Abstract
Clinical resistance to aminoglycosides in general is due to enzymatic drug modification. Mutational alterations of the small ribosomal subunit rRNA have recently been found to mediate acquired resistance in bacterial pathogens in vivo. In this study we investigated the effect of 16S rRNA heterozygosity (wild-type [wt] and mutant [mut] operons at position 1408 [1408wt/1408mut]) on aminoglycoside resistance. Using an integrative vector, we introduced a single copy of a mutated rRNA operon (1408 A→G) into Mycobacterium smegmatis, which carries two chromosomal wild-type rRNA operons; the resultant transformants exhibited an aminoglycoside-sensitive phenotype. In contrast, introduction of the mutated rRNA operon into an M. smegmatis rrnB knockout strain carrying a single functional chromosomal wild-type rRNA operon resulted in aminoglycoside-resistant transformants. Subsequent analysis by DNA sequencing and RNase protection assays unexpectedly demonstrated a homozygous mutant genotype, rRNAmut/rRNAmut, in the resistant transformants. To investigate whether RecA-mediated gene conversion was responsible for the aminoglycoside-resistant phenotype in the rRNAwt/rRNAmut strains, recA mutant strains were generated by allelic exchange techniques. Transformation of the recA rrnB M. smegmatis mutant strains with an integrative vector expressing a mutated rRNA operon (Escherichia coli position 1408 A→G) resulted in transformants with an aminoglycoside-sensitive phenotype. Subsequent analysis showed stable heterozygosity at 16S rRNA position 1408 with a single wild-type allele and a single resistant allele. These results demonstrate that rRNA-mediated mutational resistance to aminoglycosides is recessive.
Aminoglycoside antibiotics comprise a large group of antimicrobial agents which have in common structures that contain cyclic alcohols (aminocyclitols) in glycosidic linkage with amino-substituted sugars; the most common aminocyclitol is deoxystreptamine. 2-Deoxystreptamine antibiotics bind to the ribosome and affect protein synthesis by inducing codon misreading and inhibiting translation (9). Three major mechanisms of resistance to aminoglycoside antibiotics have been described in both gram-positive and gram-negative bacteria: (i) enzymatic drug modification by either plasmid-encoded or chromosomally encoded aminoglycoside-modifying enzymes, such as aminoglycoside acetyltransferases, aminoglycoside adenyltransferases, and aminoglycoside phosphoryltransferases (10, 14, 15, 19, 29, 30); (ii) reduced intracellular drug accumulation due to, for example, mutations that affect energy metabolism, such as electron transport, resulting in impaired aminoglycoside uptake or increased efflux (6, 7, 17, 20); and (iii) alterations of the antibiotic target structure.
Alterations of the antibiotic target involve a conserved region within the rRNA, i.e., methylation of an adenine at either 16S rRNA position 1405 or 16S rRNA position 1408, and have been found in aminoglycoside-producing organisms such as Streptomyces spp. and Micromonospora spp. This posttranscriptional modification has been shown to protect these organisms from their own metabolites by interfering with the binding of the aminoglycoside to the respective ribosomal target of the drug (4, 8, 13, 22, 31, 32, 36). Genetic studies with Escherichia coli have demonstrated that cis regulatory rRNA mutations that weaken base pairing between 16S rRNA positions 1409 and 1491 confer resistance to paromomycin and other aminoglycosides (11).
Aminoglycoside resistance mechanisms have been intensively studied in mycobacteria. Aminoglycoside acetyltransferases are universally present in both fast- and slow-growing mycobacteria (1, 2, 21, 37, 39). However, no correlation between the activities of these enzymes and the level of aminoglycoside resistance has been found. Recently, we have demonstrated that a single point mutation at 16S rRNA position 1408 (A→G; E. coli numbering) is associated with high-level resistance to 2-deoxystreptamine aminoglycosides in mycobacteria containing a single rRNA operon, such as M. tuberculosis, M. abscessus, and M. chelonae (24, 27).
The question of recessivity or dominance of aminoglycoside resistance mutations is a matter of debate. Apirion and Schlessinger (3) provided evidence that aminoglycoside sensitivity is dominant over resistance; De Stasio et al. (11) postulated that resistance to aminoglycosides is dominant at low drug concentrations (10 μg/ml) but recessive at high antibiotic concentrations in E. coli. Our own attempts to generate spontaneous aminoglycoside-resistant mutants of M. smegmatis, an organism containing two rRNA operons, were unsuccessful (mutation rate, <10−11); resistant mutants could be obtained only in M. smegmatis rrnA or rrnB mutant strains, genetically engineered derivatives of M. smegmatis which carry a single functional rRNA operon (27). In contrast, Taniguchi et al. (35) were able to isolate kanamycin-resistant M. smegmatis mutants upon UV irradiation, suggesting the dominance of resistance.
To address the issue of dominance versus recessivity of aminoglycoside resistance mutations, we report here on our investigations of aminoglycoside resistance in heterozygous M. smegmatis rRNAwt/rRNAmut (wt represents wild type, and mut represents mutant) strains.
MATERIALS AND METHODS
Bacterial strains and media.
