Abstract
Rotavirus remains a leading cause of diarrhoeal morbidity and mortality in young children and rotavirus vaccines are critical for reducing global disease burden. This report addresses the performance of rotavirus vaccines in countries with high child mortality. We performed a sensitivity analysis as part of a systematic review on rotavirus vaccines to inform development of World Health Organization vaccine recommendations. The efficacy of four prequalified vaccines against severe rotavirus gastroenteritis was similar across high mortality settings in Asia and Africa. Within the first year following vaccination, vaccine efficacy for the four vaccines ranged from 48% to 57% while in the second year, efficacy ranged from 29% to 54%. The four vaccines showed no increase in intussusception risk in these settings. All four vaccines appear to prevent significant numbers of severe rotavirus gastroenteritis episodes with no measurable increase in intussusception risk in high mortality settings in Africa and Asia.
Keywords: Rotavirus, Efficacy
1. Introduction
Since first being recommended by WHO in 2006, second-generation rotavirus vaccines have been introduced in over 100 countries. Four live-attenuated oral rotavirus vaccines, Rotarix™, RotaTeq™, Rotavac®, and Rotasiil™, have been prequalified by the World Health Organization (WHO) and are available for use. Prequalification indicates that a vaccine has undergone thorough evaluation by the WHO of relevant data, testing of samples, and inspection of relevant manufacturing sites [1]. The global impact of rotavirus vaccines is evident from the reductions in rotavirus mortality following the introduction of vaccination [2], as well as reductions in rotavirus hospitalisations, acute gastroenteritis hospitalisations, and gastroenteritis mortality in many countries [3], [4]. Rotavirus vaccination has consistently been found to be cost-effective and even cost-saving in most low- and middle-income countries (LMICs) when compared with no vaccination [5].
Systematic reviews of the efficacy and effectiveness of oral rotavirus vaccination have shown high efficacy against severe disease in high-income countries compared with placebo or no vaccination, while efficacy and effectiveness have been lower in many LMICs [6], [7]. Live oral rotavirus vaccines have been associated a low risk of intussusception in high- and middle-income countries [6], though relatively few data are available from low-income countries. In addition to income level, the populations, epidemiology, vaccine schedules, access to care and other factors differ substantially between the settings in which these studies were conducted. These differences affect point estimates of efficacy between high and low resource settings and create challenges for policy makers and other stakeholders who wish to assess risk-benefit analyses in low-income settings.
Efficacy studies comparing different rotavirus vaccines to each other, i.e. “head-to-head” comparisons, are lacking and the absence of a consensus immune correlate of protection also precludes immunogenicity comparisons [8]. Indirect comparisons of efficacy and safety of different rotavirus vaccines can be made by limiting meta-analyses to studies carried out in the same or similar settings. To inform vaccine policy decisions on use of various rotavirus vaccines, we undertook a systematic review and meta-analysis of efficacy and intussusception data from studies in high mortality countries. This review will complement the previously published review of effectiveness studies in these settings, and together inform the scientific community, policymakers and practitioners [3].
2. Methods
An updated systematic review on the efficacy and safety of childhood schedules of rotavirus vaccines was completed in September 2020 to inform updated vaccine recommendations of the WHO [9]. We concentrated on the most important outcomes, namely severe disease and death for efficacy, and intussusception for safety. The systematic review used evidence from randomized controlled trials (RCTs), observational studies, and unpublished data from pharmaceutical companies and trialists. The efficacy of the four prequalified rotavirus vaccines were compared in RCTs with placebo or no intervention. The safety of the vaccines was evaluated with both RCTs and observational studies to increase the available data.
Analyses were stratified by vaccine and were sub-grouped within outcomes by country under-5 year old child mortality rates [10]. Those in the lowest quartile of under-5 year old child mortality rates were considered “low mortality” countries; those in the second quartile were considered “medium mortality” countries; and “high mortality” countries were those in the highest two quartiles.
This brief report comprises of a sensitivity analysis performed as part of the systematic review. All RCTs that evaluated the efficacy and safety of rotavirus vaccines in high mortality settings in Asia and Africa and that reported on severe rotavirus gastroenteritis (RVGE), or intussusception were included. Severe RVGE cases were extracted as defined in each RCT. Effect estimates were analysed over one- and two-years of follow-up, however, the populations analysed in the two-year follow-up results are not the same as those in the one-year follow-up results.
