Abstract
The goal of this experiment was to measure the physiological response of individual laying hens exposed to heat stress (HS). Performance, egg quality, body temperature (BT), and blood chemistry of laying hens were individually recorded before and after various intervals of daily cyclic HS. In total, 407 18-week-old W-36 parent-line laying hens (Hy-Line International, Dallas Center, IA) were housed individually in battery cages. After an acclimation period, baseline data were collected from 22 to 24-wk before the hens were subjected to a daily cyclic HS consisting of 7 h at 35°C returning to 30°C for the remaining 17 h/D from 24 to 28-wk of age. Eggs were collected and individually weighed daily. Feed intake (FI), egg production (EP), egg weights, egg mass, BW, and feed efficiency (FE) (g egg/kg FI) were calculated over 2-wk time periods. Eggs were collected for quality assessment the day before HS began, the 2nd day of HS, and on a weekly basis throughout the 4-wk HS. Blood was collected and BT measured the day before heat HS was initiated, on the first day of HS, and again at 2 and 4-wk of HS. Blood PCO2 and iCa decreased, and blood pH increased within 4 to 6 h of HS (P ≤ 0.01). Shell weights decreased with acute HS, possibly due to the reduction in blood iCa (P ≤ 0.01). After 4-wk of HS the blood pH returned to pre-HS levels but iCa remained decreased (P ≤ 0.01). Shell weights remained low and Haugh units decreased after 2 and 4-wk of HS (P ≤ 0.01). Feed efficiency was increased and FI, EP, and BW decreased by 2-wk of HS and remained low through 4-wk (P ≤ 0.01). The cyclic HS had a significant effect on the performance, egg quality, and blood chemistry over the 4-wk HS.
key words: heat stress, performance, laying hen, blood chemistry, egg quality
INTRODUCTION
The optimum environmental temperature for performance of adult laying hens is between 19 and 22°C, with temperatures above and below this range requiring thermoregulation (Lin et al., 2006). High temperatures can ultimately result in heat stress (HS) causing decreased performance, altered blood chemistry, and increased mortality (Khan et al., 2011; Kilic and Simsek, 2013). Recent estimates for HS losses due to the reduced performance and increased mortality in the poultry industry were up to $98 million per year for laying hens in the US alone (Key et al., 2014).
When exposed to high environmental temperature, the laying hen will increase and deepen respiration rates to reduce body temperature (BT) through evaporative cooling (El Hadi and Sykes, 1982). The increased respiration rate can cause the partial pressure of carbon dioxide in the blood to be reduced, changing the ratio of bicarbonate to carbon dioxide and ultimately resulting in an increase in the pH of the blood, a process known as respiratory alkalosis (Franco-Jimenez et al., 2007; Koelkebeck and Odom, 1994). The elevated blood pH can cause the bioavailable form of blood calcium, ionized calcium (iCa), to bind to proteins making it unavailable for use in the shell gland to form the eggshell (Etches et al., 2008). This can result in reduced eggshell quality shortly after the hen is exposed to HS (Samara et al., 1996). Another response to elevated environmental temperatures is to reduce feed intake (FI) which limits the heat produced from digestion (Kilic and Simsek, 2013). The reduced FI will ultimately reduce egg production (EP) due to insufficient energy and nutrient intake to sustain EP at non-HS levels (Mashaly et al., 2004).
The objective of this experiment was to quantify the responses of individual laying hens in FI, EP, egg quality, BW, and blood gas parameters before and after elevated thermal exposure, quantifying both acute (4 to 6 h) and chronic (2 and 4 wk) exposure to a daily cyclic HS. The performance and physiological changes observed have also been associated with the individual hen's 600 K single nucleotide polymorphisms in a genome wide association study (Rowland et al., 2019; Rowland et al., in review). With that information it may be possible to select for heat resistant hens that are able to maintain higher EP and performance, and use them to at least partially overcome negative impacts of HS. The phenotypic data are important to understand the performance and physiological changes the laying hen experiences during acute HS and potential adaptation over chronic HS.
MATERIALS AND METHODS
Animals and Housing
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Virginia Tech. A total of 407 18-wk old W-36 female parent line laying hens were obtained from Hy-Line International (Dallas Center, IA). The hens were hatched in late February, raised during spring, and shipped overnight via truck and trailer to Virginia Tech facilities in Blacksburg, VA to minimize the environmental temperature extremes before the initiation of heat treatment. Upon arrival to Virginia Tech, the pullets were housed individually in cages (38.1 cm L × 22.9 cm W × 43.2 cm H) allowing 871 cm2 per hen, within a climate-controlled room that was maintained at approximately 23°C and 55% relative humidity. The cages were arranged in 3 tiers with 4 cages per tier and were placed on wheels. A water trough was shared by 2 neighboring hens while feed was provided via individual feeders. A plastic mesh divider was utilized between feeders to ensure that hens consumed feed from designated feeder and not surrounding feeders. Hens were given ad libitum access to a mash layer diet formulated to 2,900 kcal/kg (Table 1) and water. The lighting schedule began at 11.5 L:12.5 D and light increased by 0.5 h each wk until the birds were 24 wk of age at which point it increased by 0.25 h each wk until the end of the experiment. Light levels were measured to be 1 to 2 foot candles at the feeder and cardboard covers were used on the top tier of cages to reduce light intensity to be similar to the lower tiers.
Table 1.
