Abstract
CL 188,624, CL 190,294, and CL 191,121 are novel aminomethyl tetrahydrofuranyl (THF)-1β-methylcarbapenems. The in vitro antibacterial activities of these THF carbapenems were evaluated and compared with those of biapenem, imipenem, and meropenem against 554 recent clinical isolates obtained from geographically distinct medical centers across North America. The antibacterial activities of the THF carbapenems were equivalent to that of biapenem, and the THF carbapenems were slightly more active than imipenem and less active than meropenem against most of the members of the family Enterobacteriaceae but lacked significant activity against Pseudomonas isolates. In general, CL 191,121 was two- to fourfold more active than CL 188,624 and CL 190,294 against the staphylococcal and enterococcal isolates tested. CL 191,121 was twofold less active than imipenem against methicillin-susceptible staphylococci and was as activity as imipenem against Enterococcus faecalis isolates. Biapenem and meropenem were two- and fourfold less active than CL 191,121, respectively, against the methicillin-susceptible staphylococci and E. faecalis. All the carbapenems displayed equivalent good activities against the streptococci. Biapenem was slightly more active than the other carbapenems against Bacteroides fragilis isolates. Time-kill curve studies demonstrated that the THF carbapenems were bactericidal in 6 h against Escherichia coli and Staphylococcus aureus isolates. The postantibiotic effect exerted by CL 191,121 was comparable to or slightly longer than that of imipenem against isolates of S. aureus, E. coli, and Klebsiella pneumoniae.
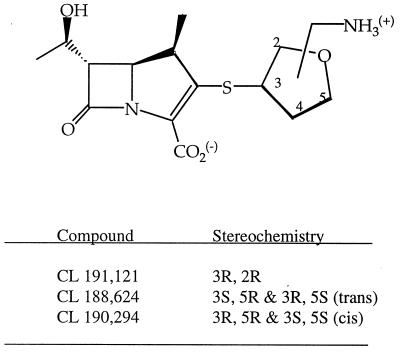
Carbapenem antibiotics have extremely potent activities against a wide range of aerobic and anaerobic gram-positive and gram-negative bacteria (9, 13, 21, 22). These activities are due to stability to hydrolysis by most β-lactamases, a high affinity for essential penicillin-binding proteins, and penetrability into most gram-negative organisms (4, 14, 28). The addition of the 1β-methyl group is responsible for the high degree of chemical stability as well as stability to renal dehydropeptidase, thereby eliminating the requirement, as with imipenem, for coadministration with cilastatin, a dehydropeptidase inhibitor, for in vivo efficacy (6). Meropenem, which was recently approved for use, contains this 1β-methyl group and also exhibits excellent activity, especially against gram-negative bacterial isolates, as do several investigational carbapenems (3, 7). As part of a carbapenem discovery program, novel 1β-methylcarbapenems with structural modification at the 2 position on the carbapenem molecule have been synthesized (12). Among them, a series of [aminomethyl(3-tetrahydrofuranylthio)]-1β-methylcarbapenems (tetrahydrofuranyl [THF] carbapenems) proved to be the most active (11). This study was performed to evaluate the in vitro activities of CL 191,121 (the 3R,2R diastereomer), CL 188,624 (a mixture of the 3R,5S and the 3S,5R diastereomers), and CL 190,294 (a mixture of the 3R,5R and the 3S,5S diastereomers) (Fig. 1) against a broad range of clinical isolates.
FIG. 1.
Structures of the aminomethylTHF 1β-methylcarbapenems.
MATERIALS AND METHODS
Organisms.
The 554 organisms (356 gram-negative and 174 gram-positive aerobes and 24 anaerobes) used for this study represent recent clinical isolates (1987 to 1993) from various medical centers and hospital outbreaks referred to the Antimicrobial Chemotherapy laboratory. The identification of the organisms in the cultures was performed by conventional methods: gram-negative rods were identified with the API 20E (Analytab Products, Plainville, N.Y.) and NF (Remel, Lenexa, Kans.) systems, the staphylococci were identified with StaphTrac (Analytab Products), and anaerobes were identified by methods described in the Wadsworth Anaerobic Bacteriology Manual (24). The susceptibility of the staphylococci to oxacillin was determined by the presence or absence of growth on an agar plate supplemented with 4% NaCl and containing 6 μg of oxacillin per ml and incubated at 35°C for 24 h (23). All isolates were stored frozen in skim milk at −70°C.
Antibiotics.
Standard powders of CL 188,624 (3,5-trans diastereomers), CL 190,294 (3,5-cis diastereomers), and CL 191,121 (3,2, optically pure) were synthesized as described elsewhere (12). Biapenem was obtained from Wyeth-Ayerst Research, Pearl River, N.Y.; imipenem was obtained from Merck Sharp & Dohme, West Point, Pa.; meropenem was obtained from Zeneca, Chesire, United Kingdom; ceftazidime was obtained from Glaxo-Wellcome, Research Triangle Park, N.C.; oxacillin was obtained from Sigma Chemical Co., St. Louis, Mo.; and penicillin was obtained from the United States Pharmacopeia.
Susceptibility tests.
