Abstract
Background
Problems in patients who could not get adequate surgical margins (SM) and good cosmetic results with breast-conserving surgery (BCS) have been overcome with the introduction of oncoplastic surgery (OPS) methods. The purpose of this study was the documentation of level II techniques and the presentation of long-term survival results.
Methods
The data on patients who had been prospectively registered in the database between 2007 and 2017 and who had been treated with level II OPS due to invasive breast cancer were examined.
Results
A total of 1,074 patients were included in the study. The most commonly applied level II oncoplastic techniques were performed in the upper outer quadrantectomy with racquet incision in 334 (31%) patients, inferior pedicle flaps in 294 (27.3%), and vertical mammoplasty in 140 (13%). Reexcision was performed in 96 patients (8.9%). Total breast conservation rate was 96%. Five-year disease-free survival (DFS) was 88%, local recurrence-free survival (LRFS) 93.6%, and overall survival (OS) 96%. Ten-year DFS was 72%, LRFS 85.4%, and OS 90.2%.
Conclusion
Level II OPS techniques have low reoperation and complication rates and a high rate of breast protection. The success of these techniques has been demonstrated in terms of long-term local control. Awareness of the fact that many patients who undergo OPS will not lose their breasts should be created, and regular training programs for OPS techniques should be conducted especially in developing countries. By revealing these results, it is hoped that the OPS and breast conservation rates will increase.
Keywords: Breast cancer, Oncoplastic surgery, Surgical techniques, Survival
Introduction
When the advances in breast cancer surgical treatment are considered, it is no longer acceptable for any woman to continue her life without her breasts. Simultaneous or delayed reconstruction should always be offered to patients after mastectomy. With the increased recognition of breast cancer at early stages, the need for mastectomy has decreased in a significant number of patients. Breast-conserving surgery (BCS) for patients who do not need mastectomy has become the standard procedure, with low rates of ipsilateral breast tumor recurrence (IBTR) in patients treated with radiotherapy (RT) [1, 2]. Although conventional BCS provides breast protection as an alternative to patients not eligible for mastectomy, it fails to maintain the natural shape of the breast and often results in deformities. Problems in patients who could not get adequate surgical margins (SM) and good cosmetic results with BCS were overcome with the introduction of oncoplastic surgery (OPS) methods. With these techniques, that combine oncological and plastic surgery methods, breast conservation with wider SM and better cosmetic results has been possible for larger tumors. Level I OPS techniques may be sufficient, especially in the treatment of tumors >2 cm. Less than 20% of the breast volume is removed in level I techniques, and resection area is filled with simple advancement or rotation flaps [3]. Tumor to breast size, tumor localization, and breast density are taken into consideration in the selection of OPS technique. Accordingly, level II OPS techniques can be applied to the vast majority of patients. Breast conservation possibilities for larger, multifocal, and multicentric tumors have been expanded with level II techniques.
More than 20% of the breast volume and a significant amount of breast skin is excised in level II techniques, and simultaneous reconstruction is performed with more complicated and sophisticated glandular flap methods. Although many level II OPS techniques have been described to date, only certain specific techniques have found worldwide acceptance [4]. To date, short- and medium-term follow-up results of different techniques have been published. Level II techniques require appropriate patient selection, a multidisciplinary approach, and a certain level of experience. For these reasons, it is necessary to know the long-term results of the centers that intensively apply level II OPC techniques. Indeed, there are only a few studies in the literature that report around 10% IBTR in the medium-term follow-up [5].
The purpose of this study was to document the level II techniques performed at 2 high-density tertiary breast surgical oncology centers and present the long-term survival results.
