Abstract
Background/Aims
The study aim was to evaluate if mTOR inhibitors can be considered as a treatment option for HR+ HER2− metastatic breast cancer (MBC) after progression on CDK4/6 inhibitors in clinical practice.
Methods
We retrospectively collected the clinicopathological data of patients with HR+ HER2− MBC treated with CDK4/6 inhibitors and subsequent therapies at our institution between 2014 and 2020. The patients were divided into 3 groups according to the type of subsequent treatment: (A) exemestane plus everolimus, (B) endocrine monotherapy, and (C) chemotherapy. Overall survival (OS) was estimated by using the Kaplan-Meier method and compared by using the log-rank test. The efficacy and adverse events (AEs) of each subsequent treatment were assessed by using Fisher's exact tests.
Results
Eighty-six patients (34 in group A, 20 in group B, 32 in group C) were included. The most common endocrine therapy in group B was fulvestrant (40%). The major chemotherapy regimen in group C was eribulin (25%). The median OS times after stopping CDK4/6 inhibitors were 34.5 months (95% confidence interval, 17.2 to NA), 13.6 months (3.9 to NA), and 19.5 months (18.8 to NA) in group A, group B, and group C, respectively. The only significant difference in OS was observed between group A and group B (20.9 months; p = 0.003). There was no difference in the incidence of grade 3 AEs between groups A and C or in the frequency of treatment discontinuation because of AEs among the 3 groups.
Conclusion
Our study shows that mTOR inhibitors might be an effective treatment option for patients with HR+ HER2− MBC previously treated with CDK4/6 inhibitors.
Keywords: Endocrine therapy, CDK4/6 inhibitors, mTOR inhibitors, Metastatic breast cancer
Introduction
The hormone receptor-positive, human epidermal growth factor 2 receptor-negative (HR+ HER2−) subtype is the most common subtype of breast cancer. The main treatment options of HR+ HER− metastatic breast cancer (MBC) are endocrine therapy and chemotherapy. The primary goals of treatment for MBC are to prolong survival and improve quality of life. HR+ HER2− MBC patients have a relatively long prognosis, and endocrine therapy is commonly the first choice for these patients unless their metastatic condition is life-threatening [1, 2]. However, continuous use of endocrine therapy eventually leads to development of endocrine resistance, making it difficult to continue subsequent endocrine treatment.
Cyclin-dependent kinase (CDK) 4/6 inhibitors were recently approved as a treatment option for MBC that has become resistant to endocrine therapy. In some clinical trials, CDK4/6 inhibitors improved the prognosis of patients with HR+ HER2− MBC in first- or second-line therapy combined with nonsteroidal aromatase inhibitors (AIs) or fulvestrant [3, 4, 5, 6]. That caused a paradigm shift in the treatment strategy for HR+ HER2− MBC. CDK4/6 inhibitors are now commonly used on early lines of treatment for HR+ HER2− MBC patients [7, 8, 9]. Optimal treatment after progression on CDK4/6 inhibitors is a current topic of discussion. There is little clinical data showing whether it is better to select another endocrine therapy or chemotherapy [10].
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is often activated in HR+ breast cancer and involves key mechanisms of endocrine resistance [11, 12]. The PI3K/Akt/mTOR pathway is also considered to be one of the resistance mechanisms of CDK4/6 inhibitors [13]. Compared with exemestane alone, everolimus, an mTOR inhibitor, combined with exemestane, improved progression-free survival (PFS) in HR+ HER2− MBC patients who showed progression on nonsteroidal AI treatment [14]. Exemestane plus everolimus may be a treatment option for CDK4/6 inhibitors resistance. However, the clinical data investigating the effect of mTOR inhibitors after using CDK4/6 inhibitors is limited [10].
The study aim was to evaluate if mTOR inhibitors can be considered as a treatment option for HR+ HER2− MBC after the administration of CDK4/6 inhibitors in clinical practice.
Materials and Methods
Patients
Eligible patients were HR+ HER2− locally advanced or MBC women who were treated with CDK4/6 inhibitors and subsequent therapies at Chiba Cancer Center (Chiba, Japan) between July 2014 and February 2020. Patients who continued CDK4/6 inhibitors at data cutoff (July 2020) or who did not undergo anti-cancer therapy after stopping the CDK4/6 inhibitors were excluded. The patients were divided into 3 groups according to the type of subsequent treatment after CDK4/6 inhibitors: (A) exemestane plus everolimus, (B) endocrine monotherapy, and (C) chemotherapy. This retrospective observational study was conducted in accordance with the latest Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan (May 29, 2017, revised edition) and approved by the institutional Research Ethics Committee in August 2020 (reference No. R02-170). Informed consent was obtained in the form of opt-out on the website.