The in vitro determination of the MICs was performed by the microtiter method as recommended by the National Committee for Clinical Laboratory Standards (16). Mueller-Hinton broth was used for assays with members of the family Enterobacteriaceae, staphylococci, and enterococci. The streptococci were tested in Mueller-Hinton broth supplemented with 5% sheep blood. Haemophilus test medium was used for assays with Haemophilus influenzae isolates. Microtiter plates in which each well contained 50 μl of twofold serial dilutions of the antimicrobial agents in the appropriate broth were inoculated with 50 μl of inoculum to yield a final density of 1 × 105 to 5 × 105 CFU/ml. Anaerobic bacteria were tested on Wilkins Chalgren agar supplemented with 5% lysed sheepblood and 0.001% vitamin K. The MICs were determined after 18 to 22 h of incubation at 35°C in ambient air for the aerobic bacteria and after 48 h in an anaerobic chamber (Coy Laboratories, Ann Arbor, Mich.) for the anaerobes. The MIC was defined as the lowest concentration of the antimicrobial agent that completely inhibits growth of the organism as detected by the unaided eye.
Time-kill curve studies.
Bactericidal activity was determined by the time-kill curve method recommended by the National Committee for Clinical Laboratory Standards (17). Flasks containing 50 ml of the appropriate antimicrobial agent were inoculated with 50 ml of each test organism in the logarithmic growth phase (adjusted to a density of approximately 1 × 106 to 5 × 106 CFU/ml) to yield a drug concentration equivalent to four times the MIC. The flasks were incubated at 35°C in a shaking water bath. Aliquots were removed at 0, 2, 4, and 6 h and diluted, and 0.1 ml was plated in duplicate onto Trypticase soy agar plates. Total bacterial counts (CFU per milliliter) were determined after 18 h of incubation at 35°C. Bactericidal activity was defined as a 99.9% (≥3 log10) reduction in the total count of the original inoculum.
PAE.
The postantibiotic effects (PAEs) of CL 191,121 and imipenem against clinical isolates of Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae were determined. Flasks containing 50 ml of the appropriate antimicrobial agent were inoculated with 50 ml of each test organism in the logarithmic growth phase (adjusted to a density of approximately 107 CFU/ml) to yield a drug concentration equivalent to four to eight times the MIC. The flasks were incubated with shaking for 2 h, followed by dilution of the culture to 1:1,000 in fresh, warm Mueller-Hinton broth. The flasks were returned to the shaker, aliquots were removed at selected time points and diluted, and 0.1 ml was plated in duplicate onto Trypticase soy agar plates. The PAE was defined as T − C, where T is the time required for the count in CFU in the test culture to increase 1 log10 above the count immediately after drug removal, and C is the corresponding time for the control culture (2).
RESULTS
The in vitro activities of CL 188,624, CL 190,294, CL 191,121, and the comparative carbapenems against a wide diversity of recent clinical gram-negative and gram-positive isolates are summarized in Tables 1 and 2, respectively.
TABLE 1.
In vitro activities of THF carbapenems and comparative agents against gram-negative bacteria
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | ||||||||
| Escherichia coli (30) | CL 188,624 | 0.06–0.12 | 0.06 | 0.12 | ||||||
