Abstract
Introduction
The clinical outcome of HER2-positive breast cancer patients changed with the use of anti-Her therapies, though it still remains an aggressive and fatal disease. Implementation of immune checkpoint inhibitors in HER2-positive Breast cancer is a concept supported by the reported biological and preclinical data.
Methods
We conducted a systematic review of the current literature involving immune checkpoint inhibitors alone or in combination with targeted therapies or chemotherapy finalized or running in HER2-positive breast cancer.
Results
Twelve clinical trials and 2 case reports were identified in our study.
Conclusion
The reported clinical trials highlight that checkpoint inhibition seems to be promising in metastatic, neoadjuvant, and adjuvant settings of HER2-positive breast cancer.
Keywords: Breast cancer, Checkpoint inhibitor, Immunotherapy, HER2-positive breast cancer, Clinical trials, Systematic review
Introduction
Breast cancer is the most common cancer in women, accounting for 15.2% of new diagnoses, and it is the second most common cause of cancer-related deaths [1]. In 2018, breast cancer was estimated to affect 2,088,849 women and men and caused nearly 626,679 deaths worldwide [2]. Furthermore, 1 out of 8 women are expected to develop invasive breast cancer over their lifetime, with a current 5-year survival rate of 89.7%. Nearly 2,550 new cases of invasive breast cancer are expected to be diagnosed in men, and the resulting lifetime risk for male breast cancer is about 4 in 1,000 [1]. The current treatment strategy for breast cancer includes chemotherapy, endocrine therapy, and targeted biologic therapy. Treatment has become more individualized based on characteristics of the tumor, including estrogen and progesteron receptor expression, expression of the human epidermal growth factor receptor (HER2), and estimation of cell proliferation with Ki67 [3, 4, 5]. Between 20 and 30% of all breast cancers overexpress HER2, accounting for 40,000–60,000 patients sharing this subtype of breast cancer [1].
HER2-positive breast cancer is an aggressive subtype, characterized by amplification or overexpression of the HER2 (erbB2) oncogene. HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family, which creates a transmembrane protein with tyrosine kinase activity [6]. Amplification or overexpression of this oncogene results in an increase in HER2 protein that has been shown to play an important role in the development and progression of the aggressive phenotype [7]. Despite the fact that HER2-targeted treatment has improved the overall survival (OS) of patients with HER2-postive metastatic breast cancer, with a median OS exceeding 3 years, there is an unmet need for better treatments [8, 9, 10].
Immunotherapeutic agents targeting the PD-1/programmed death ligand 1 (PD-L1) and CTLA-4 pathways are critical to the immune system's ability to control cancer growth [11]. Cancer cells use these pathways to escape from immune surveillance [12, 13]. Specific antibodies blocking these pathways are called immune checkpoint inhibitors. Recently, immunotherapy was added to the therapeutic armamentarium for triple-negative breast cancer. Atezolizumab, an anti-PDL1 checkpoint inhibitor, in combination with nab-paclitaxel has been approved for first-line treatment of triple-negative breast cancer patients; patients included in the Impassion130 clinical trial treated with the combination of immunotherapy plus chemotherapy presented with a median progression-free survival (PFS) of 7.2 months compared to 5.5 months of chemotherapy plus placebo for the intention-to-treat population and, PD-L1-positive patients had a median PFS of 7.5 and 5.0 months [14]. The ORR was 56% with atezolizumab/chemotherapy versus 45.9% in the chemotherapy group for the intent-to-treat population, while for the PD-L1-positive patients it was 58.9 and 42.6%, respectively [14]. In ESMO 2020 the final analysis of Impassion130 reported an improvement of 7.5 mOS in PDL-1-positive patients (21.0 [range: 19.0–23.4] vs. 18.7 months [range: 16.9–20.8] in favor of the atezolizumab and nab-paclitaxel combination) [15].
Herein, we report a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, which synthesizes all available data emerging from the current literature and evaluates the efficacy and safety of checkpoint inhibitors in metastatic, adjuvant, and neoadjuvant settings of HER2-positive breast cancer.
Methods
This systematic review was performed in accordance with PRISMA guidelines. Eligible articles were identified by a search of the Medline bibliographic database from January 1, 2010, to October 1, 2019. The search strategy included the following keywords: breast[ti] AND (neoplasm OR neoplasms OR cancer OR cancers OR carcinoma OR carcinomas) AND (pembrolizumab[ti] OR avelumab[ti] OR nivolumab[ti] OR atezolizumab[ti] OR durvalumab[ti] OR ipilimumab[ti] OR cemiplimab[ti] OR tremelimumab [ti]). Our goal was to identify all previous and ongoing clinical trials testing anti-PD1, anti-PDL1, and anti-CTLA4 agents in metastatic, neoadjuvant, and adjuvant HER2-positive breast cancer.