Composition of layer diet.
| Ingredient | (%) | Calculated composition | (%) |
|---|---|---|---|
| Corn | 44.85 | Metabolizable energy (kcal/kg) | 2900 |
| Soybean meal, 48% CP | 34.52 | Crude protein | 22.40 |
| Meat and bone meal | 2.49 | Calcium | 4.00 |
| DDGS | 1.99 | Non-phytate phosphorus | 0.45 |
| Soy oil | 4.40 | Fat | 6.85 |
| DL-Methionine | 0.27 | Digestible Met + Cys | 0.88 |
| Limestone, small | 4.41 | Digestible lysine | 1.11 |
| Limestone, large | 4.41 | Digestible threonine | 0.77 |
| Dicalcium phosphate | 1.18 | ||
| Salt | 0.42 | Analyzed composition | (%) |
| Vitamin and mineral premix1 | 0.75 | Crude protein | 22.5 |
| Titanium dioxide | 0.30 | Fat | 3.63 |
Provided per kg of diet: vitamin A, 6595.69 IU; vitamin D3, 2209.56 ICU; vitamin E, 1.65 IU; vitamin B12, 6.60 μg; menadione, 1.15 mg; riboflavin, 4.12 mg; D-pantotheic acid, 6.07 mg; niacin, 19.79 mg; choline, 381.68 mg; Co, 0.25 mg; Cu, 4.04 mg; I, 1.00 mg; Fe, 50.65 mg; Mn, 64.26 mg; Zn, 48.69 mg.
HS
From 24 to 28 wk of age the hens were subjected to a daily, cyclic HS. On the first day of exposure, 2 identical HS chambers were brought to temperature. Each room was heated using a single heater (TPI Corporation model# F3F551QT, 114 Roscoe Fitz Rd, Gray, TN 37615) in the corner of each room which is capable of heating the room to 40.5°C. Mixer fans were used to minimize the temperature difference across the room as well as among the tiers. When the room temperatures reached 35°C, 201 hens (housed within individual cages) were rolled into room 1 and the remaining 206 hens into room 2. The cages would remain in the same rooms throughout the experiment. The hens were moved into pre-heated rooms to allow for timed collection of short term HS samples for physiological measurements. Each day the heat was raised to 35°C for 6.5 h beginning at 9am and then returned to 30°C for the rest of the day. The non-HS portion of the day was maintained at a temperature above thermoneutral conditions to better mimic the conditions found in a commercial laying house. There was a transition period of approximately 30 min each day at the beginning and end of HS as room temperatures were rising or falling. The relative humidity of the rooms averaged 41% and 45% during the HS and non-HS portions of the day, respectively. On sampling days one room began the 6.5-hour HS at 6am and the other at 8am to facilitate sample collection over a specified time. Hens suffering severe heat distress, defined as unable to stand, lying on its side, and generally unresponsive (Persia et al., 2003) were euthanized by cervical dislocation. All mortalities or euthanized birds were recorded, weighed, and their feed refusal was weighed at the time of removal. Data previously collected from removed birds was not used in statistical analysis.
Sample and Data Collection
Eggs were collected and weighed between 9:00 and 10:00 am each day. Egg production, egg weights (EW), and egg mass (EM) (calculated using EP and mean EW) were all recorded individually on a daily basis and summarized over a 2-wk period. Feed provided to each hen was determined by providing feed in 90 g increments (a level cup of feed). This method was validated before the experiment and was possible as all feed was similar in density. Feed refusal was recorded every 2 wk (2 wk prior to HS, wk 1, and 2 of HS and, wk 3 and 4 of HS) to determine individual FI. Eggs were collected the day before HS, the second day of HS, and weekly for the 4-wk heat period to determine egg quality changes. The eggs were collected on the second day so that eggs measured for acute HS measurements were being formed during the first day of exposure. The collected eggs were refrigerated for approximately 1 wk to minimize changes until analysis. Body temperature was measured the day before HS exposure, the first day of HS exposure, and again at the 2 and 4 wk time point of HS exposure. To measure BT, a thermometer (DeltaTrak MDL11064) was inserted approximately 1.5 cm into the cloaca of each hen, and allowed to stabilize before the temperature was recorded. Blood was taken from the ulnar vein from the wing of each hen. A new 1 mL syringe with a 25-gauge × 1.6 cm needle was used to draw approximately 1 mL of blood for each sample. Before attaching the needle, a solution of 10,000 units lithium heparin per mL was drawn into the syringe and expelled. The blood sampled was taken within 4 to 6 h after initiation of HS.
Analyses
Eggs were analyzed for Haugh unit using a Mattox and Moore Haugh meter using the method described by Maxkwee et al. (2014). After the Haugh unit was determined, the yolk was separated from the albumen and the weights of each were recorded. The eggshells with membranes intact were placed onto individually labeled trays and dried overnight at 65°C. When the eggshells were fully dried, their weights and thicknesses were recorded. Following the methods of Emery et al. (1984), the dried membrane was removed and 3 individual measurements were taken from the equator of each eggshell to determine the shell thickness and an average value calculated per egg.
The collected blood was analyzed within 5 min of collection using an Abaxis iSTAT 1 handheld analyzer with a CG8+ cartridge (Abaxis item number 600-9001). The CG8+ cartridge measures sodium, potassium, iCa, glucose, pH, PCO2, PO2, TCO2, HCO3, BE, and sO2. Hematocrit and hemoglobin content of the blood are estimated by electrical conductance. Once collected, the needle was removed from the syringe and the first few drops of blood were discarded before a blood sample was placed into the cartridge up to the fill line of 95 μL. The filled cartridge was placed into the iSTAT analyzer. To ensure timely analysis of samples, multiple handheld units were utilized.