| CL 190,294 | 0.03–0.06 | 0.06 | 0.06 | |||||||
| CL 191,121 | ≤0.015–0.06 | 0.03 | 0.03 | |||||||
| Biapenem | 0.03–0.06 | 0.06 | 0.06 | |||||||
| Imipenem | 0.12–0.25 | 0.12 | 0.25 | |||||||
| Meropenem | ≤0.015–0.03 | 0.03 | 0.03 | |||||||
| Escherichia coli, ceft-azidime resistant (15) | CL 188,624 | 0.03–0.25 | 0.06 | 0.25 | ||||||
| CL 190,294 | 0.03–0.5 | 0.12 | 0.25 | |||||||
| CL 191,121 | 0.03–0.5 | 0.03 | 0.25 | |||||||
| Biapenem | 0.03–0.25 | 0.03 | 0.12 | |||||||
| Imipenem | 0.12–1.0 | 0.12 | 0.25 | |||||||
| Meropenem | ≤0.015–0.12 | 0.03 | 0.12 | |||||||
| Klebsiella pneumoniae (15) | CL 188,624 | 0.06–0.5 | 0.06 | 0.25 | ||||||
| CL 190,294 | 0.06–0.5 | 0.12 | 0.12 | |||||||
| CL 191,121 | 0.03–1.0 | 0.12 | 0.25 | |||||||
| Biapenem | 0.03–1.0 | 0.12 | 0.5 | |||||||
| Imipenem | 0.06–1.0 | 0.25 | 0.25 | |||||||
| Meropenem | 0.03–0.12 | 0.03 | 0.12 | |||||||
| Klebsiella pneumoniae, ceft-azidime resistant (15) | CL 188,624 | 0.06–0.5 | 0.12 | 0.25 | ||||||
| CL 190,294 | 0.12–0.5 | 0.25 | 0.25 | |||||||
| CL 191,121 | 0.03–0.25 | 0.12 | 0.25 | |||||||
| Biapenem | 0.03–0.25 | 0.12 | 0.25 | |||||||
| Imipenem | 0.12–0.5 | 0.25 | 0.5 | |||||||
| Meropenem | 0.03–0.12 | 0.06 | 0.06 | |||||||
| Klebsiella oxytoca (15) | CL 188,624 | 0.03–0.25 | 0.12 | 0.25 | ||||||
| CL 190,294 | 0.03–0.25 | 0.12 | 0.25 | |||||||
| CL 191,121 | 0.03–0.25 | 0.12 | 0.25 | |||||||
| Biapenem | 0.03–0.5 | 0.12 | 0.25 | |||||||
| Imipenem | 0.12–0.5 | 0.25 | 0.5 | |||||||
| Meropenem | 0.03–0.12 | 0.06 | 0.06 | |||||||
| Serratia marcescens (15) | CL 188,624 | 0.12–0.5 | 0.25 | 0.5 | ||||||
| CL 190,294 | 0.12–0.5 | 0.25 | 0.5 | |||||||
| CL 191,121 | 0.25–4.0 | 1.0 | 2.0 | |||||||
| Biapenem | 0.25–4.0 | 1.0 | 2.0 | |||||||
| Imipenem | 0.25–4.0 | 1.0 | 4.0 | |||||||
| Meropenem | 0.03–1.0 | 0.12 | 0.12 | |||||||
| Enterobacter cloacae (11) | CL 188,624 | 0.03–0.25 | 0.12 | 0.25 | ||||||
| CL 190,294 | 0.06–0.25 | 0.12 | 0.25 | |||||||
| CL 191,121 | 0.06–0.5 | 0.12 | 0.25 | |||||||
| Biapenem | 0.06–0.25 | 0.12 | 0.25 | |||||||
| Imipenem | 0.5–2.0 | 0.5 | 1.0 | |||||||
| Meropenem | 0.06–0.12 | 0.06 | 0.12 | |||||||
| Enterobacter aerogenes (13) | CL 188,624 | 0.06–0.5 | 0.25 | 0.5 | ||||||
| CL 190,294 | 0.06–0.5 | 0.25 | 0.5 | |||||||
| CL 191,121 | 0.06–1.0 | 0.5 | 1.0 | |||||||
| Biapenem | 0.06–1.0 | 0.25 | 0.5 | |||||||
| Imipenem | 0.5–4.0 | 1.0 | 2.0 | |||||||
| Meropenem | 0.03–0.12 | 0.06 | 0.12 | |||||||
| Citrobacter freundii (15) | CL 188,624 | 0.03–0.5 | 0.12 | 0.25 | ||||||
| CL 190,294 | 0.06–0.5 | 0.06 | 0.25 | |||||||
| CL 191,121 | 0.03–1.0 | 0.12 | 0.5 | |||||||
| Biapenem | 0.03–0.25 | 0.12 | 0.25 | |||||||
| Imipenem | 0.12–2.0 | 1.0 | 2.0 | |||||||
| Meropenem | 0.03–0.25 | 0.03 | 0.12 | |||||||
| Citrobacter diversus (15) | CL 188,624 | 0.03–0.12 | 0.03 | 0.12 | ||||||
| CL 190,294 | 0.03–0.12 | 0.03 | 0.06 | |||||||
| CL 191,121 | ≤0.015–0.06 | ≤0.015 | 0.03 | |||||||
| Biapenem | 0.03–0.12 | 0.03 | 0.06 | |||||||
| Imipenem | 0.06–0.12 | 0.12 | 0.12 | |||||||
| Meropenem | ≤0.015–0.03 | ≤0.015 | 0.03 | |||||||
| Salmonella species (15) | CL 188,624 | 0.03–0.25 | 0.06 | 0.12 | ||||||
| CL 190,294 | 0.03–0.25 | 0.06 | 0.12 | |||||||
| CL 191,121 | ≤0.015–0.06 | 0.03 | 0.06 | |||||||
| Biapenem | ≤0.06–0.12 | 0.03 | 0.06 | |||||||
| Imipenem | 0.06–0.5 | 0.12 | 0.25 | |||||||
| Meropenem | ≤0.015–0.06 | 0.03 | 0.06 | |||||||