Patients and Methods
Ankara Oncology and Gulhane Research and Application Centers of Health Sciences University are two tertiary cancer centers in Ankara, Turkey. Breast study subgroups were created in the surgical oncology clinics of these hospitals, and breast cancer treatment has been performed with multidisciplinary teams for more than 10 years. The diagnosis, treatment, and follow-up of breast cancer carried out at these two centers are similar. In this study, the data on patients who were prospectively registered in the database of these two centers between 2007 and 2017 and who were treated with level II OPS due to invasive breast cancer were examined. Patients were excluded from the study who had: de novo distant metastasis, a diagnosis of pure ductal carcinoma in situ (DCIS), undergone neoadjuvant chemotherapy (NAC), malignancies other than breast cancer, missing data, and irregular follow-ups. The level II volume displacement techniques applied were: racquet, round block, and batwing techniques, inferior and superior pedicle reduction, and radial, fusiform and vertical mammoplasty. The racquet and fusiform techniques were used for tumors located in the upper outer quadrant. Radial mammoplasties were preferred for inner quadrant tumors. Vertical mammoplasty techniques were used for tumors located in the upper or lower midline. Reduction mammoplasty with superior or inferior flap (wise pattern) were applied to patients who required the reduction. The superior flap technique was used for lower quadrant tumors and inferior flap technique for upper quadrant tumors. Batwing and round block techniques were mostly used for tumors close to the areola. Central excisions were applied in some cases with NAC involvement. However, central excisions were not included in the study because it was considered a level I technique. In another group of patients with NAC involvement, superior and inferior mammoplasties including nipple exclusion were preferred. After the completion of the adjuvant therapies, new nipples were created with different flap techniques and tattoo age was applied to these patients. Fasciocutaneous, myocutaneous, and muscular flaps, techniques combined with the implant were not included in the study. The operations were performed by 4 breast surgeons at the 2 centers participating in the study (M.A.G., L.D., N.K., and C.Ö.). Plastic surgeons did not participate in these surgeries.
In patients with nonpalpable lesions, tumors were marked preoperatively by guide-wire localization or radio-guided occult lesion localization (ROLL). Perioperative radiography was performed in these patients to confirm sufficient excision. Routine frozen-section examination or cavity shaving was not performed for SM. However, when necessary, additional resections were made from suspicious borders. Sentinel lymph node biopsy (SLNB) was examined on a frozen section. Specimen volumes were obtained by multiplying the 3 dimensions of the specimen sizes stated in the pathology report. SM were considered positive in the presence of ink on tumor. The closest SM were used to calculate the average SM. Tumor beds were marked with metallic clips. All patients received a total of 50-Gy adjuvant radiotherapy with conventional fractionation (2 Gy/day) and a booster dose to the tumor bed. Hormonotherapy was given to all patients with positive hormone receptors.
The age, body mass index (BMI), menopause status, and tumor characteristics (size, stage, grade, hormone receptor status, and CerbB2 status) of the patients were recorded. SM, reoperation, and reexcision requirements, the results of axillary intervention, ipsilateral and contralateral tumor recurrences, axillary recurrence rates, and early (within the first 2 months) and late (after 2 months) complications were recorded. Long-term local recurrence-free survival (LRFS), disease-free survival (DFS), and overall survivals (OS) were evaluated.
Wound complications were evaluated in 2 groups as minor or major complications. Seroma, hematoma, wound infection, and delayed wound-healing were evaluated. More severe complications such as incisional wound dehiscence and nipple necrosis were evaluated in the whole group. Serous fluid collections creating patient discomfort and tension were considered as seroma and treated by aspiration. Other collections which caused hemorrhagic bruises on the skin were considered as hematomas. Whether confirmed on culture or not, erythema, purulent discharge, localized temperature increase, cellulitis, pain, redness, and tenderness were considered as wound infection. While incision dehiscence repaired by simple suturing was classified as minor; the repair of the whole incision in an operating theatre was classified as major wound dehiscence. Wound dehiscence that healed without any intervention was regarded as late wound-healing. Fat necrosis and the development of granulation tissue were evaluated as late complications.
Patients were invited to attend follow-up at 3-month intervals for the first 2 years and 6-month intervals for the next 3 years. After 5 years, the patients were followed at 1-year intervals. In addition to physical examinations, annual mammography and breast ultrasonography (USG) were standard. Breast MRI was performed when needed.
Statistical Analysis
SPSS v25.0 was used for statistical analyses (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Armonk, NY, USA). The Fisher exact test was used for comparison of categorical variables. Quantitative data (patient and tumor characteristics, SM, reoperation rates, and results of surgical techniques) were evaluated by χ2 test. The Kruskal-Wallis test was used to compare the results of surgical techniques. The distribution of data was presented as mean ± SD or n (%). The Kaplan-Meier test was used to determine breast cancer recurrence rates and local and total survival time. The calculation of OS was based on the time between the date of surgery and death from any cause. The DFS calculation was based on the time between the date of surgery and the date of first relapse or death for any reason. Events ending DFS were accepted as: ipsilateral or contralateral breast recurrence (invasive or in situ) and the development of regional or distant metastasis. The events that ended LRFS were accepted as: ipsilateral breast recurrence (invasive or in situ) and regional recurrence. p ≤ 0.05 was considered statistically significant.