Statistical Analyses
All data were analyzed using ANOVA. Heat stress exposure was the response criteria with a pre-HS measurement (1 D before HS exposure for physiological and egg responses and 2 wk before HS for performance responses), an acute HS measurement (2 to 4 h post HS exposure for physiological responses, 1 D after HS exposure for egg responses and 2 wk after for performance responses) and chronic HS responses (2 to 4 h post HS exposure after 2 and 4 wk of cyclic HS exposure for physiological responses, 2 and 4 wk after HS exposure for egg responses and 2 to 4 wk after HS exposure for performance responses). Control data were generated using the same bird population immediately before HS treatment. This approach does have some limitation due to increased performance and EW over this time period of the experiment, and when this occurred breed standardized data were used to for additional comparisons. Significant differences were accepted at (P ≤ 0.05). If ANOVA was significantly different, a Tukey's test was used to separate mean effects. Coefficients of variance were calculated for all parameters. The coefficients of variance were obtained by calculating the standard deviation of each parameter at each time point and dividing by the mean from the same data set. This value was multiplied by 100 to get a percentage. These data are not statistically analyzed and are provided for observational data. Pearson correlation coefficients were calculated among all traits at all time points, were estimated using the cor.test (test for association between paired samples) function of the stats package in R.
RESULTS AND DISCUSSION
Performance
There was a drop in FI (P ≤ 0.05) after 2 wk of HS when compared to the 2 wk prior to the beginning of exposure (Table 2). During HS, hens reduce FI to decrease the heat of digestion (Kilic and Simsek, 2013). After the initial drop in FI, it increased by 14% (51.2 to 58.4 g/h/D) from 2 to 4 wk compared to the 2 wk HS exposure values (P ≤ 0.05); however, it did not fully recover to the level prior to HS. This indicates that the hens may have begun to acclimate to the high environmental temperatures although they were still limiting FI. Mashaly et al. (2004) showed that constant HS exposure had a more severe effect on FI when compared to cyclic HS. The FI over a 5 wk period for the constant HS dropped by 52% compared to the control, while the cyclic group dropped by 37% compared to the control (Mashaly et al., 2004). The FI for the 2 wk period before HS was slightly higher than average FI indicated by the Hy-Line management guide. For parent hens aged 22 to 24 wk, the Hy-Line guide indicates an average FI of 92 g/bird/D. The diet was formulated to contain 6.85% crude fat, however after analysis it was found to contain only 3.63%. Both the lower than expected crude fat content and the hens being housed individually with access to their own feeder could be responsible for the elevated pre-HS FI (95.4 g/hen/D) when compared to published values. The fact that the pre-HS FI was elevated could have led to the drop of FI during HS to be more pronounced.
Table 2.
Effect of a 4-wk, daily, cyclic heat stress on performance of laying hens.1
| Time period |
Feed intake |
Egg production |
Egg weight |
Egg mass |
Feed efficiency |
Body weight |
|---|---|---|---|---|---|---|
| (g/D) | (%) | (g) | (g/D) | (g/kg) | (kg) | |
| 2 wk before HS | 95.4a | 90.1a | 58.0b | 52.3 | 611c | 1.53a |
| 2 wk after HS | 51.2c | 84.4b | 60.9a | 51.4 | 1219a | 1.47b |
| 4 wk after HS | 58.4b | 85.6b | 60.5a | 51.8 | 1053b | 1.45c |
| Pooled SEM | 0.41 | 0.54 | 0.32 | 0.38 | 9.1 | 0.004 |
| P-value | ≤0.01 | ≤0.01 | ≤0.01 | 0.28 | ≤0.01 | ≤0.01 |
Means values of data from 398 individual hens in both rooms combined.
Values without similar letters within parameter are significantly different (P ≤ 0.05).
Egg production dropped from 90.1% prior to HS to 84.4% after 2 wk of exposure, a drop of 6.3% (P ≤ 0.05), and did not recover by the end of 4 wk, remaining low at 85.6% (Table 2). According the the Hy-Line management guide, during this time frame the hens were expected to increase in production under thermoneutral conditions (Hy-Line Int. USA, 2016). The reduced FI and hence nutrient intake resulted in insufficient nutrients to maintain EP at non-HS levels (Mashaly et al., 2004). There was an increase in EW from the pre-HS value to 2 wk HS, from 58.0 to 60.9 g/egg (P ≤ 0.05) but no further increase from the 2- to 4-wk HS period remaining at 60.5 g/egg. Other reports have demonstrated a decrease in EW within 1 wk of exposure of either cyclic or constant HS (de Andrade et al., 1977; Emery et al., 1984; Franco-Jiminez et al., 2007; Maak et al., 2003; Mashaly et al., 2004). As the hens were still young during this experiment (22 to 28 wk of age), with a natural increase in EW the treatment effect to reduce EW was delayed in comparison to previous reports.
Due to a decreased EP and an increased EM, EW measured as g egg produced/hen/D did not change during HS (P = 0.28; Table 2). Because the drop in FI was more pronounced than the drop in EP, the feed efficiency (FE) nearly doubled, from 611 to 1,219 g egg/kg FI (P ≤ 0.05), from 2 wk prior to 2 wk post HS exposure, respectively. For the 2 to 4 wk post HS period, FE dropped by 13% from 1,219 to 1,053 g/kg (P ≤ 0.05) as FI started to increase, but EP was further decreased. The pre-HS FE was lower than expected, likely caused by the high FI for that time period that might be related to the individual housing of the laying hens. Unlike the current experiment, FE was not affected by a 35°C to 16°C cyclic HS treatment (Deaton et al., 1982). This cyclic HS with a lower non-HS temperature of 16°C resulted in no significant differences in FI or EP, and no change in FE as the birds were likely able to consume adequate feed during the non-HS period in contrast to the current experiment when non-HS temperature were maintained at elevated temperatures (30°C). Before HS was initiated, the hens had an average BW of 1.53 kg, which dropped to 1.47 kg (P ≤ 0.05) by 2 wk of HS exposure and remained low after 4 wk at 1.45 kg (P ≤ 0.05) (Table 2). The increased FE noted with HS was most likely related to BW reductions over the duration of the HS period (Table 2). The loss of BW may have supported the increased FE as the hens were able to mobilize body stores to help maintain EP even during reduced FI.
