Abstract
Transient receptor potential vanilloid 1 (TRPV1) is known as a receptor of capsaicin, a spicy ingredient of chili peppers. It is also sensitive to a variety of pungent compounds and is involved in nociception. Here, we focused on the structural characteristics of capsaicin, and investigated whether vanillylmanderic acid (VMA), vanillic acid (VAcid), vanillyl alcohol (VAlc), vanillyl butyl ether (VBE), and vanillin, containing a vanillyl skeleton similar to capsaicin, affected the TRPV1 activities. For detection of TRPV1 activity, intracellular Ca2+ concentration ([Ca2+]i) was measured in HEK 293 cells heterologously expressing mouse TRPV1 (mTRPV1-HEK) and in mouse sensory neurons. Except for vanillin, four vanilloid analogues dose-dependently increased [Ca2+]i in mTRPV1-HEK. The solutions that dissolved VMA, VAcid and vanillin at high concentrations were acidic, whereas those of VAlc and VBE were neutral. Neutralized VAcid evoked [Ca2+]i increases but neutralized VMA did not. Mutation of capsaicin-sensing sites diminished [Ca2+]i responses to VAcid, VAlc and VBE. VAcid, VMA, and vanillin suppressed the activation of TRPV1 induced by capsaicin. VAcid and VMA also inhibited the acid-induced TRPV1 activation. In sensory neurons, VMA diminished TRPV1 activation by capsaicin or acids. The present data indicate that these structural characteristics of chemical compounds on TRPV1 may provide strategies for the development of novel analgesic drugs targeting nociceptive TRPV1.
Keywords: Catecholamine metabolites, Heterologous expression, Intracellular Ca2+ concentration, Mutagenesis, Sensory neurons, Vanillyl structure
Abbreviations: DMSO, Dimethyl sulfoxide; DRG, Dorsal root ganglion; HEK, Human embryonic kidney; TRPV, Transient receptor potential vanilloid 1; [Ca2+], Intracellular Ca2+ concentration; mTRPV1, Mouse TRPV1; VAcid, Vanillic acid; Valc, Vanillyl alcohol; VBE, Vanillyl butyl ether; VMA, Vanillylmandelic acid
1. Introduction
Transient receptor potential vanilloid 1 (TRPV1) is mainly expressed in primary afferent neurons and functions in pain sensation [28]. It is activated by a variety of pungent chemicals such as capsaicin (8-methyl-N-vanillyl-trans-6-noneamide) a spicy component of chili paper [2]. To relieve pain, many TRPV1 antagonists have been developed. However, these antagonists have not been applied for clinical use because they show adverse effects such as hyperthermia [26].
High doses of capsaicin alleviate noxious pain when applied to the skin [13,17]. Capsaicin activates TRPV1 resulting in the increase of [Ca2+]i and immediately causes channel desensitization [4,18]. Through TRPV1 activation, nociceptive fibers degenerate [17,33] and the depletion of pain-related neuropeptides such as CGRP and substance P occurs [10,12]. Moreover, capsaicin reduces sodium channel activity, resulting in the inhibition of action potentials in nociceptive fibers [20,29,31]. Thus, capsaicin is clinically used as an anti-nociceptive drug.
Resiniferatoxin (RTX), one of the vanilloid analogues, is much more potent agonist than capsaicin [2,21]. In sensory neurons, the EC50 value for TRPV1 activation of RTX is lower than that of capsaicin [1,21]. RTX is a promising therapeutic candidate a TRPV1-related analgesic because it can desensitize TRPV1 for a long time [3]. Interestingly, the iodinated form of this compound, iodoresiniferatoxin (iodo-RTX) acts as a potent inhibitor of TRPV1 [30]. Thus, many vanilloid analogues are expected to act as agonists or/and antagonists like RTX and iodo-RTX.
Vanillylmandelic acid (VMA), a main metabolite of catecholamines such as adrenaline and noradrenaline, is used as a marker of catecholamine-producing tumors such as neuroblastoma and pheochromocytoma [15]. Vanillin is known as the primary component of the extract of the vanilla bean [24]. Lübbert et al. showed that vanillin induced a small current in rat TRPV1 expressed in CHO cells and this current was inhibited by capsazepine, a TRPV1 antagonist. This report suggested that vanillin was a weak agonist for TRPV1 [11]. Several compounds are present as vanillin metabolites. Vanillic acid (VAcid), an oxidized form of vanillin, is found in Umbelliferae plants such as Angelicae Radix and Ligustici Rhizoma [7]. Vanillyl alcohol (VAlc), the reduced form of vanillin, has been reported to prevent oxidative stress in cell lines [23]. Vanillyl butyl ether (VBE), extracted from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) [8] is well-known as a hot feeling material utilized in cosmetics and bath products. These five vanilloid chemicals are characterized by containing a vanillyl skeleton in their structure similar to capsaicin (Fig. 1A).