The strains used in this study are listed in Table 1. E. coli XL1-Blue MRF′ (mcrA)183 (mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacqZΔM15], Tn10(Tetr) (Stratagene) was used for the cloning and propagation of the plasmids. Transformants were grown in Luria-Bertani (LB) medium containing either ampicillin (50 μg/ml) or kanamycin (50 μg/ml). Clarithromycin was kindly provided by Abbott GmbH, Wiesbaden, Germany. Selection of M. smegmatis transformants was performed by using LB medium containing either kanamycin (25 μg/ml), hygromycin B, or clarithromycin at a concentration of 50 μg/ml.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Source or reference |
|---|---|
| Strains | |
| E. coli XL 1-Blue MRF | Stratagene |
| Mycobacteria | |
| M. smegmatis mc2155 | Snapper et al. (33) |
| M. smegmatis mc2155 SMR5 rrnB mutant | Sander et al. (27) |
| M. smegmatis mc2155 SMR5 rrnB recA mutant | This work |
| Plasmids | |
| pT7-blue | Novagen |
| ptrpA-1-rpsL+ | Sander et al. (26) |
| precA(Ms)::hyg-rpsL+ | Frischkorn et al. (12) |
| pMV 261-rRNA 2058Ga | Sander et al. (28) |
| pMV 361-H-rRNA 2058Gb | Sander et al. (28) |
| pMV 261-rRNA 1408G/2058Ga | This work |
| pMV 361-H-rRNA 1408G/2058Gb | This work |
Vector confers resistance to kanamycin and clarithromycin.
Vector confers resistance to kanamycin, hygromycin, and clarithromycin.
DNA techniques.
Standard methods were used for restriction endonuclease digestion of DNA and other manipulations (25). Plasmid DNA was isolated either by the alkaline lysis method (5) or with the Qiagen preparation according to the manufacturer’s instruction (Diagen).
Nucleic acid sequencing was done manually with 32P-labelled dCTP and Sequenase (U.S. Biochemicals) or with fluorescence-labelled nucleotides and by Taq cycle sequencing (Applied Biosystems).
Analysis of 16S rRNA position 1408 was performed by amplification of genomic DNA with primer 285 and primer 261 (the primers used in this study are listed in Table 2); the amplified products were subsequently sequenced with primer 289. The peptidyltransferase region of the 23S rRNA was investigated with primer 18 and primer 92 in a PCR. Subsequent sequencing of the amplified product was performed manually with primer 99.
TABLE 2.
Primers used in this work
| Primer | Sequencea | Species | Gene | Positionb |
|---|---|---|---|---|
| 18 | AGTCGGGACCTAAGGCGAG | M. smegmatis | rrl | 1342–1360 |
| 89 | CCGCCCGTCGCGTCATGAAAG | M. smegmatis | rrs | 1399–1419 |
| 90 | CTTTCATGACGCGACGGGCGG | M. smegmatis | rrs | 1419–1399 |
| 92 | CATCCCGTCGATATGGACTC | M. smegmatis | rrl | 2710–2691 |
| 99 | GGTATTTCAACAACGACTCCA | M. smegmatis | rrl | 2174–2154 |
| 99-Sp6 | GGATTTAGGTGACACTATAGAAGGTATTTCAACAACGACTCCA | |||
| 100 | CCTGAGAGGTGATGCATAGC | M. smegmatis | rrl | 1734–1753 |
| 100-Sp6 | GGATTTAGGTGACACTATAGAACCTGAGAGGTGATGCATAGC | |||
| 261 | AAGGAGGTGATCCAGCCGCA | M. smegmatis | rrs | 1539–1514 |
| 261-Sp6 | GATTTAGGTGACACTATAGAAGGAGGTGATCCAGCCGCA | |||
| 285 | GAGAGTTTGATCCTGGCTCAG | M. smegmatis | rrs | 9–29 |
| 289 | AAGTCGGAGTCGCTAGTAAT | M. smegmatis | rrs | 1323–1342 |
| 294 | GATGCTCGCAACCACTATCC | M. smegmatis | ITS Ic | 1746–1727 |
| 297 | TTCCTTGTGGCCTGTGTGCA | M. smegmatis | rrs | 1017–1036 |
| 297-Sp6 | GATTTAGGTGACACTATAGAATCCCTTGTGGCCTGTGTGCA |
Sp6 promoter sequences are underlined. Mutagenized nucleotides are boxed.
E. coli numbering.
ITS I, internal transcribed spacer between the 16S rRNA and 23S rRNA genes.
Construction of vectors for transformation.
To introduce the 16S rRNA mutation 1408G into plasmids pMV261-rRNA 2058G and pMV361-H-rRNA 2058G, mutagenesis was performed by PCR. The mutated 16S rRNA (1408 A→G) was generated by PCR amplification of the rrn operon of M. smegmatis with primer 285 in combination with mutagenesis primer 90 and mutagenesis primer 89 in combination with primer 294. The two amplified fragments were gel purified and used as template in a fusion PCR with primer 285 and primer 294. The products were subcloned in vector pT7-blue (Novagen). Doubled-stranded sequencing confirmed that only the desired mutation was introduced. Subsequently, a SacI-Eco47III fragment of plasmids pMV261-rRNA 2058G and pMV361-H-rRNA 2058G was replaced by the corresponding fragments containing the 16S rRNA gene mutation 1408 A→G, resulting in plasmids pMV261-rRNA 1408G/2058G and pMV361-H-rRNA 1408G/2058G, respectively.
Generation of M. smegmatis rrnB recA mutant strains.
Plasmid precA(Ms)::hyg-rpsL+, which is a derivative of plasmid ptrpA-1-rpsL+ (26) and which carries the wild-type rpsL gene and the hygromycin-inactivated recA gene, was used for inactivation of the chromosomal recA gene of the M. smegmatis rrnB mutant strain. Hygromycin and streptomycin were used as positive and negative selectable markers, respectively, for gene replacement, resulting in recA mutant transformants. Plasmid precA(Ms)::hyg-rpsL+ was transformed into the streptomycin-resistant M. smegmatis rrnB mutant (27), and transformants were selected by plating on LB medium containing hygromycin (50 μg/ml) and streptomycin (50 μg/ml). Subsequent analysis of the mutant recA phenotype was performed by determination of susceptibility to DNA-damaging agents, such as ethyl methanesulfonate (0.1% [vol/vol]); the genotypes of the recA mutant strains were confirmed by Southern blot analysis.
Isolation of genomic DNA.
