Abstract
Cholinergic innervation of the hippocampus uses the neurotransmitter acetylcholine (ACh) to coordinate neuronal circuit activity while simultaneously influencing the function of non-neuronal cell types. The α7 nicotinic ACh receptor (nAChR) subtype is highly expressed throughout the hippocampus, has the highest calcium permeability compared to the other subtypes of nAChRs, and is of high therapeutic interest due to its association with a variety of neurological disorders and neurodegenerative diseases. In this review, we synthesize research describing α7 nAChR properties, function, and relationship to cognitive dysfunction within the hippocampal circuit and highlight approaches to help improve therapeutic development.
Keywords: Cognitive Dysfunction, Inflammation, Neurodegeneration, Schizophrenia, Alzheimer’s Disease, Pharmacology
α7 nAChRs: A Long Way in 100 Years?
More than a century has passed since John Newport Langley’s studies of the first identified ‘receptive substance’, later named nicotinic acetylcholine receptors (nAChRs). Since then, advances in technology have allowed for investigations of the nAChR family in specific cell populations, circuits, and living animals to better understand the connection between the structure, function, and behavior. The α7 nAChR subtype has emerged as a therapeutic target due to its association with a variety of neurological disorders and neurodegenerative diseases, especially those associated with hippocampal-dependent processes. The α7 nAChRs are expressed in all layers of the hippocampus, in which they impact declarative memory by modulating local excitation, neurotransmitter release, signal transduction, synaptic plasticity, and neurogenesis. However, due to the widespread expression of α7 nAChRs in many neuronal and non-neuronal cell populations, co-activation with other receptors, timing dependency, and desensitization sensitivity, it has been difficult to augment α7 nAChR function for clinical use while avoiding off-target effects. Here, we synthesize recent literature describing α7 nAChR properties, function, and relationship to cognitive dysfunction within the hippocampal circuit and highlight scientific approaches that have the potential to improve the development and efficacy of α7 nAChR therapeutics.
Molecular Makeup of α7 nAChRs
The nAChRs are rapidly activating, excitatory, cationic, ligand-gated ion channels in the cys-loop receptor family. These receptors are activated by the endogenous ligand ACh and various exogenous ligands (e.g., nicotine) (Fig 1). Of the twelve currently known neuronal nAChR subunits (α2-α10 and β2-β4), nine are expressed in the hippocampus (α2-α7 and β2-β4), and have been shown to form either hetero- or homopentameric nAChRs [1]. The five subunits that co-assemble into a functional receptor determine its physiological and pharmacological characteristics (for review of binding regions: [2–4]).
Figure 1. Homomeric α7 nAChRs in various conformational states.
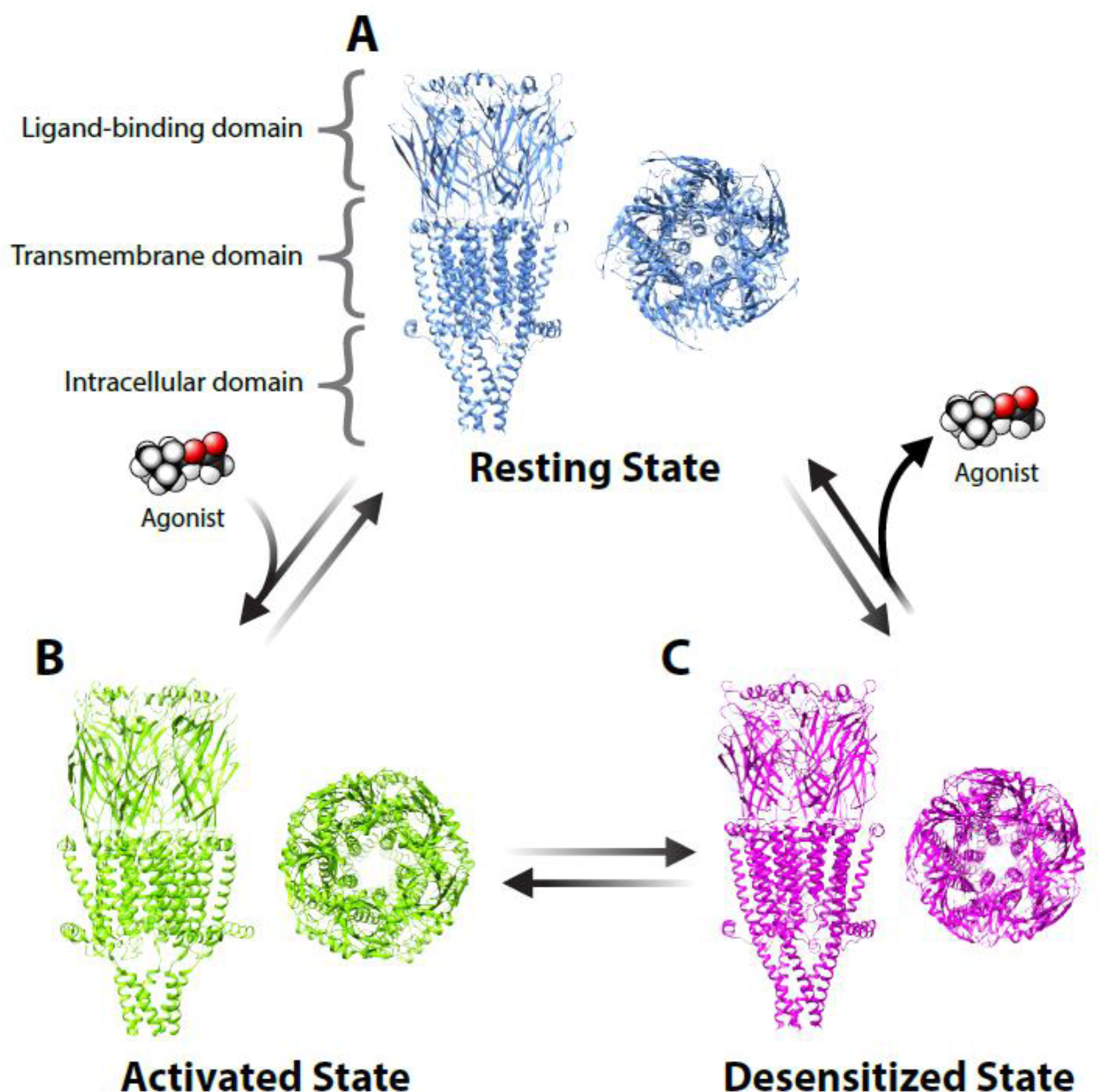
The structural images are based on data from functional electron microscopy studies [116] and visualized via Chimera software with A) the antagonist α-bungarotoxin bound in the ‘resting state’, B) ACh and the positive allosteric modulator PNU-120596 bound in the ‘activated state’, and C) long-term exposure to ACh in the ‘desensitized state’. In the activated state, the central pore allows cations (mostly sodium and calcium ions), to pass through the receptor into the cell. The long-term exposure to the agonist ACh leads to desensitization (after activation) and the closing of the pore. All ligand binding occurs within the ligand-binding domain located extracellular to the transmembrane domain. Data can be explored here: https://www.rcsb.org/structure/7KOO.
The homopentameric α7 nAChR subtype has the highest calcium permeability of all nAChR subtypes, with a relative calcium to sodium permeability at least as high as NMDA receptors [5]. α7 nAChRs have also been found to have metabotropic properties, even in the desensitized state, through G protein coupling [6]. Thus, the α7 nAChR participates in a variety of physiological processes including neuronal excitation, neurotransmitter release, signal transduction, synaptic plasticity, and neurogenesis [7–14]. In the hippocampus, α7 nAChRs are one of the most abundant subtypes and are expressed in the majority of neuronal populations [15], as well as non-neuronal cells including astrocytes [16], microglia [17], macrophages [18], as well as vascular endothelial cells and smooth muscle cells [19]
While homopentameric α7 nAChRs make up the bulk of α7 nAChR-related research, α7 and β2 nAChR subunits have been found to form functionally distinct α7β2 nAChRs, at lesser density, in the brain of rodents and humans [20]. The α7β2 nAChRs expressed in basal forebrain cholinergic neurons are more sensitive to pathologically-relevant concentrations of amyloid β (Aβ; see Glossary), which may have relevance for the cognitive deficits associated with Alzheimer’s disease [21] (this is discussed in greater detail in later sections).
