Abstract
This study aimed to estimate the productive and economic impacts caused by the withdrawal of antibiotic growth promoters (AGP) from broilers diet. Indexed publications that compared diets with or without AGP (AGP+/AGP−) for broilers (from initial to final phase) were collected and the results of feed intake, weight gain, and feed conversion were compiled in a database. A meta-analysis was performed following sequential analyses: graphical approach (to observe biological data coherence), correlation (to identify related factors), and variance-covariance (to compare groups). The annual number of broiler slaughtered in Brazil, target weight gain and feed conversion for each phase, the variation in feed conversion, feed cost, and AGP costs were used to build a model to estimate the effects of the AGP withdrawal on feeding costs. The database comprised 174 scientific articles containing 183 experiments, totaling 121,643 broilers, most of which were Ross (52% of the studies). The most frequent AGP sources/forms in the database were Avilamycin (41% of the AGP+ treatments), Flavomycin (19%), Virginiamycin (16%), and Bacitracin (14%). Higher feed intake, weight gain, and lower feed conversion were attributed (P < 0.05) to AGP+ diets during Initial phase (1 to 21 D). In Final phase (22 to 42 D) no differences were observed in performance variables. Treatments AGP+ presented higher weight gain and better feed conversion in the Total period (1 to 42 D). The results of feed conversion were improved (P < 0.05) with Avilamycin and Flavomycin; Virginiamycin improved weight gain and feed conversion. In the Total period, the economic impact was $0.03 per animal and a total of $183,560,232 per year. It was concluded that broilers fed AGP+ diets have higher weight gain and better feed conversion than those fed AGP− diets, and withdrawing AGP increases production costs.
key words: broiler chicken, feed conversion, productive cost, sensitivity analysis, weight gain
INTRODUCTION
The pressure for reducing the use of antibiotic growth promoters (AGP) in livestock is a growing and irreversible process, and several countries are adhering to the restrictions and ban on AGP usage. Sweden was the first country which changed laws of AGP usage and, in 2006, the EU imposed a complete ban of all AGP. The USA is not only limiting AGP use but also moving towards a significant reduction of general antibiotics usage in industrial food animal production (Salim et al., 2018). The most recent effort toward AGP restriction in Brazil and China was banning the use of Colistin in 2016 (Walsh and Wu, 2016; Davies and Walsh, 2018). In the same year, Vietnam announced the ban AGP by 2020 (USDA, 2016). India has introduced drug-withdrawal time for livestock production (Kahn, 2017) and Bangladesh, Bhutan, Indonesia, Myanmar, Nepal, Sri Lanka, and Thailand have announced AGP restrictions (Goutard et al., 2017). The increasing pressure to prohibit the use of these additives is based on the possibility of induction of cross-resistance of pathogenic bacterial strains in people (Baurhoo et al., 2009; Tang et al., 2017).
In broiler production, there is an estimated annual use of 148 mg/kg of AGP (Van Boeckel et al., 2015) with the objective of obtaining better results of weight gain and feed conversion. However, considerable variability in performance response to AGP has been observed, contingent on genetic potential, phase of rearing, as well as hygiene and management practices. Many studies have shown no weight gain difference in broilers fed an AGP diet in the absence of health problems (Denev, 2006; Baurhoo et al., 2007; El-Faham et al., 2015; Naveenkumar et al., 2017). However, other studies have reported the efficiency of AGP, with positive effects on broilers weight gain and feed conversion (Zhang et al., 2005; Peng et al., 2016; Wu et al., 2018).
It is clear that AGP restrictions in the production of animal food are expanding and therefore its consequences must be studied, including its effect on broiler performance and the expected economic results of such restriction. The objective of this study was to quantify the performance differences in broilers receiving diets with and without AGP and to estimate the economic impact of this withdrawal through the meta-analysis technique.
MATERIAL AND METHODS
Search and Data Filtering
Scientific literature presenting experimental results of broiler performance with AGP was searched in different online data sources (Google Scholar, ScienceDirect, Scopus, Scielo, and PubMed) using the keywords: “antibiotic growth promoter” and “performance” in addition to the terms “broiler” or “chicken.” The search terms were tested in English, Spanish, and Portuguese. References in the final publication list were also reviewed to identify any additional relevant articles. This diversity of scientific databases was intended to prevent potential publication bias. Following identification, the studies were critically evaluated according to relevance and quality in relation to the meta-analysis objectives. Abstracts were examined by two researchers, and a record was only removed from the database following agreement. At this stage, a set of information about each selected study was examined against a previously prepared checklist, and critically evaluated for eventual methodological errors. For the exclusion of pre-selected manuscripts, the following factors were considered: presence of sanitary challenge, slow-growing breeds, absence of control treatment, inconsistent methodological data, error of statistical design, and gross errors in result data. The criteria for publication selection were: (1) in vivo experimental evaluation of diets with AGP (AGP+) and without AGP (AGP−); (2) antibiotics dosage within the recommended range for growth promoters; (3) AGPs permitted in the Brazilian standard legislation of 2016 (MAPA, 2016); (4) initial, final and/or total period results expressed; (5) rates of feed intake, weight gain, and feed conversion or feed efficiency stated; (6) year of publication from 1990 until 2018.