On the first day of HS exposure (acute effects) the hen's BT rose to 42.4°C compared to 41.3°C the day before HS treatment was initiated (P ≤ 0.05) (Table 3). By 2 and 4 wk of exposure to high environmental temperature the hen's BT had decreased to 41.7°C. The hen's BT after chronic HS was significantly lower than the acute BT value, but did not decrease to the BT of the pre-HS hens. Previous research examining the effect of a cyclic HS on laying hens has shown an elevated BT through 2 wk when compared to hens maintained at thermoneutral conditions (Rozenboim et al., 2007), and similar results were seen with broilers exposed to a constant HS for 2 wk (He et al., 2018). There are few published data on BT of laying hens exposed to a HS greater than 2 wk in length. The current data could indicate that by 2 wk of exposure to HS, the hens were able to at least partially acclimate to increased environmental temperatures but that BT will remain elevated.
Table 3.
Effect of a 4-wk long, daily, cyclic heat stress over time on egg quality parameters and body temperature in laying hens.1
| Yolk weight |
Albumen weight |
Shell weight |
Shell thickness |
Body temperature |
||
|---|---|---|---|---|---|---|
| Time period | Haugh unit | (g) | (g) | (g) | (mm) | (°C) |
| Control2 | 94.4a | 14.1b | 35.0a | 6.04a | 0.39b | 41.3c |
| Acute | 94.6a | 14.7a | 34.5a,b | 5.60b | 0.39b | 42.4a |
| Chronic 2wk | 88.4b | 14.7a | 33.9b,c | 5.60b | 0.41a | 41.7b |
| Chronic 4wk | 87.2c | 14.4a,b | 33.4c | 5.48c | 0.35c | 41.7b |
| Pooled SEM | 0.36 | 0.12 | 0.24 | 0.029 | 0.003 | 0.04 |
| P-value | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
Means values of data from 398 individual hens in both rooms combined.
Control values were measured the day before first exposure to HS.
Values without similar letters within parameter are significantly different (P ≤ 0.05).
There was no mortality over the 4 wk HS period; 9 hens were euthanized due to signs of heat distress. A total of 7 of the 9 hens that were removed occurred in the first wk of HS exposure.
Egg Quality
The Haugh unit, a measure of albumen quality, significantly decreased after 2 wk of chronic HS exposure when compared to the pre-HS and acute HS values (Table 3). The Haugh unit again decreased by the end of the 4 wk HS exposure after an additional 2 wk of chronic HS treatment. Previous experiments have shown that while there was a reduction in performance parameters, no significant change occurred in either the albumen height, over a 5-wk cyclic or constant HS (Mashaly et al., 2004), or Haugh unit, during a 2-wk constant HS (Franco-Jiminez et al., 2007). Eggs collected after 1 D of exposure to the high temperatures did not have a significantly different Haugh unit when compared to those collected pre-HS. In the previous experiment by Franco-Jiminez et al. (2007), there was a drop in both overall EW as well as albumen weights, this may have led to the lack of response seen with Haugh unit measurement. Albumen weights at the acute HS time point were not significantly reduced when compared to pre-HS values, but after 2 wk of chronic exposure, the albumen weights were significantly lower than those of the pre-HS birds (Table 3). Throughout the 4 wk chronic HS the albumen weights remained low, never returning to pre-HS values. As Haugh units are calculated by albumen height (related to albumen weight) the reduced albumin weights are most likely the cause of the reduced Haugh unit noted with chronic HS exposure. The reduction in albumen weight was also noted by Mashaly et al. (2004).
The yolk weights were significantly greater in eggs measured after acute exposure to HS (14.7 g) compared to pre-HS (14.1 g) (Table 3). The yolks remained heavier through 2 wk of HS (P ≤ 0.05), but by 4 wk of HS, the yolk weights were lower, resulting in a value intermediate to the pre-HS and acute/2 wk HS values. Mashaly et al. (2004) reported yolk and overall EW were decreased with HS exposure. Shell weights were decreased by both acute and chronic HS treatments (Table 3). A similar response of eggshell weights was seen previously as hens exposed to either constant or cyclic HS had significantly reduced eggshell weights in 4 out of 5 wk HS exposure. By the fifth wk of cyclic HS treatment there was no difference in shell weight with the non-HS treatment; however, the hens exposed to a constant HS had significantly reduced eggshell weight and the overall 5 wk means were significantly reduced for both HS treatments than the control treatment (Mashaly et al., 2004). In the current experiment, the shell thickness did not change with acute HS when compared to the pre-HS treatment but was reduced after 4 wk of chronic HS resulting in the thinnest shell thickness value. Contrary to the current experiment, Franco-Jiminez et al. (2007) found that both eggshell weights and thicknesses decreased together when hens were exposed to a 2 wk constant HS. A reduction in eggshell quality of hens exposed to both cyclic and constant HS has been shown by several experiments (Balnave and Muheereza, 1997; Mahmoud et al., 1996; Mashaly et al., 2004; Odom et al., 1986). This may be due to the decrease in blood iCa caused by a change in blood chemistry or by a reduction in calcium absorption in the small intestine (Mahoud et al., 1996).