Language restrictions were applied (i.e., only articles in English, French, or German were considered eligible); 2 investigators (G.G. and A.K.), searched the literature and extracted data from each eligible study. Reviews were not eligible, while all prospective and retrospective studies, as well as case reports, were eligible for this systematic review. Papers that did not state the names of the authors were excluded. In addition, we checked all of the references of relevant reviews and eligible articles that our search retrieved, so as to identify potentially eligible conference abstracts. Additionally, we manually checked ClinicalTrials.gov in order to add ongoing trials without published data using the following words: pembrolizumab and breast cancer and HER2 positive, nivolumab and breast cancer and HER2 positive, avelumab and breast cancer and HER2 positive, atezolizumab and breast cancer and HER2 positive, durvalumab and breast cancer and HER2 positive, ipilimumab and breast cancer and HER2 positive. Only trials focused on immunotherapeutic agents in the HER2-positive subtype of breast cancer were eligible for this systematic review. Trials focused on other subtypes of breast cancer were excluded (i.e., triple negative and hormone receptor positive).
Data Reported at International Meetings Were Included as Well
All studies that examined the efficacy and safety of immunotherapeutic agents and reported the relevant frequencies, regardless of sample size, were considered eligible for this systematic review. For those studies, the following data were collected: first author, year of publication, agents, phase of the trial, number of patients treated, characteristics of the patient population (first-line treatment, second-line treatment, etc.), median age (years), complete response (CR) rate, partial response (PR) rate, stabilization of the disease (SD) rate, progression of the disease (PD) rate, median OS (months), median PFS (months), and complications. In instances where multiple (overlapping) publications stemming from the same study were identified, the larger-size study was included.
Results
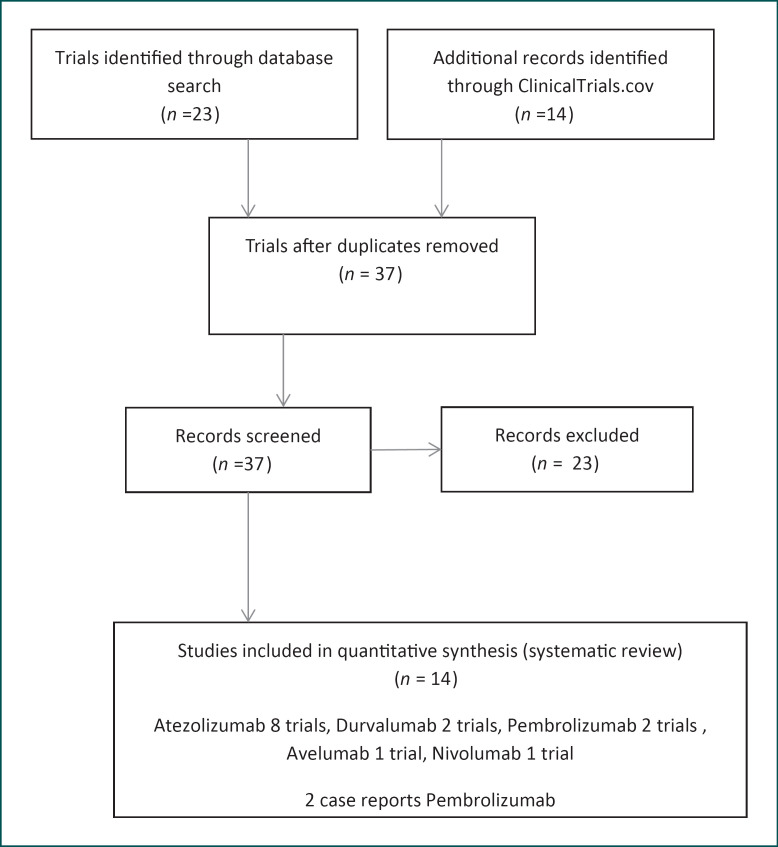
Using the above criteria, 14 clinical trials and 2 case reports were identified (Table 1). Thirteen clinical trials tested the use of immune checkpoint inhibitors in the metastatic setting and 1 trial tested it in the neoadjuvant setting (Fig. 1). Of the aforementioned clinical trials, 4 were phase Ib, 3 were phase Ib-II, 4 were phase II, and 3 were phase III clinical trials (Table 1). The exact population studied in each trial is highlighted in Table 2.
Table 1.