Mycobacteria grown on Middlebrook 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase (Difco) were harvested. The cells were suspended in 500 μl of NaCl (0.9%), heat inactivated for 10 min at 80°C, and pelleted by centrifugation (6,000 × g for 5 min). The pellet was resuspended in 400 μl of Tris-EDTA-Tween-lysozyme (10 mM, 10 mM, 0.1% [wt/vol], and 2 mg/ml, respectively) and incubated for 2 h at 37°C with constant shaking. Sodium dodecyl sulfate and proteinase K were added to final concentrations of 1% and 0.1 mg/ml, respectively. Incubation was continued for 1 h. Proteins were extracted by the addition of an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol]). The aqueous solution was extracted once with an equal volume of chloroform-isoamyl alcohol (24:1 [vol/vol]). The DNA was precipitated by adding 2.5 volumes of ethanol and 0.1 volume of 5 M NaCl. After incubation at −70°C for 30 min, the DNA was pelleted by centrifugation. The pellet was washed once with 1 ml of 70% ethanol, dried, and resuspended in TE (10 mM Tris, 1 mM EDTA).
RNase protection assay.
To generate DNA fragments as templates for in vitro transcription, standard PCR amplification reactions were performed (23). Sp6 RNA polymerase promoters were introduced into the amplified DNA fragment by using primers containing the RNA polymerase Sp6 recognition site. To amplify the peptidyltransferase region of the 23S rRNA gene, primer 99-Sp6 was used in combination with primer 100 (reaction a) and primer 100-Sp6 was used in combination with primer 99 (reaction b). Primer 261-Sp6 was used in combination with primer 297 (reaction a) and primer 297-Sp6 was used in combination with primer 261 (reaction b) to generate PCR products of the 16S rRNA gene. Amplified DNA fragments of reactions a and b were used as templates for the generation of sense and antisense transcripts, respectively. For in vitro transcription, approximately 100 ng of the PCR fragment derived from reaction a and 100 ng of the PCR fragment derived from reaction b were mixed together in a volume of 10 μl in 0.75× buffer in the presence of ribosomal nucleoside triphosphates and 20 U of Sp6 RNA polymerase according to the manufacturer’s instructions (Ambion). The mixture was then incubated for 90 min at 37°C. Afterward, an equal amount of hybridization buffer was added and the reaction mixtures were heated to 95°C for 3 min before they were slowly cooled to room temperature. An aliquot of 4 μl of the mixture was incubated either with RNase I (100-fold dilution in RNase buffer) for transcripts of the 23S rRNA gene or with a mixture of RNase II plus RNase III (100-fold dilution in RNase buffer) for transcripts of the 16S rRNA gene in RNase digestion buffer (16 μl) for 45 min at 37°C. The fragments were separated on a 2% agarose gel.
Transformation of mycobacteria.
The parental strain M. smegmatis mc2155 (33), a single rRNA allelic strain M. smegmatis mc2155 SMR5 rrnB mutant rRNA allele (27), and strain M. smegmatis mc2155 SMR5 rrnB recA mutants were used for the transformation experiments. All strains were clarithromycin sensitive. Bacteria were made electrocompetent by the method described by Jacobs et al. (16), with the exception that the bacteria were grown in brain heart infusion medium (Oxoid) with 0.05% Tween 80 as described previously (26). The transformants were primarily selected on LB agar plates containing either kanamycin (25 μg/ml), clarithromycin (50 μg/ml), or hygromycin (50 μg/ml). After 3 to 5 days of incubation, single colonies were picked and colony purified for further investigations.
RESULTS
Transformation of M. smegmatis with plasmids carrying mutated rRNA alleles.
To investigate the possible gene dosage effects of the rRNAmut and rRNAwt genes, two different M. smegmatis strains, wild-type strain M. smegmatis mc2155 with two functional rRNA operons and genetically modified strain M. smegmatis mc2155 rrnB mutant and one functional rRNA operon, were transformed with plasmids carrying a mutated rrn operon. As vectors, derivatives of the replicative plasmid pMV261 and the integrative plasmid pMV361 carrying a mutated rRNA operon (16S rRNA position 1408 A→G [E. coli numbering] and 23S rRNA position 2058 A→G [E. coli numbering]) were used. Plasmid pMV261-rRNA 1408G/2058G is based on the autonomously replicating plasmid pMV261 (34), which is present in at three to five copies per genome; plasmid pMV361-H-rRNA 1408G/2058G is based on the mycobacteriophage L5 att/int and integrates in a single copy per genome at the bacterial attB site (34). The mutation in 23S rRNA (2058 A→G) confers resistance to macrolides, such as clarithromycin, and is dominant in heterozygous strains (28). This mutation was used as a cis-selectable marker for expression of the transfected rrn operon. Transformants were selected on LB agar plates containing clarithromycin. The MICs of aminoglycosides such as amikacin, gentamicin, and tobramycin (0.1 to 200 μg/ml) were determined to investigate the effects of the mutated rRNA alleles.
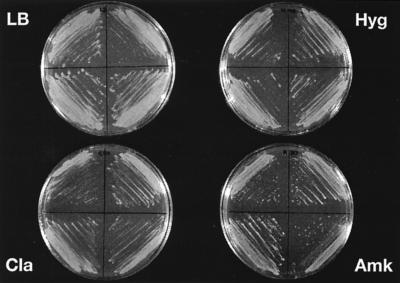
All strains obtained upon transformation of M. smegmatis mc2155 (four of four strains investigated) showed an aminoglycoside-sensitive phenotype (MIC, 0.6 μg/ml), regardless of whether the single-copy vector pMV361-H-rRNA 1408G/2058G or the multicopy vector pMV261-rRNA 1408G/2058G was used. In contrast, transformants of mutant strain M. smegmatis rrnB showed high-level aminoglycoside resistance even if vector pMV361-H-rRNA 1408G/2058G was used (Table 3 and Fig. 1). As illustrated in Fig. 1, growth of pMV361-H-rRNA 1408G/2058G transformants on amikacin was comparable to growth on LB, hygromycin (selectable marker on plasmid pMV361-H), or clarithromycin.