α7 nAChR Functions in the Hippocampal Circuit are Location, Time, and Context Dependent
α7 nAChR activation has complex effects on the hippocampal network through multiple mechanisms. For example, α7 nAChR activation can directly depolarize neurons - especially interneurons due to the high somatic expression of the receptor [22]. α7 nAChR activation can also regulate both glutamatergic and GABAergic transmission through both pre- and postsynaptic mechanisms [23]. The modulation of plasticity associated with glutamatergic transmission depends on the timing of α7 nAChR activation relative to the glutamatergic transmission [24,25]. Additionally, α7 nAChRs are likely coactivated with other cholinergic receptor subtypes (both nicotinic and muscarinic) as α7 nAChRs have relatively low affinity to ACh (EC50 ~180 µM) [26]. In this section, we emphasize the many known functions of α7 nAChRs that depend on location, timing, and context.
Location
α-bungarotoxin binding assays yield abundant α7 nAChR protein expression in hippocampal interneuron populations [27,28]. Oriens lacunosum-moleculare (OLM) interneurons located in the CA1 stratum oriens express both mAChRs and nAChRs and play an important role in mediating hippocampus-dependent memory formation [29–31]. OLM interneurons are a subset of CA1 somatostatin-positive interneurons that primarily target the distal dendrites of CA1 pyramidal neurons in the stratum lacunosum-moleculare (SLM) where CA1 pyramidal neurons also receive excitatory inputs from the entorhinal cortex (EC). OLM interneurons usually have larger α7 nAChR currents than other somatostatin interneurons and pyramidal neurons [32]. α7 nAChR activation on OLM interneurons inhibits EC to CA1 pyramidal neuron inputs through direct inhibition, but enhances Schaffer collateral (SC) inputs through indirect disinhibition [33]. This may be one of the mechanisms involved in the regulation of theta oscillations by α7 nAChRs [32,34–37]. However, activating α7 nAChRs on hippocampal CA1 stratum radiatum interneurons can have either inhibitory or disinhibitory effects on CA1 pyramidal neurons [38]. In the dentate gyrus (DG), α7 nAChR responses can be recorded from various neurons, including molecular layer interneurons and hilar interneurons [39,40].
α7 nAChR protein expression is low in the soma of primary excitatory cells [23,31], but is expressed at excitatory synapses, including pre- and postsynaptic sites [23]. While there are no obvious direct depolarizing currents generated in the soma, α7 nAChR activation can still exert important functions in regulating synaptic activities [23,41]. α7 nAChRs are also expressed in the pre- and postsynaptic sites of GABAergic inhibitory interneurons where they control inhibitory transmission [42]. Therefore, in addition to directly depolarizing interneurons, another important function of α7 nAChRs is to regulate synaptic transmission and plasticity. α7 nAChR activation generally increases presynaptic release of both glutamate and GABA [43] and promotes long-term potentiation (LTP) induction [44]. However, because α7 nAChRs are expressed in both primary excitatory cells and interneurons, the outcome in primary excitatory cells is determined by the location and timing of α7 nAChR activation. For example, α7 nAChR activation in CA1 interneurons can diminish the induction of short-term potentiation in nearby connected pyramidal neurons, while activation of CA1 dendritic α7 nAChRs in pyramidal neurons can boost short-term potentiation (STP) or LTP [41].
Timing
The outcome of α7 nAChR regulation of hippocampal excitatory synaptic transmission also depends on the timing of α7 nAChR activation relative to the activation of excitatory transmission. Exogenously applied ACh can convert STP to LTP when applied 1–5 s before electric stimulation of SCs, but convert STP to short-term depression (STD) when applied within 1 s before SC stimulation, primarily through α7 nAChR-dependent mechanisms [25]. The effects appear to depend on the depolarizing effect of ACh application on the pyramidal neurons under recording [25]. A previous study from our group demonstrated higher precision in the regulation of synaptic plasticity by concurrent single pulses of endogenous ACh release [24]. When the cholinergic input was activated 100 ms prior to SC stimulation, it resulted in α7 nAChR-dependent LTP. However, when the cholinergic input was activated 10 ms prior to SC stimulation, an α7 nAChR-dependent STD was induced instead. This millisecond precision is comparable to spike timing-dependent plasticity [45], revealing a fast and precise mechanism for neuromodulators to regulate glutamatergic transmission. In the case of cholinergic inputs, the depolarizing effect of cholinergic activation seems less relevant since most of the time there is no detectable postsynaptic currents on pyramidal neurons after single pulse cholinergic activation. Instead, α7 nAChR activation may increase calcium levels in distal synapses, thereby enhancing presynaptic neurotransmitter release. Such repeated activation may gradually strengthen the correlation of pre- and postsynaptic events and lead to Hebbian plasticity. Indeed, both pre- and postsynaptic α7 nAChRs may be involved in LTP and presynaptic α7 nAChRs may induce STD [46]. These results suggest that timing of cholinergic inputs will modulate glutamatergic transmission – such that the same cholinergic input will not influence the plasticity of inactive glutamatergic synapses, while having different effects on those activated 10 ms or 100 ms afterwards. It is worth noting that it takes 5 to 10 times of such repeated concurrent activation of cholinergic inputs and SC activation to induce synaptic plasticity, a requirement which might mimic a gradual and repeated associative learning process and that could help avoid modulating random synaptic activities.
Context
As α7 nAChRs have relatively low affinity for the endogenous ligand ACh, α7 nAChR activation is likely accompanied by co-activation with mAChRs and non-α7 nAChRs. mAChR-mediated responses may vary depending on the pattern and intensity of cholinergic activation, but strong cholinergic activation can depolarize both interneurons and pyramidal neurons through mAChRs [47,48]. Although exogenously applied ACh induces depolarizing currents in interneurons primarily through α7 nAChRs, optogenetically activated endogenous ACh release induces depolarizing currents primarily through α4β2 receptors in CA1 stratum oriens (SO) and SLM interneurons, which can be slightly enhanced by muscarinic receptor inhibition [49]. Hippocampal local infusion of antagonists of either mAChR or α7 nAChRs partially reduces theta oscillation intensity in freely moving mice alongside reductions in spatial working memory, but the combined application of mAChR and α7 nAChR antagonists does not further reduce peak theta power [32]. mAChR or α7 nAChR antagonists can also block cholinergic-pairing induced theta oscillations in slice cultures [32,50]. Moreover, SC stimulation, when paired with low concentration of ACh, can induce transient theta oscillations, while high concentrations of ACh pairing (or low concentration of ACh plus an α7 nAChR agonist) modified the network to allow theta generation by SC stimulation alone long after the pairing [32]. These studies suggest that these two receptor subtypes work together in regulating plasticity, theta oscillations strength, and cognitive function. Thus, the pairing of mAChRs and α7 nAChRs may resemble, in a way, the well-established effects of paired AMPA/NMDA glutamate receptors on synaptic transmission: mAChR activation transiently excites the target cells, while nAChR activation can induce synaptic plasticity due to its high calcium permeability.
