies have observed an increase in gut tissue weights when probiotics are supplemented in feed, which may be the cause of altered GIT weights observed in the present trial when probiotics were injected in ovo (Awad et al., 2009). It was previously proposed that this increase in tissue weight may be indicative of greater surface area in the small intestines which may lead to increased nutrient and water absorption (Awad et al., 2009). However, Coates et al. (1955) suggests that an increase in intestinal weight may be indicative of an uncharacterized infection within the gut and therefore may lead to an increase in feed intake. This may be the case for the present study, as it was found that the probiotic treatments had greater FCR along with greater jejunum and ileum weights when compared to the control on day 14. Alternatively, Jin et al. (2000) observed that the inclusion of Lactobacillus spp. in feed did not alter GIT weights nor did it negatively impact FCR. Similarly, de Oliveira et al. (2014) yielded no significant weight gain or FCR differences between injected probiotic treatments and the control even when challenged with Salmonella. This may suggest that the in ovo injection of a probiotic combination different from E. faecium and L. animalis may be capable of promoting efficient live performance through modulating the development of the GITs. Moreover, further research is necessary to understand these relationships in the future.
It is pertinent to address that treatment interactions were observed where L. animalis reduced crop pH when compared to the E. faecium and combination treatments on day 7. This may be due to its lactic acid production, although further research pertaining to microbiome analysis is necessary to directly link in ovo probiotic supplementation to the bacterial composition of the gut and tissue pH (Kashket, 1987; Hutkins and Nannen, 1993; Cintas et al., 2001). For instance, Ranjitkar et al. (2016) observed high levels of bacteria belonging to the Lactobacillaceae family in the crop, gizzard and ileum, and high levels of bacteria belonging to the Enterococcacae family in the ileum. In ovo probiotic supplementation may be capable of manipulating these populations, but further research is necessary to understand this interaction.
Therefore, this pH difference in the crop among probiotic treatments on day 7 may be demonstrative of the embryo consuming the probiotic that was injected into the amnion prior to hatch (Moran, 2007). However, the in ovo probiotic treatments were not capable of maintaining a lower pH in the gastrointestinal tract when compared to the non-injected control, even on day 7. This may indicate that the injected probiotics do not have high adherence to the epithelium of the gastrointestinal tract after injection and are sloughed off within the first week of hatch. However, Marciňáková et al. (2010) tested the survivability of an E. faecium strain isolated from chicken jejunum; in vitro, it was observed that multiple isolated E. faecium strains were capable of surviving simulated gastrointestinal conditions but exhibited low adhesion characteristics. The results obtained by Marciňáková et al. (2010) may explain why the gastrointestinal pH in the present study where any of the tissues of the E. faecium treatment consistently exhibited a higher pH than the control tissues.
Alternatively, Ehrmann et al. (2002) supplemented a one time inclusion of Lactobacillus spp. in feed and was able to recover that species from the fecal matter of ducks over a 28 d study. This may not have been observed in the present study, where the L. animalis and combination treatments observed an elevated pH in the crop when compared to the HVT control treatment by day 21. As previously stated, analysis of the microbiome of gastrointestinal tract is necessary to directly understand the relationship that the in ovo injection of probiotics may have on the development and maintenance of a chick's microbiome. It is expected that through in-feed probiotic applications coupled with in ovo probiotic injections, higher levels of lactic-acid producing probiotics may be maintained in the foregut for longer periods of time.
Bursa Weights
Along with GITs, bursa weight relative to body weight appeared to be influenced by the in ovo injection of the E. faecium and combination treatments. Even though changes in bursa weight might indicate that the in ovo injection of E. faecium and the combination induced an immune response early in the grow-out, these differences may not be indicative of a stimulated immune system (Glick, 1963; Cazaban et al., 2015; Fathi et al., 2017). In a study by Glick et al. (1963), reduced bursa weights in Pekin ducks did not decrease the level of circulating leukocytes in the blood. Additionally, Cazaban et al. (2015) observed high variations in bursa weights among broilers as the birds aged. Therefore, further analyses of circulating blood leukocyte counts, B-cell enumeration, antibody response, and immune cell expression in gut-associated lymphoid tissues are necessary to gain a comprehensive understanding of immune responses stimulated by individual probiotics and their combinations (Chrząstek et al., 2011; Madej and Bednarcyzk, 2016).