Characteristics of the clinical trials (ClinicalTrials.gov)
| Study | Type of study | Drug | Status | Patients, n | Population |
|---|---|---|---|---|---|
| PANACEA NCT02129556 [16] | Single-arm phase Ib/II study | Experimental: MK-3475 (pembrolizumab) with trastuzumab (single arm) Phase Ib: MK-3475 at a dose of 2 or 10 mg/kg (i.v.), or a fall-back dose of 1 mg/kg, together with trastuzumab at 6 mg/kg (i.v.) once every 3 weeks Phase II: MK-3475 at a flat dose of 200 mg (i.v.) together with trastuzumab at 6 mg/kg (i.v.) once every 3 weeks until progression, lack of tolerability, or 24 months of treatment A dose of 8 mg/kg trastuzumab will be used in cycle 1 if prior treatment with trastuzumab has been stopped more than 3 months prior |
Recruitment completed | 58 | Metastatic Breast cancer, central nervous system after local treatment included |
|
| |||||
| AVIATOR study NCT03414658 | Randomized phase II open label trial | Trastuzumab and vinorelbine with avelumab or avelumab and utomilumab in advanced HER2+ breast cancer | Recruiting | Estimated 100 | Patients with HER2-positive metastatic breast cancer who have progressed on prior trastuzumab and pertuzumab |
|
| |||||
| NCT02649686 [21] | Randomized phase Ib/II study | Durvalumab plus trastuzumab Durvalumab q3w until PD trastuzumab q3w ×6 | Active, not recruiting | 15 | HER2-positive metastatic breast cancer |
|
| |||||
| NCT02403271 | Phase Ib/II multicenter study | Ibrutinib in combination with durvalumab (MEDI4736) in subjects with relapsed or refractory solid tumors | Completed | 124 | Non-small cell lung cancer breast cancer Pancreatic cancer |
|
| |||||
| NCT03032107 | Phase Ib study | Pembrolizumab combined with trastuzumab emtansine | Recruiting | Estimated 27 | Metastatic breast cancer |
|
| |||||
| NCT03125928 | Single-arm phase IIA open label study | Atezolizumab in combination with paclitaxel, trastuzumab, and pertuzumab | Recruiting | Estimated 50 | HER2-positive metastatic breast cancer |
|
| |||||
| NCT02605915 | Nonrandomized phase Ib study | Atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab (with and without docetaxel) in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer | Active, not recruiting | Estimated 98 | Metastatic breast cancer |
|
| |||||
| NCT03417544 | Single-arm phase II study | Atezolizumab in combination with pertuzumab plus high-dose trastuzumab | Recruiting | Estimated 33 | HER2-positive metastatic breast cancer Central nervous system metastases |
|
| |||||
| NCT03595592 | Randomized phase III interventional clinical trial | Atezolizumab, pertuzumab, and trastuzumab with chemotherapy as neoadjuvant treatment | Not yet recruiting | Estimated 650 | Neoadjuvant HER2-positive early high-risk and locally advanced breast cancer (APTneo) |
|
| |||||
| NCT02924883 | Randomized, multicenter, double-blind, placebo-controlled phase II trial | Trastuzumab emtansine in combination with atezolizumab or atezolizumab-placebo | Active, not recruiting | Estimated 202 | HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab and taxane-based therapy |
|
| |||||
| NCT03650348 | Phase Ib open-label, dose escalation study | PRS-343 in combination with atezolizumab | Recruiting | Estimated 70 | HER2-positive advanced or metastatic solid tumors Breast, gastric, bladder, and solid tumors |
|
| |||||
| NCT03199885 | Randomized, double-blind phase III trial | Paclitaxel/trastuzumab/pertuzumab with atezolizumab or placebo | Not yet recruiting | Estimated 600 | First-line HER2-positive metastatic breast cancer |
|
| |||||
| NCT03523572 | Phase Ib, nonrandomized, dose escalation study | Trastuzumab deruxtecan with nivolumab | Recruiting | 99 | HER2-positive metastatic breast cancer, bladder cancer |
|
| |||||
| NCT03726879 | Randomized, double-blind phaseIII trial | Atezolizumab or placebo in combination with neoadjuvant doxorubicin + cyclophosphamide followed by paclitaxel + trastuzumab + pertuzumab | Recruiting | 453 | Early HER2-positive breast cancer |
Fig. 1.
Schematic presentation of the research strategy.
Table 2.