A single incoming cholinergic fiber can innervate the various layers of the SO, SR, SLM, and the ML of the DG, thus innervating a large cross section of the hippocampal neuronal population at the same time (Fig 2, Key Figure) [24], providing a potential mechanism for cholinergic inputs to coordinate hippocampal network activity. For example, while cholinergic inputs can enhance hippocampal excitatory synaptic transmission through α7 nAChR activation on both pre- and postsynaptic sites, activation of α7 nAChRs will also directly depolarize interneurons that will in turn upregulate inhibitory tone in the network. In other words, while the correctly timed excitatory synaptic events are enhanced, other events outside the time range are depressed. This may provide a mechanism for enhancing signal to noise ratio. In the DG, α7 nAChR-mediated currents are mostly observed in interneurons (with very large currents) but not granule cells (which usually exhibit very small to non-detectable currents), suggesting that α7 nAChR activation in DG mainly produce indirect inhibitory effects on granule cells [39,40]. In addition, hilar inhibitory neurons can also be excited by cholinergic inputs indirectly through hilar astrocytes, also via α7 nAChR activation. As a result, α7 nAChR activation on hilar astrocytes can result in slow and lasting inhibition of DG granule cells [51]. However, α7 nAChR activation can enhance granule cell output to CA3 by upregulating synaptic glutamate release at mossy fiber terminals through a presynaptic mechanism [52]. This allows strong and sparse mossy fiber output to CA3 and may thus support a pattern separation function (for a review on the DG’s role in pattern separation [53]). The mossy fiber to CA3 pathway may represent an important cholinergic target in the hippocampus as there is a noticeable pattern of concentrated cholinergic terminal distribution along the mossy fiber pathway in the hippocampal CA3 region [24]. For the most part, the cholinergic terminals are diffusely distributed in hippocampal subregions without a clear pattern. However, α-BTX staining is relatively higher in the CA2 of multiple mouse strains including C57BL/6, C3H/He, and ST/b [27,28]. The CA2 region has been associated with social memory and social behavior including aggression [54]. α7 nAChRs are also associated with aggression [55,56] and schizophrenia [57], although it is currently unclear whether α7 nAChR levels in the CA2 are causally linked to regulation of aggression and social behaviors.
Figure 2, Key Figure. Innervation of the Hippocampus via a Single Cholinergic Projection Neuron from the Medial Septum.
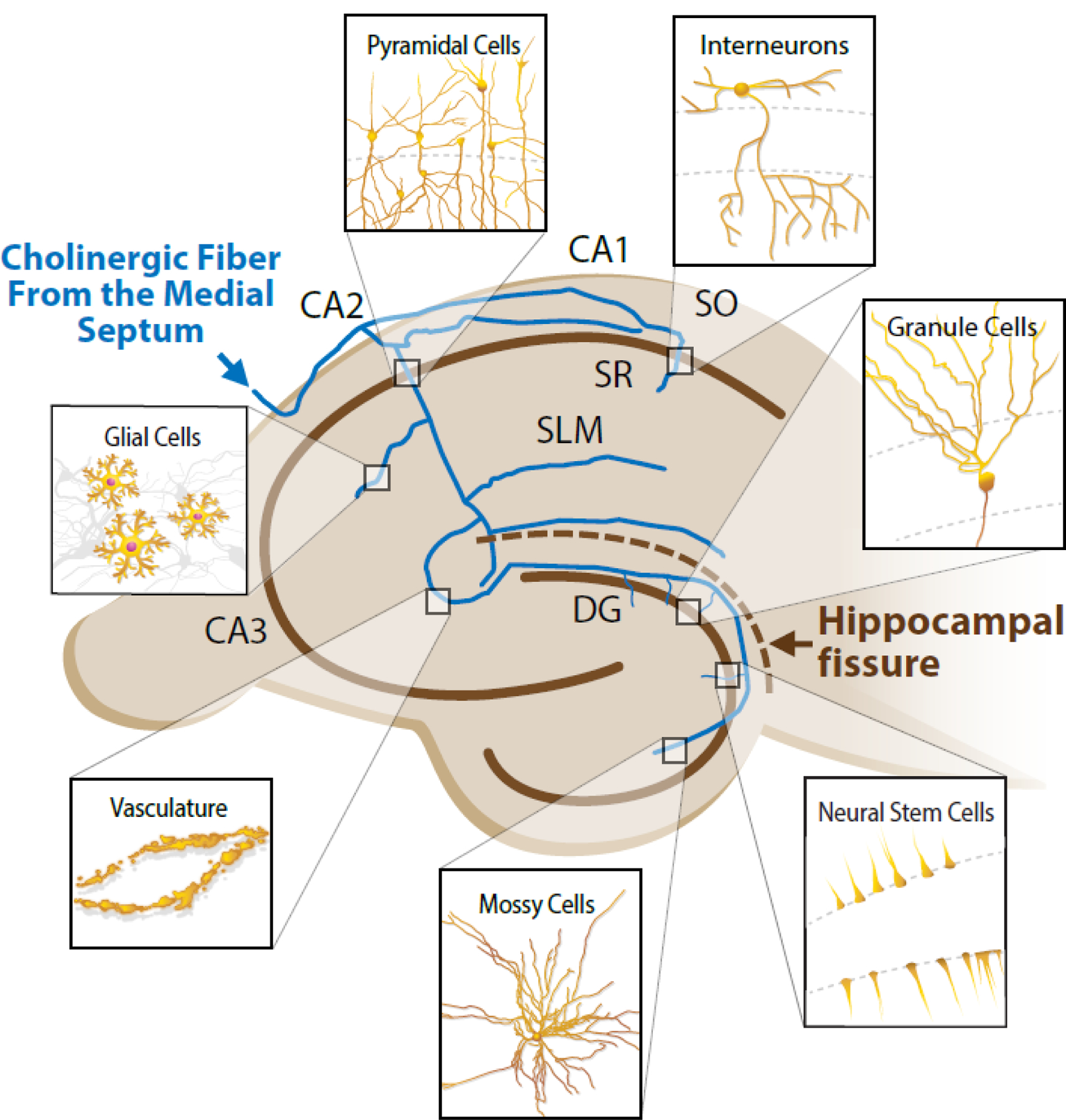
The blue lines represent the axons of a single hypothetical cholinergic projection neuron from the medial septum with bifurcations innervating various regions of the hippocampus. Each magnifying box represents one of many possible locations of various cell types that can be modified by either synaptic or volume transmission related to the cholinergic innervation. The upper left brown line indicates the pyramidal layer (somas of the pyramidal cells) while the lower right brown line represents the molecular layer (somas of the granule cells). CA1, 2, and 3 – Cornu Amonis 1, 2, and 3, respectively. DG – dentate gyrus. SO – strata oriens. SR – strata radiatum. SLM – strata lacunosum-moleculare.
Manifestation of α7 nAChR-Related Cognitive Dysfunction – What is to blame?
Alterations in cholinergic tone and altered receptor function are associated with many neurological disorders and neurodegenerative diseases including dementia/Alzheimer’s disease, schizophrenia, Parkinson’s disease, addiction, epilepsy, attention deficit hyperactivity disorder (ADHD), HIV, Rett Syndrome, autism spectrum disorder, aggression, and inflammation [55,57–66]. In this section, we focus on cognitive dysfunctions related specifically to the hippocampus and α7 nAChRs.
