Abstract
Objective
The aim of this study was to measure the expression of PD-L1, CD1a (a marker for immature dendritic cells), and CD83 (a marker for mature dendritic cells) and further examine the associations of PD-L1, CD83, and CD1a with overall survival (OS) in triple-negative breast carcinoma patients.
Methods
PD-L1, CD1a, and CD83 expression in breast carcinoma tissues and CD83 expression in lymph node tissues were examined by immunohistochemistry and tissue microarray in 159 patients. Patients were classified into the low, medium, and high PD-L1, CD1a, and CD83 levels. Pearson χ<sup>2</sup> test was used to analyze the correlations between PD-L1, CD1a, and CD83. The Kaplan-Meier method was used to calculate the OS. Multivariate analysis was used to identify determinants of 3- and 5-year OS.
Results
25.1, 25.8, and 49.1% of the patients had low, medium, and high PD-L1 levels, respectively. PD-L1 levels significantly correlated with CD1a (r = 0.30409, p < 0.001) and CD83 levels (r = 0.6146, p < 0.001) in breast carcinoma tissue, as well as CD83 levels (r = 0.17508, p = 0.027) in lymph node. The median OS was 83 months (range 12–106), and the 3- and 5-year OS rates were 94.97% (95% CI 91.57–98.37) and 86.79% (95% CI 81.53–92.06), respectively. Moreover, patients with high median CD1a levels had a significantly lower 5-year OS rate (75.6%) than those with low median CD1a levels (93.5%, p = 0.038).
Conclusion
PD-L1, CD1a, and CD83 are variably expressed in triple-negative breast carcinoma tissues, and PD-L1 expression correlates with CD1a and CD83. Higher CD1a levels correlate with PD-L1 expression and predict worse OS in triple-negative breast carcinoma.
Keywords: PD-L1, CD1a, CD83, Triple-negative breast carcinoma, Overall survival
Introduction
Triple-negative breast cancers lack the expression of estrogen and progesterone receptors and overexpression or amplification of the HER2 oncogene [1, 2]. They represent 15% of breast carcinomas and are a highly heterogeneous group of tumors, and, because of lack of the receptors and HER2, triple-negative breast cancers are not amenable to therapy directed at these molecular targets, such as endocrine or anti-HER2 therapy. On the other hand, triple-negative breast cancer patients are highly sensitive to chemotherapy and respond well to neoadjuvant chemotherapy [3, 4].
The presence of tumor-infiltrating lymphocytes correlates with responsiveness to neoadjuvant chemotherapy and improves the prognosis of breast cancer patients [5]. Currently, evaluation of tumor-infiltrating lymphocytes in triple-negative breast carcinoma tissues is not routinely done in clinical studies despite emerging evidence for an association of tumor-infiltrating lymphocytes with the improved prognosis of this subset of breast cancer patients [6, 7, 8]. A phase III clinical study comparing sequential versus concurrent docetaxel with anthracycline-based chemotherapy for breast cancer showed that a 10% increase in stromal tumor-infiltrating lymphocytes was associated with a 17% reduction in mortality of triple-negative breast carcinoma patients [9, 10]. Programmed cell death-1 receptor (PD-1) is an immune checkpoint inhibitor that is expressed on the surface of immune effector cells, and it is mainly activated by programmed death-ligand 1 (PD-L1, CD274) that is expressed in all human cells including dendritic cells. PD-L1 is expressed in 20–40% of triple-negative breast cancers [11, 12]. A study of 248 triple-negative breast cancer patients showed that PD-L1 expression correlated with high levels of tumor-infiltrating lymphocytes but was not a predictor of survival; however, positive PD-L1 expression and a low number of tumor-infiltrating lymphocytes constituted an independent negative predictor of survival of triple-negative breast cancer patients [12], suggesting the importance of studying the combination of PD-L1 expression on tumor cells and tumor-infiltrating lymphocyte presence in the tumor microenvironment.