Implications and Future Research
The results of this study indicate that individual probiotics with differing modes of action can be injected into fertilized broiler hatching eggs simultaneously without negatively impacting hatch and live performance. L. animalis may be more compatible with the in ovo injection process due to its significant improvement in percent pipped mortality. However, the present study only conducted a 21 d grow-out. Even though an increase in FCR was yielded in the in ovo injected E. faecium and combination treatments during the days 7 to 14 phase of the trial, a 49 d grow-out may observe positive differences in broiler performance in the probiotic treatments because immune system maturation does not occur until day 21 post-hatch (Dibner et al., 1998). Additionally, the differences in gut morphology among treatments that occurred in the current study may not yield positive performance differences until weeks later (Dibner et al., 1998).
Future studies using different probiotic bacteria in combination with L. animalis may demonstrate improvements not only for hatch but also improvements in live performance parameters. A more accurate analysis of gut microbiota is also necessary to understand the amount of time that an in ovo injected probiotic can be maintained in the gastrointestinal tract. Furthermore, research is also needed to understand synergistic capabilities of in ovo injection and in-feed probiotic applications, which may alter gastrointestinal pH levels during grow-out and reduce pathogen presence in the gut. Differences in probiotic applications, whether in-feed or in ovo, and the formulation of those probiotics may impact the success of any probiotic supplementation with improving bird performance, and the success of the probiotic program may rely on how those formulations interact with the existing gut microflora. Therefore, a different probiotic combination from the one proposed in the present study may have the potential to decrease FCR during grow-out. Lastly, an analysis of immune response associated with the injection of these different probiotic species and their combinations using Inovoject® equipment is necessary to understand the impacts that the differing modes of action have on the chick after hatch.
ACKNOWLEDGMENTS
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. This material is based upon work that is supported by the U. S. Department of Agriculture, Hatch projects under accession numbers of MIS-322,340 and specific Cooperative Agreements under accession number MIS-321,777.
We would like to thank Zoetis for the use of the Inovoject® equipment and for providing service during the injection process.
We would also like to thank Merial for providing diluent and HVT vaccine for the treatment applications.
REFERENCES
- Aliakbarpour H.R., Chamani M., Rahimi G., Sadeghi A.A., Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Aust. J. Anim. Sci. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo T.F., de Luces Fortes Ferreira C.L. The genus Enterococcus as probiotic: safety concerns. Braz. Arch. Biol. Technol. 2013;56:457–466. [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and symbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–55. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Bai K., Huang Q., Zhang J., He J., Zhang L., Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: How early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:1–12. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R., Lahtinen S.J., Ibrahim F., Ouwehand A.C. In: Lactic acid bacteria: Microbiological and functional aspects. Fourth edition. Lahtinen S., Auwehand A.C., Salminen S., Wright A.V., editors. Taylor & Francis Group, LLC; Boca Raton, FL: 2012. Genus Lactobacillus. [Google Scholar]
- Cazaban C., Masferrer N.M., Pascual R.D., Espadamala M.N., Costa T., Gardin Y. Proposed Bursa of Fabricius weight to body weight ratio standard in comercial broilers. Poult. Sci. 2015;94:2088–2093. doi: 10.3382/ps/pev230. [DOI] [PubMed] [Google Scholar]
- Ceccarelli G., Brenchley J.M., Cavallari E.N., Scheri G.C., Fratino M., Pinacchio C., Schietroma I., Fard S.N., Scagolari C., Mezzaroma I., Vullo V., d'Ettorre G. Impact of high-dose multi-strain probiotic supplementation on neurocognitive performance and central nervous system immune activation of hiv-1 infected individuals. Nutrients. 2017;9 doi: 10.3390/nu9111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrząstek K., Madej J.P., Mytnik E., Wieliczko A. The influence of antibiotics on B-cell number, percentage, and distribution in the bursa of Fabricius of newly hatched chicks. Poult. Sci. 2011;90:2723–2729. doi: 10.3382/ps.2011-01525. [DOI] [PubMed] [Google Scholar]