TABLE 3.
MICs for M. smegmatis strains transformed with replicative and integrative vectors
| Strain | Transformed plasmida | Aminoglycosideb MIC (μg/ml) |
|---|---|---|
| mc2155 | None | 0.6 |
| pMV361-H-rRNA 2058G | 0.6 | |
| pMV 361-H-rRNA 1408G/2058G | 0.6 | |
| pMV 261-rRNA 1408G/2058G | 0.6 | |
| mc2155 rrnB mutant | None | 0.6 |
| pMV361-H-rRNA 2058G | 0.6 | |
| pMV 361-H-rRNA 1408G/2058G | >200.0 | |
| pMV 261-rRNA 1408G/2058G | >200.0 | |
| mc2155 rrnB recA mutant | None | 0.6 |
| pMV361-H-rRNA 2058G | 0.6 | |
| pMV 361-H-rRNA 1408G/2058G | 0.6 | |
| pMV 261-rRNA 1408G/2058G | 0.6 |
Four independent transformants of each plasmid were investigated.
Amikacin, gentamicin, and tobramycin.
FIG. 1.
Drug susceptibility patterns of four independent transformants of M. smegmatis rrnB mutants with integrative vector pMV361-H-rRNA 1408G/2058G; growth on control LB agar and on LB agar containing antibiotics. Clarithromycin (Cla) and hygromycin (Hyg) were used at 50 μg/ml each. Amikacin (Amk) was used at 200 μg/ml.
Characterization of M. smegmatis rrnB mutant transformants with an aminoglycoside-resistant phenotype.
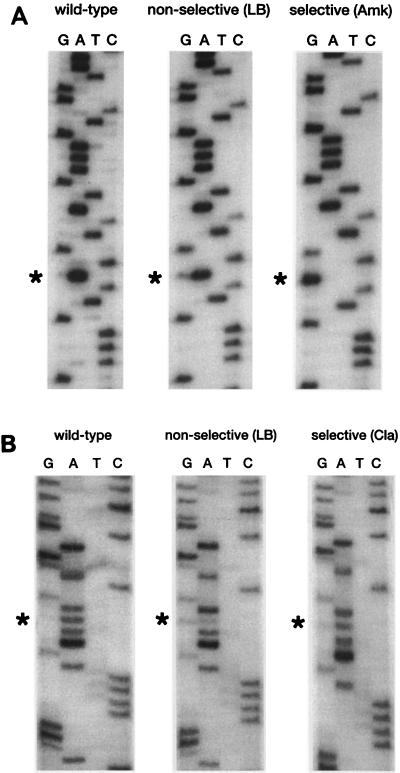
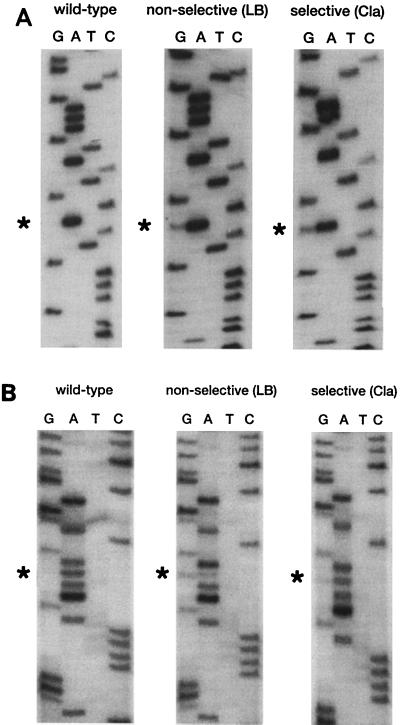
The genotype of the aminoglycoside-resistant transformants obtained by transformation of M. smegmatis mc2155 rrnB mutant with pMV361-H-rRNA 1408G/2058G was investigated by DNA sequencing and RNase protection assays. For this purpose, subcultures grown under selective conditions (on LB medium containing amikacin) and under nonselective conditions (on LB medium or LB medium containing clarithromycin) were used. Unexpectedly, the 16S rRNA genotypes of the transformants grown on medium containing aminoglycosides differed from the genotypes of the transformants grown on nonselective medium or on medium containing clarithromycin. Determination of the DNA sequences of transformants grown on LB medium revealed a heterozygous genotype with a wild-type (A) and a mutant (G) nucleotide at 16S rRNA position 1408, as demonstrated by the presence of a double band at the respective sequence position. In contrast, a homozygous genotype with a single mutant nucleotide (G) at this position was found in transformants grown in the presence of amikacin (Fig. 2A).
FIG. 2.
(A) Nucleotide sequence of a short region of the PCR-amplified 16S rRNA gene of M. smegmatis rrnB mutants transformed with integrative vector pMV361-H-rRNA 1408G/2058G. The region shown corresponds to E. coli positions 1401 to 1416. Sequencing was performed with primer 289. The sequence of a nontransformed wild-type strain is shown along with those of the transformants grown on nonselective medium and selective medium (Amk, amikacin). The mutated base is indicated by an asterisk. (B) Nucleotide sequence of a short region of the PCR-amplified 23S rRNA gene of M. smegmatis rrnB mutants transformed with integrative vector pMV361-H-rRNA 1408G/2058G. The region shown corresponds to E. coli positions 2051 to 2066. Sequencing was performed with primer 99. The sequence of a nontransformed wild-type strain is shown along with those of the transformants grown on nonselective medium and selective medium (Cla, clarithromycin). The mutated base is indicated by an asterisk.
Analysis of the 23S rRNA genes of transformants grown on LB medium and LB medium containing clarithromycin was performed and revealed the simultaneous presence of a wild-type (A) and a mutant (G) nucleotide, indicating a heterozygous genotype of the 23S rRNA gene position 2058 for both subcultures (Fig. 2B).