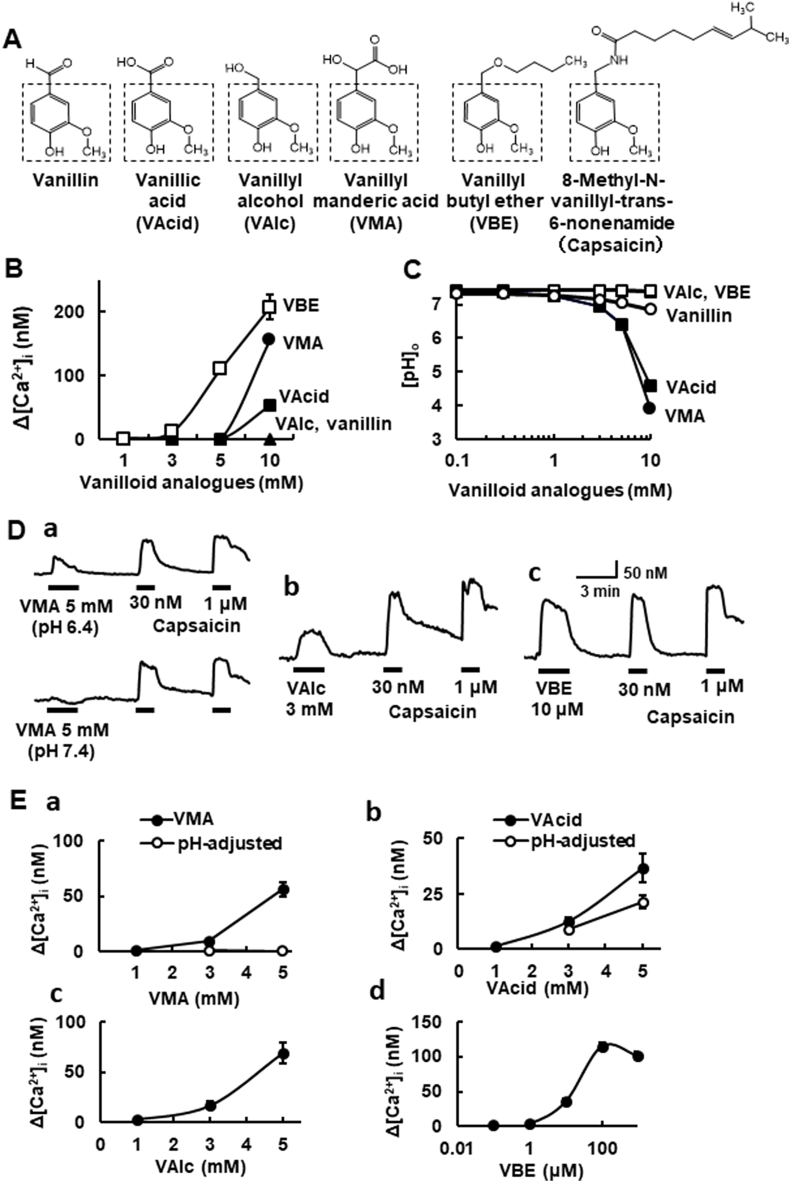
Fig. 1.
[Ca2+]i responses to vanilloid analogues in naïve HEK293 cells and those expressing mTRPV1. (A) Structures of vanilloid analogues used in the present study. The vanillyl skeleton is surrounded by a dashed square. (B) The concentration-[Ca2+]i response curves for the vanilloid analogues. Symbols with vertical lines show mean ± SEM (open squares: VBE; n = 20, closed circles: VMA; n = 100, closed squares: VAcid; n = 92). VAlc (n = 100) and vanillin (n = 100) did not change [Ca2+]i up to 10 mM (closed triangles). (C) The concentration-pH curves for the vanilloid analogue-dissolved solutions. Note that the solutions dissolved in VAcid and VMA at 10 mM are highly acidic. (D) Actual recordings of [Ca2+]i responses to the vanilloid analogues (a; VMA, b; VAlc, c; VBE) and capsaicin (30 nM, 1 μM). In VMA, upper (pH 6.4 solution) and lower (adjusted to pH 7.4). (E) The concentration-relation of four vanilloid analogues. (a) VMA (closed symbols; pH-unadjusted, open ones; pH 7.4-adjusted, n = 18–48), (b) VAcid (closed symbols; pH-unadjusted, open ones; pH 7.4-adjusted, n = 41–68), (c) VAlc (n = 8–47) and (d) VBE (n = 18–75). Symbols with vertical lines show mean ± SEM.
In the present study, the effects of five vanilloid analogues on TRPV1 were investigated. To do so, we examined whether these vanilloid analogues possessed agonistic or antagonistic activity for TRPV1 in a heterologous expression system and sensory neurons.
2. Materials and methods
2.1. Cell isolation
All protocols for experiments on animals were approved by the Committee on Animal Experimentation of Tottori University. All efforts were made to minimize the number of animals used.
We used C57BL/6 wild-type mice (adult mice of either sex; 4–12 weeks old). Euthanization was performed by inhalation of CO2 gas. Mouse dorsal root ganglion (DRG) cells were isolated and cultured as described previously [16]. DRG cells were cultured in a humidified atmosphere of 95% air and 5% CO2 at 37 °C and were used within 24 h after the isolation.
2.2. Heterologous expression
Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco's-modified Eagle's medium (DMEM, Sigma) supplemented with 10% fetal bovine serum, 100 U/ml penicillin G and 100 μg/ml streptomycin. Cells were transfected with the expression vectors [mouse TRPV1 (mTRPV1), mouse TRPV1 mutants (Y511A), and mouse TRPV1 mutants (S512Y)] using a transfection reagent (Lipofectamine 2000, Invitrogen, Japan) and used within 24 h after transfection.