Results
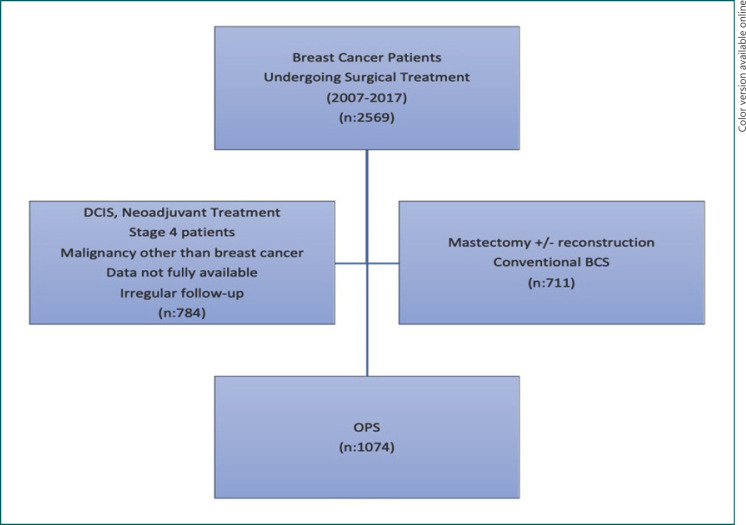
There were 1,074 patients included in the study (Fig. 1). Surgery was performed on 17% of the cases between 2007 and 2011, 36% between 2011 and 2014, and 47% between 2014 and 2017; it was observed that OPS applications continued to increase over the years. The mean age of the patients was 51.12 ± 10.51 years, mean tumor size 22.8 ± 9.3 mm, mean BMI 30 ± 4, and mean distance to the nearest SM of tumors 9.4 ± 7.5 mm. Four hundred and nineteen (39%) patients were in a premenopausal state. Three hundred and seventy-six (35%) patients were in stage I, 478 (44.6%) in stage IIA, 130 (12.1%) in stage IIB, and 90 (8.3%) in stage IIIA. Seventy-five percent of the tumors were ER-positive, 57% were PR-positive, and 19% were CerbB2-positive. Tumor grades were: grade I, 11.5%; grade II, 44.5%; and grade III, 44%. While 744 (69.2%) patients were in the pathological N0 stage, 266 (24.7%) were in the N1 stage and 64 (6%) were in the N2 stage. Invasive ductal cancers accounted for 88.2% of the tumors. Patient and tumor characteristics are summarized in Table 1.
Fig. 1.
Study flowchart.
Table 1.
General characteristics of 1,074 patients
| Mean ± SD | |
|---|---|
| Age, years | 51.12±10.51 |
| BMI | 30±4 |
| SM distance, mm | 9.4±7.5 |
| Tumor size, mm | 22.8±9.3 |
| Specimen volume, cm3 | 365±48.7 |
| n (%) | |
|---|---|
| Tumor morphology | |
| IDC | 947 (88.2) |
| ILC | 97 (9) |
| Other | 30 (2.8) |
| Premenopausal | 419 (39) |
| Postmenopausal | 655 (61) |
| Stage | |
| I | 376 (35) |
| IIA | 478 (44.6) |
| IIB | 130 (12.1) |
| IIIA | 90 (8.3) |
| Nodal stage | |
| N0 | 744 (69.2) |
| N1 | 266 (24.7) |
| N2 | 64 (6) |
| Grade | |
| I | 123 (11.5) |
| II | 478 (44.5) |
| III | 473 (44) |
| ER-positive | 805 (75) |
| ER-negative | 269 (25) |
| PR-positive | 612 (56.9) |
| PR-negative | 462 (43.1) |
| CerbB2-positive | 204 (19) |
| CerbB2-negative | 870 (81) |
| Reoperation | |
| Reexcision | 96 (8.9) |
| Mastectomy | 43 (4) |
| Relapse/recurrence | |
| IBTR | 29 (2.7) |
| Axillary recurrence | 10 (0.9) |
The most commonly applied level II oncoplastic techniques were upper outer quadrantectomy with racquet incision in 334 (31%) patients, inferior pedicle flap in 294 (27.3%), and vertical mammoplasty in 140 (13%). In addition, superior pedicle flap (6.8%), fusiform mammoplasty (6.7%), radial mammoplasty (5.5%), round block (donut) technique (5%), and batwing (4.5%) technique were also used (Table 2). One hundred and five (9.7%) patients had bilateral interventions to obtain symmetry. The average volume of specimens removed in the procedures performed on the malignant side was 365 ± 48.7 cm3. Eighty-six patients (8%) had a multifocal disease.