Blood Chemistry Parameters
The acute and chronic effects of HS on blood chemistry values of laying hens are reported in Tables 4 and 5. In this experiment, the partial pressure of carbon dioxide in the hen's blood dropped by 29% after only 4 to 6 h of acute exposure to the high environmental temperatures (P ≤ 0.01). During the same time period, the blood bicarbonate dropped by 19% (P ≤ 0.01) and the blood pH increased by 0.8% (P ≤ 0.01). Bicarbonate ions buffer the acid–base status of the blood (Franco-Jiminez et al., 2007; Odom, et al., 1986). Reference values for blood chemistry parameters were determined by Schaal et al. (2016) in first cycle Hy-Line W-36 commercial laying hens (n = 377). These reference ranges are presented in Tables 4 and 5 as the determined mean plus or minus one standard deviation to be used in comparison with current data. The pre-HS values for the partial pressure of carbon dioxide were on the lower end of the reference range while the concentration of bicarbonate was near the mean reference value. This may have contributed to the pre-HS value for pH to be slightly higher than the reference range of 7.23 to 7.37. The blood partial pressure of carbon dioxide and bicarbonate increased (P ≤ 0.01) by 2 wk of chronic HS exposure, corresponding to a decrease of 0.1% in the blood pH (P ≤ 0.01). By the end of the experiment at 4 wk, the partial pressure of carbon dioxide in the blood had increased again by 13%; however, it was still lower than that of the pre-HS value (P ≤ 0.01). The blood bicarbonate decreased again by 4% at the last measurement point after 4 wk of heat exposure (P ≤ 0.01). By the end of the experiment the blood pH had dropped by 0.9% compared to the 2 wk value (P ≤ 0.01), returning the blood pH value to slightly below the pre-HS values. The hen will pant in order to dissipate heat through evaporative cooling often causing the partial pressure of carbon dioxide to decrease (El Hadi and Sykes, 1982). This will change the ratio of carbon dioxide to bicarbonate ions in the blood, contributing to an increase in the blood pH (Franco-Jimenez et al., 2007; Koelkebeck and Odom, 1994). Calcium is typically found in a bio-available ionic form for eggshell generation. An increase in blood pH can cause the iCa to bind to protein, becoming unavailable for use in eggshell formation (Odom et al., 1986). Table 4 shows a drop of iCa in the blood of 22.7% from 1.41 to 1.09 mmol/L on the first day of HS when compared to the pre-HS value (P ≤ 0.01). By 2 wk of HS the iCa had recovered to 1.36 mmol/L (P ≤ 0.01), but was still reduced in comparison to pre-HS levels. Between 2 and 4 wk of the experiment, the blood iCa dropped again to 1.28 mmol/L (P ≤ 0.01). The drop in blood iCa with acute exposure to HS could result in an immediate reduction in eggshell quality (Samara et al., 1996). The pre-HS value of iCa of 1.41 mmol/L in this experiment was below the reference range of 1.55–1.83 for commercial laying hens (Schaal et al., 2016). The reference values were taken from hens aged 20 to 68 wk while the hens in the current experiment were 22 to 24 wk old so the reference values could be higher due to higher dietary Ca concentrations fed to older laying hens under commercial conditions. The blood glucose dropped from 231 mg/dL the day before HS to 228 mg/dL on the first day of HS exposure (P ≤ 0.05; Table 5). There were significant changes in blood glucose during this experiment (Table 5). However, they were all within the reference values. The iSTAT machine, developed for use in mammals, estimates hematocrit and hemoglobin by electrical conductance rather than standard clinical chemistry assays. Because chicken blood cells are both nucleated and elongated, comparison of the levels estimated for hematocrit and hemoglobin between prior and the current studies should be done with caution (Rowland et al., 2019). Both the iSTAT-defined hematocrit and concentration of hemoglobin decreased during exposure to HS. Within 4 to 6 h of first exposure, the hematocrit and hemoglobin concentration dropped, increasing at 2 wk but not to pre-HS level, and dropping again by the end of the experiment at 4 wk post HS, similar to results found by Yahav et al. (1997). Initially, the hematocrit and hemoglobin concentrations may have decreased due to the response to acute HS, then increased as the hens became acclimated to the high temperatures, and decreased by 4 wk because of the prolonged reduction in FI. The concentration of sodium and potassium ions in the blood followed the same trend as the hematocrit, decreasing for the acute time point, increasing by 2 wk of HS, and decreasing again by 4 wk of HS never recovering to pre-HS values. In previous experiments, the volume of plasma increased during HS possibly in order to allow for a reduction in resistance of blood flow to better dissipate heat (Yahav et al., 1997). This increase in plasma volume could cause a decrease in the concentration of ions in the blood as well as the hematocrit and hemoglobin concentration. Genetic analyses of blood chemistry components, including heritability estimation and genome-wide SNP association, are detailed in a companion paper (accepted to Poultry Science, Rowland et al., 2019).
Table 4.
Effect of a 4-wk long, daily, cyclic heat stress over time on select blood chemistry parameters in laying hens.1
| pH |
PCO22 |
PO23 |
HCO3−4 |
TCO25 |
sO26 |
iCa7 |
|
|---|---|---|---|---|---|---|---|
| Time period | (mm Hg) | (mm Hg) | (mmol/L) | (mmol/L) | (%) | (mmol/L) | |
| Control8 | 7.39c | 42.8a | 44.8b | 25.7a | 27.0a | 78.9b | 1.41a |
| Acute | 7.45a | 30.2d | 43.1c | 20.7d | 21.6d | 80.6a | 1.09d |
| 2 wk Chronic | 7.44b | 33.2c | 42.6c | 22.4b | 23.4b | 79.2b | 1.36b |
| 4 wk Chronic | 7.37d | 37.5b | 46.9a | 21.6c | 22.7c | 80.9a | 1.28c |
| Pooled SEM | 0.003 | 0.32 | 0.29 | 0.11 | 0.12 | 0.35 | 0.011 |
| P-value | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
| Reference range9 | 7.23–7.37 | 41.6–59.8 | 39.1–52.7 | 22.0–27.6 | 23.5–29.3 | 65.8–84.2 | 1.55–1.83 |
Means values of data from 398 individual hens in both rooms combined.