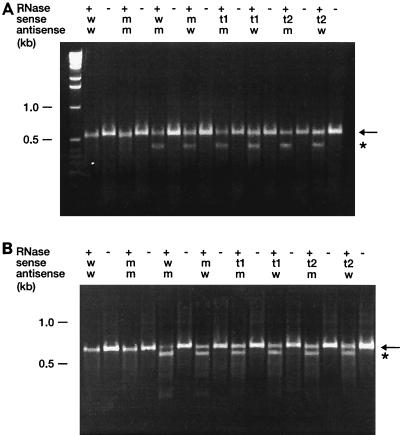
To confirm the results obtained by DNA sequencing, RNase protection assays were performed for 16S rRNA region 1408 and 23S rRNA region 2058. In this assay, the RNA probes to be tested are hybridized to complementary antisense probes whose sequences are known (e.g., wild-type or mutant sequence). Mismatches in the RNA duplex are recognized and are cleaved by RNase, and the products that are obtained are separated by gel electrophoresis. DNA amplification of the corresponding regions was performed with a primer containing an Sp6 promoter, which allows in vitro transcription. Transcripts of the coding and the noncoding strands were synthesized by in vitro transcription with phage Sp6 RNA polymerase and the PCR product as the template. Sense transcripts were generated from PCR-amplified DNA fragments derived from a wild-type strain, from a cloned mutated (1408 A→G) rrnB operon, and from M. smegmatis rrnB transformants grown on nonselective medium and selective medium. Antisense transcripts were generated from PCR-amplified fragments derived from a cloned wild-type and a cloned mutated rrnB operon. Hybridization of sense and antisense transcripts was followed by RNase digestion, and the cleavage products were separated by agarose gel electrophoresis. In this assay, homoduplexes withstand RNase cleavage and result in a RNA fragment with a molecular size of 521 bp for the 16S rRNA region. In contrast, heteroduplexes are cleaved by RNase. The cleaved products are indicated by the appearance of an RNA fragment with a smaller molecular size. A homozygous mutant genotype is present when a sense transcript of the strain to be investigated results in a noncleaved product upon hybridization to a mutated antisense probe, while a cleaved product is observed upon hybridization to a wild-type antisense probe. A heterozygous genotype is present when cleaved products are obtained upon RNase digestion when both a wild-type antisense probe and a mutated antisense probe are used.
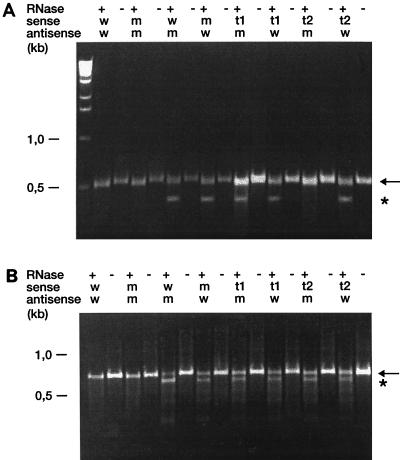
The 16S rRNA gene (rDNA) transcripts from transformants grown on selective medium were cleaved by RNase when the transcripts were hybridized to wild-type antisense probes. No digestion products were observed when those transcripts were hybridized to mutated antisense probes. In contrast, 16S rDNA transcripts from transformants grown on nonselective medium were cleaved by RNase, irrespective of whether they were hybridized to wild-type or mutated antisense probes (Fig. 3A). Controls, i.e., homoduplexes of wild-type and mutated rRNAs, gave only a single noncleaved band after RNase digestion. These results confirm the conclusions drawn from DNA sequencing: a homozygous mutated 16S rRNA genotype was found in those transformants grown on selective medium, whereas a 16S rRNA heterozygous genotype was observed in transformants grown under nonselective conditions. As expected, cleaved products were obtained when 23S rDNA transcripts from transformants grown on selective or nonselective medium were hybridized to the corresponding wild-type and mutated antisense probes, indicating the heterozygous genotype of these transformants at the 23S rRNA allele (Fig. 3B).
FIG. 3.
(A) RNase protection assay of a 16S rRNA region encompassing E. coli nucleotide position 1408. A mutant M. smegmatis rrnB strain transformed with integrative vector pMV361-H-rRNA 1408G/2058G was analyzed. RNA hybrids were incubated in the presence (+) or absence (−) of RNase at 37°C for 45 min. Sense RNAs were generated with PCR products from a cloned wild-type rRNA operon (w), from a cloned mutated rRNA operon 1408 A→G (m), or from the transformants grown on nonselective medium (t1) or selective medium (t2). Antisense RNAs were derived from cloned wild-type (w) and mutated (m) rRNA operons. The cleaved and noncleaved products are indicated by an asterisk and an arrow, respectively. (B) RNase protection assay of a 23S rRNA region encompassing E. coli nucleotide position 2058. A mutant M. smegmatis rrnB strain transformed with integrative vector pMV361-H-rRNA 1408G/2058G was analyzed. RNA hybrids were incubated in the presence (+) or absence (−) of RNase at 37°C for 45 min. Sense RNAs were generated with PCR products from a cloned wild-type rRNA operon (w), from a cloned mutated rRNA operon 2058 A→G (m), or from the transformants grown on nonselective medium (t1) or selective medium (t2). Antisense RNAs were derived from cloned wild-type (w) and mutated (m) rRNA operons. The cleaved and noncleaved products are indicated by an asterisk and an arrow, respectively.