Table 2.
Level II oncoplastic techniques
| n (%) | |
|---|---|
| Racquet technique | 334 (31) |
| Inferior pedicle flap | 294 (27.3) |
| Vertical mammaplasty | 140 (13) |
| Superior pedicle flap | 73 (6.8) |
| Fusiform mammoplasty | 72 (6.7) |
| Radial mammoplasty | 59 (5.5) |
| Round block | 54 (5) |
| Batwing | 48 (4.5) |
When early and late complications were examined, the number of patients who developed at least 1 complication was 116 (10.8%). Delayed wound-healing with 6.8% (n = 73) was the most common early complication in the minor complications group. This was followed by seroma in 5.7% (n = 61), wound infection in 4% (n = 43), and hematoma in 1.8% (n = 19). Incisional wound dehiscence and nipple necrosis rates in the major complications group were 0.74% (n = 8) and 0.55% (n = 6), respectively. Fat necrosis and granulation tissue development rates in the late complications group were 11.8% (n = 127) and 10% (n = 107), respectively. A total of 44 (4%) patients had revision procedures in the operating theatre (28 had revision surgery, 8 had hematoma drainage, and 8 had redrainage).
SM were positive in a total of 129 patients (12%). Reexcision was performed in 96 patients (8.9%), due to SM positivity (n = 86) or proximity (n = 10). Mastectomy had to be performed in 43 patients (4%) due to SM positivity. Residual invasive or in situ tumors were detected in 36 patients (83.7%) who underwent mastectomy and in 42 (43.7%) who underwent reexcision. Despite reexcision or mastectomy, there were no patients whose negative SM could not be achieved, so there was no patient who underwent >1 reexcision. Total breast conservation rate was 96%. The oncoplastic techniques used are shown in Table 2. The classification of the complications, reexcision rates, and mastectomy on the malignant side in accordance with oncoplastic techniques used are shown in Table 3.
Table 3.
Early complications and the need for reoperation according to different techniques
| Technique | Need for a mastectomy | Reexcision | Delayed healing | Seroma | Infection | Hematoma | Wound dehiscence | Nipple necrosis |
|---|---|---|---|---|---|---|---|---|
| Racquet (n = 334) | 17 | 29 | 30 | 25 | 18 | 8 | 3 | 0 |
| Inferior pedicle flap (n = 294) | 12 | 28 | 24 | 18 | 13 | 5 | 3 | 4 |
| Vertical (n = 140) | 5 | 13 | 6 | 4 | 5 | 3 | 1 | 0 |
| Superior pedicle flap (n = 73) | 3 | 4 | 4 | 4 | 3 | 1 | 1 | 1 |
| Fusiform (n = 72) | 2 | 7 | 3 | 4 | 0 | 2 | 0 | 0 |
| Radial (n = 59) | 2 | 6 | 3 | 3 | 3 | 0 | 0 | 0 |
| Round block (n = 54) | 0 | 4 | 2 | 3 | 0 | 0 | 0 | 1 |
| Batwing (n = 48) | 2 | 5 | 1 | 0 | 1 | 0 | 0 | 0 |
|
| ||||||||
| Total n | 43 | 96 | 73 | 61 | 43 | 19 | 8 | 6 |
Twenty-nine patients (2.7%) had IBTR, and 10 (0.9%) had axillary recurrence with an average of 61 months of follow-up. Nine patients had a recurrence in the contralateral breast (5 in situ and 4 invasive tumors). There was recurrence in the same quadrant in 24 (84%) of those who developed IBTR. The number of patients with distant metastases was 172 (16%). Fifty-eight patients (5.4%) died from breast cancer-related causes. The 5-year cumulative local event incidence was 2%, regional event incidence was 0.5%, and distant metastasis incidence was 12.4%. Five-year DFS was 88%, LRFS 93.6%, and OS 96%. Ten-year DFS was 72%, LRFS 85.4%, and OS 90.2%.