Cholinergic Depletion
While nicotine (via tobacco) has been used as a cognitive modulator for millennia [67], specific cognitive roles of the cholinergic system started to be substantiated during the mid-twentieth century when anticholinergics such as atropine and scopolamine were documented to impair memory. These findings helped consolidate the cholinergic hypothesis of geriatric memory dysfunction [68]. The hypothesis was based, in part, on the consistent observation of reduced choline acetyltransferase (ChAT) in post-mortem tissue from elderly people with dementia. However, as ChAT is not a rate-limiting step for the synthesis of ACh, firmer support for the hypothesis was provided only later, with the observation of significant degeneration of cholinergic neuron projections (up to 75%) from the nucleus basalis of Meynert to the cortex, as well as of cholinergic projections from the diagonal band of Broca and medial septum to the hippocampus [69]. The root cause of the cholinergic degeneration is still not certain but has many proposed theories, such as Aβ buildup or impaired neural growth factor signaling (reviewed in [70,71]). Regardless of the cause, cholinergic depletion likely alters normal α7 nAChR activation states causing disrupted cognition [72].
Development
Although age is a principal risk factor for reduced ACh production [73], hippocampal α7 nAChR expression is not consistently reduced with age like it is in people with Alzheimer’s disease [74,75]. However, the α7 nAChR is one of the first receptors to be expressed in the developing brain, including on adult born granule cells within the DG [76]. Genetic knockouts of Chrna7 (the gene that encodes the α7 nAChR) in rodents reveal a direct role in functional neurogenesis and neuron development likely through modulating GABA activity [14,77]. The importance of α7 nAChRs in developing neurons is also highlighted by the cognitive impairments in offspring of choline-deficient mothers [78] which will be discussed in more detail below.
Genetic Determinates
Variations within CHRNA7 have been linked to cognitive dysfunctions, but have low population frequencies with high phenotypic variation [79,80]. The most firmly associated genetic determinates are the linkages between schizophrenia and the abnormally high number of polymorphisms within the promoter region of CHRNA7, which helps explain the small, but often observed, reduction in α7 nAChRs expression associated with this mental disorder [81,82]. Additional associations with Alzheimer’s disease, bipolar disorder, ADHD, and epilepsy have been found with various microdeletions of CHRNA7 and a gene duplication found only in humans, CHRFAM7A [83]. These genetic patterns are indicative of an evolutionary beneficial, but not obligatory, role for CHRNA7. Supporting this, CHRNA7 mouse knockout models are viable and exhibit only partial, strain dependent deficits across many molecular and behavioral phenotypes [14,84,85] (potentially due to compensatory mechanisms during development [86]). As with any polygenic disease, epistasis and other genetic factors with small effect sizes likely contribute without robust detection via GWAS. For example, a genetic basis for α7 nAChR-related dysfunction extends beyond CHRNA7 as the receptor requires proper functioning and expression of various chaperone proteins such as RIC3, NACHO, and LY6 prototoxins to help with conformation and shuttling to the membrane [87,88].
Amyloid β
The nAChRs have a high affinity for Aβ [89], further implicating cholinergic dysfunction as a potential contributor to Alzheimer’s disease. Evidence from heterologous expression systems suggests that low levels of Aβ (picomolar to low nanomolar range) can induce α7 nAChR channel activity, while higher levels (nanomolar to low micromolar range) decreases α7 nAChR channel activation duration by ACh [90] which can lead to dysfunctional circuit activity [91]. Among the various subtypes of nAChRs, α7β2 receptors have been found to have the highest affinity for Aβ, and they can be inhibited by as little as one nanomolar of Aβ [20]. Additionally, the apolipoprotein E4 (APOE4) allele, which has the strongest genetic association with Alzheimer’s disease (heterozygotes have 2–4 fold increased disease risk and homozygotes have 8–12 fold increased risk) [92], has been found to increase the number of Aβ/nAChR complexes [93]. The binding between nAChRs and Aβ may not always be maladaptive. For example, studies in mice showed that 12 month old Chrna7 knockout mice exhibit Alzheimer’s disease-like pathology [94]. This may arise from disrupting normal, picomolar levels of Aβ’s ability to potentiate α7 nAChR presynaptic neurotransmitter release probability [95,96]. The hormetic relationship of Aβ and nAChR function is an area deserving greater attention, especially in the preclinical stages of Alzheimer’s disease.
Nicotine
High levels of nicotine delivered to the brain via inhalation activates neural nAChRs within seconds, followed by desensitization, as nicotine is not metabolized as quickly as endogenous ligands [97]. Chronic nicotine exposure leads to increased expression of multiple nAChRs and nAChR-related genes. However, the expression level of CHRNA7 is inconsistent across multiple studies [97], potentially due to α7 nAChRs relatively low binding affinity to nicotine (~90 µM). Exposure to nicotine across the lifespan lead to multiple changes in neuronal morphology and cognition depending on the specific exposure method and length [98,99]. Rates of smoking are higher among individuals with schizophrenia, for reasons that are not fully clear and are a matter of ongoing debate. While smoking has been claimed to improve negative symptoms of schizophrenia, evidence also indicates that the effect might be a result of mitigating withdrawal symptoms [100]. In any case, from a broader perspective, the negative outcomes associated with nicotine use cannot be understated. Despite nicotine’s acute ability to enhance some domains of α7 nAChR-mediated cognitive function [101], ineffective nicotine metabolism, cardiovascular disease, cancer and addiction risk outweigh the transient cognitive-enhancing aspects of its use.
Alcohol
In individuals with alcohol use disorder, excessive alcohol use has been found to correlate with reduced ChAT activity in the hippocampus [102]. Perhaps relatedly, high alcohol use (>14 drink units/week) is associated with elevated risk of dementia [103]. To our knowledge, the effect of alcohol on α7 nAChR function in humans has yet to be studied. Most work has focused on nAChRs as a modulator of mesolimbic dopamine signaling in alcohol use behaviors, which is beyond the scope of this review [105]. However, there is evidence in rodents that ethanol exposure impairs α7 nAChR function [104]. Ethanol can also enhance the sensitivity of the α7 nAChR to nicotine [105]. These studies underscore the need to consider interactions of various substances when studying therapeutics for at-risk populations.
Inflammation
As many glial cells express α7 nAChRs, cholinesterase inhibitors currently prescribed for dementia could potentially be operating at the inflammatory level. This is supported by data revealing anti-inflammatory effects of the cholinergic system, including specifically α7 nAChRs [106,107]. For example, vagal activation of α7 nAChRs expressed on macrophages prevents the synthesis of tumor necrosis factor and interleukins 1 and 6 [108]. Further influencing inflammation and recovery, ACh is essential for the vasodilation of blood vessels by relaxing smooth muscles which express a range of nAChRs including the α7 subtype [19]. Thus, modulating non-neuronal cell function by the cholinergic system via volume transmission may play a critical role in maintaining hippocampal fitness via optimizing inflammatory responses [109].
Taming the Complexity
The most widely prescribed cholinergic drugs are cholinesterase inhibitors (e.g., donepezil, galantamine, and rivastigmine) used to maintain ACh levels in patients with reduced cholinergic tone. However, many patients discontinue usage, due to limited cognitive benefits and off target side effects such as gastrointestinal issues [110]. While nAChR agonists, and even some antagonists, recover cognition in animal models and small clinical trials, none have accomplished efficacy through phase 3 clinical trials for any major cognitive dysfunction outside of tobacco use disorder (for review: [57,111,112]). The typically limited efficacy of these compounds and the adverse off-target effects underscore the need to better understand and consider the location, timing, and context-dependence in the functions of α7 nAChRs.
Non-specific binding of α7 nAChR ligands with other nAChRs and receptors such as glycine and serotonin (5-HT3) receptors provide additional complexities. However, some of these interactions may be harnessed to improve drug effectiveness. For example, the 5-HT3 receptor antagonist tropisetron is commonly used as an antiemetic through 5-HT3 receptor antagonism. Tropisetron also works as a partial agonist for α7 and α7β2 nAChRs and has been shown to improve cognition in aged Fischer rats and rhesus monkeys [113], as well as schizophrenia symptoms in non-smokers [114]. Still, the presence of nAChRs in nearly every tissue type in the body makes off target effects difficult to overcome. For this reason, basic understanding of the receptor’s unique conformation and accessory proteins across various tissues is crucial for designing cholinergic drugs with improved specificity (reviewed in [115]). The recent ability to express functional α7 nAChRs in normally nAChR null cell lines [21] provides a way to study these interactions.