Data Systematization and Coding
The methodology used for database construction and for data encoding followed the methods described in the literature (Lovatto et al., 2007; Sauvant et al., 2005). The data were entered in an electronic spreadsheet, which consisted of rows representing the treatments and columns representing the exploratory and descriptive variables. Relevant information to the objective of this study (body weight, feed intake, weight gain, and feed conversion) and other variables (genetic strain, age, sex, dietary nutritional composition, and duration of experimental period) were included to allow for a descriptive analysis of the studies. Some codes were used to create grouping criteria in the analytical models; the main codes were applied for the presence (AGP+) or absence (AGP−) of AGP and for each antibiotic, such as “A” for Avilamycin and “B” for Bacitracin. Other codes were used as moderating variables to represent the variability of the compiled trials (e.g., effect of study or trial).
Statistical Analysis
A series of graphical analyses were used to analyze the data distribution and to obtain a general view of their consistency and variance heterogeneity. Based on these analyses, correlation hypotheses were formulated to define the statistical models (Lovatto et al., 2007). During this step, the data distribution per year and the presence or absence of AGP were evaluated. The performance data of AGP− treatments were relativized according to their respective AGP+, in order to estimate the impact (percentage variation) of AGP withdrawal. Additionally, the relationships between and within studies were evaluated.
Variance-covariance analyses were conducted using the GLM procedure in the Minitab 18 statistical package (Minitab Inc., State College, PA); the effects of gender, type of facilities and year of publication were tested. However, the factors were not significant (P > 0.05) and consequently, all 3 factors were removed from the model. A mixed model was applied, considering treatments as fixed effect, while inter-study codes and mean body weight were random effects (P < 0.05). The analyses were performed considering treatments, the inter-study codes, and BW. The variables analyzed were feed intake, weight gain, and feed conversion. The analyses were grouped by the rearing phase: Initial (1 to 21 D), Final (22 to 42 D), and Total phase (1 to 42 D). The Total phase analysis was carried out considering the entire database, including both rearing phases. Individual analyses were also performed for the main AGP in the database. Residual analysis was performed, and it was observed that the residuals were normally distributed.
Economic Impact
The parameters obtained by meta-analysis were used to build a model that estimates the withdrawal of AGP on the production costs, particularly feeding costs in Brazilian scenario. Brazil was chosen for this simulation as it is a large producer and exporter of broiler meat. Moreover, AGP withdrawal is an important and current debate in Brazilian meat industry. The simulation (Table 1) considered the annual slaughtering rate, reported at 5,840,000,000 broilers in 2017 (IBGE, 2017), the target weight gain and feed conversion for each phase (AGP+), the variation in feed conversion (obtained from the meta-analysis), as well as feed and AGP costs (information provided by a local feed factory).
Table 1.
Inputs for estimating the economic impact caused by the withdrawal of antibiotic growth promoters (AGP) from broiler diets.
| Initial phase |
Total Period |
|
|---|---|---|
| Inputs | (1 to 21 D) | (1 to 42 D) |
| Annual slaughterings, heads | 5.840.000.000 | 5.840.000.000 |
| Weight gain, kg | 0.80 | 2.50 |
| Feed conversion target (APC+), kg/kg | 1.47 | 1.66 |
| Feed conversion variation1, % | 2.64 | 3.48 |
| Feed cost, $/ton | 232.00 | 230.00 |
Feed conversion variation: variation between diets with and without AGP obtained by the meta-analysis.
Equations for economic impact simulation:
| (1) |
where: α: feed intake of the phase (kg/D) and β: cost of feed ($/ton).
| (2) |
where: T: weight gain of the phase (kg) and FCR: feed conversion (kg/kg).
| (3) |
where: µ: price of feed ($/ton) and y: price of AGP ($/ton).
| (4) |
where: N: number of slaughters in the year (head).
The performance data of animals that received and did not receive AGP was used in the equations, and the difference between the results was calculated to estimate the economic impact.
The currency conversion rate used for the calculation of production costs in USD was: $1.00 = R$3.75.
A sensitivity analysis was performed using the key variables “feed conversion” and “AGP price.” It indicates whether one or more variables can have an impact on the economic results of a production system and influence its profitability (Saltelli et al., 2000). To define the scenarios in which the withdrawal of AGP would have a negative economic impact, the range between feed conversion in AGP+ and AGP− treatments was increased from 0.0 to 5.0%, and the price of AGP was halved and increased up to 5 times. When the value of the economic impact was greater than zero, AGP withdrawal was judged as an economically sound strategy, and when the impact value was less than zero, AGP use was judged as a better economic decision to avoid financial loss. The price of feed used to evaluate the Total phase was utilized in the sensitivity analysis. In this study, only the feed cost was considered; therefore, other factors such as management and sanitary costs were not included in the economic impact model.