Outcome Assessments
Demographics and clinical characteristics, such as age, tumor size, nodal involvement, sites of metastases, and the patient's treatment history, were collected from the institutional database. The primary outcome of this study was overall survival (OS) after stopping CDK4/6 inhibitor treatment, defined as the time between the date of stopping CDK4/6 inhibitors to the date of any death or the last follow-up. The treatment response, PFS, and adverse events (AEs) of each subsequent treatment were also assessed. PFS was defined as the time from the date that subsequent treatment was started to the date that the first disease progression was confirmed by imaging. Treatment response was assessed using RECIST version 1.1 [15]. AE were recorded and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 5.0).
Statistical Analysis
The associations among various clinicopathological features were evaluated by Fisher's exact test or the Mann-Whitney U test if appropriate. OS and PFS were estimated by using the Kaplan-Meier method and compared by using the log-rank test. Cox proportional hazard models were used to estimate the effect of clinical features and subsequent treatments on OS. Cases with unknown values in any of the covariates were excluded. The objective response rate (ORR), clinical benefit rate (CBR), and AEs were compared by using Fisher's exact tests. All statistical analyses were two-sided, and values of p < 0.05 were considered to be indicative of statistical significance. All statistical analyses were performed by using R version 4.0.0 (https://www.r-project.org/).
Results
Patients
A total of 189 patients with ER+ HER2− MBC were treated with CDK4/6 inhibitors at our institution. Forty-five patients were excluded because they had continued CDK4/6 inhibitors at the data cutoff. Fifty-eight patients were excluded because they did not have anti-cancer therapies after stopping the CDK4/6 inhibitor. As a result, 86 patients (34 in group A, 20 in group B, 32 in group C) were included in this study. The most common endocrine therapy in group B was fulvestrant (40%) followed by letrozole (20%). The major chemotherapy regimens in group C were eribulin (25%), S-1 (25%), and vinorelbine (16%). The mean follow-up after stopping the CDK4/6 inhibitors was 12.6 months (interquartile range, 5.6–17.3).
The patient and tumor characteristics are shown in Table 1. The patients in group B had a higher median age, worse performance status (PS), and a higher percentage of Stage VI MBC than those of the other groups. The percentage of visceral metastases or metastatic sites >3 was higher for the patients in group C than for the patients in the other 2 groups. Palbociclib was used as a CDK4/6 inhibitor in about three-quarters of all cases. In the remaining cases, abemaciclib was used. Ribociclib was not used because it had not been approved in Japan. A CDK4/6 inhibitor was used in the first- or second-line treatment in almost half of the cases in groups A and B, whereas in group C, a CDK4/6 inhibitor was used after the third-line treatment in about two-thirds of the cases. The median PFS with CDK4/6 inhibitors was 5.4 months (95% confidence interval [CI], 3.7–9.5) in group A, 6.4 months (95% CI, 3.7–10.1) in group B, and 6.1 months (95% CI, 4.1–7.7) in group C, respectively. The response rate, PFS, and reason for stopping the CDK4/6 inhibitors did not differ between the 3 groups.
Table 1.