2.3. Measurement of [Ca2+]i
The intracellular Ca2+ concentrations ([Ca2+]i) in individual cells were measured by using a fluorescent-imaging system (Aqua Cosmos, Hamamatsu Photonics, Japan) as described previously [19]. To load the Ca2+-indicator, fura-2, cells were incubated for 40 min at 37 °C with 10 μM fura-2 AM (Molecular Probes) in HEPES-buffered solution (in mM:134 NaCl, 6 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose, and 10 HEPES, pH 7.4). Fura-2 loaded cells were illuminated with lights at 340 and 380 nm, and the respective fluorescence signals at 500 nm were detected. [Ca2+]i was calculated from the ratio of fluorescent signals (F340/F380) with a calibration kit (Invitrogen) [16]. Cells were continuously superfused with the external solution at a flow rate of ∼2 ml/min. All experiments were carried out at room temperature (22–25 °C).
2.4. Chemicals
The following drugs were used (vehicle and concentration for stock solution). Capsaicin (ethanol, 1 mM) and vanillylmandelic acid (VMA; DL-4-hydroxy-3-methoxymandelic acid, DMSO, 2 M) were from Sigma-Aldrich. Vanillic acid (VAcid; 4-hydroxy-3-methoxybenzoic acid, DMSO, 2 M), vanillyl alcohol (VAlc; 4-hydroxy-3-methoxybenzyl alcohol, DMSO, 2 M), vanillyl butyl ether (VBE, 4-butoxymethyl-2-methoxyphenol, DMSO, 5 M) and vanillin (vanillin; 4-hydroxy-3-methoxybenzaldehyde, DMSO, 2 M) were from TCl Chemicals (Tokyo, Japan). All other drugs used were obtained from Wako Pure Chem (Tokyo, Japan).
2.5. Data analysis
The data are shown as the mean ± SEM (n = number of cells). For comparison of two groups, data were analyzed using the unpaired Student's t-test, and for multiple comparisons, one-way ANOVA was used, followed by the Dunnet T3 or Tukey-Kramer test. Differences with a P-value of less than 0.05 were considered significant.
3. Results
3.1. Effects of vanilloid analogues on [Ca2+]i in HEK293 cells expressing mTRPV1
The structures of the vanilloid analogues used in the present study are depicted in Fig. 1A. We first examined the effects of these vanilloids on [Ca2+]i in untransfected naïve HEK293 cells (naïve-HEK). Vanillyl butyl ether (VBE) from 5 mM, and vanillylmandelic acid (VMA) and vanillic acid (VAcid) at 10 mM increased [Ca2+]i in naïve-HEK (Fig. 1B). Vanillyl alcohol (Valc) and vanillin had no effect. The pHs of the solutions that dissolved some vanilloids were acidic. At 10 mM, the pHs of VMA- and VAcid-solutions were 3.9 and 4.6, respectively (Fig. 1C). The pHs of VBE- and Valc-solutions were neutral (pH 7.4, 1–10 mM), but the pH of 10 mM vanillin-solution was slightly acidic.
Next, we examined whether vanilloid analogues could activate TRPV1. VMA evoked [Ca2+]i increases at 5 mM in HEK293 cells expressing mTRPV1 (mTRPV1-HEK). Since the VMA-containing solution was acidic, the effect of VMA solution adjusted to pH 7.4 was further examined. [Ca2+]i responses to VMA disappeared with neutralization (Fig. 1Da and Ea). Although 5 mM VAcid-solution was acidic (pH 6.4), VAcid solution adjusted to pH 7.4 continued to evoke [Ca2+]i increases (Fig. 1Eb). VAlc from 3 mM (Fig. 1Db and Dc) and VBE from 10 μM (Fig. 1Dc and Dd) evoked [Ca2+]i increases in a dose-dependent manner. Vanillin failed to increase the [Ca2+]i in mTRPV1-HEK up to 5 mM (data not shown). Among all five vanilloids tested, VBE was the most effective (EC50 = 14.1 μM) to activate TRPV1.