Dendritic cells are central regulators of the adaptive immune response and necessary for T cell-mediated cancer immunity. Dendritic cells transport tumor antigens to the draining lymph nodes and cross-present tumor antigens to activate cytotoxic T lymphocytes; however, maturation of dendritic cells is required to provide co-stimulatory signals to activate T lymphocytes. During the maturation process, the cell surface expression of the CD83 protein is upregulated in dendritic cells, which plays an important role in the regulation of dendritic cells-mediated adaptive immune responses [13]. A study of 130 breast carcinoma patients showed that the number of CD83+ tumor-infiltrating dendritic cells was inversely correlated with lymph node metastasis and significantly associated with longer overall survival (OS) of breast carcinoma patients [14]. However, the prognostic importance of tumor-infiltrating dendritic cells in triple-negative breast cancer remains undefined. The expression of PD-L1 is increased in triple-negative breast carcinomas versus in hormone-driven breast tumors, and the role of the interaction between PD-L1 expression and tumor-infiltrating dendritic cells in triple-negative breast cancer has not been examined. Given the appeal of the PD-1/PD-L1 axis as a therapeutic target, it is warranted to elucidate the interaction between PD-L1 and tumor-infiltrating dendritic cells, particularly with regards to OS in triple-negative breast cancer. This will improve our understanding of the unique biologic characteristics of tumor-infiltrating dendritic cells in triple-negative breast cancer and the role of the combination of PD-L1 expression on tumor cells and tumor-infiltrating dendritic cell presence in the tumor microenvironment.
In the current retrospective study, we measured the expression of PD-L1, CD1a, a marker of immature dendritic cells, and CD83, a marker of mature dendritic cells, in triple-negative breast carcinoma tissues and further examined the association of PD-L1, CD1a, and CD83 with OS of triple-negative breast carcinoma patients.
Patients and Methods
Patients
We retrospectively reviewed the clinical pathological data of triple-negative breast cancer patients who underwent curative surgical resection of breast carcinoma at Liaoning Cancer Hospital and Institute, Shenyang, China, between January 2010 and December 2012. Triple-negative breast cancer was defined as <1% tumor cell expression of estrogen and progesterone receptors and negative HER2 status (fluorescence or chromogenic in situ hybridization [FISH/CISH] HER2/CEP17 ratio <2.0 and HER2 copy number/cell <4, or locally assessed immunohistochemistry 0 or 1+ [or 2+ but negative by FISH/CISH]). Patients with treatment-naïve pathologically proven breast cancer were included. Eligible patients were women aged 18 years or older, with Eastern Cooperative Oncology Group performance status (ECOG-PS) 0 or 1. Patients with concurrent tumors or who had received neoadjuvant therapy were excluded.
The study protocol was approved by the local Ethics Committee of Liaoning Cancer Hospital and Institute (No. 20190546). No patient consent was required because of the retrospective nature of the study. Patient data were anonymized in the report.
Patient Evaluation
The following clinical parameters were recorded from electronic records by retrospective collection: patient demographics and baseline characteristics including ECOG-PS score, adjuvant chemotherapy, and patient survival data. OS was defined as the time from the date of surgery to the date of death from any cause. Tumor stages were determined according to the 7th Edition of the TNM classification. All patients were evaluated monthly with physical examination, including ECOG-PS and routine laboratory investigations. Routine surveillance imaging included chest computed tomography scan and brain magnetic resonance imaging.
Tissue Acquisition
Archived surgical carcinoma tissue specimens were obtained of treatment-naïve pathologically proven triple-negative breast cancer patients. Axillary lymph node dissection included all level I–III axillary nodes. For all nodes acquired by axillary lymph node dissection, routine paraffin pathological examination was performed. All nodes were HE stained and reviewed by 2 experienced pathologists (Y.Z. and B.H.).
Human tissue acquisition was carried out in strict accordance with institutional and state regulations and guidelines on the use of human tissues for experimental purposes.
Immunohistochemistry
The tissues were paraffin embedded, cut into 4-μm-thick sections, and dewaxed using xylene, followed by rehydration through gradient ethanol. Antigen retrieval was done by heating in a pressure cooker. Tissue slides were immersed in 3% hydrogen peroxide for 15 min. Nonspecific binding was blocked by normal goat serum. Tissue slides were incubated overnight with rabbit polyclonal anti-CD83 antibody (catalog No.: bs-4826R, dilutions 1:200), rabbit polyclonal anti-CD1a (catalog No.: bs-3786R, dilutions 1:200), or mouse anti-PD-L1/CD274 antibody (catalog No.: 66248-1-Ig, dilutions 1:200; all from Bioss Antibodies Beijing, China) at 4°C and then rinsed with phosphate-buffered saline (PBS). Subsequently, the sections were incubated with secondary antibodies for 40 min at 37°C, washed with PBS, and then stained with diaminobenzidine, followed by counterstaining with hematoxylin. Finally, the sections were air dried, dehydrated, and mounted.