Clinicopathological characteristics and treatment status of patients treated with CDK4/6 inhibitors
| Group A (n = 34) | Group B (n = 20) | Group C (n = 32) | |||||
|---|---|---|---|---|---|---|---|
| Median age, years (IQR)* | 63 | (58–69) | 70 | (61–77)** | 63 | (53–71) | |
|
| |||||||
| Performance status, n (%)* | 0 | 25 | (73) | 9 | (45) | 19 | (60) |
| 1 | 8 | (24) | 7 | (35) | 12 | (37) | |
| 2 | 1 | (3) | 4 | (20) | 0 | (0) | |
| 3 | 0 | (0) | 0 | (0) | 1 | (3) | |
|
| |||||||
| Breast cancer type, n (%) | Invasive ductal carcinoma | 29 | (85) | 19 | (95) | 31 | (97) |
| Invasive lobular carcinoma | 4 | (12) | 0 | (0) | 1 | (3) | |
| Others | 1 | (3) | 1 | (5) | 0 | (0) | |
|
| |||||||
| Stage, n (%) | I | 3 | (9) | 2 | (10) | 5 | (16) |
| II | 13 | (38) | 4 | (20) | 10 | (31) | |
| III | 10 | (29) | 2 | (10) | 5 | (16) | |
| IV | 8 | (24) | 12 | (60)*** | 12 | (37) | |
|
| |||||||
| Nodal involvement, n (%) | N0 | 7 | (21) | 2 | (10) | 9 | (28) |
| N1 | 11 | (32) | 10 | (50) | 10 | (31) | |
| N2 | 3 | (9) | 1 | (5) | 7 | (22) | |
| N3 | 12 | (35) | 7 | (35) | 6 | (19) | |
| Not evaluated | 1 | (3) | 0 | (0) | 0 | (0) | |
|
| |||||||
| Histological grade, n (%) | I | 7 | (21) | 5 | (25) | 6 | (19) |
| II | 18 | (53) | 11 | (55) | 15 | (47) | |
| III | 3 | (9) | 3 | (15) | 3 | (9) | |
| Not evaluated | 6 | (17) | 1 | (5) | 8 | (25) | |
|
| |||||||
| Metastasis sites, n (%)* | Nonvisceral metastases | 16 | (47) | 7 | (35) | 6 | (19)*** |
| Visceral metastases | 18 | (53) | 13 | (65) | 26 | (81)*** | |
| Metastatic sites ≥3 | 5 | (15) | 6 | (30) | 15 | (47)*** | |
|
| |||||||
| History of chemotherapy, n (%)* | Neoadjuvant or adjuvant therapy | 17 | (50) | 5 | (20) | 12 | (38) |
| Chemotherapy for MBC | 4 | (12) | 6 | (30) | 18 | (56)*** | |
|
| |||||||
| History of endocrine therapy, n (%)* | Neoadjuvant or adjuvant therapy | 19 | (51) | 8 | (40) | 19 | (59) |
| Endocrine therapy for MBC | 26 | (76) | 13 | (65) | 32 | (100)*** | |
|
| |||||||
| Type of CDK4/6 inhibitors, n (%)* | Palbociclib | 24 | (71) | 16 | (80) | 25 | (78) |
| Abemaciclib | 10 | (29) | 4 | (20) | 7 | (22) | |
|
| |||||||
| Treatment line of CDK4/6 inhibitors, n (%)* | 1 | 8 | (23) | 7 | (35) | 0 | (0)*** |
| 2 | 8 | (23) | 4 | (20) | 6 | (19) | |
| 3 | 11 | (33) | 2 | (10)*** | 7 | (22) | |
| ≥4 | 7 | (21) | 7 | (35) | 19 | (59)*** | |
|
| |||||||
| Best overall response for CDK4/6 inhibitors, n (%) | Partial response | 5 | (15) | 4 | (20) | 3 | (9) |
| Stable disease | 23 | (68) | 11 | (55) | 23 | (72) | |
| Progressive disease | 6 | (17) | 2 | (10) | 5 | (16) | |
| Not evaluated | 0 | (0) | 3 | (15) | 1 | (3) | |
|
| |||||||
| PFS of CDK4/6 inhibitors, n (%) | ≥6 months | 15 | (44) | 11 | (55) | 18 | (56) |
| <6 months | 19 | (56) | 9 | (45) | 14 | (44) | |
|
| |||||||
| Reason for stopping CDK4/6 inhibitors, n (%) | Disease progression | 30 | (88) | 17 | (85) | 30 | (94) |
| Adverse events | 4 | (12) | 3 | (15) | 2 | (6) | |
IQR, interquartile range; MBC, metastatic breast cancer; PFS, progression-free survival.
At the point of stopping CDK4/6 inhibitors.
Mann-Whitney U test, p < 0.05.
Fisher's exact test, p < 0.05.