3.2. Amino acid residues sensitive to vanilloid analogues for TRPV1 activation
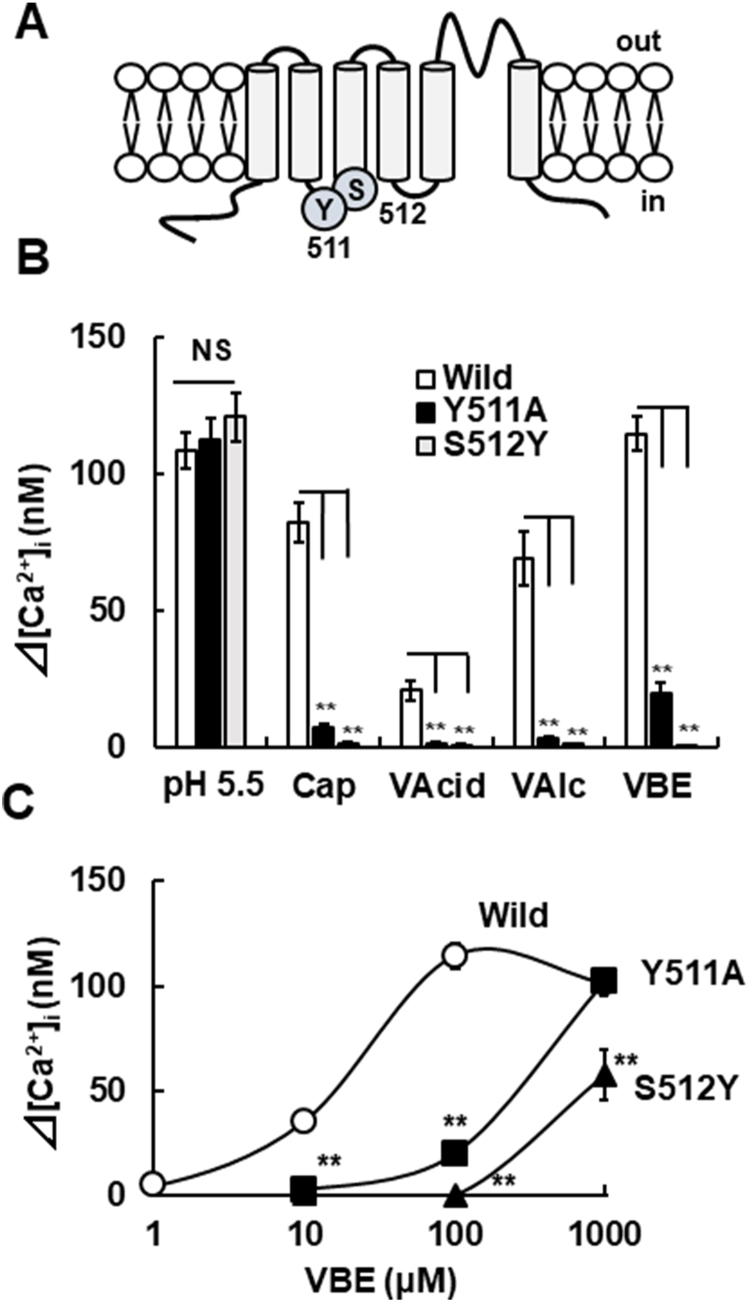
Capsaicin has been reported to interact with specific amino acids located in the intracellular region to activate TRPV1 [21]. Since VAcid, VAlc and VBE evoked increases of [Ca2+]i in mTRPV1-HEK (Fig. 1), we explored the amino acid residues involved in TRPV1 activation induced by these analogues. Mutated mTRPV1 was produced by the replacement of the tyrosine residue at 511 with alanine (Y511A) and the serine residue at 512 with tyrosine (S512Y) (Fig. 2A). The [Ca2+]i responses to capsaicin, but not acids, were strongly suppressed by the mutation of these two amino acids. The [Ca2+]i responses to VAcid (5 mM) adjusted to pH 7.4 and VAlc (5 mM) also disappeared in both mTRPV1 mutants. The [Ca2+]i responses to VBE (100 μM) decreased in mTRPV1 (Y511A) and mTRPV1 (S512Y), but in the former the [Ca2+]i response remained (Fig. 2B).
Fig. 2.
Interactions of vanilloid analogues to capsaicin-sensing sites for the channel activation. (A) A schematic of the mTRPV1 structure including the capsaicin-sensitive amino acid residues tyrosine 511 (Y511) and serine 512 (S512). (B) The amplitudes of [Ca2+]i increases induced by pH 5.5, capsaicin (300 nM), VAcid (5 mM)-adjusted pH 7.4, VAlc (5 mM) and VBE (100 nM) in wild (open columns), Y511A (closed columns) and S512Y (grey columns). Columns with vertical lines show mean ± SEM (open column: wild, pH 5.5; n = 71, cap; n = 31, VAcid; n = 24, VAlc; n = 26, VBE; n = 75, closed column: Y511A, pH 5.5; n = 22, cap; n = 22, VAcid; n = 19, VAlc; n = 27, VBE; n = 28, and grey column: S512Y, pH 5.5; n = 30, cap; n = 30, VAcid; n = 35, VAlc; n = 47, VBE; n = 38). (C) The concentration-[Ca2+]i response curves for VBE [open circles; Wild mTRPV1-HEK, closed squares; mTRPV1 (Y511A)-HEK, closed triangles; mTRPV1 (S512A)-HEK]. Symbols with vertical lines show mean ± SEM (Wild, 10 μM; n = 40, 100 μM; n = 75, 1000 μM; n = 63, mTRPV1 (Y511A)-HEK, 10 μM, 100 μM; n = 28, 1000 μM; n = 29, mTRPV1 (S512Y) -HEK, 10 μM, 100 μM; n = 38, 1000 μM; n = 21). **, p < 0.01 vs. Wild (control) by one-way ANOVA with Tukey-Kramer test or Dunnett T3 test, NS, not significant.