In addition to RCT evidence, the systematic review also included observational studies using a self-controlled case series method [11] to determine the risk of intussusception following a dose of rotavirus vaccine. RCTs often do not have sufficient statistical power to detect rare adverse events such as intussusception. From these studies, the risk of intussusception over the first week (1–7 days) and the second and third weeks (8–21 days) following each dose of vaccine is compared to a control interval. Self-controlled case series carried out in high mortality settings in Africa and Asia were also included in this sensitivity analysis.
3. Results
Thirteen RCTs were included that evaluated the effect of rotavirus vaccine on severe RVGE in high mortality countries in Africa or Asia. Five trials each evaluated RotaTeq™ [12], [13] or Rotarix™ [14], [15], [16], [17] one trial evaluated Rotavac® [18], and two trials evaluated Rotasiil™ [19], [20].
Fifteen RCTs were included that evaluated the incidence of intussusception following rotavirus vaccine. Five trials evaluated RotaTeq™ [13], [21], [22], [23],four each evaluated Rotarix™ [24], [15], [16], [17] or Rotavac® [18], [25], [26], [27], and two trials evaluated Rotasiil™ [19], [20].
Five self-controlled case series on intussusception were included, one unpublished study from Africa (data provided by authors), while four were published [28], [29], [30], [31].
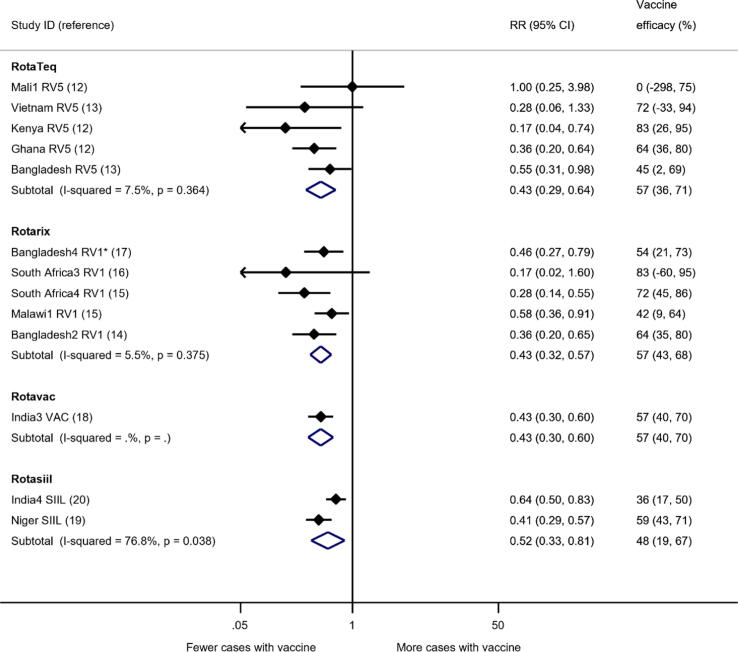
At one-year follow-up, the sensitivity analysis for efficacy against severe RVGE in high mortality countries showed reductions of 57% (95% confidence interval [CI] 36% to 71%) for RotaTeq™; 57% (95% CI 43% to 68%) for Rotarix™; 57% (95% CI 40% to 70%) for Rotavac®; and 48% (95% CI 19% to 67%) for Rotasiil™, all compared with placebo (Fig. 1).
Fig. 1.
Analyses of RCTs for the effect of rotavirus vaccine on severe RVGE in high mortality countries, up to one-year follow-up. For this analysis only high mortality studies from Africa and Asia were included. *Bangladesh4 RV1 was a cluster-RCT, villages were randomised to Rotarix or to no intervention, adjusted using ICC = 0.02. CI = confidence interval; RR = risk ratio.
Over a two-year follow-up, the corresponding reduction in severe RVGE compared with placebo for RotaTeq™ was 44% (95% CI 23% to 59%); Rotarix™ 29% (95% CI 8% to 45%), Rotavac® 54% (95% CI 40% to 65%), and Rotasiil™ 44% (95% CI 26% to 58%) (Table 1).
Table 1.