Drugs, studies, and populations
| Drug | Study | Population |
|---|---|---|
| Atezolizumab | 1. NCT03125928 (phase IIA) 2. NCT02605915 (nonrandomized phase Ib study) 3. NCT03417544 (single-arm phase II study) 4. NCT03595592 (randomized phase III interventional clinical trial) 5. NCT02924883 (randomized, multicenter, double-blind, placebo-controlled phase II) 6. NCT03650348 (phase Ib, open-label, dose escalation study) 7. NCT03199885 (randomized, double-blind phase III trial) |
1. HER2-positive metastatic breast cancer 2. Metastatic breast cancer 3. HER2-positive metastatic breast cancer, central nervous system metastases 4. Neoadjuvant HER2-positive early high-risk and locally advanced breast cancer (APTneo) 5. HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab and taxane-based therapy 6. HER2-positive advanced or metastatic solid tumors; breast, gastric, bladder, solid tumors 7. First-line HER2-positive metastatic breast cancer |
|
| ||
| Pembrolizumab | 1. PANACEA NCT02129556 (randomized phase Ib/II [16]) | 1. Metastatic breast cancer, central nervous system after local treatment included |
| 2. NCT03032107 (phase Ib study) | 2. Metastatic breast cancer | |
|
| ||
| Durvalumab | 1. NCT02649686 (randomized phase Ib/II [21]) | 1. HER2-positive metastatic breast cancer |
| 2. NCT02403271 (phase Ib/II multicenter study) | 2. Non-small cell lung cancer, breast cancer, pancreatic cancer | |
|
| ||
| Avelumab | AVIATOR study (NCT03414658 randomized phase II trial) | Patients with HER2-positive metastatic breast cancer who have progressed on prior trastuzumab and pertuzumab |
Atezolizumab is explored in 8 clinical trials, pembrolizumab in 2 clinical trials, durvalumab in 2 clinical trials, and avelumab or avelumab with utomilumab in 1 trial, each (Fig. 1).
Atezolizumab
Atezolizumab is an Fc-engineered, humanized immunoglobulin G1 (IgG1) monoclonal antibody that directly binds to PD-L1 and provides a dual blockade of the PD-1 and B7.1 receptors. Atezolizumab spares the PD-L2/PD-1 interaction allowing PD-L2/PD-1-mediated inhibitory signals to persist [16]. Currently, there are 7 ongoing clinical trials exploring atezolizumab in Her-2-positive breast cancer. The NCT03595592 or APTneo trial is a randomized phase III Interventional clinical trial evaluating the use of atezolizumab, pertuzumab, and trastuzumab with chemotherapy as neoadjuvant treatment and it is estimated to enroll 650 patients with Her-2-positive, early high-risk, and locally advanced breast cancer. NCT02924883-Kate2 is a randomized, multicenter, double-blind, placebo-controlled phase II clinical trial exploring the role of trastuzumab emtansine in combination with atezolizumab or atezolizumab-placebo. It is estimated to enroll 202 participants with Her2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab and taxane-based therapy. At the ESMO 2019 congress, investigators reported that, in the intent-to-treat population, the 1-year OS was 89% in both arms; however, in PD-L1-positive patients, the 1-year OS was greater in the atezolizumab-plus-trastuzumab emtansine arm at 94.3 versus 87.9% (stratified HR = 0.55; 95% CI 0.22–1.38), while at the date of cut-off the median OS was not reached in either arm, with an absence of statistical significance in the early analysis [17]. NCT03125928 − Impassion50 is a single-arm phase IIA open-label clinical trial exploring the use of atezolizumab in combination with paclitaxel, trastuzumab, and pertuzumab in HER2-positive metastatic breast cancer and it is estimated to enroll 50 patients. NCT02605915 is a nonrandomized phase Ib study exploring the use of atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab (with and without docetaxel) in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer and it is estimated to enroll 98 patients. NCT03417544 is a single-arm phase II study where the researchers are combining atezolizumab with pertuzumab plus high-dose trastuzumab in HER2-positive metastatic breast cancer and it is estimated to include 33 patients with central nervous system metastases. NCT03650348 is a phase Ib open-label, dose escalation study combining atezolizumab with PRS-343 in HER2-positive advanced or metastatic solid tumors, including breast, gastric, and bladder cancer and is estimated to enroll 70 patients. NCT03199885 is a randomized, double-blind phase III trial evaluating the use of paclitaxel/trastuzumab/pertuzumab with atezolizumab or placebo, and it is estimated to enroll 600 patients with first-line HER2-positive metastatic breast cancer. NCT03726879 − IMpassion050 (BO40747) is a phase III randomized, double-blind trial evaluating the efficacy and safety of atezolizumab compared with placebo when given in combination with neoadjuvant dose-dense anthracycline (doxorubicin) + cyclophosphamide followed by paclitaxel + trastuzumab + pertuzumab in patients with early HER2-positive breast cancer.