To further examine the interaction of VBE with the two amino acid residues, we evaluated the relationships between the VBE concentration and the [Ca2+]i responses in mTRPV1 (Y511A) and mTRPV1 (S512Y). VBE evoked [Ca2+]i increases from 100 μM in mTRPV1 (Y511A)-HEK and its amplitude at 1000 μM increased to the same level as the wild-type mTRPV1 (Fig, 2C). In mTRPV1 (S512Y), VBE at 10 and 100 μM failed to induce [Ca2+]i increases but increased the [Ca2+]i at 1000 μM. These results indicate that the vanilloid analogues used activate mTRPV1 via interaction with the capsaicin-binding sites.
3.3. Effects of vanilloid analogues on the activity of heterologously expressed TRPV1
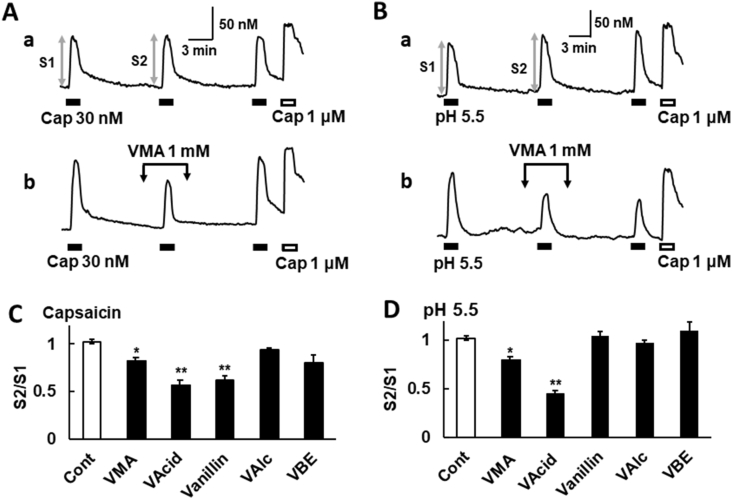
Since some ligands possessing a vanillyl skeleton such as capsazepine and iodoresiniferatoxin [21,30] show antagonistic activity, we investigated the effects of vanilloid analogues on the TRPV1 activity in mTRPV1-HEK. Repetitive applications of capsaicin (30 nM) evoked [Ca2+]i increases with similar amplitudes in mTRPV1-HEK (Fig. 3Aa). VMA at 1 mM (∼pH 7.3) inhibited the capsaicin-induced [Ca2+]i increases (Fig. 3Ab). VAcid and vanillin at 1 mM also attenuated the capsaicin-induced responses, but VAlc at 1 mM and VBE at 10 μM did not (Fig. 3C).
Fig. 3.
Effects of vanilloid analogues on the TRPV1 activation by capsaicin or acids in mTRPV1-HEK. (A, B) Actual recordings of [Ca2+]i responses induced by the sequential application of capsaicin (30 nM, A) or acids (pH 5.5, B) and capsaicin (1 μM) as a control (a). VMA (1 mM) was added before, during and after the second stimuli (b). (C, D) The relative amplitude of [Ca2+]i increases induced by the second application of capsaicin or pH 5.5 (S2) to that of the first one (S1) (Cont; control, VAcid; 1 mM, VMA; 1 mM, vanillin; 1 mM, VAlc; 1 mM, and VBE; 10 μM). Columns with vertical lines show mean ± SEM (C: control; n = 100, VAcid; n = 83, VMA; n = 53, vanillin; n = 23, VAlc; n = 91, and VBE n = 9, D: control; n = 94, VAcid; n = 44, VMA; n = 27, vanillin; n = 21, VAlc; n = 40, and VBE; n = 8), *, p < 0.05, **, p < 0.01 vs. Control by one-way ANOVA with Tukey-Kramer test.
We verified the effects of vanilloids on the acid-induced TRPV1 activation. Repetitive applications of acids (pH 5.5) increased [Ca2+]i with similar amplitudes in mTRPV1-HEK (Fig. 3Ba). VMA at 1 mM (∼pH 7.3) inhibited the pH 5.5-induced [Ca2+]i increases in mTRPV1-HEK (Fig. 3Bb). VAcid at 1 mM also attenuated the pH 5.5-induced responses, but vanillin and VAlc at 1 mM, and VBE at 10 μM did not (Fig. 3D). These results suggest that some vanilloid analogues have inhibitory effects on the TRPV1 activation.