PD-L1, CD1a, and CD83 expression in breast carcinoma tissues was assessed by 2 independent pathologists who were blinded to patient data. The density of positive staining was measured using a computerized image system composed of a Leica CCD camera DFC420 (Leica Microsystems Imaging Solutions, Ltd., Cambridge, UK) connected to a Leica DM IRE2 microscope. If both agreed with a scoring result, the value was selected. CD83 expression in axillary lymph nodes was also examined, and the H score of CD83 expression in axillary lymph nodes was calculated. A staining index obtained as the intensity of positive staining (low, 1; moderate, 2; high, 3) and the proportion of immune-positive cells of interest (0–25%, 1; 25–50%, 2; 50–75%, 3; 75–100%, 4) was calculated. The 2 scores were multiplied to yield the H score of the tissue using the following equation [15, 16]:
H score = ∑ (PI × I) = (percentage of cells of weak intensity ×1) +
(percentage of cells of moderate intensity ×2) + (percentage of cells of strong intensity ×3)
where PI represents the percentage of positively stained cells of all cells in the tissue section examined and I stands for staining intensity.
The patients were classified using the interquartile range (IQR) of H scores into the low expression group, the medium expression group, and the high expression group.
Tissue Microarray
Construction of tissue microarrays was undertaken as previously described [17]. One-millimeter cores from paraffin blocks of breast tumors were used to generate tissue microarrays. Before staining, microarrays were baked overnight, after which they were deparaffinized and rehydrated. Nonspecific binding was blocked, and then the sections were incubated for 60 min with rabbit polyclonal anti-CD83 antibody (catalog No.: bs-4826R, 1:200), rabbit polyclonal anti-CD1a (catalog No.: bs-3786R, 1:200), or mouse anti-PD-L1/CD274 antibody (catalog No.: 66248–1-Ig, 1:200; all from Bioss Antibodies Beijing). Slides were washed and incubated with horseradish peroxidase-labeled goat anti-mouse IgG, Cy3-conjugated goat anti-rabbit IgG (Catalog No.: GB21303, Servicebio, Wuhan, China), and Alexa Fluor® 488-conjugated AffiniPure goat anti-mouse IgG (Catalog No.: GB25301, 1:400, Servicebio). Slides were visualized with diaminobenzidine. Tissue sections were scanned using a tissue chip scanner (Pannoramic MIDI, 3D HISTECH) and analyzed using the Quant center and Pannoramic viewer software. The DensitoQuant software in the QuantCenter automatically recognized nuclear dark brown staining as strongly positive, brown-yellow staining as moderately positive, light yellow staining as weakly positive, and blue staining as negative. The area and percentage of strongly, moderately, and weakly positive were calculated, and finally, H scores of CD83 expression in axillary lymph nodes were obtained. Furthermore, the integrated optical density of PD-L1, CD1a, and CD83 expression in breast carcinoma tissues was determined using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical Analysis
All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA). Count data were presented as median and IQR and compared using the Wilcoxon two-samples test between 2 groups and Kruskal-Wallis test among 3 or more groups. Categorical data were expressed as number and frequency (%) and analyzed using a χ2 test or Fisher's exact test. The Kaplan-Meier method was used to calculate the OS. Data were censored for patients who were lost to follow-up. Multivariate analysis using forced entry was used to identify determinants of 3- and 5-year OS. Pearson χ2 test was used to analyze the correlation between PD-L1 expression and CD1a and CD83 levels. For all analyses, p < 0.05 (two-sided) was considered statistically significant.
Results
Patient Demographics and Baseline Characteristics
Totally, 3,500 women underwent surgical resection of primary breast cancer during the study period. Among them, 2,905 women with non-triple-negative breast cancer were excluded; 306 noninfiltrating breast cancer patients were also not included. Furthermore, 80 patients who received neoadjuvant chemotherapy and 29 women with bilateral primary breast cancer were excluded, and 21 women were excluded due to poor quality of paraffin-embedded specimens. Finally, 159 breast cancer patients were eligible for the study. The demographic and baseline characteristics of these women are shown in Table 1. Their median age was 51 years (range 26–83). The median tumor size was 2 cm (IQR 1.5–2.5). One hundred forty-nine (93.7%) women had infiltrating duct carcinoma of the breast. Fifty-nine (37.1%) women had metastasis to the ipsilateral lymph nodes, and 14 (8.8%) patients had stage III breast cancer.
Table 1.