Pembrolizumab
Pembrolizumab is a humanized monoclonal antibody that binds to the programmed cell death-1 (PD-1) receptor and blocks its interaction with the ligands PD-L1 and PD-L2. Pembrolizumab potentiates T-cell responses, including antitumor responses, which are expressed in antigen-presenting cells and may be expressed by tumors or other cells in the tumor microenvironment [18]. Two trials are currently evaluating the role of pembrolizumab in HER2-positive breast cancer. PANACEA (2018) or NCT02129556 is a single-arm phase Ib/II clinical trial that has completed the enrollment of 58 patients with metastatic breast cancer and included patients with CNS metastases after local treatment [19]. Patients in the experimental arm receive pembrolizumab with trastuzumab in an attempt to find the optimal dose (single arm) [19]. In phase Ib patients will be given pembrolizumab at a dose of 2 or 10 mg/kg (i.v.), or a fall-back dose of 1 mg/kg, together with trastuzumab 6 mg/kg by (i.v.) once every 3 weeks. In phase II, pembrolizumab will be administered at a flat dose of 200 mg (i.v.) together with trastuzumab 6 mg/kg (i.v.) once every 3 weeks until disease progression, a lack of tolerability, or 24 months of treatment. A dose of 8 mg/kg trastuzumab will be used in cycle 1 if prior treatment with trastuzumab was stopped at least 3 months earlier. The results of this study are very encouraging since, in the PD-L1+ patients, the median OS was 16.1 months (90% CI 13.1 to not reached) vs. 7.0 months (90% CI 4.9–9.8) in the PD-L1-negative group (p = 0.0006). The median PFS among all of the PD-L1+ patients was 2.7 (90% CI 2.6–4.0) versus 2.5 months (90% CI 1.4–2.7) in PD-L1-negative patients (p = 0.07). The 12-month PFS rate was 13 (90% CI 6–22) versus 0%. The 12-month OS rate was 65 (90% CI 52–76) versus 12% (90% CI 1–36). Objective response rates (ORR) of 15.2% in the 40 PD-L1-positive breast cancer patients and 0% in the 12 PD-L1-negative patient cohort were reported. The number of tumor-infiltrating lymphocytes (TIL) was correlated with the ORR. Most of the patients had low numbers of TIL in the metastatic niche. In the patients with TIL levels ≥5%, the ORR was 39 vs. 5% in patients with TIL levels <5%. Safety was evaluated across all 58 patients. Most adverse effects (AE) were grade 1/2. The most common grade 1 AE included fatigue (n = 7), diarrhea (n = 6), arthralgia (n = 6), headache (n = 4), nausea (n = 6), dyspnea (n = 2), and myalgia (n = 5). Grade 2 AE included fatigue (n = 5), diarrhea (n = 2), arthralgia (n = 2), headache (n = 2), and dyspnea (n = 1). There was 1 case with grade 3 and 1 with grade 4 dyspnea. No cardiac events were reported. There were 11 all-grade immune-related AE reported, 6 of which were grade ≥3. Four immune-related AE resulted in discontinuation. Thyroid dysfunction across all grades occurred in 4 patients. There were 4 cases of pneumonitis across all grades, including 2 cases with grade ≥3 severity [19].
Pembrolizumab is also being studied in trial NCT03032107, which is an ongoing phase Ib study exploring the role of pembrolizumab in combination with trastuzumab emtansine in an estimated number of 50 patients with HER2-positive metastatic breast cancer.
In 2 cases of HER2-positive metastatic breast cancer where multi-anti-HER2-targeted therapy had failed, pembrolizumab was combined with albumin-bound paclitaxel. Both patients achieved partial remission, and serum ECD levels showed a remarkable decrease compared to the baseline of 75 and 60%, respectively [20].
Durvalumab
Durvalumab is a fully human, IgG1κ monoclonal antibody that selectively blocks the interaction of PD-L1 with PD-1 and CD80 (B7.1). Durvalumab does not induce antibody-dependent cell-mediated cytotoxicity [21]. Selective blockade of PD-L1/PD-1 and PD-L1/CD80 interactions enhances antitumor immune responses and increases T-cell activation [22]. There are 2 trials exploring the role of durvalumab in HER2-positive breast cancer. NCT02649686 is a randomized phase Ib/II using durvalumab plus trastuzumab. In this setting, patients will receive durvalumab q3w until PD and trastuzumab q3w for 6 administrations. The researchers opted to enroll 15 participants with HER2-positive metastatic breast cancer. No responses in RECIST were seen, with 4 out of 14 patients (29%) demonstrating stable disease as the best response at week 6 (median duration: 2.7 months). No dose-limiting toxicities were observed at dose level 1 (n = 6) or with dose expansion (n = 9) during cycle 1. One patient developed ≥grade 3 immune related AE (grade 4 diabetes mellitus) [23, 24].