Survival Analyses
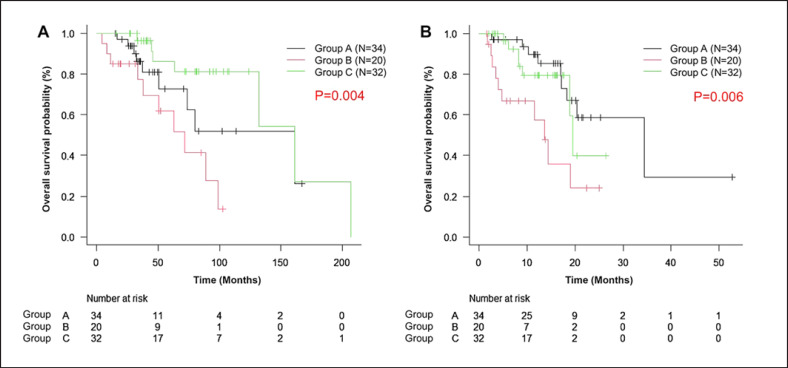
Figure 1 shows OS after recurrence and after stopping the CDK4/6 inhibitors. The OS after recurrence was similar in groups A and C (median, 161.1 months) and was significantly shorter in group B (median, 71.7 months). The median OS after stopping CDK4/6 inhibitors was 34.5 months (95% CI, 17.2 to NA), 13.6 months (95% CI, 3.9 to NA), and 19.5 months (95% CI, 18.8 to NA) in group A, group B, and group C, respectively. The OS was significantly better in group A than in group B by 20.9 months (p = 0.003), but no significant difference was observed between groups A and C (p = 0.42). We performed a subanalysis according to the previous type of CDK4/6 inhibitors used but found no significant difference in the OS results (data not shown).
Fig. 1.
Overall survival rate after recurrence (A) and after stopping CDK4/6 inhibitors (B). Group A: exemestane plus everolimus (black line), group B: endocrine monotherapy (red line), group C: chemotherapy (green line). Survival rate was estimated by using the Kaplan-Meier method and compared by using the log-rank test. Symbols indicate censored data. The median OS was 161.1 months in groups A and C and 71.7 months in group B. The median OS after stopping CDK4/6 inhibitors was 34.5 months in group A, 13.6 months in group B, and 19.5 months in group C.
Table 2 shows the results of univariate and multivariate analysis of factors associated with OS after stopping CDK4/6 inhibitors. Compared with exemestane plus everolimus, endocrine monotherapy (as a subsequent treatment regimen) was an independent prognostic factor for poor OS (hazard ratio, 3.99; 95% CI, 1.53–10.44; p < 0.01). Poor PS and PFS <6 months after CDK4/6 inhibitors were also independent prognostic factors for poor OS. No significant difference in OS was found according to the type of CDK4/6 inhibitors used.
Table 2.
Cox proportional hazards model for survival after stopping CDK4/6 inhibitors
| n | Univariate analyses |
Multivariate analyses |
||||||
|---|---|---|---|---|---|---|---|---|
| hazard ratio | 95% CI | p value | hazard ratio | 95% CI | p value | |||
| Age | <70 years | 29 | ||||||
| ≥70 years | 57 | 0.60 | 0.27–1.31 | 0.20 | ||||
|
| ||||||||
| Performance status | 0 | 33 | ||||||
| 1–3 | 53 | 4.69 | 1.85–11.87 | <0.01 | 4.43 | 1.70–11.54 | <0.01 | |
|
| ||||||||
| Stage | 1–3 | 54 | ||||||
| 4 | 32 | 1.314 | 0.61–2.85 | 0.48 | ||||
|
| ||||||||
| Visceral metastases | No | 29 | ||||||
| Yes | 57 | 1.07 | 0.46–2.49 | 0.87 | ||||
|
| ||||||||
| Metastatic sites ≥3 | No | 60 | ||||||
| Yes | 26 | 0.66 | 0.26–1.65 | 0.37 | ||||
|
| ||||||||
| Number of regimens for MBC | 1–2 | 33 | ||||||
| ≥3 | 53 | 1.31 | 0.56–3.04 | 0.53 | ||||
|
| ||||||||
| Type of CDK4/6 inhibitors | Palbociclib | 65 | ||||||
| Abemaciclib | 21 | 0.55 | 0.23–1.28 | 0.17 | ||||
|
| ||||||||
| PFS of CDK4/6 inhibitors | ≥6 months | 44 | ||||||
| <6 months | 42 | 2.59 | 1.08–6.22 | 0.03 | 2.69 | 1.12–6.51 | 0.03 | |
|
| ||||||||
| Subsequent treatment | Exemestane and everolimus | 34 | ||||||
| Endocrine monotherapy | 20 | 3.88 | 1.52–9.90 | <0.01 | 3.99 | 1.53–10.44 | <0.01 | |
| Chemotherapy | 32 | 1.37 | 0.49–3.82 | 0.55 | 1.51 | 0.53–4.28 | 0.44 | |
MBC, metastatic breast cancer; PFS, progression-free survival; CI, confidence interval.