Demographic and baseline characteristics of the study population (n = 159)
| Age at onset, years | |
| Median | 51.0 |
| Range | 26–83 |
| Tumor size | |
| Median (IQR), cm | 2 (1.5–2.5) |
| <1.5 cm, n (%) | 23 (14.47) |
| ≥1.5 and <2 cm, n (%) | 41 (25.79) |
| ≥2 and <2.5 cm, n (%) | 39 (24.53) |
| ≥2.5, n (%) | 56 (35.22) |
| Pathological type, n (%) | |
| Duct infiltrating | 149 (93.7) |
| Others | 10 (6.3) |
| TNM stage, n (%) | |
| T1 ≤2 cm | 92 (57.86) |
| T2 >2 cm, but ≤5 | 67 (42.14) |
| N0 | 100 (62.89) |
| N1 | 45 (28.30) |
| N2 | 14 (8.81) |
| IA | 62 (38.99) |
| IIA | 58 (36.48) |
| IIB | 25 (15.72) |
| III | 14 (8.81) |
CD1a+ Immature Tumor-Infiltrating Dendritic Cells and CD83+ Mature Tumor-Infiltrating Dendritic Cells
Immunohistochemical analysis showed that the median CD1a level in the breast carcinoma tissue was 30.2 (IQR 13.6–45.9) in the study population (Table 2). Totally, 28.9% of the women had low CD1a levels and 25.8% had high CD1a levels, while 45.3% had medium CD1a levels. The median CD83 level in breast carcinoma tissue was 1,073.9 (IQR 374.3–2,110.9), and 25.79% of the women had low CD83 levels, while 26.42% had high CD83 levels. In addition, the median H score of CD83 in the axillary lymph node was 28.5 (IQR 10.5–58.0); 24.5% of patients had a low median CD83 level and 23.9% had a high median CD83 level, while 51.6% had a medium median CD83 level.
Table 2.
PD-L1, CD1a, and CD83 levels in breast carcinoma tissues and CD83 levels in axillary lymph nodes
| Breast carcinoma tissue PD-L1 IOD | |
| Median (IQR) | 882.6 (184.5–4,136.2) |
| Low | 40 (25.16) |
| Medium | 78 (49.06) |
| High | 41 (25.79) |
| Breast carcinoma tissue CD1a IOD | |
| Median (IQR) | 30.2 (13.6–45.9) |
| Low | 46 (28.93) |
| Medium | 72 (45.28) |
| High | 41 (25.79) |
| Breast carcinoma tissue CD83 IOD | |
| Median (IQR) | 1,073.9 (374.3–2,110.9) |
| Low | 41 (25.79) |
| Medium | 76 (47.80) |
| High | 42 (26.42) |
| Axillary lymph node CD83 H score | |
| Median (IQR) | 28.5 (10.5–58.0) |
| Low | 39 (24.53) |
| Medium | 82 (51.57) |
| High | 38 (23.90) |
Values are n (%) unless otherwise specified. IOD, integrated optical density.
PD-L1 Levels
Immunohistochemical analysis of breast carcinoma tissues revealed that the median PD-L1 level was 882.6 (IQR 184.5–4,136.2; Table 2). Moreover, 25.1% of the women had low PD-L1 levels, 25.8% had high PD-L1 levels, and 49.1% had medium PD-L1 levels. Furthermore, patients with a larger tumor size had significantly lower median PD-L1 levels than those with a smaller tumor size (≥2.5 cm, median [IQR] 610.8 [107.4–2407.4] vs. <1.5 cm, 1,365.0 [246.9–7,797.9]; p = 0.02; Table 3 and online suppl. Table 1, see www.karger.com/doi/10.1159/000513502). Stage T2 patients also had significantly lower median PD-L1 levels than stage T1 patients (T2: median [IQR] 684.5 [92.6–2,687.8] vs. T1: 1,250.0 [357.1–5,915.5]; p = 0.009).
Table 3.