The NCT02403271 trial is a phase Ib/2 multicenter study where ibrutinib in combination with durvalumab (MEDI4736) was used in 124 subjects with relapsed or refractory solid tumors including non-small cell lung cancer, breast cancer, and pancreatic cancer. The trial has been closed and the results are highly awaited.
Avelumab
Avelumab is a human IgG1 monoclonal antibody directed against PD-L1. Avelumab removes the suppressive effects of PD-L1 on cytotoxic CD8+ T cells, resulting in the restoration of anti-tumor T-cell responses. Avelumab has also been shown to induce natural killer cell-mediated direct tumor cell lysis via antibody-dependent cell-mediated cytotoxicity [25]. In the AVIATOR study (NCT03414658), a randomized phase II open-label trial, 100 patients are estimated to receive trastuzumab and vinorelbine with avelumab or avelumab and utomilumab for advanced HER2-positive breast cancer with progression on prior trastuzumab and pertuzumab.
Nivolumab
Nivolumab is a human monoclonal antibody (IgG4) that binds to the receptor of PD1. Nivolumab is being tested in a phase Ib clinical trial (NCT03523572) in metastatic HER2-positive breast cancer patients who have progressed after 2 prior therapies and receive trastuzumab deruxtecan with or without nivolumab.
Discussion
Checkpoint inhibition is approved for triple-negative breast cancer with the combination of atezolizumab and nab-paclitaxel used in the first-line setting [14]. The combination of atezolizumab with nab-paclitaxel and anthracycline chemotherapy improved the pathologic CR in the neoadjuvant setting [26]. Further, the combination of pembrolizumab and chemotherapy has shown promising activity both in the first-line setting and in the neoadjuvant setting for triple-negative breast cancer [27, 28]. HER2-positive breast cancer, though, remains an aggressive breast cancer subtype with an unmet need for new therapeutic choices despite the fact that anti-HER treatment has changed the natural history of this disease. Several reports have discussed the implementation of immunotherapy in HER2-positive breast cancer treatment [23, 29, 30]. Immunotherapeutic agents are used alone or in combination with chemotherapy or other drugs. Despite the fact that checkpoint inhibitors are proven to be an affective option in the therapeutic armamentarium against HER2 breast cancer, it is not yet clear from phase II/III clinical trials whether they can make a breakthrough in the treatment of this breast cancer subtype. Ongoing randomized clinical trials are comparing immune checkpoint inhibitors with standard treatments in a quest to define the exact role of these agents in the clinical management of HER2 breast cancer. In parallel, biomarker analysis may optimize patient selection.
The tumor mutational burden (TMB) has been studied in metastatic HER2-positive breast cancer and it has been proven to be a statistically significant prognostic factor. Patients with a high TMB had a longer mOS compared to low-TMB patients (85.8 vs. 44.9 months, respectively; p = 0.016) [31]. The results of this study suggest that a high TMB may be a prognostic marker predictive of a good OS for patients undergoing conventional HER2-directed treatments and chemotherapy. HER2-positive breast cancer tumors are considered to be more “inflamed” than luminal A tumors, and the presence of TIL is prognostic according to the analysis of the NeoALTTo trial [32]. In patients whose tumors presented with a higher proportion of TIL, there was an increased pathologic CR rate [32]. Another analysis from the FinHER trial showed that the number of TIL was predictive of the benefit from trastuzumab [33]. Researchers reported that each 10% increase in lymphocytic infiltration was significantly associated with a decreased distant recurrence in patients randomized to the trastuzumab arm (distant disease free survival; pinteraction = 0.025) [33]. Furthermore, preclinical studies have shown that the therapeutic benefit of anti-HER therapies is increased in combination with anti-PD-1 drugs. Preclinical data with the combination of anti-HER2 treatment and interferon-γ have shown promising results in vivo in xenografts [34]. In a phase I trial combining trastuzumab with CD8+ T-cell-eliciting HER peptide vaccines provided results suggesting a synergistic immunotherapeutic benefit, which is now being studied in a phase II clinical trial [35]. All of the above data are indicative of a solid rationale to study the action of immunotherapy regimens in HER2-positive breast cancer.