| Shigella species (15) | CL 188,624 | 0.12–0.5 | 0.25 | 0.25 | ||||||
| CL 190,294 | 0.12–0.5 | 0.25 | 0.25 | |||||||
| CL 191,121 | 0.25–0.5 | 0.25 | 0.5 | |||||||
| Biapenem | 0.12–0.5 | 0.25 | 0.25 | |||||||
| Imipenem | 0.25–0.5 | 0.25 | 0.5 | |||||||
| Meropenem | ≤0.015–0.25 | 0.03 | 0.03 | |||||||
| Morganella morganii (15) | CL 188,624 | 0.5–2.0 | 1.0 | 2.0 | ||||||
| CL 190,294 | 0.5–1.0 | 1.0 | 1.0 | |||||||
| CL 191,121 | 0.25–2.0 | 1.0 | 2.0 | |||||||
| Biapenem | 0.25–2.0 | 1.0 | 2.0 | |||||||
| Imipenem | 2.0–4.0 | 4.0 | 4.0 | |||||||
| Meropenem | 0.06–0.5 | 0.25 | 0.5 | |||||||
| Proteus mirabilis (15) | CL 188,624 | 0.25–2.0 | 1.0 | 2.0 | ||||||
| CL 190,294 | 0.25–2.0 | 1.0 | 2.0 | |||||||
| CL 191,121 | 0.06–2.0 | 1.0 | 2.0 | |||||||
| Biapenem | 0.06–4.0 | 2.0 | 4.0 | |||||||
| Imipenem | 0.12–4.0 | 2.0 | 4.0 | |||||||
| Meropenem | 0.03–0.12 | 0.06 | 0.12 | |||||||
| Providencia species (15) | CL 188,624 | 0.25–8.0 | 0.5 | 4.0 | ||||||
| CL 190,294 | 0.12–4.0 | 0.5 | 2.0 | |||||||
| CL 191,121 | 0.25–2.0 | 1.0 | 2.0 | |||||||
| Biapenem | 0.25–2.0 | 1.0 | 2.0 | |||||||
| Imipenem | 0.5–4.0 | 1.0 | 2.0 | |||||||
| Meropenem | 0.06–1.0 | 0.06 | 0.12 | |||||||
| Moraxella catarrhalis (15) | CL 188,624 | ≤0.015–0.06 | 0.03 | 0.03 | ||||||
| CL 190,294 | ≤0.015–0.06 | 0.03 | 0.06 | |||||||
| CL 191,121 | ≤0.015 | ≤0.015 | ≤0.015 | |||||||
| Biapenem | ≤0.015–0.06 | ≤0.015 | 0.06 | |||||||
| Imipenem | ≤0.015–0.06 | ≤0.015 | 0.06 | |||||||
| Meropenem | ≤0.015 | ≤0.015 | ≤0.015 | |||||||
| Burkholderia cepacia (12) | CL 188,624 | 2.0–8.0 | 8.0 | 8.0 | ||||||
| CL 190,294 | 4.0–16.0 | 8.0 | 16.0 | |||||||
| CL 191,121 | 4.0–16.0 | 8.0 | 16.0 | |||||||
| Biapenem | 0.25–8.0 | 2.0 | 8.0 | |||||||
| Imipenem | 1.0–16.0 | 8.0 | 16.0 | |||||||
| Meropenem | 0.25–8.0 | 2.0 | 8.0 | |||||||
| Stenotrophomonas malto-philia (20) | CL 188,624 | 256.0–>256.0 | >256.0 | >256.0 | ||||||
| CL 190,294 | 256.0–>256.0 | >256.0 | >256.0 | |||||||
| CL 191,121 | 256.0–>256.0 | >256.0 | >256.0 | |||||||
| Biapenem | 256.0–>256.0 | >256.0 | >256.0 | |||||||
| Imipenem | 256.0–>256.0 | 256.0 | >256.0 | |||||||
| Meropenem | 64.0–>256.0 | 256.0 | 256.0 | |||||||
| Haemophilus influ-enzae (30) | CL 188,624 | 0.12–2.0 | 0.5 | 1.0 | ||||||
| CL 190,294 | 0.12–2.0 | 0.5 | 1.0 | |||||||
| CL 191,121 | ≤0.015–4.0 | 0.25 | 0.5 | |||||||
| Biapenem | ≤0.015–4.0 | 0.5 | 1.0 | |||||||
| Imipenem | 0.06–4.0 | 0.5 | 1.0 | |||||||
| Meropenem | ≤0.015–0.12 | 0.06 | 0.06 | |||||||
| Bacteroides fragilis (24) | CL 188,624 | 0.5–32.0 | 0.5 | 1.0 | ||||||
| CL 190,294 | 0.25–16.0 | 0.5 | 1.0 | |||||||
| CL 191,121 | 0.06–8.0 | 0.12 | 0.5 | |||||||
| Biapenem | 0.06–0.5 | 0.25 | 0.25 | |||||||
| Imipenem | 0.06–2.0 | 0.25 | 0.5 | |||||||
| Meropenem | 0.06–4.0 | 0.25 | 1.0 | |||||||
| Acinetobacter calco-aceticus (15) | CL 188,624 | 0.12–1.0 | 0.25 | 1.0 | ||||||
| CL 190,294 | 0.12–2.0 | 0.25 | 1.0 | |||||||
| CL 191,121 | 0.06–1.0 | 0.25 | 0.5 | |||||||
| Biapenem | 0.03–0.25 | 0.12 | 0.25 | |||||||
| Imipenem | 0.12–0.5 | 0.25 | 0.5 | |||||||
| Meropenem | 0.12–1.0 | 0.5 | 1.0 | |||||||
| Pseudomonas aeru-ginosa (30) | CL 188,624 | 2.0–>16.0 | 8.0 | 16.0 | ||||||
| CL 190,294 | 2.0–>16.0 | 8.0 | 16.0 | |||||||
| CL 191,121 | 1.0–16.0 | 4.0 | 8.0 | |||||||
| Biapenem | 0.12–16.0 | 0.25 | 4.0 | |||||||
| Imipenem | 0.5–16.0 | 1.0 | 4.0 | |||||||
| Meropenem | 0.06–>16.0 | 0.5 | 8.0 | |||||||
TABLE 2.