PD-L1 levels stratified by patient pathologic variables
| Variables | Median (IQR) | Statistical test | Statistical volume | p value |
|---|---|---|---|---|
| Tumor size | ||||
| <1.5 cm | 1,365.0 (246.9–7,797.9) | Kruskal-Wallis test | 9.802 | 0.020 |
| ≥1.5 and <2 cm | 1,265.6 (443.6–5,872.0) | |||
| ≥2 and <2.5 cm | 1,075.3 (115.1–4,245.3) | |||
| ≥2.5 cm | 610.8 (107.4–2,407.4) | |||
| Pathological stage | ||||
| T1 | 1,250.0 (357.1–5,915.5) | Wilcoxon two-samples test | −2.6 | 0.009 |
| T2 | 684.5 (92.6–2,687.8) | |||
| N0 | 932.5 (209.2–5,225.1) | Kruskal-Wallis test | 0.7 | 0.705 |
| N1 | 934.24 (170.1–4,032.1) | |||
| N2 | 807.36 (115.1–2,637.3) | |||
| IA | 1,341.9 (455.3–6,980.5) | Kruskal-Wallis test | 7.174 | 0.067 |
| IIA | 756.4 (137.4–2,871.4) | |||
| IIB | 794.1 (77.0–3,742.0) | |||
| III | 807.4 (115.1–2,637.3) | |||
| Pathological type | ||||
| Duct infiltrating | 860.1 (175.0–4,136.2) | Wilcoxon two-samples test | 1.203 | 0.229 |
| Others | 1,669.7 (828.2–3,413.8) |
Furthermore, Pearson correlation analysis revealed that PD-L1 levels significantly correlated with breast carcinoma tissue CD1a levels (r = 0.30409, p < 0.001), breast carcinoma tissue CD83 levels (r = 0.6146, p < 0.001), and the H score of axillary lymph node CD83 (r = 0.17508, p = 0.0273). Meanwhile, CD1a levels showed no correlation with the H score of axillary lymph node CD83 (r = 0.067, p = 0.4011) but significantly correlated with breast carcinoma tissue CD83 levels (r = 0.38244, p < 0.001; Table 4).
Table 4.
The relation between PD-L1 and CD1a and CD83 levels in carcinoma tissues of triple-negative breast cancer patients
| PD-L1 |
Sum | Statistical test | Statistical volume | p value | |||
|---|---|---|---|---|---|---|---|
| low | medium | high | |||||
| Breast carcinoma tissue CD1a IOD | |||||||
| Low | 20 (50.00) | 21 (26.92) | 5 (12.20) | 46 (28.93) | χ2 | 16.116 | 0.003 |
| Medium | 14 (35.00) | 38 (48.72) | 20 (48.78) | 72 (45.28) | |||
| High | 6 (15.00) | 19 (24.36) | 16 (39.02) | 41 (25.79) | |||
| Breast carcinoma tissue CD83 IOD | |||||||
| Low | 25 (62.50) | 16 (20.51) | 0 (0.00) | 41 (25.79) | χ2 | 51.785 | 0.000 |
| Medium | 13 (32.50) | 43 (55.13) | 20 (48.78) | 76 (47.80) | |||
| High | 2 (5.00) | 19 (24.36) | 21 (51.22) | 42 (26.42) | |||
| Axillary lymph node CD83 H score | |||||||
| Low | 17 (42.50) | 19 (24.36) | 3 (7.32) | 39 (24.53) | χ2 | 15.791 | 0.003 |
| Medium | 16 (40.00) | 37 (47.44) | 29 (70.73) | 82 (51.57) | |||
| High | 7 (17.50) | 22 (28.21) | 9 (21.95) | 38 (23.90) | |||
Values are n (%). IOD, integrated optical density.
Overall Survival
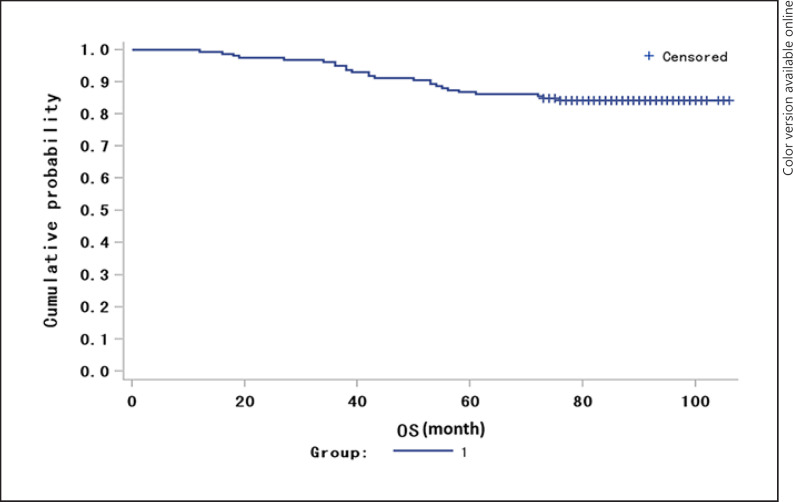
The patients were followed up for a median duration of 86.6 months (range 73–106). The median OS for the study population was 83 months (range 12–106; Fig. 1). Eight patients died within 3 years, and the 3-year OS rate was 94.97% (95% CI 91.57–98.37). Twenty-one patients died within 5 years, and the 5-year OS rate was 86.79% (95% CI 81.53–92.06). Though women who died had numerically higher median PD-L1 levels than those who survived, no statistically significant difference in median PD-L1 levels was observed (Table 5). At 5 years, women who died had significantly higher median CD83 levels (median [IQR] 1,287.6 [875.5–3,297.3]) in the breast carcinoma tissues than those who survived (median [IQR] 995.0 [335.8–1,982.8]; Wilcoxon two-samples test, p = 0.048; Table 5). Though patients with high median CD83 levels in the breast carcinoma tissues had a numerically lower 5-year OS rate (81.0%) than those with low median CD83 levels (95.1%), no statistical difference was found (χ2 test, p = 0.147; Table 6). There was no difference in the median CD83 H score in the axillary lymph nodes between those who survived and those who died. Moreover, patients with high median CD1a levels in breast carcinoma tissues had a significantly lower 5-year OS rate (75.6%) than those with low median CD1a levels (93.5%, χ2 test, p = 0.038; Table 6).