Treatment Efficacy and AEs
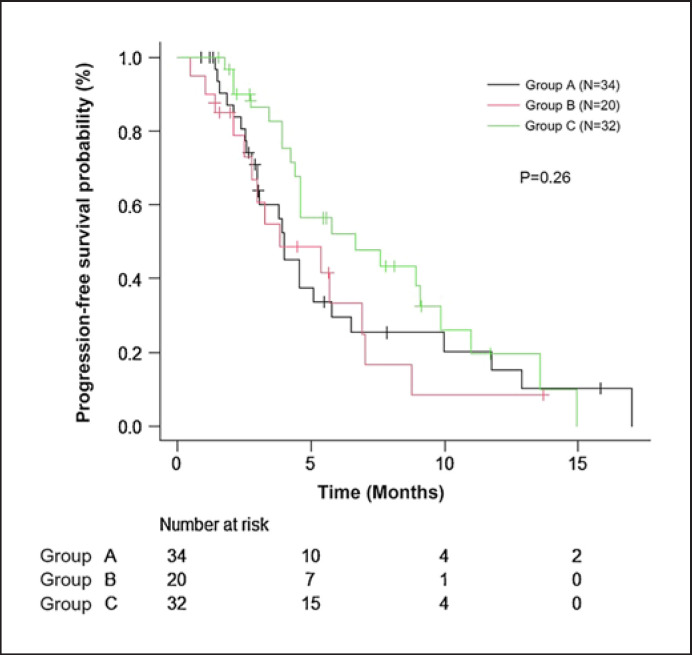
The treatment efficacies of subsequent therapies are shown in Figure 2 and Table 3. The median PFS in group A was 4.0 months (95% CI, 3.0–5.7). PFS was longer in group C (median, 6.6 months; 95% CI, 4.4–9.9) than in group A, but the difference was not significant (p = 0.23). No cases of complete response or partial response were observed in group B. The ORR tended to be better in group C than in group A (21.9 vs. 8.8%, p = 0.18) and had a significantly better CBR (43.8 vs. 20.6%, p < 0.05). Within the cases in which a CDK4/6 inhibitor was used as the first or second line of treatment, the ORRs were 12.5, 0, and 0% and the CBRs were 25, 9, and 16.6% in groups A, B, and C, respectively (data not shown).
Fig. 2.
Progression-free survival of subsequent treatments. Group A: exemestane plus everolimus (black line), group B: endocrine monotherapy (red line), group C: chemotherapy (green line). Symbols indicate censored data. The median PFS was 4.0 months in group A, 3.8 months in group B, and 6.6 months in group C. Survival rate was estimated by using the Kaplan-Meier method and compared by using the log-rank test.
Table 3.
Efficacy of subsequent therapies
| Group A (n = 34) | Group B (n = 20) | Group C (n = 32) | ||
|---|---|---|---|---|
| PFS, median (95% CI) | 4.0 (3.0–5.7) | 3.8 (2.1–6.9) | 6.6 (4.4–9.9) | |
|
| ||||
| Best overall response, n (%) | CR | 0 (0) | 0 (0) | 1 (3) |
| PR | 3 (9) | 0 (0) | 6 (19) | |
| SD | 21 (62) | 12 (60) | 19 (59) | |
| (Long SD) | 4 (12) | 4 (20) | 7 (22) | |
| PD | 7 (20) | 7 (35) | 6 (19) | |
| NE | 3 (9) | 1 (5) | 0 (0) | |
|
| ||||
| ORR, % | 8.8 | 0.0 | 21.9 | |
|
| ||||
| CBR, % | 20.6 | 20.0 | 43.8* | |
PFS, progression-free survival; CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluated. Long SD means maintaining SD for ≥6 months. ORR, objective response rate = CR + PR. CBR, clinical benefit rate = CR + PR + long SD.
Fisher's exact test, p < 0.05, compared with group A or group B, respectively.
Table 4 shows the AEs of subsequent therapies. The frequencies of stomatitis and hyperglycemia as nonhematological AEs were higher in group A (23.5 and 14.7%, respectively) than in the other 2 groups. In group C, there was 1 case of grade 3 renal dysfunction and 1 case of grade 3 pneumonia. No difference in the incidences of grade 3 AEs was observed between groups A and C. The frequency of treatment discontinuation because of AEs did not differ between the 3 groups.