Our analysis demonstrated that immune checkpoint inhibitors are now under investigation in HER2-positive breast cancer. All of the available molecules − avelumab, atezolizumab, durvalumab, and pembrolizumab − are being tested alone or in combination in several settings including neoadjuvant, adjuvant, and metastatic settings of HER2-positive breast cancer. The results of the few reported trials and case reports are indicative of a promising therapeutic choice [19, 20]. Immunotherapy in combination with anti-HER therapy seems to be effective. Results from PANACEA show an OS of 13.1 months for PDL1+ patients compared to 7.0 months for PDL1− patients (p = 0.006), highlighting the usefulness of this biomarker in HER2-positive breast cancer [19, 23]. However, ORR according to TIL (39% in patients with ≥5 vs. 5% in patients with TIL <5%) depicts that the ideal biomarker predicting the response to immune checkpoint inhibitors is not well defined [19, 23].
Regarding safety, immune checkpoint inhibitors in HER2-positive breast cancer patients present AE similar to those found in other tumor types, making it a relatively tolerable treatment. There are no reports of grade 4 toxicities or treatment discontinuation due to severe AE [19, 24]. Thyroid dysfunction and pneumonitis were the most common immune related AE reported [19, 24].
HER2-positive breast cancer remains a tough disease for both clinicians and patients, and if it becomes metastatic it is still incurable. Despite the fact that clinical trials and case reports have demonstrated promising results, there are several ongoing trials expected to provide solid data regarding the use of immunotherapeutic agents in the neoadjuvant, adjuvant, and metastatic settings of HER2-positive breast cancer.
Statement of Ethics
The authors declare that this systematic review was conducted according to the laws of Greece. This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest Statement
F.Z. has received honoraria from Roche, Novartis, and Lilly. M.A.D. has received honoraria and participates in advisory boards from TAKEDA, Janssen, BMS, Cellgene, and Amgen.
Funding Sources
There are no funding sources to declare.
Author Contributions
F.Z. and A.K. are the writers of this article. G.G. and A.K. are 2 independent investigators who performed the literature search and data extraction from all of the studies examined. Data acquisition was done by A.K., G.G., and E.Z. The statistical analysis was performed by M.L., M.K., and R.Z. M.A.D., F.Z., and A.K. contributed to the conception and design of this study. Editing and revision of this work were performed by M.A.D., M.L., E.Z., and F.Z. All of the authors read and approved the final version of this paper.
A.K. and F.Z. contributed equally to this work.
References
- 1. https://seer.cancer.gov/statfacts/html/breast.html .
- 2. https://gco.iarc.fr/ GLOBAL CANCER OBSERVATORY.
- 3.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017 Jan;28((1)):16–33. doi: 10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017 Apr;15((4)):433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 5.Loi S. The ESMO clinical practise guidelines for early breast cancer: diagnosis, treatment and follow-up: on the winding road to personalized medicine. Ann Oncol. 2019 Aug;30((8)):1183–1184. doi: 10.1093/annonc/mdz201. [DOI] [PubMed] [Google Scholar]
- 6.Mrhalová M, Kodet R, Kalinová M, Hilská I. Relative quantification of ERBB2 mRNA in invasive duct carcinoma of the breast: correlation with ERBB-2 protein expression and ERBB2 gene copy number. Pathol Res Pract. 2003;199((7)):453–461. doi: 10.1078/0344-0338-00445. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014 Mar;21((2)):100–107. doi: 10.1097/PAP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 8.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. Herceptin Adjuvant (HERA) Trial Study Team 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017 Mar;389((10075)):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017 Jun;18((6)):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. APHINITY Steering Committee and Investigators Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017 Jul;377((2)):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter P. The fourth pillar: despite some setbacks in the clinic, immunotherapy has made notable progress toward becoming an additional therapeutic option against cancer. EMBO Rep. 2017 Nov;18((11)):1889–1892. doi: 10.15252/embr.201745172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun DA, Burke KP, Van Allen EM. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin Cancer Res. 2016 Dec;22((23)):5642–5650. doi: 10.1158/1078-0432.CCR-16-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milano G. Resistance to immunotherapy: clouds in a bright sky. Invest New Drugs. 2017 Oct;35((5)):649–654. doi: 10.1007/s10637-017-0456-x. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. IMpassion130 Trial Investigators Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018 Nov;379((22)):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 15.L.A. Emens1 SA, C.H. Barrios3, V.C. Dieras4, H. Iwata5, S. Loi6, H.S. Rugo7, A. Schneeweiss8, E.P. Winer9, S. Patel, V. Henschel, A. Swat, M. Kaul, L. Molinero, S.Y. Chui, P. Schmid. LBA. IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2020;31(suppl_4):S1142–215. [Google Scholar]