Our results indicate that in a heterozygous strain and with growth under selective conditions, the wild-type 16S rRNA position 1408 acquired the respective drug resistance-conferring mutation. We determined the frequency of this event by plating serial dilutions of transformants on nonselective LB agar and on LB medium containing amikacin (50 μg/ml). By counting the numbers of CFU, we determined that the frequency of resistance in a heterozygous strain is approximately 10−4. Apparently, mere streaking of strains on solid medium in order to investigate a drug-resistant phenotype grossly overestimates the proportion of resistant cells in a population (Fig. 1). The frequency that was determined (10−4) is much higher than the frequency of spontaneous 16S rRNA mutations conferring aminoglycoside resistance in a strain with a single rRNA allele (10−8) (27).
Characterization of M. smegmatis recA mutant transformants carrying resistant rRNA alleles.
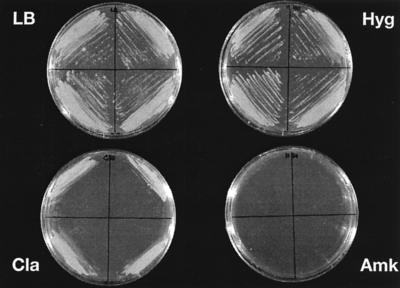
We hypothesized that RecA-mediated gene conversion is responsible for converting the wild-type allele into the resistant allele in our transformants. To prove this hypothesis, recA knockout derivatives from a strain M. smegmatis rrnB mutant were generated. Investigations with DNA-damaging agents indicated that mutant M. smegmatis rrnB recA strains were highly susceptible to DNA-damaging agents, a phenotype characteristic of mutant recA strains (12, 18, 38). The mutated recA genotype was confirmed by Southern blot analysis (data not shown). To investigate the effect of RecA on aminoglycoside resistance in a heterozygous rRNAwt/rRNAmut strain, an M. smegmatis rrnB and recA mutant was transformed with plasmids pMV261-rRNA 1408G/2058G and pMV361-H-rRNA 1408G/2058G, and transformants were selected by plating on clarithromycin. Subsequently, the MICs of different aminoglycosides were determined, and an aminoglycoside-sensitive phenotype was demonstrated for these transformants (Table 3 and Fig. 4). No evidence of a gene dosage effect was observed, because transformants with the replicative vector carrying approximately five copies of the mutated operon showed a sensitive phenotype. The drug susceptibilities of the transformants that were obtained were identical to that of the nontransformed parental strain (amikacin MICs, 0.6 μg/ml).
FIG. 4.
Drug susceptibility patterns of four independent transformants of M. smegmatis rrnB recA mutants with integrative vector pMV361-H-rRNA 1408G/2058G; growth on control LB agar and on LB agar containing antibiotics. Clarithromycin (Cla) and hygromycin (Hyg) were used at 50 μg/ml each. Amikacin (Amk) was used at 200 μg/ml.
DNA sequencing of the recA mutant transformants demonstrated sequence ambiguities in terms of double bands that indicated a mixed genotype at 16S rRNA position 1408 (Fig. 5A). The mixed genotype was also confirmed by an RNase protection assay after PCR-mediated DNA amplification of the 16S rRNA gene (Fig. 6A). As a control, DNA sequencing and RNase protection assays of the 23S rRNA gene were performed and showed a mixed genotype at 23S rRNA position 2058 in these transformants (Fig. 5B and 6B).
FIG. 5.
(A) Nucleotide sequence of a short region of the PCR-amplified 16S rRNA gene of M. smegmatis rrnB recA mutants transformed with integrative vector pMV361-H-rRNA 1408G/2058G. The region shown corresponds to E. coli positions 1401 to 1416. Sequencing was performed with primer 289. The sequence of a nontransformed wild-type strain is shown along with those of the transformants grown on nonselective medium and selective medium (Cla, clarithromycin). The mutated base is indicated by an asterisk. (B) Nucleotide sequence of a short region of the PCR-amplified 23S rRNA gene of M. smegmatis rrnB recA mutants transformed with integrative vector pMV361-H-rRNA 1408G/2058G. The region shown corresponds to E. coli positions 2051 to 2066. Sequencing was performed with primer 99. The sequence of a nontransformed wild-type strain is shown along with those of the transformants grown on nonselective and selective medium (Cla, clarithromycin). The mutated base is indicated by an asterisk.
FIG. 6.
(A) RNase protection assay of a 16S rRNA region encompassing E. coli nucleotide position 1408. A mutant strain of M. smegmatis rrnB recA transformed with integrative vector pMV361-H-rRNA 1408G/2058G was analyzed. RNA hybrids were incubated in the presence (+) or absence (−) of RNase at 37°C for 45 min. Sense RNAs were generated with PCR products from a cloned wild-type rRNA operon (w), from a cloned mutated rRNA operon 1408 A→G (m), or from the transformants grown on nonselective medium (t1) or selective medium (t2). Antisense RNAs were derived from cloned wild-type (w) and mutated (m) rRNA operons. The cleaved and noncleaved products are indicated by an asterisk and an arrow, respectively. (B) RNase protection assay of a 23S rRNA region encompassing E. coli nucleotide position 2058. A mutant strain of M. smegmatis rrnB recA transformed with integrative vector pMV361-H-rRNA 1408G/2058G was analyzed. RNA hybrids were incubated in the presence (+) or absence (−) of RNase at 37°C for 45 min. Sense RNAs were generated with PCR products from a cloned wild-type rRNA operon (w), from a cloned mutated rRNA operon 2058 A→G (m), or from the transformants grown on nonselective medium (t1) or selective medium (t2). Antisense RNAs were derived from cloned wild-type (w) and mutated (m) rRNA operons. The cleaved and noncleaved products are indicated by an asterisk and an arrow, respectively.
These results indicate (i) that aminoglycoside sensitivity is dominant over resistance in a heterozygous strain and (ii) that the resistant phenotype of a previously heterozygous rRNAwt/rRNAmut strain is due to RecA-dependent gene conversion.