Fig. 1.
The Kaplan-Meier overall survival curve of the study population.
Table 5.
Median PD-L1, CD1a, and CD83 levels and survival outcome of triple-negative breast cancer patients
| Median (IQR) | Statistical test | Statistical volume | p value | |
|---|---|---|---|---|
| PD-L1 | ||||
| 3-year outcome | Wilcoxon two-samples test | 0.122 | 0.903 | |
| Survival | 870.7 (184.5–4,245.3) | |||
| Death | 1,500.1 (281.7–2,948.7) | |||
| 5-year outcome | Wilcoxon two-samples test | 0.872 | 0.383 | |
| Survival | 859.1 (173.8–4,245.3) | |||
| Death | 1,304.7 (695.4–2,888.1) | |||
|
| ||||
| Breast carcinoma CD1a | ||||
| 3-year outcome | Wilcoxon two-samples test | 0.725 | 0.468 | |
| Survival | 30.1 (13.6–43.8) | |||
| Death | 44.1 (11.32–66.3) | |||
| 5-year outcome | Wilcoxon two-samples test | 1.811 | 0.070 | |
| Survival | 29.5 (12.5–43.0) | |||
| Death | 39.5 (20.9–70.9) | |||
|
| ||||
| Breast carcinoma CD83 | ||||
| 3-year outcome | Wilcoxon two-samples test | −0.012 | 0.991 | |
| Survival | 1,067.0 (374.3–2,110.9) | |||
| Death | 1,270.1 (588.4–1,918.3) | |||
| 5-year outcome | Wilcoxon two-samples test | 1.976 | 0.048 | |
| Survival | 995.0 (335.8–1,982.8) | |||
| Death | 1,287.6 (875.5–3,297.3) | |||
|
| ||||
| Axillary lymph node CD83 | ||||
| 3-year outcome | Wilcoxon two-samples test | −0.453 | 0.650 | |
| Survival | 28.5 (10.9–58.0) | |||
| Death | 27.6 (6.4–53.9) | |||
| 5-year outcome | Wilcoxon two-samples test | 0.048 | 0.961 | |
| Survival | 26.4 (10.5–58.8) | |||
| Death | 32.4 (17.6–54.6) | |||
Table 6.