An approach to overcome specificity issues, which has been receiving growing attention, is to enhance endogenous ACh action via allosteric modulators. There are many types of allosteric modulators that vary in their ability to alter the target receptor’s activation, affinity to other ligands, and the efficacy of other ligands when binding to the receptor. Type I nAChR PAMs for instance alter the amplitude of currents, and Type 2 PAMs alter the temporal kinetics of the current. Thus far, only two α7 nAChR-specific Type 1 PAMs (to our knowledge) have made it to clinical trials, AVL-3288i and JNJ-39393406ii; both intended to treat symptoms of schizophrenia. Despite benefits in preclinical and Phase 1a trials, AVL-3288 failed to demonstrate effectiveness in Phase 1b trials while JNJ-39393406 was abandoned in Phase 2 trials due to lack of efficacy. Despite these challenges, PAMs remain a promising avenue for future research as recent high resolution structures of the α7 nAChR in various conformational states [116] can be combined with advances in protein structure folding and binding [117] to discover novel PAMs. During this process, it will be critical to understand the role of α7 nAChRs dysfunction in each specific condition. For example, the ability of silent allosteric modulators to leverage the metabotropic properties of α7 nAChRs via stimulating Jak2/STAT3 and PI3K pathways with little to no ionotropic activity could be useful for specifically reducing inflammatory states [118]. As new drugs emerge, the ability to test their effects using innovative in vivo techniques (e.g., fiber photometry and miniature microscopes) and translationally-relevant behavioral tasks (e.g., touchscreen cognitive testing [119] and improved recognition assays [120]) could accelerate the preclinical development process [121,122] (Box 1).
Text Box 1. Investigating α7 nAChR Function in Vivo.
The study of α7 nAChRs in vivo can help improve the translational relevance of the findings, given the substantial differences between circuit properties in vitro and in vivo. Below, we describe various techniques that can be utilized for studying circuit function in vivo and highlight some of the recent studies that employed these techniques in the context of the cholinergic system.
Fiber Photometry –
An optic fiber is implanted into a specific brain region to capture fluorescent emissions from virally-expressed biosensors when bound to specific substrates such as calcium [132], acetylcholine [129], or nicotine [133] on a sub-second time scale in a freely moving animal.
Miniature Microscope –
A technique similar to fiber photometry except that a cylindrical lens is implanted into a specific brain region, and the lense is reversibly attached to a miniature microscope allowing the imaging of fluorescence from individual cells instead of bulk fluorescence [133].
Optogenetics/Electrophysiology –
Specific wavelengths of light are exposed to virally-expressed potassium channels combined with light sensitive rhodopsins that in turn activate/inactivate their host cell on a millisecond timescale. As the optic fiber is small, the implanted apparatus can be combined with an array of electrodes [134].
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) –
Modified muscarinic G-protein coupled receptors are virally-expressed in specific brain regions/cells and can activate/inactivate their host cell when bound to biologically inert, exogenous ligands (e.g., clozapine-N-oxide). DREADDs can be activated through delivery of the ligand via intraperitoneal injection for acute exposure or drinking water for chronic exposure [135].
Positron Emission Tomography (PET) –
Ligands labeled with radioactive isotopes are infused systemically and imaged in real time to visualize the location and degree of binding to specific endogenous receptors. High affinity tracers for α7 nAChRs, such as 18F-ASEM, can be imaged with minimal displacement of endogenous ligands preventing disruption of normal function [72].
Nanosensors –
By combining acetylcholine-catalyzing enzymes and pH-sensitive gadolinium contrast agents on the surface of nanoparticles, low micromolar levels of acetylcholine can be detected real time via MRI. However, in most current approaches, the nanoparticles cannot cross the blood-brain barrier, which limits the use in humans [136].
Touchscreen Operant Chamber –
In order to improve translation from rodent behavior models to humans, a touchscreen task was created to mimic the Cambridge Neuropsychological Test Automated Battery. Both tests require subjects to touch symbols on a screen in specific patterns and receive immediate feedback of performance. Multiple tests are available to test specific domains of memory and behavior [122].
Non-pharmacological approaches, such as diet, exercise, and sleep, may also provide synergistic therapeutic support for α7 nAChR-related dysfunction. For example, choline is an essential nutrient that is required for ACh synthesis and can function as an α7 nAChR agonist. In the United States, according to a 2016 assessment, only approximately 10% of the population achieved adequate intake of choline [123]. Insufficient choline intake during pregnancy can have particularly severe adverse effects, as a deficiency can disrupt offspring’s fetal neural development [78]. Exercise offers a potential therapeutic benefit as physical activity is associated with increased hippocampal size and improved memory [124–127]. While causal links remain to be tested, and mechanistic understanding of these associations represents an important gap in knowledge [128], findings from rodent models indicate that physical activity can increase hippocampal ACh release and expression of α7 nAChRs [129,130]. Lastly, animal model studies have shown that sleep loss can impair ACh-mediated hippocampal function and hippocampal-dependent memory consolidation [131].
Concluding Remarks and Future Perspectives
α7 nAChRs influence a wide variety of physiological processes in the hippocampal circuit, via both ionotropic and metabotropic signaling pathways. Relatedly, hippocampal α7 nAChRs are implicated in multiple neurological disorders and neurodegenerative diseases. The specific role of α7 nAChRs depends on cell type and expression location, timing of activation relative to other excitatory/inhibitory signals, and the context in which ligands are bound. As such, α7 nAChR-related cognitive dysfunction is complex, and addressing these impairments will likely require a comprehensive therapeutic approach consisting of early detection of symptoms, effective biomarkers, lifestyle adjustments (e.g. adequate nutrition, avoiding chronic substance use, limiting neuroinflammatory states), consideration of genetic background, and application of highly specific pharmaceutical neuromodulators (e.g., PAMs). Improving the effectiveness of current therapeutics will also require addressing fundamental questions regarding α7 nAChRs’ function in the hippocampal circuit (see Outstanding Questions). To best achieve these goals, focused collaborations between scientists, engineers, clinicians, and pharmaceutical companies will be necessary.
Outstanding Questions.
Are there ligands that bind to α7 nAChRs in specific cell types?
Are there α7 nAChR PAMs that can alter firing threshold and/or amplitudes while maintaining temporal activation, desensitization, and/or expression of CHRNA7?
How do lifestyle and environmental factors such as physical activity, diet, sleep, or toxicant exposure modulate α7 nAChR function?
In what context are α7 nAChRs activated in relationship to excitatory and inhibitory signaling in various cell types?
How are hippocampal subregions’ activity co-coordinated via α7 nAChRs?
How does Aβ interact with α7 nAChRs in vivo?
What are the fundamental differences in α7 nAChR function between rodent models and humans?
Highlights.
α7 nAChR expression is abundant in the hippocampus and located pre- and postsynaptically in most neuronal types as well as in non-neuronal cells such as astrocytes, microglia, macrophages, and endothelial cells.
α7 nAChRs have low affinity for acetylcholine (EC50 ~180 µM), indicating they are likely coactivated with other cholinergic receptor subtypes.
Timing of α7 nAChR activation related to excitatory inputs alters the effect on postsynaptic plasticity, influencing theta oscillations.
A single basal forebrain cholinergic neuron can innervate cells in all layers of the hippocampus, possibly coordinating hippocampal activities.