Summary of findings for rotavirus efficacy against severe RVGE in high mortality countries in Africa and Asia.
| Vaccine | Absolute risk reduction (95% CI)* | Vaccine efficacy (95% CI) | N° of participants & studies | Certainty of the evidence (GRADE)† |
|---|---|---|---|---|
| One-year follow-up | ||||
| RotaTeq™ | 17 (11 to 21) fewer cases per 1000 children | 57% (36 to 71) | 6775 participants in 5 RCTs [12], [13] | ⊕⊕⊕⊕ HIGH |
| Rotarix™ | 17 (13 to 20) fewer cases per 1000 children | 57% (43 to 68) | 8623 participants in 5 RCTs [14], [15], [16], [17] | ⊕⊕⊕⊕ HIGH |
| Rotavac® | 17 (12 to 21) fewer cases per 1000 children | 57% (40 to 70) | 6799 participants in 1 RCT [18] | ⊕⊕⊕⊕ MODERATE a |
| Rotasiil™ | 14 (6 to 20) fewer cases per 1000 children | 48% (19 to 67) | 11,008 participants in 2 RCTs [19], [20] | ⊕⊕⊕⊕ HIGH |
| Two-years follow-up | ||||
| RotaTeq™ | 22 (12 to 30) fewer cases per 1000 children | 44% (23 to 59) | 6744 participants in 5 RCTs [12], [13] | ⊕⊕⊕⊕ HIGH |
| Rotarix™ | 15 (4 to 23) fewer cases per 1000 children | 29% (8 to 45) | 6183 participants in 3 RCTs [15], [17] | ⊕⊕⊕⊕ HIGH |
| Rotavac® | 27 (20 to 33) fewer cases per 1000 children | 54% (40 to 65) | 6541 participants in 1 RCT [18] | ⊕⊕⊕⊕ MODERATE a |
| Rotasiil™ | 22 (13 to 29) fewer cases per 1000 children | 44% (26 to 58) | 11,008 participants in 2 RCTs [19], [20] | ⊕⊕⊕⊕ HIGH |
Assumed risk of severe RVGE in the control groups of 30 per 1000 children in the first year and 50 per 1000 children in the second year. Numbers in brackets represent 95% confidence intervals.
The GRADE approach considers the following factors for downgrading the certainty of the evidence: limitations in the study design; inconsistency of results; indirectness of evidence; imprecision; and publication bias.
Downgraded by one level for indirectness: single trial conducted in one country, so estimate may not apply to other high-mortality countries.
The sensitivity analysis for safety included RCTs that reported on incidence of intussusception following rotavirus vaccination in high mortality countries in Africa and Asia. Of 15 RCTs included in the analysis (38,116 participants), only five reported any cases of intussusception during the trial follow-up. The evidence suggested that all four rotavirus vaccines resulted in little to no difference in intussusception risk compared with placebo at up to two years follow-up.
In the analysis of self-controlled case series studies for Rotarix™, two studies were included [29], [31] which did not identify any cases of intussusception in the first week following the first dose. In the second and third week, one study reported an increased risk (RI 4.01, 95% CI 1.15 to 13.97) while the other reported no increased risk (RI 1.01, 95% CI 0.34 to 2.96). No increased risk was seen following the second dose.
For Rotavac®, two studies were included and no increase in risk was seen following the first, second, or third doses. No self-controlled case series for Rotasiil™ were identified for inclusion in the systematic review.
4. Discussion
Diarrhoea remains a leading cause of morbidity and mortality in young children worldwide, and the introduction of rotavirus vaccines is a critical step to reduce this global burden. The results of this sensitivity analysis suggest that the efficacy of the four prequalified rotavirus vaccines against severe RVGE is similar in high mortality settings in Africa and Asia. Within the first year following vaccination, vaccine efficacy ranged from 48% to 57% while in the second year following vaccination, efficacy ranged from 29% to 54%. In these epidemiologic settings, for every 1000 children vaccinated, 14 to 17 severe rotavirus cases were prevented in the first year of life and 15 to 27 severe rotavirus cases were prevented in the first two years of life. From a policy perspective, early administration of rotavirus vaccines is recommended by WHO to optimize protection against disease occurring early in life.
Rotavirus vaccine efficacy is lower in high mortality settings than in low mortality settings and the reasons for the difference in vaccine performance remain unknown, however factors such as nutritional status and maternal antibodies have been suggested as potential explanations [3]. Although point estimates of efficacy in the current analysis were substantially lower when compared to low mortality countries, each rotavirus vaccine would be expected to lead to substantial and similar reductions in severe rotavirus disease outcomes across a range of high mortality settings. While high efficacy is maintained in low mortality countries into the second year of life, in high mortality settings the efficacy tends to be lower in the second year of life. Strategies to improve vaccine efficacy in high mortality settings should be explored, such as an additional dose of vaccine or alternative formulations of vaccine [32].