Discussion
It has been demonstrated in many studies comparing OPS with conventional BCS that OPS provides a wider SM, higher resection volume, less need for reoperation, and indisputably superior cosmetic results [6, 7, 8]. Currently, the period to compare OPS with conventional BCS has come to a close. Fewer SM problems and local recurrences after mastectomy and immediate reconstruction have directed the attention of breast surgeons from OPS to mastectomy and immediate reconstruction. However, both autologous tissue and implant-based reconstructions have their own disadvantages and complications [9, 10]. SM status, reoperation, breast protection, complication rates, and long-term survival results are the main safety parameters of OPS, and they all should be discussed to show that OPS is still a good alternative to mastectomy and immediate reconstruction.
SM are the main determinant of the need for reoperation and IBTR. If tumor cells are seen at the SM with ink, the need for reoperation arises. Reoperations adversely affect the patient's psychological state and cosmetic results. When the need for reoperation arises, reexcision or completion mastectomy can be performed. An essential criticism about OPS is that the tumor bed is displaced during remodeling, and this provides limited opportunities for reexcision. It is possible to excise specimens that reach quite large volumes with OPS [8]. This means wider SM.
The average specimen weight was 198 g in Rietjens et al. [11] and 187 g in Fitoussi et al. [12]. In a Swiss study, the volume of specimens was 270 cm3 and the average distance to the SM was 6.9 mm [13]. In these series, the average tumor size varied between 2.2 and 2.9 cm. The given specimen weights and volumes support the idea that much more tissue can be removed. On the other hand, the SM positivity rate in OPS varies between 3.3 and 23% in tumors between 2 and 3 cm in size. The numbers seem better in high-volume centers and in the hands of experienced surgeons.
In previous studies, it was seen that reoperation is applied not only to SM positive cases but also to cases with close SM. In the series of Clough et al. [14], 44 (12.6%) patients needed to be reoperated, and 28 of them underwent mastectomy. While the reoperation rate in Fitoussi et al. [12] was 18.9%, approximately half of the patients underwent mastectomy. In the meta-analysis of Losken et al. [8], the reoperation rate was 12.3% and the mastectomy rate 6.5%. In the meantime, it should be remembered that the need for reoperation with BCS is >20% for similar-sized tumors [15, 16].
If specimen markings and surgery documentation after OPS is done in detail, in cases of SM positivity, reexcision is possible and patients can avoid a mastectomy. In our series, with an average tumor size of 22.8 mm, it was possible to excise an average specimen of 365 cm3 with level II techniques. In contrast, an average of a 9.4-mm SM was obtained. While the need for reoperation was 12.9%, the mastectomy rate was only 4%. Over time, it was seen that both incomplete resection and reoperation rates in patients with close SM decreased. Tumor-to-breast ratio and localization are among the factors affecting incomplete resection [17]. The risk is higher, especially in tumors close to the areola and located in the inner quadrant. However, appropriate selection of patients and techniques eliminates the difference in incomplete resection rates between techniques. The possibility of reexcision increases due to the domination and orientation of the reconstruction technique in operations performed by breast surgeons experienced in OPS. In this way, it has been possible to provide breast preservation rates of >96%.
Now, if the goal is to compare OPS with mastectomy and reconstruction, our IBTR rates should be as low as possible. When large patient series and studies with >5 years' follow-up are examined, local recurrence rates appear to vary between 2.2 and 6.8% [12, 14, 18, 19, 20]. Considering the tumor size and the results obtained with BCS, it can be said that these figures are highly acceptable. It appears that the rate of IBTR obtained with OPS are close to that of mastectomy for tumors <2 cm. The results are significantly better than BCS for tumors >2 cm. Risk factors other than SM positivity for IBRT after BCS were found to be young age, multicentricity, and lymphovascular invasion.