In vitro activities of THF carbapenems and comparative agents against gram-positive bacteria
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Streptococcus pneumoniae, penicillin susceptible (15) | CL 188,624 | ≤0.015–0.06 | ≤0.015 | ≤0.015 |
| CL 190,294 | ≤0.015–0.06 | ≤0.015 | ≤0.015 | |
| CL 191,121 | ≤0.015 | ≤0.015 | ≤0.015 | |
| Biapenem | ≤0.015–0.06 | ≤0.015 | ≤0.015 | |
| Imipenem | ≤0.015–0.03 | ≤0.015 | ≤0.015 | |
| Meropenem | ≤0.015–0.06 | ≤0.015 | ≤0.015 | |
| Streptococcus pneumoniae, penicillin intermediate (12) | CL 188,624 | 0.03–0.25 | 0.12 | 0.12 |
| CL 190,294 | 0.03–0.25 | 0.12 | 0.12 | |
| CL 191,121 | 0.03–0.25 | 0.12 | 0.12 | |
| Biapenem | 0.03–0.25 | 0.06 | 0.12 | |
| Imipenem | ≤0.015–0.12 | 0.03 | 0.12 | |
| Meropenem | 0.06–0.25 | 0.12 | 0.25 | |
| Streptococcus pneumoniae, penicillin resistant (15) | CL 188,624 | 0.06–2.0 | 1.0 | 2.0 |
| CL 190,294 | 0.12–2.0 | 1.0 | 2.0 | |
| CL 191,121 | 0.12–1.0 | 0.5 | 1.0 | |
| Biapenem | 0.12–1.0 | 0.5 | 1.0 | |
| Imipenem | 0.12–1.0 | 0.25 | 1.0 | |
| Meropenem | 0.5–2.0 | 0.5 | 2.0 | |
| Streptococcus pyogenes (15) | CL 188,624 | ≤0.06–0.12 | ≤0.06 | ≤0.06 |
| CL 190,294 | ≤0.06–0.12 | ≤0.06 | ≤0.06 | |
| CL 191,121 | ≤0.015 | ≤0.015 | ≤0.015 | |
| Biapenem | ≤0.015 | ≤0.015 | ≤0.015 | |
| Imipenem | ≤0.015 | ≤0.015 | ≤0.015 | |
| Meropenem | ≤0.015 | ≤0.015 | ≤0.015 | |
| Staphylococcus aureus, methicillin susceptible (15) | CL 188,624 | 0.25–0.5 | 0.25 | 0.25 |
| CL 190,294 | 0.06–0.25 | 0.12 | 0.12 | |
| CL 191,121 | 0.03–0.06 | 0.06 | 0.06 | |
| Biapenem | 0.03–0.12 | 0.06 | 0.12 | |
| Imipenem | ≤0.015–0.03 | ≤0.015 | 0.03 | |
| Meropenem | 0.12–0.25 | 0.12 | 0.25 | |
| Coagulase-negative staphylococcus, methicillin susceptible (15) | CL 188,624 | 0.12–1.0 | 0.25 | 0.5 |
| CL 190,294 | 0.03–0.5 | 0.12 | 0.25 | |
| CL 191,121 | ≤0.015–0.12 | 0.03 | 0.12 | |
| Biapenem | 0.03–0.25 | 0.06 | 0.12 | |
| Imipenem | ≤0.015–0.06 | ≤0.015 | 0.06 | |
| Meropenem | 0.06–0.5 | 0.12 | 0.5 | |
| Staphylococcus aureus, methicillin resistant (29) | CL 188,624 | 1.0–>16.0 | 16.0 | >16.0 |
| CL 190,294 | 0.5–>16.0 | 8.0 | >16.0 | |
| CL 191,121 | 0.06–>16.0 | >16.0 | >16.0 | |
| Biapenem | 0.12–>16.0 | >16.0 | >16.0 | |
| Imipenem | ≤0.015–>16.0 | >16.0 | >16.0 | |
| Meropenem | 0.25–>16.0 | >16.0 | >16.0 | |
| Coagulase-negative staphylococcus, methicillin resistant (15) | CL 188,624 | 2.0–>16.0 | >16.0 | >16.0 |
| CL 190,294 | 2.0–>16.0 | >16.0 | >16.0 | |
| CL 191,121 | 0.5–>16.0 | 16.0 | >16.0 | |
| Biapenem | 2.0–>16.0 | >16.0 | >16.0 | |
| Imipenem | 0.25–>16.0 | >16.0 | >16.0 | |
| Meropenem | 4.0–>16.0 | >16.0 | >16.0 | |
| Streptococcus agalactiae (15) | CL 188,624 | ≤0.015–0.06 | 0.03 | 0.03 |
| CL 190,294 | 0.03–0.06 | 0.06 | 0.06 | |
| CL 191,121 | ≤0.015–0.03 | ≤0.015 | ≤0.015 | |
| Biapenem | ≤0.015–0.03 | ≤0.015 | 0.03 | |
| Imipenem | ≤0.015 | ≤0.015 | ≤0.015 | |
| Meropenem | 0.03–0.12 | 0.06 | 0.06 | |
| Enterococcus faecalis (15) | CL 188,624 | 1.0–8.0 | 2.0 | 8.0 |
| CL 190,294 | 1.0–8.0 | 1.0 | 8.0 | |
| CL 191,121 | 0.25–2.0 | 0.5 | 1.0 | |
| Biapenem | 0.5–8.0 | 2.0 | 2.0 | |
| Imipenem | 0.25–2.0 | 1.0 | 1.0 | |
| Meropenem | 1.0–16.0 | 4.0 | 4.0 | |
| Enterococcus faecium (13) | CL 188,624 | 0.5–>128.0 | 8.0 | 128.0 |
| CL 190,294 | 0.5–>128.0 | 8.0 | 128.0 | |
| CL 191,121 | 0.25–128.0 | 8.0 | 64.0 | |
| Biapenem | 0.5–>128.0 | 32.0 | >128.0 | |
| Imipenem | 0.25–>128.0 | 8.0 | 64.0 | |
| Meropenem | 1.0–>128.0 | 32.0 | 128.0 | |
The THF carbapenems demonstrated comparable activity against most of the gram-negative isolates tested. CL 191,121 (2-aminomethyl substitution) was slightly more active (twofold) than CL 188,624 and CL 190,294 (5-aminomethyl substitution) against E. coli, Citrobacter diversus, Salmonella spp., Moraxella catarrhalis, Haemophilus influenzae, Acinetobacter calcoaceticus, and Bacteroides fragilis and was two- to fourfold more active against methicillin-susceptible Staphylococcus aureus and coagulase-negative staphylococci, Enterococcus faecalis, Enterococcus faecium, Streptococcus agalactiae, and penicillin-resistant Streptococcus pneumoniae.