Table 4.
Adverse events of subsequent therapies
| Group A (n = 34) | Group B (n = 20) | Group C (n = 32) | |
|---|---|---|---|
| Hematological AE | 2 (6) | 0 (0) | 3 (9) |
| Nonhematological AE | 19 (56) | 2 (10) | 9 (28) |
| Grade 3 AE | 2 (6) | 0 (0) | 3 (9) |
| Discontinuation due to AE | 2 (6) | 2 (10) | 2 (6) |
Values are n (%). AE, adverse events.
Discussion
This retrospective observational study investigated the survival benefit and safety of mTOR inhibitors, compared with chemotherapy or endocrine monotherapy, for subsequent treatment in HR+/HER2− MBC patients on CDK4/6 inhibitors who showed progression.
There were 3 important findings in this study. First, the patients treated with exemestane plus everolimus showed good OS. Second, both mTOR inhibitors and chemotherapy showed good treatment activity with acceptable tolerability. Third, we identified PS, treatment duration of CDK4/6 inhibitors, and endocrine monotherapy as prognostic factors for OS of patients on CDK4/6 inhibitors who showed progression.
In this study, the median OS after stopping CDK4/6 inhibitor treatment was 34.5 months for the patients treated with exemestane plus everolimus, 14.3 months for endocrine monotherapy, and 19.5 months for chemotherapy, and the median PFS of those 3 subsequent treatments was 4.0, 3.8, and 6.6 months, respectively. The subset analyses of the PALOMA-3 study [16], which investigated the efficacy of palbociclib in combination with fulvestrant in a second-line setting, showed that the median duration of subsequent treatment after progression with palbociclib was 4.3 months (95% CI, 2.5–7.6) for patients treated with everolimus, 4.0 months (95% CI, 3.2–5.7) for endocrine monotherapy, and 5.6 months (95% CI, 4.3–6.1) for chemotherapy. Although about 60% of patients in our study used CDK4/6 inhibitors in treatment lines over 3, PFS after treatment was similar to that in the PALOMA-3 study. There was no prospective data about OS of patients treated with mTOR inhibitors as a subsequent treatment after progression on CDK4/6 inhibitors. In the BOLERO 2 study, the median OS of patients who were receiving exemestane plus everolimus was 31.0 months (95% CI, 28.0–34.6) as compared with 26.6 months (95% CI, 22.6–33.1) in the patients who were receiving exemestane, indicating no improvement in OS with mTOR inhibitors [17]. In our study, the median OS of the patients treated with exemestane plus everolimus was similar to that of the patients in the BOLERO 2 study. On the other hand, the OS of the patients treated with endocrine monotherapy was relatively short. The high rate of poor PS in the patients treated with endocrine monotherapy might have influenced the results. Palumbo et al. [18] reported prospective data about the effectiveness of fulvestrant in unselected real-life cases. In their report; the median OS of patients treated with fulvestrant monotherapy was 22.0 months in the second-line treatment and 13.7 months in the subsequent lines of treatment.
On the other hand, no difference was observed in OS between exemestane plus everolimus and chemotherapy. In the BOLERO-6 study [19] which investigated the clinical benefit of exemestane plus everolimus versus everolimus or capecitabine monotherapy for patients with HR+ HER2− MBC who showed progression on nonsteroidal AIs, the hazard ratio for OS was 1.33 (90% CI, 0.99–1.79) for exemestane plus everolimus versus capecitabine. However, there was an imbalance in the baseline characteristics between the 2 groups, and the results of the multivariate Cox regression model gave a hazard ratio of 1.19 (90% CI, 0.88–1.62), showing no clear superiority of capecitabine. Only a small number of patients in the BOLERO-6 study were pretreated with CDK4/6 inhibitors. A clinical trial comparing mTOR inhibitors and chemotherapy as subsequent treatment for post-CDK4/6 inhibitors progression is needed.
The main purpose of treatment for MBC is to improve quality of life and prognosis. The main causes of poor quality of life in patients are symptoms associated with disease progression and AEs associated with treatment. Chemotherapy showed the best activity in our study. However, in about one-third of the cases in which a CDK4/6 inhibitor was used in early lines, there were no differences in the ORR and CBR of each subsequent treatment. Since the CDK4/6 inhibitors are mainly used in the first or second lines in clinical practice, we need to examine whether the activity is better for chemotherapy than for mTOR inhibitors with sufficient cases. Our study suggested that chemotherapy may provide a better response than that of mTOR inhibitors in the late lines. However, the combination of data from patients treated with different drugs makes it difficult to interpret the clinical utility of chemotherapy.