- 16.Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: A PD-L1-Blocking Antibody for Bladder Cancer. Clin Cancer Res. 2017 Apr;23((8)):1886–1890. doi: 10.1158/1078-0432.CCR-16-1417. [DOI] [PubMed] [Google Scholar]
- 17.Sanglier T, Fabi A, Flores C, Flahavan E, Lindegger N, Montemurro F. Use of trastuzumab emtansine (T-DM1; K) after pertuzumab + trastuzumab (PH) in patients with HER2-positive metastatic breast cancer (mBC): challenges in assessing effectiveness of treatment sequencing in the real world (RW) Ann Oncol. 2019;30(suppl_5):v104–42. [Google Scholar]
- 18.Savina M, Le Cesne A, Blay JY, Ray-Coquard I, Mir O, Toulmonde M, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017 Apr;15((1)):78. doi: 10.1186/s12916-017-0831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. International Breast Cancer Study Group and the Breast International Group Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019 Mar;20((3)):371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 20.Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. ESMO Guidelines Committee and EURACAN Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct;29(Suppl 4):iv268–9. doi: 10.1093/annonc/mdy321. [DOI] [PubMed] [Google Scholar]
- 21.Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res. 2015 Sep;3((9)):1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 22.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. PACIFIC Investigators Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017 Nov;377((20)):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 23.Bartsch R, Bergen E. ASCO 2018: highlights in HER2-positive metastatic breast cancer. Memo. 2018;11((4)):280–283. doi: 10.1007/s12254-018-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chia S, Bedard PL, Hilton J, Amir E, Gelmon K, Goodwin R, et al. A Phase Ib Trial of Durvalumab in Combination with Trastuzumab in HER2-Positive Metastatic Breast Cancer (CCTG IND.229) Oncologist. 2019 Nov;24((11)):1439–1445. doi: 10.1634/theoncologist.2019-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, Grob JJ, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018 Sep;4((9)):e180077. doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020 Oct;396((10257)):1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 27.Javier Cortes DW, Hope S. Rugo, Zbigniew Nowecki, Seock-Ah Im, Mastura Md Yusof, Carlos Gallardo, Oleg Lipatov, Carlos Henrique Barrios, Esther Holgado, Hiroji Iwata, Norikazu Masuda, Marco Torregroza Otero, Erhan Gokmen, Sherene Loi, Zifang Guo, Jing Zhao, Gursel Aktan, Vassiliki Karantza, Peter Schmid. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020 May;38((15 _suppl)):1000–1000. [Google Scholar]
- 28.Schmid P, Cortés J, Dent R, Pusztai L, McArthur HL, Kuemmel S, et al. KEYNOTE-522: Phase 3 study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo as neoadjuvant treatment, followed by pembro vs pbo as adjuvant treatment for early triple-negative breast cancer (TNBC) Annals of Oncology. 2019;30((suppl_5)):v853–v854. [Google Scholar]
- 29.Holgado E, Perez-Garcia J, Gion M, Cortes J. Is there a role for immunotherapy in HER2-positive breast cancer? NPJ Breast Cancer. 2018 Aug;4((1)):21. doi: 10.1038/s41523-018-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoub NM, Al-Shami KM, Yaghan RJ. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer (Dove Med Press) 2019 Jan;11:53–69. doi: 10.2147/BCTT.S175360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SE, Park K, Lee E, Kim JY, Ahn JS, Im YH, et al. Clinical implication of tumor mutational burden in patients with HER2-positive refractory metastatic breast cancer. OncoImmunology. 2018 May;7((8)):e1466768. doi: 10.1080/2162402X.2018.1466768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015 Jul;1((4)):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014 Aug;25((8)):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Lam L, Nagai Y, Zhu Z, Chen X, Ji MQ, et al. A targeted immunotherapy approach for HER2/neu transformed tumors by coupling an engineered effector domain with interferon-γ. OncoImmunology. 2018 Feb;7((4)):e1300739. doi: 10.1080/2162402X.2017.1300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifton GT, Litton JK, Arrington K, Ponniah S, Ibrahim NK, Gall V, et al. Results of a Phase Ib Trial of Combination Immunotherapy with a CD8+ T Cell Eliciting Vaccine and Trastuzumab in Breast Cancer Patients. Ann Surg Oncol. 2017 Aug;24((8)):2161–2167. doi: 10.1245/s10434-017-5844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]