RESULTS
Composition of Database
The database consisted of 174 articles (listed in Supplementary Table 1) containing 183 experiments. The majority of selected papers (98%) were published between 1998 and 2018 (Figure 1), and studies were developed in Brazil (14%), Korea (12%), Canada (9%), USA (9%) and other countries (56%), such Africa, Egypt, China, France, Israel, among others. The total number of broilers used in all trials was 121,640, with the initial and final BW of 0.45 and 2.3 kg, respectively. The most used strains were Ross (52% of the treatments), Cobb (28%), and Arbor Acres (10%). Groups with mixed sexes were used in 20% of the database studies, while treatments with males and females separated, represented 60% and 2% of the total, respectively. The sex of the animals was not described in 18% of the studies. The Initial phase was described in 16% of the studies, and the Final phase and Total in 7 and 22%. The majority of the studies (55%) described the performance per week of production. The most frequent antibiotics in the database were Avilamycin (41% of the AGP+), Flavomycin (19%), Virginiamycin (16%), and Bacitracin (14%). Pens and battery housing systems were used in 75% and 15% of the database studies, respectively. A summary of the manuscripts is available in Supplementary Table 2.
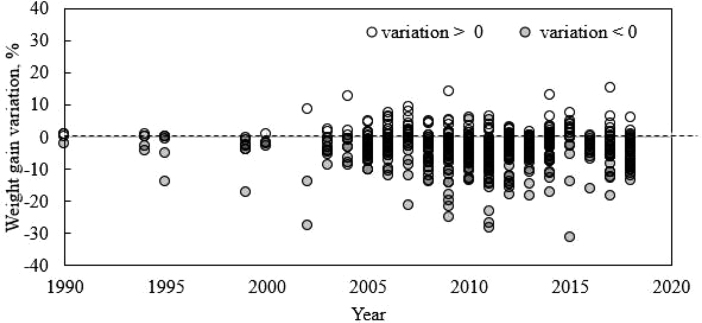
Figure 1.
Percentage change calculated for each treatment containing antibiotic growth promoter (AGP) in relation to its respective treatment without AGP in studies included in the database according to year of publication.
Performance
The AGP+ presented a positive impact on broiler performance. A negative variation in weight gain was observed in 76% of comparisons between AGP +/AGP− treatments in the database (Figure 1). Likewise, better feed conversion was observed in 84% of individual comparisons among treatments. However, it is important to point out that some of these variations were not statistically significant in the original studies.
In the meta-analyze, feed intake showed better result (P < 0.05) to AGP+ in the Initial phase (1 to 21 D), but no effects (P > 0.05) were observed in the Final (22 to 42 D) and Total phases (1 to 42 D) (Table 2). The weight gain presented a higher result (P < 0.05) when AGP+ diets were used in the Initial and Total phases, but no difference was observed in the Final phase (P > 0.05), with 2.79 and 3.84% differences between AGP+ and AGP−. Feed conversion had better results (P < 0.05) in AGP+ in Initial and Total phases, and no difference was observed in Final phase. The biggest difference between AGP+ and AGP− was 3.48% in Total phase, followed by Initial with 2.64%.
Table 2.
Performance -obtained by meta-analysis- of broilers fed diets with (AGP+) or without antibiotic growth promoters (AGP−).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Variable | AGP+ | AGP− | P | SER | R2 | % |
| Initial (1 to 21 D) | ||||||
| Feed intake, g/D | 55 | 56 | 0.005 | 1.059 | 99.15 | 1.78 |
| (n:86) | (n:75) | |||||
| Weight gain, g/D | 38 | 37 | <0.001 | 1.043 | 98.29 | 2.70 |
| (n:87) | (n:76) | |||||
| Feed conversion, g/g | 1.47 | 1.51 | <0.001 | 0.049 | 92.66 | 2.64 |
| (n:86) | (n:75) | |||||
| Final (22 to 42 D) | ||||||
| Feed intake, g/D | 161 | 162 | 0.111 | 2.952 | 98.21 | 0.61 |
| (n:37) | (n:34) | |||||
| Weight gain, g/D | 82 | 82 | 0.561 | 1.841 | 97.70 | 0.00 |
| (n: 39) | (n:36) | |||||
| Feed conversion, g/g | 1.96 | 1.99 | 0.128 | 0.056 | 96.01 | 1.50 |
| (n:37) | (n:34) | |||||
| Total (1 to 42 D) | ||||||
| Feed intake, g/D | 90 | 91 | 0.127 | 15.707 | 87.85 | 1.09 |
| (n:476) | (n:452) | |||||
| Weight gain, g/D | 54 | 52 | 0.040 | 8.886 | 82.62 | 3.84 |
| (n:513) | (n:489) | |||||
| Feed conversion, g/g | 1.66 | 1.72 | <0.001 | 0.157 | 74.61 | 3.48 |
| (n:482) | (n:458) | |||||
P: Probability of treatment effect. The models included the study effect (P < 0.001).
RSE: Residual standard error.
R2: Coefficient of determination of the model.
%: Percentage change between AGP+ and AGP−.
n: Number of studies observations in each mean.
Analyzing each AGP individually (Table 3), feed intake was not influenced by the treatments (P > 0.05), regardless of the AGP analyzed. The addition of Avilamycin and Flavomycin did not result in any detectable difference between AGP+ and AGP− (P > 0.05) in weight gain. Virginiamycin showed positive effect on weight gain (P < 0.05) in AGP+ compared to AGP−. Avilamycin, Flavomycin, and Virginiamycin showed better results (P < 0.05) of feed conversion in AGP+.