CL 191,121 was twofold more active than biapenem and two- to fourfold more active than imipenem against E. coli, K. pneumoniae, Klebsiella oxytoca, C. diversus, Proteus mirabilis, and H. influenzae isolates but was two- to fourfold less active than meropenem against K. pneumoniae, K. oxytoca, P. mirabilis, and H. influenzae. Against Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii, Morganella morganii, and Salmonella isolates, CL 191,121 was twofold more active than imipenem, as active or twofold less active than biapenem, and four to eight times less active than meropenem. CL 191,121, biapenem, and imipenem demonstrated equivalent activities against clinical Serratia, Shigella, and Providencia isolates (MICs at which 90% of isolates are inhibited [MIC90s], 2, 0.5, and 2 μg/ml, respectively). Meropenem was 2- to 16-fold more active than the other carbapenems against these isolates. Biapenem was two times more active than CL 191,121 and imipenem and four times more active than CL 188,624, CL 190,294, and meropenem against Acinetobacter calcoaceticus strains. None of the carbapenems demonstrated activity against the Stenotrophomonas maltophilia isolates tested. For ceftazidime-resistant K. pneumoniae isolates susceptibilities to all the carbapenems were comparable to those for the ceftazidime-susceptible isolates (twofold or lower increase in the MIC). Biapenem, imipenem, and meropenem were much more active (MIC50s, 0.25, 1.0, and 0.5 μg/ml, respectively) than the THF carbapenems (MIC50s, 4 to 8 μg/ml) against Pseudomonas aeruginosa isolates. The carbapenem MICs were elevated (MICs, ≥8 μg/ml) for 10% of the Pseudomonas strains. CL 191,121, biapenem, and imipenem demonstrated equivalent activities (MIC90s, 0.5 μg/ml) and were twofold more active than CL 188,624, CL 190,294, and meropenem against 24 clinical isolates of Bacteroides fragilis. Imipenem was twofold more active than CL 191,121, fourfold more active than biapenem, and four- to eightfold more active than meropenem against methicillin-susceptible staphylococci (S. aureus as well as coagulase-negative staphylococci) (Table 2). None of the carbapenems exhibited activity against methicillin-resistant staphylococci (MIC90s, >16 μg/ml). The susceptibilities of the S. pneumoniae strains varied with their susceptibilities to penicillin. The penicillin-resistant strains were less sensitive to the carbapenems than the penicillin-susceptible strains. The THF carbapenems, biapenem, and imipenem all had similar activities against penicillin-susceptible (MIC90s, ≤0.015 μg/ml) and penicillin-intermediate (MIC90s, 0.12 μg/ml) strains. CL 191,121, imipenem, and biapenem were twofold more active (MIC90s, 1 μg/ml) than meropenem against penicillin-resistant strains of S. pneumoniae. All the carbapenems had excellent activities (MIC90s, ≤0.06 μg/ml) against Streptococcus pyogenes. CL 191,121 and imipenem were twofold more active than biapenem and fourfold more active than meropenem against S. agalactiae. CL 191,121 and imipenem had moderate activities (MIC90s, 1 μg/ml) against E. faecalis isolates. Biapenem and meropenem were two- and fourfold less active, respectively, than CL 191,121 and imipenem. All the carbapenems displayed poor activities (MIC90s, 64 to >128 μg/ml) against the E. faecium isolates tested.
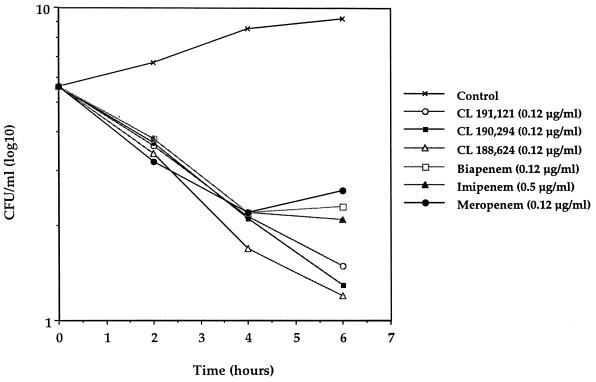
Each of the carbapenems demonstrated fairly rapid cidal activities against E. coli 311 and S. aureus Smith by time-kill curve studies (Fig. 2 and 3, respectively). At 6 h the THF carbapenems demonstrated bactericidal activity (>1 log10) greater than those of biapenem, imipenem, and meropenem against E. coli 311. The use of CL 191,121 and CL 188,624 resulted in slightly greater reductions in viable cell counts (<1 log10) than the use of CL 190,294, biapenem, meropenem, and imipenem against the S. aureus strain.