One the other hand, our study showed no difference in AEs between exemestane plus everolimus and chemotherapy. The predominant AEs in the exemestane plus everolimus group were nonhematological toxicity, with frequencies of 23.5% in stomatitis and 14.7% in hyperglycemia. In the BOLERO-2 study, which investigated the efficacy of exemestane plus everolimus in a second-line setting, 40% of the patients in the exemestane plus everolimus group had stomatitis and 13% had hyperglycemia of all grades. In contrast, the frequency of nonhematological AEs we observed in the chemotherapy group was 28%, which was lower than that reported in clinical trials (54% in the EMBRACE study with eribulin, and 49% in the SELECT-BC study in S-1) [20, 21]. In our study, 56% of the patients in the chemotherapy group had a history of chemotherapy for MBC. Since it was real-world data, symptoms related to past AEs may not have been fully counted. The frequency of grade 3 AEs and discontinuation because of AEs were similar in the exemestane plus everolimus group and in the chemotherapy group. Subsequent treatment after CDK4/6 inhibitors with 1 of the 3 possible types of drugs we investigated did not appear likely to increase the frequency of AEs.
Multivariate analysis revealed that the prognostic factors for worse OS after progression in patients on CDK4/6 inhibitors were poor PS, PFS <6 months after CDK4/6 inhibitors treatment, and endocrine monotherapy for subsequent treatment. To the best of our knowledge, no previous report has examined the correlation between CDK4/6 response rate and subsequent-treatment effect. The number of pretreatment regimens and types of CDK4/6 inhibitors did not affect OS after progression on CDK4/6 inhibitors. Our results suggest that patients who progressed with CDK4/6 inhibitors within <6 months will have a poor prognosis and that mTOR inhibitors as a subsequent treatment would not be affected by short PFS for CDK4/6 inhibitors.
Several studies on the mechanism of resistance and biomarkers for CDK4/6 inhibitors have been reported. Possible mechanisms of resistance include upstream signaling, such as via the PI3K/AKT/mTOR pathway [22]. CDK4/6 inhibitors-resistant breast cancer activates the PI3K/AKT/mTOR pathway and is sensitive to inhibition of mTORC1/2 [13, 23]. The SOLAR-1 study evaluated the efficacy of an α-specific PI3K inhibitor, alpelisib, plus fulvestrant in patients with PIK3CA-mutated HR+ HER2− MBC [24]. About 5% of cases had been previously treated with CDK4/6 inhibitors in SOLAR-1, and the study reported that a consistent benefit of treatment with alpelisib plus fulvestrant was observed in subgroup analyses. The ESMO guideline recommended alpelisib plus endocrine therapy for patients with PIK3CA-mutated tumors or everolimus plus endocrine therapy for those with PIK3CA wild type or unknown tumors as the best subsequent treatment [2]. Furthermore, clinical trials examining the effect of PI3K inhibitors and AKT inhibitors on post-CDK4/6 inhibitors are now ongoing [25].
Our study had some limitations. This was an exploratory study, and it was not designed to confirm an absolute effect. Our study was also retrospective and conducted at a single institution with a relatively small sample size, and a variety of patients were compared without randomization. The patients' characteristics were different between the groups and might have influenced the prognosis and performance of the treatments used. Therefore, careful interpretation regarding generalization of the findings is necessary. Basic research may be necessary to conclusively prove an association between CDK4/6 inhibitors resistance and the effect of mTOR inhibitors. Finally, because this study was conducted under daily clinical conditions, discontinuation decided by the patient or attending physician was possible regardless of whether the effects of treatment were sustained. However, OS is a robust endpoint, and we consider our OS results to be close to their true value because they were achieved in patients treated under routine clinical conditions. CDK4/6 inhibitors are now commonly used in early lines of treatment for HR+ HER2− MBC patients. However, the selection of subsequent treatment after progression with CDK4/6 inhibitors has become a clinical issue. Our study showed that mTOR inhibitors might be an effective treatment option for patients with HR+ HER2− MBC previously treated with CDK4/6 inhibitors in the real-world setting. A prospective study is needed to validate our findings.