Therapies for α7 nAChR-related dysfunction should preserve the function of cholinergic projection neurons and/or utilize modulators that enhance function while preserving response kinetics to maintain the circuit’s temporal fidelity.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors would like to thank Drs. Colleen Noviello and Ryan Hibbs for providing the ribbon diagrams shown in Figure 1, Donna Corcoran from the National Institute of Environmental Health Sciences’ Image Associates Multimedia Services for generating Figure 1 and 2, and Pattie Lamb, Dr. Joanne Damborsky, Dr. Oliver Goral, and Diane Youngstrom for providing feedback throughout the review.
Glossary
- α-Bungarotoxin (α-BTX):
a molecule derived from the Taiwanese banded krait snake which can antagonistically and irreversibly bind to the α7 nicotinic acetylcholine receptor. Because of the lack of quality antibodies for α7 nAChRs, α-BTX has been widely used to study the α7 nAChR at the protein level
- allosteric modulators:
substances that can bind to a non-orthosteric site of a receptor altering that receptor’s typical response to other ligands
- amyloid β (Aβ):
peptides implicated in Alzheimer’s disease that are derived from the amyloid precursor protein
- anticholinergics:
any drug that blocks the action of acetylcholine
- apolipoprotein:
a class of proteins responsible for the binding and transport of lipids in the blood, lymph, and cerebrospinal fluid as well as functioning as enzyme cofactors involved in metabolism of lipoproteins
- choline acetyltransferase (ChAT):
an enzyme that synthesizes acetylcholine
- cholinesterase inhibitors:
a class of drugs that inhibit the enzyme acetylcholinesterase and therefore prevent the breakdown of acetylcholine. Also referred to as anticholinesterases
- CHRNA7:
the gene encoding the α7 nicotinic acetylcholine receptor subunit
- compensatory mechanisms:
alternative actions taken by the body to maintain physiological function despite an alteration in natural function
- epistasis:
a phenomenon in which the effect of one gene depends on the effects of one or more other genes
- genome wide association study (GWAS):
a study in which many genetic variants, called single nucleotide polymorphisms, are statistically tested for associations with a specific phenotype
- long term potentiation (LTP):
an increase in synaptic strength between two neurons due to repeated stimulations that has a time course lasting from minutes to hours
- Schaffer collateral (SC):
axon collaterals given off by CA3 pyramidal cells that project to the CA1
- short term depression (STD):
a decrease in synaptic strength between two neurons due to repeated stimulations that has a time course lasting from milliseconds to minutes
- short term potentiation (STP):
an increase in synaptic strength between two neurons due to repeated stimulations that has a time course lasting from milliseconds to minutes
- theta oscillations:
electrical rhythms with a frequency of 6–10 Hz hypothesized to represent coordination of neurons within a circuit
- volume transmission:
a non-specific diffusion of neurotransmitters into the extracellular space with a longer time course of action than synaptic transmission
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no conflicts of interest.
References
- 1.Zoli M et al. (2015) Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 96, 302–311 [DOI] [PubMed] [Google Scholar]
- 2.Papke RL and Lindstrom JM (2020) Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 168, 108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharpure A et al. (2020) Progress in nicotinic receptor structural biology. Neuropharmacology 171, 108086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcombe J et al. (2018) Diversity of Nicotinic Acetylcholine Receptor Positive Allosteric Modulators Revealed by Mutagenesis and a Revised Structural Model. Mol. Pharmacol 93, 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro NG and Albuquerque EX (1995) alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J 68, 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabbani N and Nichols RA (2018) Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci 39, 354–366 [DOI] [PubMed] [Google Scholar]
- 7.Chung BYT et al. (2016) Postsynaptic nicotinic acetylcholine receptors facilitate excitation of developing CA1 pyramidal neurons. J. Neurophysiol 116, 2043–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King JR et al. (2017) A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio 7, 1350–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza CM et al. (2019) JAK2/STAT3 Pathway is Required for α7nAChR-Dependent Expression of POMC and AGRP Neuropeptides in Male Mice. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 53, 701–712 [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Varela D and Berg DK (2013) Lateral Mobility of Presynaptic α7-Containing Nicotinic Receptors and Its Relevance for Glutamate Release. J. Neurosci 33, 17062–17071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend M et al. (2016) α7-nAChR agonist enhances neural plasticity in the hippocampus via a GABAergic circuit. J. Neurophysiol 116, 2663–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papke RL et al. (2018) NS6740, an α7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci. Lett 677, 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djemil S et al. (2020) Activation of nicotinic acetylcholine receptors induces potentiation and synchronization within in vitro hippocampal networks. J. Neurochem 153, 468–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nacer SA et al. (2021) Loss of α7 nicotinic acetylcholine receptors in GABAergic neurons causes sex-dependent decreases in radial glia-like cell quantity and impairments in cognitive and social behavior. Brain Struct. Funct 226, 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudweeks SN and Yakel JL (2000) Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J. Physiol 527 Pt 3, 515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papouin T et al. (2017) Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron 94, 840–854.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T et al. (2006) Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res 83, 1461–1470 [DOI] [PubMed] [Google Scholar]
- 18.Báez-Pagán CA et al. (2015) Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol 10, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehead AK et al. (2021) Nicotine and vascular dysfunction. Acta Physiol 231, e13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J et al. (2016) Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharmacol. Sci 37, 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George AA et al. (2021) Implications of Oligomeric Amyloid-Beta (oAβ42) Signaling through α7β2-Nicotinic Acetylcholine Receptors (nAChRs) on Basal Forebrain Cholinergic Neuronal Intrinsic Excitability and Cognitive Decline. J. Neurosci 41, 555–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khiroug L et al. (2003) Functional Mapping and Ca2+ Regulation of Nicotinic Acetylcholine Receptor Channels in Rat Hippocampal CA1 Neurons. J. Neurosci 23, 9024–9031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabian-Fine R et al. (2001) Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. Off. J. Soc. Neurosci 21, 7993–8003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z and Yakel JL (2011) Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 71, 155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge S and Dani JA (2005) Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J. Neurosci. Off. J. Soc. Neurosci 25, 6084–6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papke RL et al. (2007) The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J. Neurochem 101, 160–167 [DOI] [PubMed] [Google Scholar]
- 27.Adams CE et al. (2006) Development of hippocampal alpha7 nicotinic receptors in C3H and DBA/2 congenic mice. Brain Res 1122, 27–35 [DOI] [PubMed] [Google Scholar]
- 28.Stevens KE et al. (1996) Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 15, 152–162 [DOI] [PubMed] [Google Scholar]
- 29.Haam J et al. (2018) Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc. Natl. Acad. Sci. U. S. A 115, E1886–E1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Goethem NP et al. (2019) Antagonizing α7 nicotinic receptors with methyllycaconitine (MLA) potentiates receptor activity and memory acquisition. Cell. Signal 62, 109338. [DOI] [PubMed] [Google Scholar]
- 31.Siwani S et al. (2018) OLMα2 Cells Bidirectionally Modulate Learning. Neuron 99, 404–412.e3 [DOI] [PubMed] [Google Scholar]
- 32.Gu Z et al. (2020) Hippocampal Interneuronal α7 nAChRs Modulate Theta Oscillations in Freely Moving Mice. Cell Rep 31, 107740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leão RN et al. (2012) OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat. Neurosci 15, 1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoiljkovic M et al. (2016) Selective activation of α7 nicotinic acetylcholine receptors augments hippocampal oscillations. Neuropharmacology 110, 102–108 [DOI] [PubMed] [Google Scholar]