Low, medium, and high PDL1, CD1a, and CD83 levels and survival outcome of triple-negative breast cancer patients
| Low | Medium | High | Statistical test | Statistical volume | p value | |
|---|---|---|---|---|---|---|
| PD-L1 | ||||||
| 3-year outcome | Fisher's exact test | − | 0.811 | |||
| Survival | 38 (95.00) | 73 (93.59) | 40 (97.56) | |||
| Death | 2 (5.00) | 5 (6.41) | 1 (2.44) | χ2 test | ||
| 5-year outcome | 3.092 | 0.213 | ||||
| Survival | 37 (92.50) | 64 (82.05) | 37 (90.24) | |||
| Death | 3 (7.50) | 14 (17.95) | 4 (9.76) | |||
|
| ||||||
| CD1a | ||||||
| 3-year outcome | Fisher's exact test | − | 0.260 | |||
| Survival | 44 (95.65) | 70 (97.22) | 37 (90.24) | |||
| Death | 2 (4.35) | 2 (2.78) | 4 (9.76) | |||
| 5-year outcome | χ2 test | 6.543 | 0.038 | |||
| Survival | 43 (93.48) | 64 (88.89) | 31 (75.61) | |||
| Death | 3 (6.52) | 8 (11.11) | 10 (24.39) | |||
|
| ||||||
| Breast carcinoma CD83 | ||||||
| 3-year outcome | Fisher's exact test | − | 1.000 | |||
| Survival | 39 (95.12) | 72 (94.74) | 40 (95.24) | |||
| Death | 2 (4.88) | 4 (5.26) | 2 (4.76) | |||
| 5-year outcome | χ2 test | 3.837 | 0.147 | |||
| Survival | 39 (95.12) | 65 (85.53) | 34 (80.95) | |||
| Death | 2 (4.88) | 11 (14.47) | 8 (19.05) | |||
|
| ||||||
| Axillary lymph node CD83 | ||||||
| 3-year outcome | Fisher's exact test | − | 0.574 | |||
| Survival | 36 (92.31) | 79 (96.34) | 36 (94.74) | |||
| Death | 3 (7.69) | 3 (3.66) | 2 (5.26) | χ2 test | ||
| 5-year outcome | 0.389 | 0.823 | ||||
| Survival | 34 (87.18) | 70 (85.37) | 34 (89.47) | |||
| Death | 5 (12.82) | 12 (14.63) | 4 (10.53) | |||
Values are n (%).
Our multivariate stepwise logistic regression analysis showed that the T stage was a significant determinant of OS (T1 vs. T2, HR 0.285, 95% CI 0.120–0.677; p = 0.0045; Table 7).
Table 7.
Multivariate stepwise logistic regression analysis of determinants of overall survival of triple-negative breast cancer patients
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Pathological stage | |||
| T1 vs. T2 | 0.285 | 0.120–0.677 | 0.005 |
| N0 vs. N2 | 0.218 | 0.073–0.651 | 0.006 |
| N1 vs. N2 | 0.488 | 0.160–1.488 | 0.207 |
| PD-L1 levels | 1.694 | 0.409–7.026 | 0.467 |
| High vs. low | 1.694 | 0.409–7.026 | 0.467 |
| Medium vs. low | 3.277 | 1.089–9.858 | 0.035 |
| CD1a levels | |||
| High vs. low | 4.896 | 1.152–20.807 | 0.030 |
| Medium vs. low | 2.280 | 0.526–9.880 | 0.957 |
Discussion
Emerging evidence has demonstrated that tumor-infiltrating lymphocytes correlate with improved prognosis of triple-negative breast cancer patients [6, 7, 8]. Dendritic cells are central regulators of the adaptive immune response, but little literature is available on the presence of infiltrating immature and mature dendritic cells in triple-negative breast carcinoma tissues. In the current study, we demonstrated that immature and mature dendritic cells were variably present in all the breast carcinoma tissues examined, and mature dendritic cells were also present in axillary lymph nodes. Approximately 25% of the breast carcinoma tissues showed high expression of PD-L1, CD1a, or CD83. Furthermore, PD-L1 levels significantly correlated with breast carcinoma tissue CD1a and CD83 levels and axillary lymph node CD83 levels.
The number of CD83+ tumor-infiltrating dendritic cells was significantly associated with a longer OS of breast carcinoma patients [14]. However, no literature is available on the correlation between tumor-infiltrating mature (CD83+) dendritic cells and the OS of breast cancer patients. Our study showed that women who died had significantly higher median CD83 levels in breast carcinoma tissues than those who survived at 5 years. Our findings are different from the study on breast cancer patients [14], suggesting that tumor-infiltrating mature dendritic cells may play different roles in mediating adaptive antitumor immune response in the breast cancer microenvironment in triple-negative and non-triple-negative breast cancer patients. However, our multivariate logistic regression analysis failed to establish breast carcinoma CD83 as an independent, immunologic prognostic determinant of OS of triple-negative breast cancer patients. Meanwhile, we found that patients with high median CD1a levels in breast carcinoma tissues had a significantly lower 5-year OS rate than those with low median CD1a levels. Our multivariate logistic regression analysis further showed that high CD1a levels were associated with an approximately 5-fold higher risk of death than low CD1a levels.