Partial pressure of carbon dioxide in the blood.
Partial pressure of oxygen in the blood.
Concentration of bicarbonate ions in the blood.
Total concentration of carbon dioxide in the blood.
Saturation of oxygen in the blood.
Concentration of ionized calcium in the blood.
Control values were measured the day before first exposure to HS.
Blood chemistry reference ranges calculated from mean values plus or minus one standard deviation determined by Schaal et al. (2016) for first cycle Hy-Line W-36 laying hens.
Values without similar letters within parameter are significantly different (P ≤ 0.05).
Table 5.
Effects of a 4-wk, daily, cyclic heat stress on select blood chemistry parameters in laying hens.1
| Na2 |
K3 |
Glu4 |
Hct5 |
Hb6 |
|
|---|---|---|---|---|---|
| Time period | (mmol/L) | (mmol/L) | (mg/dL) | (% PCV) | (mmol/L) |
| Control7 | 142.0a | 4.74a | 231.5a | 22.6a | 7.69a |
| Acute | 132.0d | 4.15d | 228.3b | 19.2c | 6.54c |
| 2 wk chronic | 138.5b | 4.56b | 227.6b | 20.9b | 7.11b |
| 4 wk chronic | 135.7c | 4.40c | 218.6c | 19.4c | 6.60c |
| Pooled SEM | 0.42 | 0.022 | 0.79 | 0.13 | 0.043 |
| P-value | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
| Reference range8 | 146.6–152.2 | 4.3–5.3 | 212.4–236.4 | 21.0–26.4 | 7.2–9.0 |
Means values of data from 398 individual hens in both rooms combined.
Concentration of sodium ions in the blood.
Concentration of potassium ions in the blood.
Concentration of glucose in the blood.
Percent packed cell volume of hematocrit in the blood.
Concentration of hemoglobin in the blood.
Control values were measured the day before first exposure to HS.
Blood chemistry reference ranges calculated from mean values plus or minus one standard deviation determined by Schaal et al. (2016) for first cycle Hy-Line W-36 laying hens.
Values without similar letters within parameter are significantly different (P ≤ 0.05).
Variation
Tables Table 6, Table 7, Table 8 show the coefficients of variation of the data collected during the experiment. The variation of FI increased for the first 2 wk of HS compared to the pre-HS values but was decreased from 2 to 4 wk post HS compared to the first 2 wk. The variation in EW increased between the pre-HS and 2 wk measurements but decreased between the 2 and 4 wk measurements. The variation in albumen weight increased during the last 2 wk of the experiment, when the overall EW returned to pre-HS variation. The yolk weights however decreased in variation for measurements taken at 4 wk of HS exposure. This may indicate that the increase in variation of overall EW was associated with the variation in weights of albumen and not the yolk. Increasing the uniformity of egg sizes and weights in commercial EP is beneficial due to processing and packaging as well as value of the egg as a product (Wolc et al., 2012). The eggshell thickness variation increased for the acute time point, then decreased for the first 2 wk of HS. By the last 2 wk of the experiment, the eggshell thickness variation had decreased again. As expected, the variance in BT of hens increased for the acute period when compared to the pre-HS values and decreased at the 2 wk time point and again at the 4 wk time point. This could indicate that for a short term HS exposure, some hens are better able to control BT during acute HS, although over time it appears that adaptation to chronic HS exposure might mitigate some of these differences. The partial pressure of carbon dioxide and the concentration of bicarbonate in the blood both reached their highest variance for the acute time point and decreased for both the 2 and 4 wk time points. The blood pH variance increased throughout the 4 wk of HS. This could indicate that other mechanisms may affect the blood pH besides the ratio of carbon dioxide and bicarbonate. While the amount of calcium found as iCa in the blood is affected by pH, the variance of blood iCa increased at the acute time point, but decreased for the 2 and 4 wk measurements unlike the pH variance.
Table 6.
Coefficient of variance of performance parameters over a 4-wk, daily, cyclic heat stress treatment (data collected from 398 individual laying hens).1
| Time period | Feed intake | Egg production | Egg weight | Egg mass | Feed efficiency | Body weight |
|---|---|---|---|---|---|---|
| Pre-HS2 | 8.1 | 13.6 | 5.4 | 15.0 | 9.0 | 5.1 |
| Wk22 | 14.9 | 10.4 | 6.2 | 12.2 | 15.7 | 5.8 |
| Wk42 | 10.8 | 12.8 | 5.2 | 11.7 | 15.2 | 6.1 |
Calculated by dividing the standard deviation of data at each time point by corresponding averages and multiplying by 100.
Pre-HS: data collected for the 2-wk period before heat stress; wk2: data collected from the first 2 wk of heat stress; wk4: data collected from weeks 2 through 4 of heat stress.
Table 7.
Coefficient of variance of body temperature and egg quality parameters over a 4-wk, daily, cyclic heat stress treatment (data collected from 398 individual laying hens).1
| Time period | Body temperature | Haugh unit | Yolk weight | Albumen weight | Shell weight | Shell thickness |
|---|---|---|---|---|---|---|
| Pre-HS2 | 0.59 | 5.9 | 16.4 | 10.2 | 7.9 | 10.3 |
| Acute2 | 0.83 | 6.5 | 16.3 | 12.8 | 11.2 | 14.6 |
| Wk22 | 0.73 | 6.8 | 15.5 | 9.9 | 7.4 | 10.4 |
| Wk42 | 0.67 | 6.9 | 9.5 | 13.1 | 8.3 | 8.1 |
Calculated by dividing the standard deviation of data at each time point by corresponding averages and multiplying by 100.