Statement of Ethics
This retrospective observational study was approved by the institutional Research Ethics Committee of the Chiba Cancer Center, Chiba, Japan, in August 2020 (reference No. R02-170). Informed consent was obtained in the form of opt-out on the website.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Conception and design: Rikiya Nakamura. Acquisition of data: Shouko Hayama, Toshiko Miyaki, Makiko Itami, Naohito Yamamoto. Analysis and interpretation of data: Shouko Hayama. Drafting the article: Shouko Hayama. Revision of the manuscript: Shouko Hayama. Final approval of the version to be submitted: Rikiya Nakamura.
Acknowledgments
The authors express their sincere thanks to all participating patients and staff members at the study institution for their contributions. The author also would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016 Sep;34((25)):3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020 Dec;31((12)):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017 Sep;35((25)):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 4.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017 Nov;35((32)):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016 Nov;375((20)):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17((4)):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 7.Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: a systematic review and meta-analysis. Int J Mol Sci. 2020 Sep;21((17)):6400. doi: 10.3390/ijms21176400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Wang J, Wang Z, Xu B. Comparative efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors in women with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and network meta-analysis. Curr Probl Cancer. 2020 Dec;44((6)):100606. doi: 10.1016/j.currproblcancer.2020.100606. [DOI] [PubMed] [Google Scholar]
- 9.Serra F, Lapidari P, Quaquarini E, Tagliaferri B, Sottotetti F, Palumbo R. Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs Context. 2019;8:212579. doi: 10.7573/dic.212579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi J, Ma CX. Sequencing endocrine therapy for metastatic breast cancer: what do we do after disease progression on a CDK4/6 inhibitor? Curr Oncol Rep. 2020 Jul;22((6)):57. doi: 10.1007/s11912-020-00917-8. [DOI] [PubMed] [Google Scholar]
- 11.Araki K, Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2018 Jul;25((4)):392–401. doi: 10.1007/s12282-017-0812-x. [DOI] [PubMed] [Google Scholar]
- 12.Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2− metastatic breast cancer: biological mechanisms and new treatments. Cancers (Basel) 2019 Aug;11((9)):1242. doi: 10.3390/cancers11091242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey K, An HJ, Kim SK, Lee SA, Kim S, Lim SM, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer. 2019 Sep;145((5)):1179–1188. doi: 10.1002/ijc.32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012 Feb;366((6)):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45((2)):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018 Nov;379((20)):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 17.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014 Dec;25((12)):2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palumbo R, Sottotetti F, Quaquarini E, Gambaro A, Ferzi A, Tagliaferri B, et al. Patterns of treatment and outcome with 500-mg fulvestrant in postmenopausal women with hormone receptor-positive/HER2-negative metastatic breast cancer: a real-life multicenter Italian experience. Ther Adv Med Oncol. 2019 Jun;11:1758835919833864–13. doi: 10.1177/1758835919833864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerusalem G, de Boer RH, Hurvitz S, Yardley DA, Kovalenko E, Ejlertsen B, et al. Everolimus plus exemestane vs everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-negative advanced breast cancer: the BOLERO-6 randomized clinical trial. JAMA Oncol. 2018 Oct;4((10)):1367–1374. doi: 10.1001/jamaoncol.2018.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016 Jan;17((1)):90–98. doi: 10.1016/S1470-2045(15)00411-8. [DOI] [PubMed] [Google Scholar]
- 21.Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011 Mar;377((9769)):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 22.Piezzo M, Cocco S, Caputo R, Cianniello D, Gioia GD, Lauro VD, et al. Targeting cell cycle in breast cancer: CDK4/6 inhibitors. Int J Mol Sci. 2020 Sep;21((18)):6479. doi: 10.3390/ijms21186479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Occhipinti G, Romagnoli E, Santoni M, Cimadamore A, Sorgentoni G, Cecati M, et al. Sequential or concomitant inhibition of cyclin-dependent Kinase 4/6 before mTOR pathway in hormone-positive HER2 negative breast cancer: biological insights and clinical implications. Front Genet. 2020 Apr;11:349. doi: 10.3389/fgene.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019 May;380((20)):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi MN, Anandan A, Grogan P, O'Regan RM. Therapy after cyclin-dependent kinase inhibition in metastatic hormone receptor-positive breast cancer: Resistance mechanisms and novel treatment strategies. Cancer. 2020 Aug;126((15)):3400–3416. doi: 10.1002/cncr.32931. [DOI] [PubMed] [Google Scholar]