Table 3.
Total performance* -obtained by meta-analysis- of broilers fed diets containing specifics antibiotic growth promoters (AGP+) or not (AGP−).
| Treatments |
|||||
|---|---|---|---|---|---|
| Variable | AGP+ | AGP− | P | SER | R2 |
| Avilamycin | |||||
| Feed intake, g/D | 91 | 93 | 0.108 | 15.786 | 87.51 |
| (n:200) | (n:199) | ||||
| Weight gain, g/D | 54 | 53 | 0.248 | 8.952 | 82.47 |
| (n:219) | (n:218) | ||||
| Feed conversion, g/g | 1.63 | 1.71 | <0.001 | 0.118 | 82.17 |
| (n:206) | (n:205) | ||||
| Flavomycin | |||||
| Feed intake, g/D | 90 | 91 | 0.743 | 24.082 | 78.41 |
| (n:102) | (n:93) | ||||
| Weight gain, g/D | 51 | 51 | 0.622 | 9.921 | 78.13 |
| (n:104) | (n:95) | ||||
| Feed conversion, g/g | 1.72 | 1.76 | 0.037 | 0.312 | 67.39 |
| (n:102) | (n:93) | ||||
| Virginiamycin | |||||
| Feed intake, g/D | 83 | 85 | 0.566 | 14.406 | 88.45 |
| (n:65) | (n:64) | ||||
| Weight gain, g/D | 49 | 47 | 0.042 | 9.117 | 78.77 |
| (n:70) | (n:69) | ||||
| Feed conversion, g/g | 1.68 | 1.75 | 0.015 | 0.165 | 76.63 |
| (n:65) | (n:64) | ||||
P: Probability of treatment effect. The models included the study effect (P < 0.001).
RSE: Residual standard error.
R2: Coefficient of determination of the model.
%: Percentage change between AGP+ and AGP−.
n: Number of studies observations in each mean.
The model considered the effect of the study (P < 0.001) and the mean weight of animals as adjusted variable (P < 0.001).
Economic Impact
AGP withdrawal in Initial phase and Total period increased the production cost in $0.01 and $0.03 per animal, totalizing an amount of $38,950,725 and $183,560,232 per year in Brazil (Table 4).
Table 4.
Simulation of the economic impact of the antibiotic growth promoter withdrawal from broiler diets containing antibiotic growth promoters (AGP+) or not (AGP−).
| Treatments |
|||
|---|---|---|---|
| AGP+ | AGP− | Variation | |
| Initial (1 to 21 D) | |||
| Feed conversion, g/g | 1.47 | 1.51 | 2.64 |
| Feed intake, g/animal | 1155 | 1176 | 1.78 |
| Feeding cost, $/animal | 0.27 | 0.28 | 0.01 |
| Feeding cost1, $/yr | 1,596,452,300 | 1,635,403,026 | 38,950,725 |
| Total (1 to 42 D) | |||
| Feed conversion, g/g | 1.66 | 1.72 | 3.48 |
| Feed intake, g/animal | 4.150 | 4.290 | 0.14 |
| Feeding cost, $/animal | 0.96 | 0.99 | 0.03 |
| Feeding cost, $/yr | 5,601,424,320 | 5,784,984,552 | 183,560,232 |
Feeding cost: considering 5,840,000,000 slaughtered broilers/yr (IBGE, 2017).
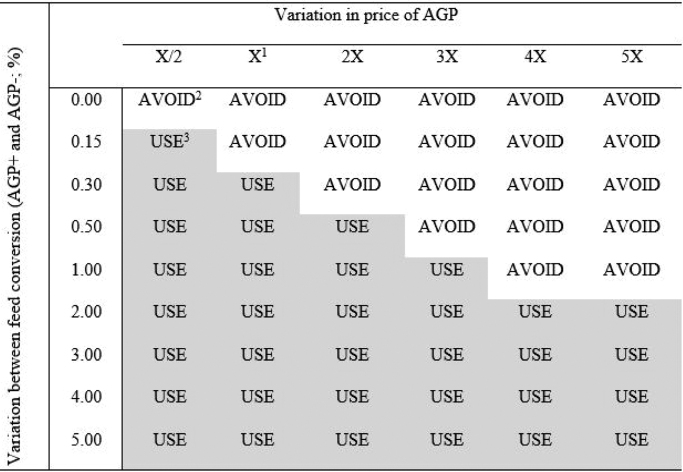
In the sensitivity analysis (Figure 2), “AVOID” indicates when it is possible not to use AGP without having a negative economic impact and “USE” indicates when the use of AGP usage is important to avoid economic losses. There is little elasticity between the variables not to use the AGP without economic losses. If the price of AGP increases 5 times compared to the current price, and the variation between the feed conversion of AGP+ and AGP− is at a maximum of 0.5%, there will be no economic losses taking out AGP. If the price of AGP is half of the current price with zero variation in feed conversion, there will be no negative economic impact. However, in the situation of the half of current price, a variation in feed conversion higher than 0.15% indicates the use of the additive. With the current AGP price and the results of feed conversion found in the meta-analysis (2.64% in Initial, 1.50% in the Final phase, and 3.48% in Total period), there will be economic losses withdrawing AGP.