Pre-HS: data collected for the 2-wk period before heat stress; acute: data collected within 4 to 6 h of first heat stress exposure; wk2: data collected from the first 2 wk of heat stress; wk4: data collected from weeks 2 through 4 of heat stress.
Table 8.
Coefficient of variance of blood gas parameters over a 4-wk, daily, cyclic heat stress treatment (data collected from 398 individual laying hens).1
| Time period | pH | PCO2 | PO2 | HCO3 | TCO2 | sO2 | Na | iCa | Glu | Hct | Hb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-HS2 | 0.69 | 15.2 | 12.2 | 8.4 | 8.5 | 8.5 | 5.7 | 15.0 | 5.7 | 10.1 | 10.1 |
| Acute2 | 0.89 | 22.1 | 13.0 | 12.3 | 12.8 | 9.1 | 8.5 | 22.4 | 8.9 | 15.4 | 15.4 |
| Wk22 | 0.90 | 16.7 | 14.3 | 8.4 | 8.4 | 9.4 | 5.0 | 14.0 | 6.2 | 11.3 | 11.2 |
| Wk42 | 0.90 | 16.8 | 11.4 | 9.2 | 9.2 | 6.6 | 4.4 | 12.6 | 5.9 | 11.7 | 11.7 |
Calculated by dividing the standard deviation of data at each time point by corresponding averages and multiplying by 100.
Pre-HS: data collected for the 2-wk period before heat stress; acute: data collected within 4 to 6 h of first heat stress exposure; wk2: data collected from the first 2 wk of heat stress; wk4: data collected from weeks 2 through 4 of heat stress.
Correlations
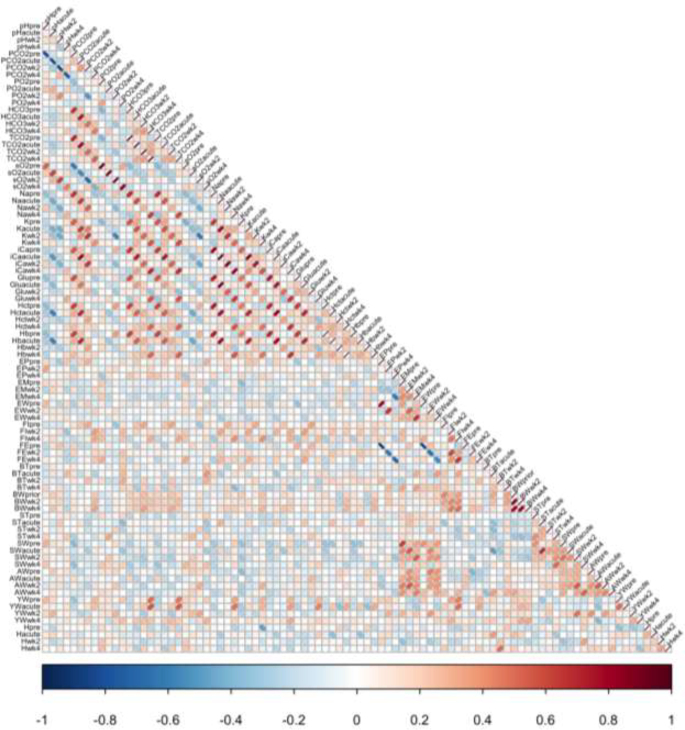
Correlation coefficients were estimated among all traits and are displayed in a correlation matrix (Figure 1). The strongest correlations are between blood chemistry components. In general, correlations (positive and negative) appear between blood components measured at the same time point and correlation patterns between components were consistent across time points. A strong correlation between a blood component measured pre heat exposure (relatively quick and easy to measure) and a trait such as FE or EP measured after heat exposure (relatively difficult and time consuming to measure) would be a useful indicator trait for a hard to measure trait, and could potentially be used for genetic selection to improve performance under HS.
Figure 1.
Correlations between all parameters over a 4-wk, daily, cyclic heat stress treatment. A correlation of 0.1 represents an approximate P -value of 0.001.
Conclusions
Cyclic HS when hens were not returned to thermoneutral temperatures after HS, negatively impacted the performance of laying hens, reducing FI, EP and BW over time. Feed intake did seem to at least partially recover, but both EP and BW were not recovered over the 4 wk period. The changes in shell quality seen at the acute time point agree with blood chemistry changes including the ratio of bicarbonate to carbon dioxide reducing blood buffering capability, increasing the blood pH and reducing bioavailable Ca in the blood. By the end of the 4 wk HS, blood pH, PCO2, and PO2 had returned to near pre-HS levels. These data could indicate that the hens began to acclimate to the high environmental temperatures for these measurements, although other egg quality and blood chemistry measurements did not result in similar recovery. This experiment generated a substantial amount of new data following the exposure of laying hens to a daily, cyclic HS for a longer period of time than previously measured.
ACKNOWLEDGMENTS
This research was funded by the USDA-NIFA-AFRI Climate Change Award number 2011-67003-30228. The authors would like to thank Hy-Line International for donating the pullets used in the experiment. They would also like to thank the Persia and Dalloul labs for setup, management, and sample analysis during this experiment.