3.4. Effect of VMA on the TRPV1 activation in mouse sensory neurons
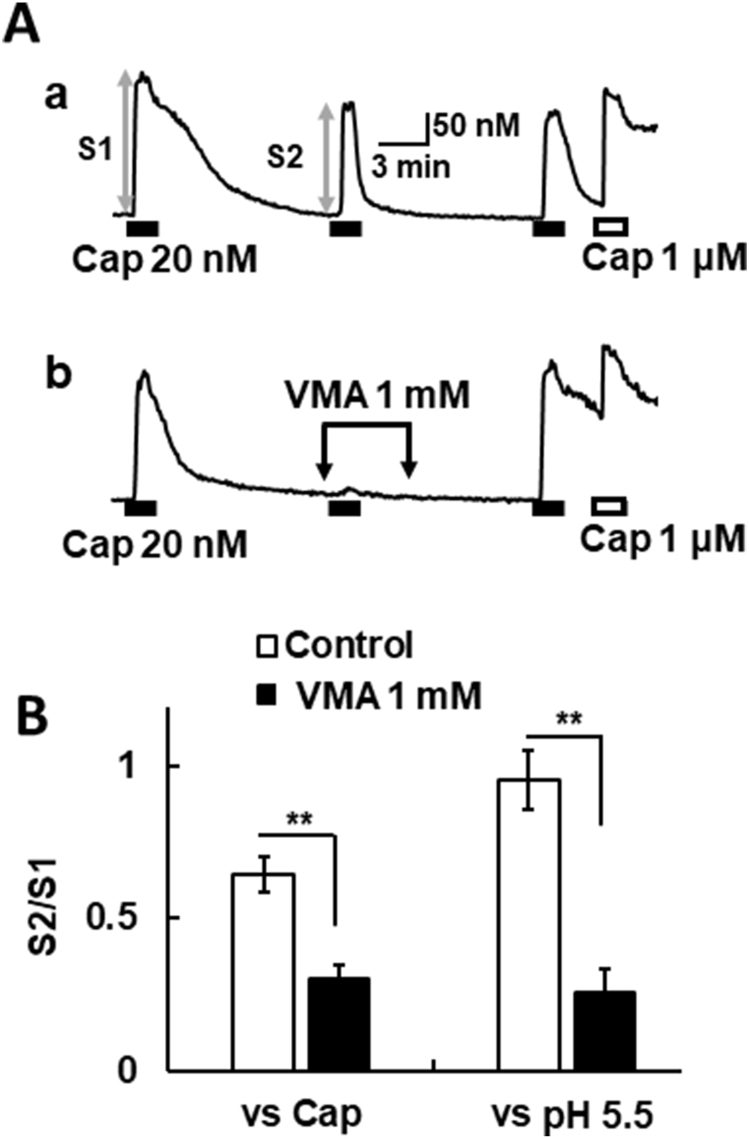
Finally, we investigated the effect of VMA in mouse sensory neurons, since VMA is a major endproduct of catecholamine metabolism [15], showed inhibitory effects on heterologously expressed TRPV1 (Fig. 3). Repetitive application of capsaicin at 20 nM evoked [Ca2+]i increases in sensory neurons (Fig. 4Aa). VMA reversibly attenuated the [Ca2+]i increases induced by capsaicin in capsaicin (1 μM)-sensitive neurons, i.e., endogenously TRPV1-expressing neurons (Fig. 4Ab). VMA also decreased the [Ca2+]i responses to pH 5.5 in TRPV1-expressing neurons (Fig. 4B). These results suggest that VMA can modulate intrinsic TRPV1 channels expressed in sensory neurons.
Fig. 4.
Effects of VMA on the TRPV1 activation in mouse sensory neurons. (A) Actual recordings of [Ca2+]i responses induced by the sequential application of capsaicin (20 nM), capsaicin (1 μM, 1 min), and KCl (80 mM, 1 min) in mouse DRG neurons as a control (a). VMA (1 mM) was added before, during and after the second application of capsaicin (b). (B) The relative amplitude of [Ca2+]i increases (S2) induced by the second application of capsaicin or pH 5.5 to that of the first one (S1). Columns with vertical lines show mean ± SEM (control of capsaicin; n = 41, VMA vs. capsaicin; n = 31, control of pH 5.5; n = 51, VMA vs. pH 5.5; n = 30), **, p < 0.01 vs Control by un-paired t-test.
4. Discussion
In this report, to explore novel reagents modulating TRPV1 activation, we examined the effects of five vanilloid analogues on TRPV1 channels using heterologous expression systems and mouse sensory neurons.
First, we investigated the effects of these vanilloid analogues on [Ca2+]i in naïve HEK293 cells (naïve-HEK). VMA, VAcid and VBE evoked [Ca2+]i increases in naïve-HEK. HEK293 cells have been reported to possess various acid-sensitive channels, including acid-sensitive ion channels (ASICs) [6,27]. The [Ca2+]i increases induced by VMA and VAcid in naïve-HEK were mainly due to the activation of these channels because these compound-dissolved solutions were acidic enough to activate ASICs. In contrast, VBE was able to evoke [Ca2+]i increases in naïve-HEK cells despite its neutral pH, suggesting that VBE seems not to activate ASICs but rather modulate other targets expressed endogenously.
VAcid, VAlc and VBE acted as agonists of mouse TRPV1 (mTRPV1). In addition, the mutation of capsaicin-binding sites of mouse TRPV1 (Y511A and S512Y) [21] eliminated their agonistic activities (Fig. 2). Therefore, these analogues enclosed a vanilloid group that seemed to affect the capsaicin-binding pocket of mTRPV1. The differences of agonistic activities may be derived from the types of side chains. It is reported that the sensitivity of TRPV1 to capsaicin is decreased by shortening of the carbon chain [32]. The highest sensitivity of VBE among the five analogues used supported this chemical characteristic because it had the longest side chain among analogues used in the present study (Fig. 1A). VBE at a high dose (1000 μM) was able to activate both mutated mTRPV1s (Fig. 2). It has been reported that various amino acids including tyrosine 511 (Y511) and serine 512 (S512) are the vanilloid-binding sites [21]. Thus, for the agonistic action of VBE, other residues may be important in addition to Y511 and S512. Our findings further suggest that not only the length of the carbon chain, but also slight structural changes are involved in the TRPV1 modulation since vanillin showed no agonistic action, but VAcid and VAlc did. The former had monooxygen added to vanillin and the latter was reduced from VAcid. The structure mechanism of TRPV1 for the agonist function is determined from cryoEM analysis [32]. To determine the detailed structural characteristics, future investigations are required.