FIG. 2.
Antibacterial activities of carbapenems against E. coli 311.
FIG. 3.
Antibacterial activities of carbapenems against S. aureus Smith.
Both CL 191,121 and imipenem exerted similar PAEs (0.7 and 0.9 h, respectively) against S. aureus PT4308, while the PAE of CL 191,121 was approximately 40% longer than that of imipenem (1.3 and 0.9 h, respectively) against the other S. aureus clinical isolate tested (Table 3). The PAEs of CL 191,121 and imipenem against the gram-negative isolates E. coli 311 and K. pneumoniae PT4696 were comparable, with the duration of the effect being between 1.3 and 1.6 h.
TABLE 3.
PAEs of CL 191,121 and imipenem
| Organism | Strain | PAE (h)
|
|
|---|---|---|---|
| CL 191,121 | Imipenem | ||
| Staphylococcus aureus | PT4308 | 0.7 | 0.9 |
| Staphylococcus aureus | PT5635 | 1.3 | 0.9 |
| Escherichia coli | 311 | 1.6 | 1.4 |
| Klebsiella pneumoniae | PT4696 | 1.3 | 1.3 |
DISCUSSION
The carbapenems represent highly potent antimicrobial agents. Imipenem, biapenem, and meropenem have been extensively investigated and demonstrate excellent in vitro activities (7, 15, 20). In addition, several investigational carbapenems with dithiocarbamate, bicyclic imidazole, carboxyphenyl, and pyrrolidine side chains have been reported to have potent activities against gram-negative and gram-positive organisms (5, 8, 15, 19, 25, 26). CL 188,624, CL 190,294, and CL 191,121 are 1β-methylcarbapenems with a novel aminomethyl-substituted tetrahydrofuranylthio moiety at the 2 position of the carbapenem molecule. They have good affinities of binding to penicillin-binding proteins in gram-positive and gram-negative bacteria, as noted by Bush et al. (1), and demonstrate excellent in vitro activities against a wide range of gram-negative and gram-positive isolates with the exception of Pseudomonas and Stenotrophomonas isolates, methicillin-resistant staphylococci, and E. faecium. The THF carbapenems, like other carbapenems, are stable to hydrolysis by the majority of common β-lactamases (10, 27). It was noted that the THF carbapenems had comparable activities against ceftazidime-susceptible and ceftazidime-resistant, extended-spectrum β-lactamase-producing E. coli and K. pneumoniae isolates. In general, among the THF carbapenems, the 3,2-substituted THF carbapenem CL 191,121 was slightly more active than the 3,5-substituted compounds CL 188,624 and CL 190,294 against gram-positive isolates and Moraxella isolates. There were no significant differences in activity between the cis- and trans-3,5-substituted isomers. In comparison with other carbapenem antibiotics, the THF carbapenems were more active than imipenem, as active as biapenem, and less active than meropenem against gram-negative isolates. One of these compounds, CL 191,121, was more active than biapenem and meropenem and was as active as imipenem against gram-positive isolates and was slightly more active than imipenem against most gram-negative isolates.
The excellent in vitro activities of the THF carbapenems, in particular, CL 191,121, against a wide range of gram-negative and gram-positive isolates, combined with their rapid cidal activities, moderate PAEs, and stability to β-lactamases and dehydropeptidases, make them excellent candidates for further investigations.
ACKNOWLEDGMENTS
We thank Lynetta Lieberman, Mary Whatley, and Teri Popkave for superb technical assistance.
REFERENCES
- 1.Bush K, Bhachech N, Yang Y, Weiss W, Lin Y, Testa R, Tally F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Binding to penicillin-binding proteins (PBPs) and permeability of novel THF carbapenems, abstr. F76; p. 128. [Google Scholar]
- 2.Craig W, Gudmundsson S. The postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 515–536. [Google Scholar]
- 3.Fukuoka T, Ohya S, Utsui Y, Domon H, Takenouchi T, Koga T, Masuda N, Kawada H, Kakuta M, Kubota M, Ishii C, Ishii C, Sakagawa E, Harasaki T, Hirasawa A, Abe T, Yasuda H, Iwata M, Kuwahara S. In vitro and in vivo antibacterial activities of CS-834, a novel oral carbapenem. Antimicrob Agents Chemother. 1997;41:2652–2663. doi: 10.1128/aac.41.12.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham D, Ashton W, Barash L, Canning L, Chen A, Springer J, Rogers E. Inhibition of the mammalian β-lactamase renal dipeptidase by (Z)-2-(acyclamido)-3-substituted-propenoic acids. J Med Chem. 1987;30:1074–1090. doi: 10.1021/jm00389a018. [DOI] [PubMed] [Google Scholar]
- 5.Hashizume T, Shibata K, Nagano R, Adachi Y, Nakamura K, Fuse A, Kato Y, Hazumi N, Asano K, Naito T, Ishihara A, Sawaasaki Y, Nishino M, Uchida M, Nagami K, Samura K. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro and in vivo evaluation of BO-3482, a novel dithiocarbamate carbapenem, abstr. F118; p. 120. [Google Scholar]