DISCUSSION
Methylation of 16S rRNA positions 1405 and 1408 in aminoglycoside-producing organisms, such as Streptomyces spp. and Micromonospora purpurea, confers resistance to 2-deoxystreptamine aminoglycosides. Posttranscriptional modification of these nucleotides by methylation prevents binding of the antibiotics to their targets (4, 8, 22, 31, 32, 36). Our previous work has demonstrated that ribosomal proteins play no role in acquired resistance to 2-deoxystreptamine aminoglycosides but that mutational target alterations are limited to the rRNA of the small ribosomal subunit. Specifically, a mutation at 16S rRNA position 1408 (A→G) conferred resistance to 2-deoxystreptamine aminoglycosides (27). Spontaneous aminoglycoside-resistant mutants were found only for organisms which carry a single rRNA operon. Efforts to generate aminoglycoside-resistant mutants of organisms with two rRNA operons were unsuccessful (27), suggesting that the dominance of aminoglycoside sensitivity over resistance prevented the isolation of resistant mutants.
The question of whether recessivity or dominance of mutational target alterations causes aminoglycoside resistance has been a matter of debate (3, 11, 35). Mutations in the E. coli 16S rRNA-decoding region that confer resistance to aminoglycosides were demonstrated to be dominant in cells growing in the presence of low antibiotic concentrations but recessive in cells growing in the presence of high drug concentrations (11). Apirion and Schlessinger (3) demonstrated that kanamycin resistance in E. coli is recessive. Using a conjugation system, Taniguchi et al. (35) suggested that aminoglycoside susceptibility is dominant in heterogenomic strains of M. smegmatis. In contrast, upon UV irradiation the same investigators (35) obtained aminoglycoside-resistant mutants at a frequency of approximately 10−9 for organisms carrying two wild-type rRNA operons, indicating a dominance of aminoglycoside resistance (35).
Targeted inactivation of the recA gene in merodiploids carrying a wild-type rRNA operon and a drug-resistant mutated rRNA operon allowed us to establish that resistance due to mutational target alteration is recessive. Interestingly, rRNAwt/rRNAmut heterozygous strains with a recA+ genotype were genetically unstable under selective pressure. Transformants able to grow on aminoglycoside-containing medium demonstrated a homozygous mutant genotype at 16S rRNA position 1408. RecA-mediated gene conversion was shown to be responsible for conversion of the wild-type allele into the mutant allele in these transformants. In organisms with a limited number of rRNA operons and a heterozygous rRNA genotype, RecA-mediated gene conversion promotes the generation of homozygous strains at a high frequency. Cells obtained by transformation of the mutant M. smegmatis rrnB recA strain containing one chromosomal wild-type and one plasmid-encoded mutated rRNA operon were genetically stable and highly sensitive to aminoglycoside antibiotics. From these results one must draw the conclusion that aminoglycoside resistance is recessive in heterozygous rRNAwt/rRNAmut strains in the presence of high as well as low drug concentrations.
Our results and the conclusions based thereon explain why aminoglycoside resistance due to mutational rRNA alterations has not been found in organisms that carry more than one rRNA operon, such as members of the family Enterobacteriaceae, staphylococci, and streptococci. The data presented here provide the experimental evidence for the observed rarity of acquired aminoglycoside resistance during drug therapy in such organisms.
ACKNOWLEDGMENTS
We thank C. K. Stover for plasmids pMV261 and pMV361 and W. R. Jacobs, Jr., for providing M. smegmatis mc2155. Clarithromycin was a generous gift from Abbott. We are grateful to K. Teschner for excellent technical assistance.
This work was supported in part by grants from the Commission of the European Community (grants BioMed 2–BMH4-CT96-1241) and the Bundesministerium für Bildung, Wissenschaft, Forschung und Technik (Verbund Mykobakterielle Infektionen). E.C.B. is supported by a Hermann und Lilly Schilling Professorship and T. Prammananan is supported by the Deutscher Akademischer Austauschdienst (DAAD).
REFERENCES
- 1.Aínsa J A, Martin C, Gicquel B, Gomez-Lus R. Characterization of the aminoglycoside 2′-N-acetyltransferase gene from Mycobacterium fortuitum. Antimicrob Agents Chemother. 1996;40:2350–2355. doi: 10.1128/aac.40.10.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aínsa J A, Pérez E, Pelicic V, Berthet F, Gicquel B, Martin C. Aminoglycoside 2′-N-acetyltransferase genes are universally present in mycobacteria: characterization of the aac(2′)-Ic gene from Mycobacterium tuberculosis and the aac(2′)-Id gene from Mycobacterium smegmatis. Mol Microbiol. 1997;24:431–441. doi: 10.1046/j.1365-2958.1997.3471717.x. [DOI] [PubMed] [Google Scholar]
- 3.Apirion D, Schlessinger D. Coresistance to neomycin and kanamycin by mutations in an Escherichia coli locus that affects ribosomes. J Bacteriol. 1968;96:768–776. doi: 10.1128/jb.96.3.768-776.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauclerk A A D, Cundliffe E. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J Mol Biol. 1987;193:661–671. doi: 10.1016/0022-2836(87)90349-4. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan L E, Kwan S. Aminoglycoside-resistant mutants of Pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob Agents Chemother. 1981;19:958–964. doi: 10.1128/aac.19.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan L E, Nicas T, Holloway B W, Crowther C. Aminoglycoside-resistant mutation of Pseudomonas aeruginosa defective in cytochrome c522 and nitrate reductase. Antimicrob Agents Chemother. 1980;17:71–79. doi: 10.1128/aac.17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 9.Davies J, Davis B D. Misreading of RNA code words induced by aminoglycoside antibiotics. J Biol Chem. 1968;243:3312–3316. [PubMed] [Google Scholar]