Figure 2.
-
1X: current price of antibiotic growth promoter.
-
2AVOID: the situation where it is possible to raise broilers without AGP with no economic losses.
-
3USE: the situation where it is indicated the use of AGP to have no economic losses.
DISCUSSION
A reduction in the effectiveness of AGP in the last 30 yr was suggested by Laxminarayan et al. (2015), which may be linked to optimization of production conditions, increasing in the baseline weight gain of animals, increasing level of resistance, and potential switch in the type of molecules used. In contrast, the “year” effect was not significant in the current meta-analysis. This result may be associated to the fact that the studies selected to compose the database did not have any type of sanitary challenge.
Our results showed a clear connection between AGP feed supplementation and broiler performance, which was particularly evident in feed conversion and weight gain in Initial (1 to 21 D) and Total (1 to 42 D) phases and feed intake in the Initial phase. Instead, no relation between AGP usage and growth was found in the Final (22 to 42 D) phase. A multitude of factors can influence the performance results, including the environment, stress, and diet characteristics. Different mechanisms of action have been proposed and several studies have been carried out to explain AGP function: a growth-promoting effect may be associated to modification of some intestinal characteristics in the first week of life of chickens, as deeper crypts in the ileum, indicating faster tissue development (Miles et al., 2006). In addition, the studies show the ability of AGP to reduce normal early microbial proliferation, and hence competition for nutrients during the gut maturation, which takes approximately 6 to 9 D in chicks (Geyra et al., 2001). These alterations may be related to better nutrient absorption, resulting in lower feed intake and greater weight gain when compared to chickens that do not receive AGP in the Initial phase. Nutrient absorption is not the intestines’ sole function, as they perform an immunological role as well (Round et al., 2010). The close and intermittent contact of the gastrointestinal mucosa with the enteric microbiota results in a constant state of inflammation (Biancone et al., 2002) and can influence macromolecular intestinal permeability (MacDonald and Monteleone, 2005). AGP accumulates in inflammatory cells and enhance the intracellular killing of bacteria, inhibiting the innate immune response (Labro, 1998, 2000). Therefore, the use of AGPs decreases the catabolic costs of maintaining an immune response by allowing more resources to be dedicated to anabolic processes (Niewold, 2007). The first days of life of a chicken can be considered stressful, since it happens the vaccination management, transportation, setting in new place, and microbial exposures resulting from living on litter, as well as the introduction of a diet with anti-nutritional factors (Willis and Reid, 2008; Yassin et al., 2009). Considering the hypothesis of a non-antibiotic action of AGP, which results in a reduced intestinal inflammatory response (Niewold, 2007), this may be an explanation why AGP results in positive effects on the Initial phase. On the other hand, broilers in Final phase are much more able to cope with stressors, because the first contact with microorganisms has already occurred and it results in a lower level of immune response (Koutsos and Klasing,2014), and there is a reduction on total stress, since the adaptation of the animal to the environment already occurred.
Also has been proposed that AGP growth-promoting effect does coincide with a decrease in bile salt hydrolase (BSH) activity in the gut (Knarreborg et al., 2002, 2004; Guban et al., 2006; Lin, 2014). The lactobacilli are present in the crop and in the digestive tract (Lu et al., 2003; Hilmi et al., 2007), and this genus is responsible for BSH production, active in the small intestine (Moser and Savage, 2001), impairing the emulsification and absorption of dietary lipid. Other authors affirm that AGP is the most common dietary intervention to modulate the gut microflora (Dibner and Richards, 2005) and the activity of the intestinal microbiota, including both pathogenic and commensal bacteria (Lin et al., 2013). Some differences in the spectrum of activities, differing gut microbiota effects could be expected between different AGP, and this has been demonstrated in some studies (Neumann and Suen, 2015; Costa et al., 2017). As an example, Zinc bacitracin increased the diversity of the cecal microbiota of Cobb broilers, with increases in Faecalibacterium and Ruminococcus torques phylotype, and reductions in Lactobacillus salivarius phylotype and Eubacterium (Crisol-Martínez et al., 2017). All these mechanisms acting as interrelated multi factors may explain the best results observed in the Total period of rearing.