From the OPS perspective, it seems that these risk factors lose their importance, and that the only significant factor, other than SM, is tumor size [8]. Tumor bed displacement that occurs during wide glandular flap mobilization and difficulties in the calculation of target boost volume have caused concern about local recurrence [21]. In subsequent radiotherapy studies, it has been shown that if a cavity has been marked after glandular displacement, the tumor bed can receive a sufficient booster dose [22]. The radiotherapist should be orientated by placing at least 3 metallic clips on the tumor bed. It has been observed that the results are getting better with the establishment of multidisciplinary working principles and the increase of coordination among the teams. In our series, the IBTR rate was 2.7%. The majority of these relapses (84%) appeared in the same quadrant of the primary tumor. In the series of De Lorenzi et al. [18], the rate was 77.3%. Although follow-up times and numbers of patients vary across studies, the rate of regional recurrence is around 1% and the rate of distant metastasis between 12 and 18%. Reports are that 5-year OS rate is >92% [11, 12, 19, 23]. In our series, the regional recurrence rate was 0.9%, 5-year DFS 88%, LRFS 93.6%, and OS 96%. The 10-year DFS was 72%, LRFS 85.4%, and OS 90.2%.
It was thought that complication rates could be higher than expected during the development process of OPS. OPS is mainly characterized by wide excision, dissection, and displacement of the glandular tissue. Incisions are longer, and skin excision is done excessively. These conditions may increase the risk of necrosis by causing ischemia in the tissues. Axillary intervention is performed through the breast incision in most OPS techniques. Sometimes, quadranectomy and axillary intervention pouches are combined. While this may increase the risk of infection, seroma, hematoma, and abscess may possibly spread to larger areas. In a series of 350 patients under study by Clough et al. [14], 31 (8.9%) patients developed at least 1 complication. Fat necrosis drew attention as the most common complication and then minor wound-healing problems. Only 5 patients required reoperation due to complications. When data from the Swiss study were analyzed, the complication rate was found to be 7.7% in patients treated with OPS, and this rate was not higher than that in the patients treated with BCS in the same series [13].
The complication rates of OPS vary between 7.5 and 30% in the literature [23, 24]. The most crucial factor that increases the risk of complications is the resection volume. When evaluated in this sense, complication rates are slightly higher in patients undergoing reduction mammoplasty [25]. The higher BMI of patients undergoing reduction mammoplasty can also be considered a risk factor [26]. Diabetes and smoking are other factors that increase the risk of complications [27]. It was observed that the complication rate did not increase in patients where axillary intervention was performed through breast incision [12].
In the series comparing OPS and BCS, it was observed that the complication rates of OPS were not higher than BCS [28]. In fact, in the meta-analysis of Losken et al. [8], the complication rate of BCS was higher. Most OPS complications are minor, and can be corrected with simple interventions without the need for operating theatre conditions. The proportion of patients requiring intervention in operating theatre conditions is between 1 and 4%. Delayed wound-healing due to ischemic problems in the early period and fat necrosis in the late period are the most common complications. The complication rate in patients undergoing mastectomy and immediate reconstruction is up to 22%. Two-stage operations with tissue-expander and silicone implants are the most hazardous interventions in terms of complications [29, 30, 31, 32]. One of the most critical factors to disrupt cosmetic results and increase complication rates after breast surgery is the nonobliteration of the excision pouch and the large dead space left behind. Although wide excision and dissection are performed in OPS, the underlying philosophy of OPS is to reconstruct without leaving a dead space. The complication rates are not as high as feared since there is not much dead space left behind with OPS. Complications in our series (10.8%) and a need for revision (4%) were found to be compatible with rates in the literature.
OPS has not become widespread, especially in developing countries. Mastectomy rates are still high, and early or late reconstruction rates after mastectomy are low. Conventional lumpectomies are frequently applied to patients eligible for BCS. However, OPS is performed on >70% of all patients in important cancer centers, with documented successful oncological and cosmetic results. It is seen in many published series that the techniques applied on this subject are not homogeneous. The results of the techniques like fasciocutaneous, myocutaneous, and muscular flaps and implant are presented together.
Our investigation involved a homogeneous series of level II OPS techniques and surgery was performed by surgeons who allocate a significant part of their daily routine practice to breast surgery. This series is also valuable in that it contained a high number of patients from a developing country with a relatively long follow-up. It can be seen that OPS techniques are being used increasingly over the years. In this series, OPS techniques were shown to have low reoperation and complication rates. We revealed that most of the complications that occur are minor and without management difficulties. Breast protection rates are very high. The rate of patients who had to undergo mastectomy due to SM failure was only 4%. Considering the long-term follow-up results, it was seen that OPS techniques can successfully achieve local control. The 5-year cumulative incidence of local events was 2%.