- Cintas L.M., Casaus M.P., Herranz C., Nes I.F., Hernández P.E. Review: Bacteriocins of lactic acid bacteria. Food Sci. Tech. Int. 2001;7:281–305. [Google Scholar]
- Coates M.E., Davies M.K., Kon S.K. The effect of antibiotics on the intestines of the chick. Br. J. Nutr. 1955;9:110–119. doi: 10.1079/bjn19550016. [DOI] [PubMed] [Google Scholar]
- Cox N.A., Bailey J.S., Blankenship L.C., Gildersleeve R.P. Research note: in ovo administration of a competitive exclusion culture treatment to broiler embryos. Poult. Sci. 1992;71:1781–1784. doi: 10.3382/ps.0711781. [DOI] [PubMed] [Google Scholar]
- de Oliveira J.E., van der Hoeven-Hangoor E., van de Linde I.B., Montjin R.C., van der Vossen J.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Knight C.D., Kitchell M.L., Atwell C.A. Early feeding and development of the immune system in neonatal poultry. J Appl. Poult. Res. 1998;7:425–436. [Google Scholar]
- Ehrmann M.A., Kurzak P., Bauer J., Vogel R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002;92:966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- Fathi M.M., Ebeid T.A., Al-Homidan I., Soliman N.K., Abou-Emera O.K. Influence of probiotic supplementation on immune response in broilers raised under hot climate. Brit. Poult. Sci. 2017;58:512–516. doi: 10.1080/00071668.2017.1332405. [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies . 3rd edition. Committees to revise the Guide for the Care and Use of Agricultural Animals in Research and Teaching; 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. [Google Scholar]
- Fuller R. Probiotics in man and animals. J Appl. Microbiol. 1989;66:365–378. [PubMed] [Google Scholar]
- Franz C.M., van Belkum M.J., Holzapfel W.H., Abriouel H., Gálvez A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 2007;31:293–310. doi: 10.1111/j.1574-6976.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- Gendron R.L., Baines M.G. Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol. 1988;113:261–267. doi: 10.1016/0008-8749(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Gildersleeve R.P., Hoyle C.M., Miles A.M., Murray D.L., Ricks C.A., Secrest M.N., Williams C.J., Womack C.L. Developmental performance of an egg injection machine for administration of Marek's disease vaccine. J. Appl. Poult. Res. 1993;2:337–346. [Google Scholar]
- Glick B. The effect of surgical and chemical bursectomy in the White Pekin Duck. Poult. Sci. 1963;42:1106–1113. [Google Scholar]
- Guerra N.P., Bernárdez P.F., Méndez J., Cachaldora P., Castro L.P. Production of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. An Feed Sci. Tech. 2007;134:89–107. [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B., Chambers R., Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vac. Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H., Rompola M., Glaser A.W., Kinsey S.E., Phillips R.S. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. 2018;26:2503–2509. doi: 10.1007/s00520-018-4216-z. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Wong M.H., Thelin A., Hansson L., Falk P.G., Gordon J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Aviagen; Huntsville, Alabama, USA: 2017. “How to … Break Out and Analyse Hatch Debris.”. [Google Scholar]
- Hutkins R.W., Nannen N.L. pH homeostasis in Lactic acid bacteria. J. Dairy Sci. 1993;76:2354–2365. [Google Scholar]
- Jansen C.A., van de Haar P.M., van Haarlem D., van Kooten P., de Wit S., van Eden W., Viertlböck B.C., Göbel T.W., Verveldea L. Identification of new populations of chicken natural killer (NK) cells. Dev. Comp. Immunol. 2010;34:759–767. doi: 10.1016/j.dci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Kim I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Marquardt R.R., Zhao X. A Strain of Enterococcus faecium (18C23) Inhibits Adhesion of Enterotoxigenic Escherichia coli K88 to Porcine Small Intestine Mucus. Appl. Environ. Microbiol. 2000;66:4200–4204. doi: 10.1128/aem.66.10.4200-4204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P.A., Liu H., O'Connell T., Phelps P., Bland M., Tyczkowski A., Kemper A., Harding T., Avakian A., Haddad E., Whitfill C., Gildersleeve R., Ricks C.A. Applications in in ovo technology. Poult. Sci. 1997;76:165–178. doi: 10.1093/ps/76.1.165. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E.R. Review: bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987;3:233–244. [Google Scholar]
- Kim J.H., Kim K.S. Hatchery hygiene evaluation by microbiological examination of hatchery samples. Poult. Sci. 2010;89:1389–1398. doi: 10.3382/ps.2010-00661. [DOI] [PubMed] [Google Scholar]