When AGP was analyzed individually, there is variation in performance responses. However, there is an improvement in feed conversion regardless of the AGP used. Avilamycin is an oligosaccharide classified as an orthosomysin. The Virginiamycin is a streptogramin combination that is a fermentation product of Streptomyces virginiae and Flavomycin is a polypeptide obtained from Streptomyces bambergiensis and Streptomyces ghanaensis (Giguère, 2013). The different results may be associated with the effectiveness of AGP in different mechanisms of action in the organism and the rearing phase of the animal. Niewold (2007) reported that AGP compounds can essentially be divided into groups based on their interaction with inflammatory cells, as non-accumulating, accumulating without inhibition of function, and accumulating with inhibition of function; for example, cyclines, macrolides (Tylosin) and peptides (Bacitracin) showed phagocyte-inhibition function (Labro, 2000). In another proposed mode of action, tetracycline antibiotics were consistently potent inhibitors of BSH. Both Roxarsone and Oxytetracycline inhibited BSH activity by over 95%. The β-lactam and lincosamide displayed a relatively lower inhibitory effect on BSH activity, and macrolide and peptides had a weak effect on BSH (Smith et al., 2014). In another point of view, there are different metabolites changes by dietary supplementation with AGP. Many long chain fatty acids in the intestine of bacitracin-supplemented were increased, but not in virginiamycin-supplemented chicks. At the same time, levels of amino acid metabolites (lysine and tryptophan) were substantially altered by dietary supplementation with virginiamycin or bacitracin (Gadde et al., 2018). The mechanisms of action of AGP are not yet completely clear, so it is not possible to attribute which one of them is linked to the differences in results.
The productive impact considered in this study was focused on the broilers feeding, and this can become part of a more complete economic impact analysis, taking into account aspects such as sanity, productive structure, labor, housing period, among others. Although it is based in a very simple approach, the model may provide relevant information for nutritionists and producers while facilitating the decision-making process. Some costs associated with the production system are difficult to measure and have not been included in the economic calculations (Kjeldsen and Callesen, 2006). Different authors report that the economic impact will affect producers differently, since there is a variation in the factors considered in the characterization of the productive scenario, such as farm size, contracting arrangements, and production practices (McBride et al., 2008; McDonald and Wang, 2011). On the other hand cost of feed is estimated to be more than 70%.
To better understand our findings in relation to production costs, it is safe to consider that the sum of feed and AGP cost increases total feeding costs, which conversely reduces feed cost by improving feed conversion ratio. The meta-analysis highlighted a reduction in broiler performance when fed AGP− diets, which may lead to an increase in production costs. Considering the current situation (AGP prices and 3.48 % of better feed conversion using AGP), the results from Total performance showed an increase of 0.8% in the cost of production per animal with the withdrawal of AGP. However, if techniques to reduce close to zero the difference in feed conversion between AGP+ and AGP− are used, it will be possible to raise broilers without AGP, as can be observed in the sensitivity analysis. To reduce the variation in feed conversion it is necessary to implement new management, using the biosecurity as a tool to reduce diseases (Gelaude et al., 2014) and vaccination to improve the overall health status, reducing the risk of secondary infections (M'Sadeq et al., 2015). As coccidiosis is considered a great risk factor to broilers reared without AGP, it is of foremost importance to understand Eimeria spp. cycle and the immunization process in flocks to benefit intestinal health (Parent et al., 2018). Also, the optimization of the climate and housing conditions and nutritional strategies are necessary to reduce the variation between AGP+ and AGP−. Various feed additives such as essential oils, copper sulfate, zinc oxide, probiotics, and prebiotics have been studied (Huyghebaert et al., 2011; Ali et al., 2017; Moraes et al., 2019) aimed to replace AGP. The use of organic acids in broiler diet can have a beneficial effect on the performance by decreasing pathogenic bacteria, like Salmonella, Campylobacter and Escherichia coli (Gharib Naseri et al., 2012). Polycarpo et al. (2017) observed a significant interaction between organic acids and microbiological challenge on FCR. Under experimental conditions carried out without microorganism inoculation, the organic acids improved broilers' FCR and presented similar results to antibiotics. Adding organic acid to the drinking water helps to regulate gut microflora and to increase the digestion of feed (Açıkgöz et al., 2011; Hamed and Hassan, 2013). Although it is possible to reduce the variation in feed conversion between AGP+ and AGP−, there is still a long way. It is necessary to evaluate strategies to improve productivity without the use of AGP and analyze other factors that directly interfere in the production cost besides the feeding.
CONCLUSION
Broilers fed diets with AGP show better performance in the total period of rearing, mainly because of its effect on the initial phase. The AGP withdrawal from broiler diet presents different results of performance in total phase according to the AGP used. The worse performance of broilers with withdrawal of AGP becomes a significant increase in production costs.
ACKNOWLEDGMENTS
The authors thank Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for funding this study.