- 10.Davies J, Wright G D. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 11.De Stasio E A, Moazed D, Noller H F, Dahlberg A E. Mutations in 16S ribosomal RNA disrupt antibiotic-RNA interactions. EMBO J. 1989;8:1213–1216. doi: 10.1002/j.1460-2075.1989.tb03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frischkorn K, Sander P, Scholz M, Teschner K, Prammananan T, Böttger E C. Investigation of mycobacterial recA function: protein introns in the RecA of pathogenic mycobacteria do not affect competency for homologous recombination. Mol Microbiol. 1998;29:1203–1214. doi: 10.1046/j.1365-2958.1998.01003.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes D J, Cundliffe E. Analysis of a ribosomal RNA methylase gene from Streptomyces tenebrarius which confers resistance to gentamicin. Mol Gen Genet. 1991;229:229–237. doi: 10.1007/BF00272160. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Ishikawa J, Ogata T, Mizuno S. Secondary aminoglycoside resistance in aminoglycoside-producing strains of Streptomyces. Gene. 1992;115:113–117. doi: 10.1016/0378-1119(92)90548-4. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa J, Hotta K. Nucleotide sequence and transcriptional start point of the kan gene encoding an aminoglycoside 3-N-acetyltransferase from Streptomyces griseus SS-1198PR. Gene. 1991;108:127–132. doi: 10.1016/0378-1119(91)90497-y. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs W R, Jr, Kalpana G V, Cirillio J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 17.Karlowsky J A, Saunders M H, Harding G A, Hoban D L, Zhanel G G. In vitro characterization of aminoglycoside adaptive resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:1387–1393. doi: 10.1128/aac.40.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Cabrera M, Pérez-Gonzalez J A, Heinzel P, Piepersberg W, Jiménez A. Isolation and nucleotide sequencing of an aminocyclitol acetyltransferase gene from Streptomyces rimosus forma paramomycinus. J Bacteriol. 1989;171:2093–2100. doi: 10.1128/jb.171.1.321-328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller M H, Edberg S C, Mandel L J, Behar C F, Steigbeigel N H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980;18:722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsuhashi S, Tanaka T, Kawabe H, Umezawa H. Biochemical mechanism of kanamycin resistance in Mycobacterium tuberculosis. Microbiol Immunol. 1977;21:325–327. doi: 10.1111/j.1348-0421.1977.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakano M M, Mashiko H, Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984;157:79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash K A, Inderlied C B. Rapid detection of mutations associated with macrolide resistance in Mycobacterium avium complex. Antimicrob Agents Chemother. 1996;40:1748–1750. doi: 10.1128/aac.40.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prammananan T, Sander P, Brown B A, Frischkorn K, Onyi G O, Zhang Y, Böttger E C, Wallace R J. A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycosides in Mycobacterium abscessus and Mycobacterium chelonae. J Infect Dis. 1998;177:1573–1581. doi: 10.1086/515328. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sander P, Meier A, Böttger E C. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol Microbiol. 1995;16:991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 27.Sander P, Prammananan T, Böttger E C. Introducing mutations into a chromosomal rRNA gene using a genetically modified eubacterial host with a single rRNA operon. Mol Microbiol. 1996;22:841–848. doi: 10.1046/j.1365-2958.1996.01532.x. [DOI] [PubMed] [Google Scholar]
- 28.Sander P, Prammananan T, Böttger E C. The role of ribosomal RNAs in macrolide resistance. Mol Microbiol. 1997;26:496–480. doi: 10.1046/j.1365-2958.1997.5811946.x. [DOI] [PubMed] [Google Scholar]
- 29.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw K J, Rather P, Sabatelli F, Mann P, Munayyer H, Mierzwa R, Petrikkos G, Hare R S, Miller G H, Bennett P, Downey P. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992;36:1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skeggs P A, Holmes D J, Cundliffe E. Cloning of aminoglycoside-resistance determinants from Streptomyces tenebrarius and comparison with related genes from other actinomycetes. J Gen Microbiol. 1987;133:915–923. doi: 10.1099/00221287-133-4-915. [DOI] [PubMed] [Google Scholar]
- 32.Skeggs P A, Thompson J, Cundliffe E. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol Gen Genet. 1985;200:415–421. doi: 10.1007/BF00425725. [DOI] [PubMed] [Google Scholar]
- 33.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 34.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi H, Chang B, Abe C, Nikaido Y, Mizuguchi Y, Yishida S. Molecular analysis of kanamycin and viomycin resistance in Mycobacterium smegmatis by use of the conjugation system. J Bacteriol. 1997;179:4795–4801. doi: 10.1128/jb.179.15.4795-4801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J, Skeggs P A, Cundliffe E. Methylation of 16S ribosomal RNA and resistance to the aminoglycoside antibiotics gentamicin and kanamycin determined by DNA from gentamicin-producer, Micromonospora purpurea. Mol Gen Genet. 1985;201:168–173. doi: 10.1007/BF00425655. [DOI] [PubMed] [Google Scholar]
- 37.Udou T, Mizuguchi Y, Yamada T. Biochemical mechanisms of antibiotic resistance in a clinical isolate of Mycobacterium fortuitum. Presence of beta-lactamase and aminoglycoside-acetyltransferase and possible participation of altered drug transport on resistance mechanism. Am Rev Respir Dis. 1986;133:653–657. doi: 10.1164/arrd.1986.133.4.653. [DOI] [PubMed] [Google Scholar]
- 38.Walker G C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- 39.Wallace R J, Jr, Hull S I, Bobey D G, Price K E, Swenson J A, Steele L C, Christensen L. Mutational resistance as the mechanism of acquired drug resistance to aminoglycoside and antibacterial agents in Mycobacterium fortuitum and Mycobacterium chelonei: evidence is based on plasmid analysis, mutational frequencies and aminoglycoside-modifying enzyme assay. Am Rev Respir Dis. 1985;132:409–416. doi: 10.1164/arrd.1985.132.2.409. [DOI] [PubMed] [Google Scholar]