REFERENCES
- Balnave D., Muheereza S.K. Improving eggshell quality at high temperatures with dietary sodium bicarbonate. Poult. Sci. 1997;76:558–593. doi: 10.1093/ps/76.4.588. [DOI] [PubMed] [Google Scholar]
- de Andrade A.N., Rogler J.C., Featherston W.R., Alliston C.W. Interrelationships between diet and elevated temperatures (cyclic and constant) on egg production and shell quality. Poult. Sci. 1977;56:1178–1188. [Google Scholar]
- Deaton J.W., McNaughton J.L., Lott B.D. Effect of heat stress on laying hens acclimated to cyclic versus constant temperatures. Poult. Sci. 1982;61:875–878. [Google Scholar]
- El Hadi H., Sykes A.H. Thermal panting and respiratory alkalosis in the laying hen. Br. Poult. Sci. 1982;23:49–57. doi: 10.1080/00071688208447928. [DOI] [PubMed] [Google Scholar]
- Emery D.A., Vohra P., Ernst R.A., Morrison S.R. the effect of cyclic and constant ambient temperatures on feed consumption, egg production, egg weight, and shell thickness of hens. Poult. Sci. 1984;63:2027–2035. doi: 10.3382/ps.0632027. [DOI] [PubMed] [Google Scholar]
- Etches R., John T., Gibbins A. V., Daghir N. 2008. Behavioural, physiological, neuroendocrine and molecular responses to heat stress. Poultry production in hot climates: second edtion 31–66.
- Franco-Jimenez D.J., Scheideler S.E., Kittok R.J., Brown-Brandl T.M., Robeson L.R., Taira H., Beck M.M. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 2007;16:628–634. [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018;98:4471–4478. doi: 10.1002/jsfa.8971. [DOI] [PubMed] [Google Scholar]
- Hy-Line International . Hy-Line Int.; USA: 2016. Hy-Line W-36 Parent Stock Management Guide. [Google Scholar]
- Key N., Sneeringer S., Marquardt D. 2014. Climate change, heat stress, and us dairy production. USDA-ERS Economic Research Report(175).
- Khan R.U., Naz S., Nikousefat Z., Tufarelli V., Javdani M., Rana N., Laudadio V. Effect of vitamin E in heat-stressed poultry. Worlds Poult. Sci. J. 2011;67:469–477. [Google Scholar]
- Kilic I., Simsek E. The effects of heat stress on egg production and quality of laying hens. J. Anim. Vet. Adv. 2013;12:42–47. [Google Scholar]
- Koelkebeck K.W., Odom T.W. Laying hen responses to acute heat stress and carbon dioxide supplementation: I. Blood gas changes and plasma lactate accumulation. Comp. Biochem. Physiol. Comp. Physiol. 1994;107:603–606. doi: 10.1016/0300-9629(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H.C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Maak S., Melesse A., Schmidt R., Schneider F., Von Lengerken G. Effect of long-term heat exposure on peripheral concentrations of heat shock protein 70 (Hsp70) and hormones in laying hens with different genotypes. Br. Poult. Sci. 2003;44:133–138. doi: 10.1080/0007166031000085319. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Beck M.M., Scheideler S.E., Forman M.F., Anderson K.P., Kachman S.D. Acute high environmental temperature and calcium-estrogen relationship in the hen. Poult. Sci. 1996;75:1555–1562. doi: 10.3382/ps.0751555. [DOI] [PubMed] [Google Scholar]
- Mashaly M.M., Hendricks G.L., Kalama M.A., 3rd, Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Maxkwee E.N., Perry J.J., Lee K. Flavor and appearance of whole shell eggs made safe with ozone pasteurization. Food Sci. Nutr. 2014;2:578–584. doi: 10.1002/fsn3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom T.W., Harrison P.C., Bottje W.G. Effects of thermal-induced respiratory alkalosis on blood ionized calcium levels in the domestic hen. Poult. Sci. 1986;65:570–573. doi: 10.3382/ps.0650570. [DOI] [PubMed] [Google Scholar]
- Persia M., Utterback P., Biggs P., Koelkebeck K., Parsons C. Interrelationship between environmental temperature and dietary nonphytate phosphorus in laying hens. Poult. Sci. 2003;82:1763–1768. doi: 10.1093/ps/82.11.1763. [DOI] [PubMed] [Google Scholar]
- Rowland K., Persia M.E., Rothschild M.F., Schmidt C., Lamont S.J. Venous blood gas and chemistry components are moderately heritable in commercial white egg-laying hens under acute or chronic heat exposure. Poult. Sci. 2019;98:3426–3430. doi: 10.3382/ps/pez204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., A J., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Samara M.H., Robbins K.R., Smith M.O. Environmental heat stress does not reduce blood ionized calcium concentration in hens acclimated to elevated temperatures. Poult. Sci. 1996;75:197–200. doi: 10.3382/ps.0750197. [DOI] [PubMed] [Google Scholar]
- Schaal T.P., Arango J., Wolc A., Brady J.V., Fulton J.E., Rubinoff I., Ehr I.J., Persia M.E., O'Sullivan N.P. Commercial Hy-Line W-36 pullet and laying hen venous blood gas and chemistry profiles utilizing the portable i-STAT® 1 analyzer. Poult. Sci. 2016;95:466–471. doi: 10.3382/ps/pev350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolc A., Arango J., Settar P., Fulton J.E., O'Sullivan N.P., Preisinger R., Habier D., Fernando R., Garrick D.J., Hill W.G., Dekkers J.C.M. Genome‐wide association analysis and genetic architecture of egg weight and egg uniformity in layer chickens. Anim. Gen. 2012;43:87–96. doi: 10.1111/j.1365-2052.2012.02381.x. [DOI] [PubMed] [Google Scholar]
- Yahav S., Straschnow A., Plavnik I., Hurwitz S. Blood system response of chickens to changes in environmental temperature. Poult. Sci. 1997;76:627–633. doi: 10.1093/ps/76.4.627. [DOI] [PubMed] [Google Scholar]