Footnotes
Supplementary Material
REFERENCES
- Aclkgoz Z., Bayraktar H., Altan O. Effects of formic acid administration in the drinking water on performance, intestinal microflora and carcass contamination in male broilers under high ambient temperature. Asian Australas. J. Anim. Sci. 2011;24:96–102. [Google Scholar]
- Ali A., Rane A., Haq Z., Khan N., Bala S., Gupta M., Wani J. Comparative effect of polyherbal agents and organic acid to substitute antibiotic growth promoters in broilers for combating drus resistance. Environ. Ecol. 2017;35:372–376. [Google Scholar]
- Baurhoo B., Ferket P., Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Biancone L., Monteleone I., Blanco G.D.V., Vavassori P., Pallone F. Resident bacterial flora and immune system. Dig. Liver Dis. 2002;34:37–43. doi: 10.1016/s1590-8658(02)80162-1. [DOI] [PubMed] [Google Scholar]
- Costa M.C., Bessegatto J.A., Alfieri A.A., Weese J.S., João Filho A., Oba A. Different antibiotic growth promoters induce specific changes in the cecal microbiota membership of broiler chicken. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0171642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microb. Biotech. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Davies M., Walsh T.R. A colistin crisis in India. Lancet Infect. Dis. 2018;18:256–257. doi: 10.1016/S1473-3099(18)30072-0. [DOI] [PubMed] [Google Scholar]
- Denev S. Effect of different growth promoters on the cecal microflora and performance of broiler chickens. Bulgarian J. Agri. Sci. 2006;12:461. [Google Scholar]
- Dibner J., Richards J. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- El-Faham A., Ahmed A., El-Sanhoury M. Thyme leaves or its extracted oil for enhancing productive and physiological status of broiler chickens. Egyptian Poult. Sci. J. 2015;35:215–236. [Google Scholar]
- Gadde U.D., Oh S., Lillehoj H.S., Lillehoj E.P. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-22004-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gelaude P., Schlepers M., Verlinden M., Laanen M., Dewulf J. Biocheck. UGent: a quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult. Sci. 2014;93:2740–2751. doi: 10.3382/ps.2014-04002. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. Br. J. Nut. 2001;86:53–61. doi: 10.1079/bjn2001368. [DOI] [PubMed] [Google Scholar]
- Gharib Naseri K., Rahimi S., Khaki P. Comparison of the effects of probiotic, organic acid and medicinal plant on Campylobacter jejuni challenged broiler chickens. J. Agri. Sci. Tech. 2012;14:1485–1496. [Google Scholar]
- Giguère S. In: Antimicrobial Therapy in Veterinary Medicine. Giguère Steeve, Prescott John F., Dowling Patricia M., editors. 2013. Ames, Iowa. [Google Scholar]
- Goutard F.L., Bordier M., Calba C., Erlacher-Vindel E., Góchez D., de Balogh K., Benigno C., Kalpravidh W., Roger F., Vong S. Antimicrobial policy interventions in food animal production in South East Asia. BMJ. 2017;358 doi: 10.1136/bmj.j3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guban J., Korver D.R., Allison G.E., Tannock G.W. Relationship of dietary antimicrobial drug administration with broiler performance, decreased population levels of Lactobacillus salivarius, and reduced bile salt deconjugation in the ileum of broiler chickens. Poult. Sci. 2006;85:2186–2194. doi: 10.1093/ps/85.12.2186. [DOI] [PubMed] [Google Scholar]
- Hamed D.M., Hassan A.M.A. Acids supplementation to drinking water and their effects on Japanese quails experimentally challenged with Salmonella enteritidis. Res Zool. 2013;3:15–22. [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Hilmi H.T.A., Surakka A., Apajalahti J., Saris P.E. Identification of the most abundant Lactobacillus species in the crop of 1-and 5-week-old broiler chickens. Appl. Envir. Micr. 2007;73:7867–7873. doi: 10.1128/AEM.01128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Brasileiro De Geografia E Estatística. 2017. Indicadores IBGE: estatística da produção pecuária. Rio de Janeiro. Accessed: Jan. 2018.
- Kahn L.H. Antimicrobial resistance: a One Health perspective. Trans. R. Soc. Trop. Med. Hyg. 2017;111:255–260. doi: 10.1093/trstmh/trx050. [DOI] [PubMed] [Google Scholar]
- Kjeldsen N., Callesen J. In: BOKU-Symposium Tierernährung ohne Antibiotische Leistungsförderer. Barug D., de Jong D., Kies A.K., Verstegen M.W.A., editors. Universität für Bodenkultur Wien: The National Committee for Pig Production; Vienna: 2006. Terminated use of antimicrobial growth promoters in pig production in Denmark: effects on pig welfare and productivity; pp. 127–135. [Google Scholar]
- Knarreborg A., Engberg R.M., Jensen S.K., Jensen B.B. Quantitative determination of bile salt hydrolase activity in bacteria isolated from the small intestine of chickens. Appl. Envir. Micr. 2002;68:6425–6428. doi: 10.1128/AEM.68.12.6425-6428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knarreborg A., Lauridsen C., Engberg R.M., Jensen S.K. Dietary antibiotic growth promoters enhance the bioavailability of α-tocopheryl acetate in broilers by altering lipid absorption. J. Nutr. 2004;134:1487–1492. doi: 10.1093/jn/134.6.1487. [DOI] [PubMed] [Google Scholar]
- Koutsos E.A., Klasing K.C. Avian Immunology. 2nd ed. Elsevier; 2014. Factors modulating the avian immune system; pp. 299–313. [Google Scholar]
- Labro M.-T. Antibacterial agents—phagocytes: new concepts for old in immunomodulation. Int. J. Antimic. Agents. 1998;10:11–21. doi: 10.1016/s0924-8579(98)00012-0. [DOI] [PubMed] [Google Scholar]