- 35.Buhler AV and Dunwiddie TV (2001) Regulation of the activity of hippocampal stratum oriens interneurons by alpha7 nicotinic acetylcholine receptors. Neuroscience 106, 55–67 [DOI] [PubMed] [Google Scholar]
- 36.Siok CJ et al. (2009) Anxiolytic profile of pregabalin on elicited hippocampal theta oscillation. Neuropharmacology 56, 379–385 [DOI] [PubMed] [Google Scholar]
- 37.Mikulovic S et al. (2018) Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat. Commun 9, 3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji D and Dani JA (2000) Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J. Neurophysiol 83, 2682–2690 [DOI] [PubMed] [Google Scholar]
- 39.Jones S and Yakel JL (1997) Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J. Physiol 504, 603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazier CJ et al. (2003) Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J. Neurophysiol 89, 3018–3028 [DOI] [PubMed] [Google Scholar]
- 41.Ji D et al. (2001) Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31, 131–141 [DOI] [PubMed] [Google Scholar]
- 42.Alkondon M and Albuquerque EX (2001) Nicotinic Acetylcholine Receptor α7 and α4β2 Subtypes Differentially Control GABAergic Input to CA1 Neurons in Rat Hippocampus. J. Neurophysiol 86, 3043–3055 [DOI] [PubMed] [Google Scholar]
- 43.Radcliffe KA et al. (1999) Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann. N. Y. Acad. Sci 868, 591–610 [DOI] [PubMed] [Google Scholar]
- 44.Cheng Q and Yakel JL (2015) The effect of α7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem. Pharmacol 97, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dan Y and Poo M-M (2004) Spike timing-dependent plasticity of neural circuits. Neuron 44, 23–30 [DOI] [PubMed] [Google Scholar]
- 46.Gu Z et al. (2012) Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci 32, 12337–12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagger-Vaughan N and Storm JF (2019) Synergy of Glutamatergic and Cholinergic Modulation Induces Plateau Potentials in Hippocampal OLM Interneurons. Front. Cell. Neurosci 13, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robert V et al. (2020) The mechanisms shaping CA2 pyramidal neuron action potential bursting induced by muscarinic acetylcholine receptor activation. J. Gen. Physiol 152, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell LA et al. (2013) Synaptic muscarinic response types in hippocampal CA1 interneurons depend on different levels of presynaptic activity and different muscarinic receptor subtypes. Neuropharmacology 73, 160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu Z et al. (2017) Hippocampus and Entorhinal Cortex Recruit Cholinergic and NMDA Receptors Separately to Generate Hippocampal Theta Oscillations. Cell Rep 21, 3585–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pabst M et al. (2016) Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron 90, 853–865 [DOI] [PubMed] [Google Scholar]
- 52.Cheng Q and Yakel JL (2014) Presynaptic α7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J. Neurosci. Off. J. Soc. Neurosci 34, 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hainmueller T and Bartos M (2020) Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci 21, 153–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudek SM et al. (2016) Rediscovering area CA2: unique properties and functions. Nat. Rev. Neurosci 17, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis AS et al. (2018) Bidirectional Regulation of Aggression in Mice by Hippocampal Alpha-7 Nicotinic Acetylcholine Receptors. Neuropsychopharmacology 43, 1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillentine MA et al. (2017) CHRNA7 Deletions are Enriched in Risperidone-Treated Children and Adolescents. J. Child Adolesc. Psychopharmacol 27, 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terry AV and Callahan PM (2020) α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 170, 108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hampel H et al. (2019) Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimers Dis 6, 2–15 [DOI] [PubMed] [Google Scholar]
- 59.Ztaou S and Amalric M (2019) Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem. Int 126, 1–10 [DOI] [PubMed] [Google Scholar]
- 60.Papke RL et al. (2020) Cholinergic Receptors and Addiction. Curr. Top. Behav. Neurosci 45, 123–151 [DOI] [PubMed] [Google Scholar]
- 61.Wang Y et al. (2021) Cholinergic Signaling, Neural Excitability, and Epilepsy. Mol. Basel Switz 26, 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eck SR et al. (2020) Stress Regulation of Sustained Attention and the Cholinergic Attention System. Biol. Psychiatry 88, 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang L et al. (2020) Independent and Combined Effects of Nicotine or Chronic Tobacco Smoking and HIV on the Brain: A Review of Preclinical and Clinical Studies. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol 15, 658–693 [DOI] [PubMed] [Google Scholar]
- 64.Ballinger EC et al. (2019) Mecp2 Deletion from Cholinergic Neurons Selectively Impairs Recognition Memory and Disrupts Cholinergic Modulation of the Perirhinal Cortex. eNeuro 6, ENEURO.0134–19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deutsch SI and Burket JA (2020) An Evolving Therapeutic Rationale for Targeting the α7 Nicotinic Acetylcholine Receptor in Autism Spectrum Disorder. Curr. Top. Behav. Neurosci 45, 167–208 [DOI] [PubMed] [Google Scholar]
- 66.Halder N and Lal G (2021) Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol 12, 660342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tushingham S et al. (2018) Biomolecular archaeology reveals ancient origins of indigenous tobacco smoking in North American Plateau. Proc. Natl. Acad. Sci 115, 11742–11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartus RT et al. (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–417 [DOI] [PubMed] [Google Scholar]
- 69.Whitehouse PJ et al. (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239 [DOI] [PubMed] [Google Scholar]
- 70.Pepeu G and Grazia Giovannini M (2017) The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res 1670, 173–184 [DOI] [PubMed] [Google Scholar]
- 71.Geula C et al. (2021) Basal forebrain cholinergic system in the dementias: Vulnerability, resilience, and resistance. J. Neurochem 158, 1394–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coughlin JM et al. (2020) High Availability of the α7-Nicotinic Acetylcholine Receptor in Brains of Individuals with Mild Cognitive Impairment: A Pilot Study Using 18 F-ASEM PET. J. Nucl. Med 61, 423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson GE and Peterson C (1981) Aging Decreases Oxidative Metabolism and the Release and Synthesis of Acetylcholine. J. Neurochem 37, 978–984 [DOI] [PubMed] [Google Scholar]
- 74.Coughlin JM et al. (2018) The distribution of the alpha7 nicotinic acetylcholine receptor in healthy aging: An in vivo positron emission tomography study with [18F]ASEM. NeuroImage 165, 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Counts SE et al. (2007) α7 Nicotinic Receptor Up-regulation in Cholinergic Basal Forebrain Neurons in Alzheimer Disease. Arch. Neurol 64, 1771–1776 [DOI] [PubMed] [Google Scholar]
- 76.Broide RS et al. (2019) Distribution of α7 Nicotinic Acetylcholine Receptor Subunit mRNA in the Developing Mouse. Front. Neuroanat 13, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morley BJ and Mervis RF (2013) Dendritic spine alterations in the hippocampus and parietal cortex of alpha7 nicotinic acetylcholine receptor knockout mice. Neuroscience 233, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derbyshire E and Obeid R (2020) Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 12, E1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaaf CP (2014) Nicotinic acetylcholine receptors in human genetic disease. Genet. Med 16, 649–656 [DOI] [PubMed] [Google Scholar]
- 80.Gillentine MA and Schaaf CP (2015) The human clinical phenotypes of altered CHRNA7 copy number. Biochem. Pharmacol 97, 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freedman R et al. (2003) The genetics of sensory gating deficits in schizophrenia. Curr. Psychiatry Rep 5, 155–161 [DOI] [PubMed] [Google Scholar]
- 82.Leonard S et al. (2002) Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry 59, 1085–1096 [DOI] [PubMed] [Google Scholar]
- 83.Sinkus ML et al. (2015) The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 96, 274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin J et al. (2017) Chrna7 deficient mice manifest no consistent neuropsychiatric and behavioral phenotypes. Sci. Rep 7, 39941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freund RK et al. (2016) Genetic knockout of the α7 nicotinic acetylcholine receptor gene alters hippocampal long-term potentiation in a background strain-dependent manner. Neurosci. Lett 627, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young JW et al. (2004) Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 29, 891–900 [DOI] [PubMed] [Google Scholar]