- 6.Hikida M, Kawashima K, Yoshida M, Mitsuhashi S. Inactivation of new carbapenem antibiotics by dehydropeptidase-I from porcine and human renal cortex. J Antimicrob Chemother. 1992;30:129–134. doi: 10.1093/jac/30.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Hoban D, Jones R, Yamane N, Frei R, Trilla A, Pignatari A. In vitro activity of three carbapenem antibiotics. Diagn Microbiol Infect Dis. 1993;17:299–305. doi: 10.1016/0732-8893(93)90039-a. [DOI] [PubMed] [Google Scholar]
- 8.Hohmura M, Tanaka M, Ishida H, Akasaka T, Mori S, Sato K, Hayano T, Hayakawa I. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. DZ-2640, a new oral carbapenem antibiotic, abstr. F134; p. 136. [Google Scholar]
- 9.Jones R, Barry A, Thornsberry C. In-vitro studies of meropenem. J Antimicrob Chemother. 1989;24(Suppl. A):9–29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 10.Labia R, Morand A, Tiwari K, Sirot D, Chanal C. Interactions of meropenem, with β-lactamases, including enzymes with extended-spectrum activity against third-generation cephalosporins. J Antimicrob Chemother. 1989;24(Suppl. A):219–223. doi: 10.1093/jac/24.suppl_a.219. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Bitha P, Sakya S, Strohmeyer T, Yang Y, Weiss W, Jacobus N, Bush K, Testa R, Tally F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Novel THF carbapenems II: Structure activity relationships, abstr. F72; p. 128. [Google Scholar]
- 12.Lin Y, Bitha P, Sakya S, Strohmeyer T, Li Z, Lee V, Lang S, Yang Y, Bhachech N, Weiss W, Petersen P, Jacobus N, Bush K, Testa R, Tally F. Synthesis and structure activity relationships of novel THF 1β-methylcarbapenems. Bioorg Med Chem Lett. 1997;7:1671–1676. [Google Scholar]
- 13.Moellering R, Eliopoulos G, Sentochnik D. The carbapenems: new broad spectrum β-lactam antibiotics. J Antimicrob Chemother. 1989;24(Suppl. A):1–7. doi: 10.1093/jac/24.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 14.Murray B. Problems and dilemmas of antimicrobial resistance. Pharmacotherapy. 1992;12(6 Pt 2):86S–93S. [PubMed] [Google Scholar]
- 15.Nakagawa S, Hashizume T, Matsuda K, Sanada M, Okamoto O, Fukatsu H, Tanaka N. In vitro activity of a new carbapenem antibiotic, BO-2727, with potent antipseudomonal activity. Antimicrob Agents Chemother. 1993;37:2756–2759. doi: 10.1128/aac.37.12.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 17. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Proposed guideline M26-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 18.Neu H, Gu J, Fang W, Chin N. In vitro activity and β-lactamase stability of LJC 10,627. Antimicrob Agents Chemother. 1992;36:1418–1423. doi: 10.1128/aac.36.7.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelak B, Gerckens L, Scott P, Gill C, Pacholok C, Lynch L, Dorso H, Kohler J, Shungu D, Rosen H, Kropp H. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Antibacterial profile of L-749,345 (ZD-4433), a new potent 1-β-methyl carbapenem, abstr. F119; p. 120. [Google Scholar]
- 20.Petersen P, Jacobus N, Weiss W, Testa R. In vitro and in vivo activities of LJC 10,627, a new carbapenem with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1991;35:203–207. doi: 10.1128/aac.35.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitkin D, Sheikh W, Nadler H. Comparative in vitro activity of meropenem versus other extended-spectrum antimicrobials against randomly chosen and selected resistant clinical isolates tested in 26 North American Centers. Clin Infect Dis. 1997;24(Suppl 2):S238–S248. doi: 10.1093/clinids/24.supplement_2.s238. [DOI] [PubMed] [Google Scholar]
- 22.Sader H, Jones R. Antimicrobial activity of the new carbapenem biapenem compared to imipenem, meropenem and other broad spectrum beta-lactam drugs. Eur J Clin Microbiol Infect Dis. 1993;12:384–391. doi: 10.1007/BF01964439. [DOI] [PubMed] [Google Scholar]
- 23.Stratton C, Cooksey R. Susceptibility tests: special tests. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 1161–1162. [Google Scholar]
- 24.Sutter V, Citron D, Edelstein M, Finegold S. Wadsworth anaerobic bacteriology manual. Belmont, Calif: Star Publishing Co.; 1985. pp. 30–67. [Google Scholar]
- 25.Tanaka M, Hohmura M, Nishi T, Sato K, Hayakawa I. Antimicrobial activity of DU-6681a, a parent compound of novel oral carbapenem DZ-2640. Antimicrob Agents Chemother. 1997;41:1260–1268. doi: 10.1128/aac.41.6.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji M, Ishi Y, Ohno A, Miyazaki S, Yamaguchi K. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob Agents Chemother. 1998;42:94–99. doi: 10.1128/aac.42.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss W, Yang Y, Petersen P, Shelofsky A, Bush K, Jacobus N, Bitha P, Lin Y, Testa R, Tally F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. In vitro activity and β-lactamase stability of novel THF carbapenems, abstr. F74; p. 128. [Google Scholar]
- 28.Zhou X, Kitzis M, Gutmann L. Role of cephalosporinase in carbapenem resistance of clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1387–1389. doi: 10.1128/aac.37.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]