The weakness of this study was a lack of data on cosmetic results, long-term patient satisfaction, and quality of life; these were evaluated by objective or subjective methods. It is a well-known fact that the cosmetic results of OPS are superior, and this study focused entirely on oncological safety. Awareness of the fact that many patients with OPS will not lose their breasts should be created, and regular training programs for OPS techniques should be conducted. By revealing these results, it is hoped that the OPS and breast conservation rates will increase.
Statement of Ethics
Research approval was obtained from University of Health Science, Oncology Hospital Clinical Research Ethics Committee- Ankara-Turkey. Ethics Committee approval was received with number AOH-31–2012. As it was a retrospective study, it was not necessary to obtain informed consent from the patients.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
There was no funding for the research stages of this study. There are no sponsors supporting this study.
Author Contributions
M.A.G. planning the study, L.D. wrote the article, and all authors contributed to the collection and evaluation of data.
References
- 1.Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012 Apr;13((4)):412–419. doi: 10.1016/S1470-2045(12)70042-6. [DOI] [PubMed] [Google Scholar]
- 2.Bosma SC, van der Leij F, van Werkhoven E, Bartelink H, Wesseling J, Linn S, et al. Very low local recurrence rates after breast-conserving therapy: analysis of 8485 patients treated over a 28-year period. Breast Cancer Res Treat. 2016 Apr;156((2)):391–400. doi: 10.1007/s10549-016-3732-0. [DOI] [PubMed] [Google Scholar]
- 3.Clough KB, Ihrai T, Oden S, Kaufman G, Massey E, Nos C. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg. 2012 Oct;99((10)):1389–1395. doi: 10.1002/bjs.8877. [DOI] [PubMed] [Google Scholar]
- 4.Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol. 2010 May;17((5)):1375–1391. doi: 10.1245/s10434-009-0792-y. [DOI] [PubMed] [Google Scholar]
- 5.Emiroglu M, Salimoglu S, Karaali C, Sert I, Gungor O, Sert F, et al. Oncoplastic reduction mammoplasty for breast cancer in women with macromastia: oncological long-term outcomes. Asian J Surg. 2017 Jan;40((1)):41–47. doi: 10.1016/j.asjsur.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003 Jan;237((1)):26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulcelik MA, Dogan L, Yuksel M, Camlibel M, Ozaslan C, Reis E. Comparison of outcomes of standard and oncoplastic breast-conserving surgery. J Breast Cancer. 2013 Jun;16((2)):193–197. doi: 10.4048/jbc.2013.16.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2014 Feb;72((2)):145–149. doi: 10.1097/SAP.0b013e3182605598. [DOI] [PubMed] [Google Scholar]
- 9.Anbiyaiee A, Abouali Galeh Dari M, Anbiyaee O, Anbiyaiee A. Breast Reconstruction after Mastectomy in Women with Breast Cancer: A Systematic and Meta-Analysis Review. World J Plast Surg. 2020 Jan;9((1)):3–9. doi: 10.29252/wjps.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Li CZ, Wu CT, Jiao GM, Yan F, Zhu HC, et al. Comparison of immediate breast reconstruction after mastectomy and mastectomy alone for breast cancer: A meta-analysis. Eur J Surg Oncol. 2017 Feb;43((2)):285–293. doi: 10.1016/j.ejso.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, Garusi C, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast. 2007 Aug;16((4)):387–395. doi: 10.1016/j.breast.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Fitoussi AD, Berry MG, Famà F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article] Plast Reconstr Surg. 2010 Feb;125((2)):454–462. doi: 10.1097/PRS.0b013e3181c82d3e. [DOI] [PubMed] [Google Scholar]