A recent meta-analysis showed that PD-L1 overexpression in breast cancer was associated with a poor outcome and an increased risk of mortality [18]. Positive PD-L1 expression and a low number of tumor-infiltrating lymphocytes were also shown to be independent negative predictors of survival of triple-negative breast cancer patients [12]. Consistently, we found that higher PD-L1 levels were associated with increased risk of death compared to low PD-L1 levels, and women who had low PD-L1 levels had a numerically longer OS than women with high PD-L1 levels. Our findings are different from the study by Li et al. [19] who found that PD-L1 expression was associated with better disease-free survival (DFS). The 2 studies examined 2 different outcomes, OS and DFS. Another major difference between the 2 studies is that our study examined the expression of PD-L1 in tumoral tissues, including the tumor-host interface, while the study by Li et al. [19] studied the expression of PD-L1 in stromal tissues. Interestingly, Catacchio et al. [20] showed that intratumoral CD8+ T cell overexpression exhibited a trend towards a worse 5-year DFS, while those with stromal CD8+ T cell overexpression displayed a trend towards a better 5-year DFS.
Our study has several limitations. This is a retrospective study and cannot establish a causal relation. Another weakness is that there is no strong relationship between PD-L1, CD1a, and CD83 and patient OS, probably due to the small size of the study population and the low number of deaths. Moreover, the major part of our patients had favorable clinical pathological characteristics and had mostly stage I or II breast cancer. Our findings require confirmation in a large cohort of triple-negative breast cancer patients.
Conclusion
PD-L1, CD1a, and CD83 are variably expressed in triple-negative breast carcinoma tissues, and PD-L1 expression correlates with CD1a and CD83 levels in breast carcinoma tissues. Higher CD1a levels correlate with PD-L1 expression and predict worse OS in triple-negative breast carcinoma.
Statement of Ethics
Ethical approval was given by the Ethics Committee of Liaoning Cancer Hospital and Institute, Shengyang, China (No. 20190546). No patient consent was required because of the retrospective nature of the study. Patient data were anonymized in the report.
Conflict of Interest Statement
All authors declare that they have no conflicts of interest.
Funding Sources
This work was supported by grants obtained from Shenyang Science and Technology Project (grant No. 19-112-4-087).
Author Contributions
J.Z. and Y.J. contributed to the study conception and design. All authors collected the data and performed the data analysis. All authors contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer—current status and future directions. Ann Oncol. 2009 Dec;20((12)):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 2.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010 Dec;7((12)):683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 3.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar;26((8)):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 4.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010 Nov;363((20)):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 5.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. European journal of cancer (Oxford, England: 1990) 1992;28a:859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 6.Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical Validity and Utility of Tumor-Infiltrating Lymphocytes in Routine Clinical Practice for Breast Cancer Patients: Current and Future Directions. Front Oncol. 2017 Aug;7:156. doi: 10.3389/fonc.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014 Sep;32((27)):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stovgaard ES, Nielsen D, Hogdall E, Balslev E. Triple negative breast cancer - prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018 Jan;57((1)):74–82. doi: 10.1080/0284186X.2017.1400180. [DOI] [PubMed] [Google Scholar]
- 9.Francis P, Crown J, Di Leo A, Buyse M, Balil A, Andersson M, et al. BIG 02-98 Collaborative Group Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008 Jan;100((2)):121–133. doi: 10.1093/jnci/djm287. [DOI] [PubMed] [Google Scholar]
- 10.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013 Mar;31((7)):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 11.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014 Apr;2((4)):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017 Feb;8((9)):15584–92. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto Y, Tedder TF. CD83: a regulatory molecule of the immune system with great potential for therapeutic application. J Med Dent Sci. 2006 Jun;53((2)):85–91. [PubMed] [Google Scholar]
- 14.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, et al. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003 Mar;104((1)):92–97. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 15.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995 Sep;48((9)):876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol. 2014 Nov;9((1)):221. doi: 10.1186/s13000-014-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998 Jul;4((7)):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Zhu H, Zhou Y, Mao F, Lin Y, Pan B, et al. Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast J. 2017 Jul;23((4)):436–443. doi: 10.1111/tbj.12753. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Wetherilt CS, Krishnamurti U, Yang J, Ma Y, Styblo TM, et al. Stromal PD-L1 Expression Is Associated With Better Disease-Free Survival in Triple-Negative Breast Cancer. Am J Clin Pathol. 2016 Oct;146((4)):496–502. doi: 10.1093/ajcp/aqw134. [DOI] [PubMed] [Google Scholar]
- 20.Catacchio I, Silvestris N, Scarpi E, Schirosi L, Scattone A, Mangia A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl Oncol. 2019 Mar;12((3)):585–595. doi: 10.1016/j.tranon.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.