- 87.Wu M et al. (2021) Unbalanced Regulation of α7 nAChRs by Ly6h and NACHO Contributes to Neurotoxicity in Alzheimer’s Disease. J. Neurosci 41, 8461–8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu S et al. (2016) Brain α7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 89, 948–955 [DOI] [PubMed] [Google Scholar]
- 89.Cecon E et al. (2019) Quantitative assessment of oligomeric amyloid β peptide binding to α7 nicotinic receptor. Br. J. Pharmacol 176, 3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lasala M et al. (2019) Molecular Modulation of Human α7 Nicotinic Receptor by Amyloid-β Peptides. Front. Cell. Neurosci 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoiljkovic M et al. (2016) Hippocampal network dynamics in response to α7 nACh receptors activation in amyloid-β overproducing transgenic mice. Neurobiol. Aging 45, 161–168 [DOI] [PubMed] [Google Scholar]
- 92.Belloy ME et al. (2019) A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 101, 820–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H-Y et al. (2017) Increased Aβ42-α7-like nicotinic acetylcholine receptor complex level in lymphocytes is associated with apolipoprotein E4-driven Alzheimer’s disease pathogenesis. Alzheimers Res. Ther 9, 54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Tropea MR et al. (2021) Genetic deletion of α7 nicotinic acetylcholine receptors induces an age-dependent Alzheimer’s disease-like pathology. Prog. Neurobiol 206, 102154. [DOI] [PubMed] [Google Scholar]
- 95.Lazarevic V et al. (2017) Physiological Concentrations of Amyloid Beta Regulate Recycling of Synaptic Vesicles via Alpha7 Acetylcholine Receptor and CDK5/Calcineurin Signaling. Front. Mol. Neurosci 10, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gulisano W et al. (2019) Neuromodulatory Action of Picomolar Extracellular Aβ42 Oligomers on Presynaptic and Postsynaptic Mechanisms Underlying Synaptic Function and Memory. J. Neurosci. Off. J. Soc. Neurosci 39, 5986–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picciotto MR et al. (2008) It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol 84, 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polli FS and Kohlmeier KA (2020) Prenatal Nicotine Exposure in Rodents: Why Are There So Many Variations in Behavioral Outcomes? Nicotine Tob. Res 22, 1694–1710 [DOI] [PubMed] [Google Scholar]
- 99.Deal JA et al. (2020) Relationship of Cigarette Smoking and Time of Quitting with Incident Dementia and Cognitive Decline. J. Am. Geriatr. Soc 68, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lucatch AM et al. (2018) Neurobiological Determinants of Tobacco Smoking in Schizophrenia. Front. Psychiatry 9, 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majdi A et al. (2021) Effects of transdermal nicotine delivery on cognitive outcomes: A meta-analysis. Acta Neurol. Scand 144, 179–191 [DOI] [PubMed] [Google Scholar]
- 102.Nordberg A et al. (1983) Changes in cholinergic activity in human hippocampus following chronic alcohol abuse. Pharmacol. Biochem. Behav 18, 397–400 [DOI] [PubMed] [Google Scholar]
- 103.Wiegmann C et al. (2020) Alcohol and Dementia – What is the Link? A Systematic Review. Neuropsychiatr. Dis. Treat 16, 87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDaid J et al. (2016) Ethanol-Induced Motor Impairment Mediated by Inhibition of 7 Nicotinic Receptors. J. Neurosci 36, 7768–7778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waeiss RA et al. (2019) Peri-adolescent alcohol consumption increases sensitivity and dopaminergic response to nicotine during adulthood in female alcohol-preferring (P) rats: Alterations to α7 nicotinic acetylcholine receptor expression. Behav. Brain Res 376, 112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallowitsch-Puerta M and Tracey KJ (2005) Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann. N. Y. Acad. Sci 1062, 209–219 [DOI] [PubMed] [Google Scholar]
- 107.Tracey KJ (2009) Reflex control of immunity. Nat. Rev. Immunol 9, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H et al. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 109.Disney AA and Higley MJ (2020) Diverse Spatiotemporal Scales of Cholinergic Signaling in the Neocortex. J. Neurosci 40, 720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaduszkiewicz H et al. (2005) Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 331, 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang L-K et al. (2020) Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci 27, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Papke RL and Horenstein NA (2021) Therapeutic Targeting of α 7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev 73, 1118–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Callahan PM et al. (2017) Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology 117, 422–433 [DOI] [PubMed] [Google Scholar]
- 114.Xia L et al. (2020) One-day tropisetron treatment improves cognitive deficits and P50 inhibition deficits in schizophrenia. Neuropsychopharmacology 45, 1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matta JA et al. (2021) Nicotinic acetylcholine receptor redux: Discovery of accessories opens therapeutic vistas. Science 373, eabg6539. [DOI] [PubMed] [Google Scholar]
- 116.Noviello CM et al. (2021) Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 184, 2121–2134.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.AlQuraishi M (2020) A watershed moment for protein structure prediction. Nature 577, 627–628 [DOI] [PubMed] [Google Scholar]
- 118.Godin J-R et al. (2020) A silent agonist of α7 nicotinic acetylcholine receptors modulates inflammation ex vivo and attenuates EAE. Brain. Behav. Immun 87, 286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bussey TJ et al. (2008) The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn. Mem. Cold Spring Harb. N 15, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spry KP et al. (2021) 3D-Printed Capacitive Sensor Objects for Object Recognition Assays. eNeuro 8, ENEURO.0310–20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hvoslef-Eide M et al. (2015) Facilitation of spatial working memory performance following intra-prefrontal cortical administration of the adrenergic alpha1 agonist phenylephrine. Psychopharmacology (Berl.) 232, 4005–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sullivan JA et al. (2021) New frontiers in translational research: Touchscreens, open science, and the mouse translational research accelerator platform. Genes Brain Behav 20, e12705. [DOI] [PubMed] [Google Scholar]
- 123.Wallace TC and Fulgoni VL (2016) Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr 35, 108–112 [DOI] [PubMed] [Google Scholar]
- 124.Erickson KI et al. (2011) Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci 108, 3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brinke LF ten et al. (2015) Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Sports Med 49, 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stillman CM et al. (2018) Cardiorespiratory fitness is associated with enhanced hippocampal functional connectivity in healthy young adults. Hippocampus 28, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pereira AC et al. (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci 104, 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Voss MW et al. (2019) Exercise and Hippocampal Memory Systems. Trends Cogn. Sci 23, 318–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jing M et al. (2020) An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat. Methods 17, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keyworth H et al. (2018) Wheel running during chronic nicotine exposure is protective against mecamylamine-precipitated withdrawal and up-regulates hippocampal α7 nACh receptors in mice. Br. J. Pharmacol 175, 1928–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Delorme J et al. (2021) Sleep loss drives acetylcholine- and somatostatin interneuron–mediated gating of hippocampal activity to inhibit memory consolidation. Proc. Natl. Acad. Sci 118, e2019318118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng Q et al. (2021) Differential signalling induced by α7 nicotinic acetylcholine receptors in hippocampal dentate gyrus in vitro and in vivo. J. Physiol 599, 4687–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shivange AV et al. (2019) Determining the pharmacokinetics of nicotinic drugs in the endoplasmic reticulum using biosensors. J. Gen. Physiol 151, 738–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma X et al. (2020) The Firing of Theta State-Related Septal Cholinergic Neurons Disrupt Hippocampal Ripple Oscillations via Muscarinic Receptors. J. Neurosci 40, 3591–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patel JM et al. (2019) Sensory perception drives food avoidance through excitatory basal forebrain circuits. eLife 8, e44548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo Y et al. (2018) Nanosensors for the Chemical Imaging of Acetylcholine Using Magnetic Resonance Imaging. ACS Nano 12, 5761–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]