- Lai H.C., Chang C.J., Yang C.H., Hsu Y.J., Chen C.C., Lin C.S., Tsai Y.H., Huang T.T., Ojcius D.M., Tsai Y.H., Lu C.C. Activation of NK cell cytotoxicity by the natural compound 2,3-butanediol. J. Leukoc Biol. 2012;92:807–814. doi: 10.1189/jlb.0112024. [DOI] [PubMed] [Google Scholar]
- de Lange C.F.M., Pluske J., Gong J., Nyachoti C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010;134:124–134. [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. App. Environ. Micro. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madej J.P., Stefaniak T., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs' morphology in chickens. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev076. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016;95:19–29. doi: 10.3382/ps/pev291. [DOI] [PubMed] [Google Scholar]
- Marciňáková M., Klingberg T.D., Lauková A., Budde B.B. The effect of pH, bile and calcium on the adhesion ability of probiotic enterococci of animal origin to the porcine jejunal epithelial cell line IPEC-J2. Anaerobe. 2010;16:120–124. doi: 10.1016/j.anaerobe.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meijerhof R., Hulet R.M. In ovo injection of competitive exclusion culture in broiler hatching eggs. J. Appl. Poult. Res. 1997;6:260–266. [Google Scholar]
- Moran E.T., Jr. Nutrition of the developing embryo. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Mountzoris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Biridobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Ness I.F., Diep D.B., Ike Y. In: Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Gilmore MS, Clewell DB, Ike Y, et al., editors. Massachusetts Eye and Ear Infirmary; Boston: 2014. “Enterococcal Bacteriocins and Antimicrobial Proteins that Contribute to Niche Control.”. [PubMed] [Google Scholar]
- Nicholson W.L. The Bacillus subtilis ydjL (bdhA) Gene Encodes Acetoin Reductase/2,3-Butanediol Dehydrogenase. Appl Environ Microbiol. 2008;74:6832–6838. doi: 10.1128/AEM.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oggioni M.R., Pozzi G., Valensin P.E. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J. Clin. Microbiol. 1998;36:325–326. doi: 10.1128/jcm.36.1.325-326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017;96:1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- Ploweic A., Slawińska A., Siwek M.Z., Bednarczyk M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015;76:975–982. doi: 10.2460/ajvr.76.11.975. [DOI] [PubMed] [Google Scholar]
- Pruszynska-Oszmalek E., Kolodziejski P.A., Stadnicka K., Sassek M., Chalupka D., Kuston B., Nogowski L., Mackowiak P., Maiorano G., Jankowski J., Bednarczyk M. In ovo injection of prebiotics and synbiotics affect the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015;94:1909–1916. doi: 10.3382/ps/pev162. [DOI] [PubMed] [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R.M. Bacterial succession in the broiler gastrointestinal tract. App. Environ. Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen; Huntsville, Alabama, USA: 2009. “Ross Broiler Management Manual.”. [Google Scholar]
- Aviagen; Huntsville, Alabama, USA: 2014. “Ross 708 Nutrition Specifications.”. [Google Scholar]
- Shokryazdan P., Kalavathy R., Sieo C.C., Alitheen N.B., Liang J.B., Jahromi M.F., Ho Y.W. Isolation and characterization of Lactobacillus strains as potential probiotics for chickens. Pentanika J. Trop. Agric. Sci. 2014;37:141–157. ISSN: 1511–3701. [Google Scholar]
- Slawińska A., Siwek M.Z., Bednarczyk M. EF. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie J.H. McGraw-Hill, Inc; New York, NY: 1980. Principal and Procedures of Statistics: A Biometrical Approach. [Google Scholar]
- Tannock G.W. In: The Lactic Acid Bacteria in Health & Disease. Wood B.J.B., editor. Elsevier Science Publishers Ltd.; Essex, England: 1992. “2. The lactic microflora of pigs, mice and rats.”. [Google Scholar]
- Teague K.D., Graham L.E., Dunn J.R., Cheng H.H., Anthony N., Latorre J.D., Menconi A., Wolfenden R.E., Wolfenden A.D., Mahaffey B.D., Baxter M., Hernandez-Velasco X., Merino-Guzman R., Bielke L.R., Hargis B.M., Tellez G. In ovo evaluation of FloraMax®-B11 on Marek's disease HVT vaccine protective efficacy, hatchability, microbiota, composition, morphometric analysis, and Salmonella enteritidis infection in broiler chickens. Poult. Sci. 2017;96:2074–2082. doi: 10.3382/ps/pew494. [DOI] [PubMed] [Google Scholar]
- Triplett M.D., Zhai W., Peebles E.D., McDaniel C.D., Kiess A.S. Investigating commercial in ovo technology as a strategy for introducing probiotic bacteria to broiler embryos. Poult Sci. 2018;97:658–666. doi: 10.3382/ps/pex317. [DOI] [PubMed] [Google Scholar]
- Yeo J., Kim K.I. Effect of feeding diets containing and antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poult. Sci. 1997;76:381–385. doi: 10.1093/ps/76.2.381. [DOI] [PubMed] [Google Scholar]