Some vanilloid analogues also had inhibitory action. Among the three analogues with stimulatory actions (VAcid, VAlc and VBE), only VAcid suppressed the capsaicin-induced TRPV1 activation. Capsaicin is known to desensitize TRPV1 [4,18]. However, capsaicin at 30 nM activated TRPV1 repetitively without desensitization. Moreover, VMA and vanillin at the concentrations that did not show agonistic effects inhibited the capsaicin-induced TRPV1 activation. Thus, the inhibitory actions of the two chemicals are not related to the desensitization of TRPV1. These vanilloid analogues may act at the capsaicin-binding pocket, resulting in competition with capsaicin. These data may provide further insights into the manner of TRPV1 inhibition.
In the acid-induced TRPV1 activation, VMA and VAcid inhibited [Ca2+]i responses to pH 5.5 in mTRPV1-HEK. Notably, the inhibitory effect of VAcid was higher than those of the other two. The residues involved in proton-mediated activation (E648) and sensitization (E600) have been reported [21]. VAcid may act at these residues, resulting in inhibition of the TRPV1 activation induced by acids. Alternatively, there is a possibility of its inhibitory action through an allosteric mechanism because low pH sensitizes capsaicin-induced TRPV1 activation allosterically [25]. Furthermore, it has been reported that BCTC, a TRPV1 antagonist, inhibits both capsaicin and proton responses through changes in channel conformation [5].
Since VMA is the only one vanilloids used in the present study that is produced endogenously through catecholamine metabolism, we focused on the effect of VMA in mouse sensory neurons. VMA inhibited TRPV1 activation induced by capsaicin or acids (Fig. 4). VMA is used as a diagnostic marker for certain diseases such as neuroblastoma and pheochromocytoma. Manickum [14] reported that the VMA levels in normal urine samples and pathological ones were 20 μM and 77–79 μM, respectively. In the present experiment, a relatively high concentration of VMA was needed to inhibit TRPV1. Peripheral nerve injury induces sympathetic nerve fiber sprouting to the DRG [9,22]. Though the VMA level in naïve and peripheral injured DRGs has not determined, it might be possible that VMA affects TRPV1 activity endogenously. Further research is needed to elucidate the possible role of VMA on pain sensation under pathophysiological conditions.
In conclusion, we found that the five vanilloid analogues used in this study showed various activities with regard to TRPV1 activation. Of particular interest, VMA demonstrates an inhibitory effect against capsaicin- and acid-induced TRPV1 activation in mouse sensory neurons. Further investigation of the modulating mechanisms of these vanilloid analogues may be useful for the development of analgesics for TRPV1-related pain.
Declaration of competing interest
We have no conflict-of-interest to declare.
Acknowledgements
This work was supported, in whole or part, by JSPS KAKENHI (Grant No. 18H02345, T.O.).
Data availability
No data was used for the research described in the article.
References
- 1.Acs G., Palkovits M., Blumberg P.M. Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci. 1996;59:1899–1908. doi: 10.1016/s0024-3205(96)00537-1. [DOI] [PubMed] [Google Scholar]
- 2.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The Capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Choi H.K., Choi S., Lee Y., Kang D.W., Ryu H.C., Maeng H.J., Chung S.J., Pavlyukovetsc V.A., Pearcec L.V., Tothc A., Tran R., Wang Y., Morgan M.A., Blumberg P.M., Lee J. Non-vanillyl resiniferatoxin analogues as potent and metabolically stable transient receptor potential vanilloid 1 agonists. Bioorg. Med. Chem. 2009;17:690–698. doi: 10.1016/j.bmc.2008.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty R.J., Yeats J.C., Bevan S., Boddeke H.W. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 5.Gavva N.R., Tamir R., Klionsky L., Norman M.H., Louis J.C., Wild K.D., Treanor J.J.S. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol. Pharmacol. 2005;68:1524–1533. doi: 10.1124/mol.105.015727. [DOI] [PubMed] [Google Scholar]
- 6.Gunthorpe M.J., Smith G.D., Davis J.B., Randall A.D. Characterisation of a human acid-sensing ion channel (hASIC1a) endogenously expressed in HEK293 cells. Pflügers Archiv. 2001;442:668–674. doi: 10.1007/s004240100584. [DOI] [PubMed] [Google Scholar]
- 7.Huang W.Y., Sheu S.J. Separation and identification of the organic acids in Angelicae Radix and Ligustici Rhizoma by HPLC and CE. J. Separ. Sci. 2006;29:2616–2624. doi: 10.1002/jssc.200600136. [DOI] [PubMed] [Google Scholar]
- 8.Ivanović J., Đjilas S., Jadranin M., Vajs V., Babović N., Petrović S., Žižović I. Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) J. Serb. Chem. Soc. 2009;74:717–732. [Google Scholar]
- 9.Kim H.J., Na H.S., Nam H.J., Park K.A., Hong S.K., Kang B.S. Sprouting of sympathetic nerve fibers into the dorsal root ganglion following peripheral nerve injury depends on the injury site. Neurosci. Lett. 1996;212:191–194. doi: 10.1016/0304-3940(96)12811-1. [DOI] [PubMed] [Google Scholar]