- Labro M.-T. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin. Microbiol. Rev. 2000;13:615–650. doi: 10.1128/cmr.13.4.615-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R., Van Boeckel T., Teillant A. OECD Food, Agriculture and Fisheries Papers; Paris: 2015. The Economic Costs of Withdrawing Antimicrobial Growth Promoters from the Livestock Sector. No. 78. [DOI] [Google Scholar]
- Lin J. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front. Microbiol. 2014;5:33. doi: 10.3389/fmicb.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y.-J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Lovatto P., Lehnen C., Andretta I., Carvalho A., Hauschild L. Meta-análise em pesquisas científicas-enfoque em metodologias. Rev. Soc. Bras. Zoot. 2007;36:285–294. [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Envir. Micr. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W.D., Key N., Mathews K.H. Subtherapeutic antibiotics and productivity in U.S. hog production. Appl. Econ. Perspect. Policy. 2008;30:270–288. [Google Scholar]
- MacDonald J.M., Wang S.L. Foregoing sub-therapeutic antibiotics: the impact on broiler grow-out operations. Appl. Econ. Perspect. Policy. 2011;33:79–98. [Google Scholar]
- MacDonald T.T., Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- MAPA – Ministério da Agricultura, Pecuária e Abastecimento Instrução Normativa n°. 13/04. Regulamento técnico sobre aditivos para produtos destinados à alimentação animal. Diário Oficial da União, Brasília-DF. 2016. Access: http://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos.pecuarios/alimentacao-animal/aditivos
- Miles R., Butcher G., Henry P., Littell R. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Moser S.A., Savage D.C. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Appl. Envir. Micr. 2001;67:3476–3480. doi: 10.1128/AEM.67.8.3476-3480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes P.O., Cardinal K.M., Gouvêa F.L., Schroeder B., Ceron M.S., Lunedo R., Ribeiro A.M.L. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poult. Sci. 2019;0:1–9. doi: 10.3382/ps/pez345. [DOI] [PubMed] [Google Scholar]
- Naveenkumar S., Karthikeyan N., Babu R.N., Veeramani P., Sivaramakrishnan S. Effect of organic acid salts as an alternative to antibiotic growth promoters on the production performance of commercial broiler chicken. Int. J. Curr. Microbiol. App. Sci. 2017;6:3470–3480. [Google Scholar]
- Neumann A., Suen G. Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate. J. Appl. Microb. 2015;119:1515–1526. doi: 10.1111/jam.12960. [DOI] [PubMed] [Google Scholar]
- Niewold T. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- Parent E., Fernandez D., Boulianne M. The use of a live non-attenuated coccidiosis vaccine modifies Eimeria spp. excretion in commercial antibiotic-free broiler chicken flocks compared to conventional shuttle anticoccidial programs. Poult. Sci. 2018;97:2740–2744. doi: 10.3382/ps/pey140. [DOI] [PubMed] [Google Scholar]
- Peng Q., Li J., Li Z., Duan Z., Wu Y. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016;214:148–153. [Google Scholar]
- Polycarpo G.V., Andretta I., Kipper M., Cruz-Polycarpo V.C., Dadalt J.C., Rodrigues P.H.M., Albuquerque R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017;96:3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., O'Connell R.M., Mazmanian S.K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 2010;34:220–225. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H., Huque K.S., Kamaruddin K.M., Beg M. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018;101:52–75. doi: 10.3184/003685018X15173975498947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltelli A., Chan K., Scott E.M. Wiley; New York: 2000. Sensitivity Analysis. [Google Scholar]
- Sauvant D., Schmidely P., Daudin J. Les méta-analyses des données expérimentales: applications en nutrition animale. INRA Productions Animales. 2005;18:23–33. [Google Scholar]
- Smith K., Zeng X., Lin J. Discovery of bile salt hydrolase inhibitors using an efficient high-throughput screening system. Plos ONE. 2014;9 doi: 10.1371/journal.pone.0085344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. The Lancet Planet. Health. 2017;1:316–327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA . USDA GAIN; Washington: 2016. MARD Phases out growth promotion usage of antibiotics in feed.https://gain.fas.usda.gov/Recent%20GAIN%20Publications/MARD%20phases%20out%20of%20growth%20promotion%20usage%20of%20antibiotics%20in%20feed_Hanoi_Vietnam_7-6-2016.pdf [Google Scholar]
- Walsh T.R., Wu Y. China bans colistin as a feed additive for animals. Lancet Infect. Dis. 2016;16:1102–1103. doi: 10.1016/S1473-3099(16)30329-2. [DOI] [PubMed] [Google Scholar]
- Willis W.L., Reid L. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci. 2008;87:606–611. doi: 10.3382/ps.2006-00458. [DOI] [PubMed] [Google Scholar]
- Wu S., Li T., Niu H., Zhu Y., Liu Y., Duan Y., Sun Q., Yang X. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult. Sci. 2018;98:828–841. doi: 10.3382/ps/pey393. [DOI] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G., Boerjan M., van Riel J. Field study on broilers' first-week mortality. Poult. Sci. 2009;88:798–804. doi: 10.3382/ps.2008-00292. [DOI] [PubMed] [Google Scholar]
- Zhang K., Yan F., Keen C., Waldroup P. Evaluation of microencapsulated essential oils and organic acids in diets for broiler chickens. Int. J. Poult. Sci. 2005;4:612–619. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.