- 13.Behluli I, Le Renard PE, Rozwag K, Oppelt P, Kaufmann A, Schneider A. Oncoplastic breast surgery versus conventional breast-conserving surgery: a comparative retrospective study. ANZ J Surg. 2019 Oct;89((10)):1236–1241. doi: 10.1111/ans.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clough KB, van la Parra RF, Thygesen HH, Levy E, Russ E, Halabi NM, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg. 2018 Jul;268((1)):165–171. doi: 10.1097/SLA.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 15.Jeevan R, Cromwell DA, Trivella M, Lawrence G, Kearins O, Pereira J, et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ. 2012 Jul;345(jul12 2):e4505. doi: 10.1136/bmj.e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery(SM) database. Ann Surg Oncol. 2014 Oct;21((10)):3185–3191. doi: 10.1245/s10434-014-3905-1. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995 Apr;332((14)):907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 18.De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. Eur J Surg Oncol. 2016 Jan;42((1)):71–77. doi: 10.1016/j.ejso.2015.08.160. [DOI] [PubMed] [Google Scholar]
- 19.Grubnik A, Benn C, Edwards G. Therapeutic mammaplasty for breast cancer: oncological and aesthetic outcomes. World J Surg. 2013 Jan;37((1)):72–83. doi: 10.1007/s00268-012-1786-7. [DOI] [PubMed] [Google Scholar]
- 20.Calabrese C, Casella D, Di Taranto G, Marcasciano M, Kothari A, Sordi S, et al. Oncoplastic conservative surgery for breast cancer: long-term outcomes of our first ten years experience. Eur Rev Med Pharmacol Sci. 2018 Nov;22((21)):7333–7342. doi: 10.26355/eurrev_201811_16270. [DOI] [PubMed] [Google Scholar]
- 21.Eaton BR, Losken A, Okwan-Duodu D, Schuster DM, Switchenko JM, Mister D, et al. Local recurrence patterns in breast cancer patients treated with oncoplastic reduction mammaplasty and radiotherapy. Ann Surg Oncol. 2014 Jan;21((1)):93–99. doi: 10.1245/s10434-013-3235-8. [DOI] [PubMed] [Google Scholar]
- 22.Pezner RD, Tan MC, Clancy SL, Chen YJ, Joseph T, Vora NL. Radiation therapy for breast cancer patients who undergo oncoplastic surgery: localization of the tumor bed for the local boost. Am J Clin Oncol. 2013 Dec;36((6)):535–539. doi: 10.1097/COC.0b013e318256efba. [DOI] [PubMed] [Google Scholar]
- 23.Meretoja TJ, Svarvar C, Jahkola TA. Outcome of oncoplastic breast surgery in 90 prospective patients. Am J Surg. 2010 Aug;200((2)):224–228. doi: 10.1016/j.amjsurg.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Losken A, Elwood ET, Styblo TM, Bostwick J., 3rd The role of reduction mammaplasty in reconstructing partial mastectomy defects. Plast Reconstr Surg. 2002 Mar;109((3)):968–975. doi: 10.1097/00006534-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Nizet JL, Maweja S, Lakosi F, Lifrange E, Scagnol I, Seidel L, et al. Oncological and surgical outcome afteroncoplastic breast surgery. Acta Chir Belg. 2015;115((1)):33–41. doi: 10.1080/00015458.2015.11681064. [DOI] [PubMed] [Google Scholar]
- 26.de Blacam C, Ogunleye AA, Momoh AO, Colakoglu S, Tobias AM, Sharma R, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012 Mar;255((3)):551–555. doi: 10.1097/SLA.0b013e318246c294. [DOI] [PubMed] [Google Scholar]
- 27.Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012 May;38((5)):375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 28.Rose M, Manjer J, Ringberg A, Svensson H. Surgical strategy, methods of reconstruction, surgical margins and postoperative complications in oncoplastic breast surgery. Eur J Plast Surg. 2014;37((4)):205–214. doi: 10.1007/s00238-013-0922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Momeni A, Kanchwala S, Sbitany H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast Reconstr Surg. 2020 Apr;145((4)):914–920. doi: 10.1097/PRS.0000000000006657. [DOI] [PubMed] [Google Scholar]
- 30.Economides JM, Graziano F, Tousimis E, Willey S, Pittman TA. Expanded Algorithm and Updated Experience with Breast Reconstruction Using a Staged Nipple-Sparing Mastectomy following Mastopexy or Reduction Mammaplasty in the Large or Ptotic Breast. Plast Reconstr Surg. 2019 Apr;143((4)):688e–97e. doi: 10.1097/PRS.0000000000005425. [DOI] [PubMed] [Google Scholar]
- 31.Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013 Nov;132((5)):1043–1054. doi: 10.1097/PRS.0b013e3182a48b8a. [DOI] [PubMed] [Google Scholar]
- 32.Reverberi C, Marinelli L, Campanella B, Scalabrino G, Nicosia L, Anzellini D, et al. Post-mastectomy immediate breast reconstruction and adjuvant radiotherapy: long term results of a mono institutional experience. Radiol Med (Torino) 2020 Sep;125((9)):887–893. doi: 10.1007/s11547-020-01161-7. [DOI] [PubMed] [Google Scholar]