- 10.Kashiba H., Ueda Y., Senba E. Systemic capsaicin in the adult rat differentially affects gene expression for neuropeptides and neurotrophin receptors in primary sensory neurons. Neuroscience. 1997;76:299–312. doi: 10.1016/s0306-4522(96)00334-x. [DOI] [PubMed] [Google Scholar]
- 11.Lübbert M., Kyereme J., Schöbel N., Beltrán L., Wetzel C.H., Hatt H. Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggi C.A. Therapeutic potential of capsaicin-like molecules: studies in animals and human. Life Sci. 1992;51:1777–1781. doi: 10.1016/0024-3205(92)90047-s. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg A.B., Mizisin A.P., Calcutt N.A., von Stein T., Robbins W.R., Bley K.R. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111:360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Manickum T. Simultaneous analysis of neuroendocrine tumor markers by HPLC-electrochemical detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:4140–4146. doi: 10.1016/j.jchromb.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Němečková-Makrlíková A., Navrátil T., Barek J., Štenclová P., Kromka A., Vyskočil V. Determination of tumour biomarkers homovanillic and vanillylmandelic acid using flow injection analysis with amperometric detection at a boron doped diamond electrode. Anal. Chim. Acta. 2019;1087:44–50. doi: 10.1016/j.aca.2019.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Nishizawa Y., Takahashi K., Oguma N., Tominaga M., Ohta T. Possible involvement of transient receptor potential ankyrin 1 in Ca2+ signaling via T-type Ca2+ channel in mouse sensory neurons. J. Neurosci. Res. 2018;96:901–910. doi: 10.1002/jnr.24208. [DOI] [PubMed] [Google Scholar]
- 17.Nolano M., Simone D.A., Wendelschafer-Crabb G., Johnson T., Hazen E., Kennedy W.R. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 18.Numazaki M., Tominaga T., Takeuchi K., Murayama N., Toyooka H., Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohta T., Imagawa T., Ito S. Novel gating and sensitizing mechanism of capsaicin receptor (TRPV1): tonic inhibitory regulation of extracellular sodium through the external protonation sites on TRPV1. J. Biol. Chem. 2008;283:9377–9387. doi: 10.1074/jbc.M709377200. [DOI] [PubMed] [Google Scholar]
- 20.Petsche U., Fleischer E., Lembeck F., Handwerker H.O. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983;265:233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- 21.Pingle S.C., Matta J.A., Ahern G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb. Exp. Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 22.Ramer M.S., Bisby M.A. Normal and injury-induced sympathetic innervation of rat dorsal root ganglia increases with age. J. Comp. Neurol. 1998;394:38–47. [PubMed] [Google Scholar]
- 23.Rosa A., Atzeri A., Deiana M., Melis M.P., Incani A., Corona G., Loru D., Appendino G., Dessì M.A. Protective effect of vanilloids against tert-butyl hydroperoxide-induced oxidative stress in vero cells culture. J. Agric. Food Chem. 2008;56:3546–3553. doi: 10.1021/jf073448t. [DOI] [PubMed] [Google Scholar]
- 24.Sharp M.D., Kocaoglu‐Vurma N.A., Langford V., Rodriguez‐Saona L.E., James Harperv W.J. Rapid discrimination and characterization of vanilla bean extracts by attenuated total reflection infrared spectroscopy and selected ion flow tube mass spectrometry. J. Food Sci. 2012;77:C284–C292. doi: 10.1111/j.1750-3841.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 25.Sujung R., Liu B., Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J. Gen. Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szallasi A., Sheta M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expet Opin. Invest. Drugs. 2012;9:1351–1369. doi: 10.1517/13543784.2012.704021. [DOI] [PubMed] [Google Scholar]
- 27.Thomas P., Smart P.T. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Tominaga M. Nociception and TRP channels. Handb. Exp. Pharmacol. 2007;179:489–505. doi: 10.1007/978-3-540-34891-7_29. [DOI] [PubMed] [Google Scholar]
- 29.Tomohiro D., Mizuta K., Fujita T., Nishikubo Y., Kumamoto E. Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves. Life Sci. 2013;92:368–378. doi: 10.1016/j.lfs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Wahl P., Foged C., Tullin S., Thomsen T. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Yamanaka K., Kigoshi S., Muramatsu I. Conduction-block induced by capsaicin in crayfish giant axon. Brain Res. 1984;300:113–119. doi: 10.1016/0006-8993(84)91345-3. [DOI] [PubMed] [Google Scholar]
- 32.Yang F., Xiao X., Cheng W., Yang W., Yu P., Song Z., Yarov-Yarovoy V., Zheng J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015;11:518–524. doi: 10.1038/nchembio.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K., Furue H., Fujita T., Kumamoto E., Yoshimura M. Alterations in primary afferent input to substantia gelatinosa of adult rat spinal cord after neonatal capsaicin treatment. J. Neurosci. Res. 2003;74:928–933. doi: 10.1002/jnr.10818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.