The evidence on safety of rotavirus vaccines, in terms of intussusception risk, from studies in high mortality settings was limited. Few RCTs reported any cases of intussusception during the follow-up so further analysis was performed using observational self-controlled case series. While limited, the evidence suggested that there was little to no difference in risk of intussusception across the three rotavirus vaccines evaluated. There are currently no self-controlled case series of intussusception following vaccination with Rotasiil™.
Prior reviews by the Global Advisory Committee on Vaccine Safety (GACVS) emphasised that the benefit of Rotarix™ and RotaTeq™ vaccines is greater than the small risk of intussusception [5], which has been supported by modelling studies [6]. During the December 2019 GACVS Meeting, the safety of RotaTeq™ in sub-Saharan Africa and of Rotavac® in India were reviewed and it was noted that the data did not indicate a significantly higher risk of intussusception during the post-vaccination periods than in the control period for either vaccine. The current sensitivity analysis supports this finding, with the updated systematic review from 2020 including more recent and larger studies, as well as unpublished data [9].
Based on the updated systematic review for the WHO, the GACVS reports, and other available information, the Strategic Advisory Group of Experts on Immunization (SAGE) concluded that Rotavac® and Rotasiil™ are safe and effective [5]. Following the October 2020 SAGE Meeting, WHO recommends all four oral rotavirus vaccines (Rotarix™, RotaTeq™, Rotavac®, and Rotasiil™) for use.
Product-specific supply shortages have slowed the introduction of rotavirus vaccines in low resource countries. The availability of four prequalified vaccines, of which three - Rotarix™, Rotavac®, and Rotasiil™ - are currently available through Gavi support to eligible countries, should provide broader options for rotavirus vaccines and thus reduce supply constraints as a barrier to rotavirus vaccine introduction. However, improvements in supply alone will not yield higher coverage without political will, detailed planning, demand generation, and attention to country-specific financial constraints [33]. Our analysis supports that any of the four rotavirus vaccines will be safe and efficacious in high child mortality settings. Presentation, storage and shelf-life, schedule and other considerations are likely to inform country-decision-making for specific product selection.
These data strongly support WHO recommendations to introduce rotavirus vaccines to infants in all countries. While post-introduction monitoring of the effectiveness and safety of rotavirus vaccines, particularly Rotavac® and Rotasiil™ should remain a priority, the anticipation of additional data should not impede the current introduction of rotavirus vaccines. This is particularly important in high mortality settings, where widespread vaccine use promises to bring further reductions in diarrhoeal morbidity and mortality among children living in high disease burden countries across Africa and Asia.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NH: no competing financial interests. HB: no competing financial interests. DH: reports grants on the topic of rotavirus vaccines, outside of the submitted work, from GlaxoSmithKline Biologicals, Sanofi Pasteur and Merck and Co (Kenilworth, NJ, USA) after the closure of Sanofi Pasteur-MSD in December 2016. NAC: has received investigator-initiated grant support for rotavirus research from GlaxoSmithKline Biologicals. He has received honoraria for participation in Independent Data Monitoring Committee meetings for rotavirus vaccines from GlaxoSmithKline Vaccines, and honoraria for participation in rotavirus vaccine advisory board meetings from Sanofi Pasteur. RFG: no competing financial interests. GK: no competing financial interests. UDP: no competing financial interests. SAW: no competing financial interests. KN: no competing financial interests.
References
- 1.Organization WH. List of Prequalified Vaccines; 2021 [Available from: https://extranet.who.int/pqweb/vaccines/list-prequalified-vaccines.
- 2.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, Network WHOCGRS, Agocs M, et al. Global, regional, and national estimates of rotavirus mortality in children < 5 years of age, 2000–2013. Clinical Infectious Diseases 2016;62(suppl_2):S96-S105. [DOI] [PubMed]
- 3.Burnett E., Parashar U.D., Tate J.E. Real-world effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Global Health. 2020;8(9):e1195–e1202. doi: 10.1016/S2214-109X(20)30262-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hungerford D., Smith K., Tucker A., Iturriza-Gómara M., Vivancos R., McLeonard C., et al. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2017;17(1) doi: 10.1186/s12879-017-2613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization = Organisation mondiale de la S. Weekly Epidemiological Record, 2020, vol. 95, 48 [full issue]. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire. 2020;95(48):585-608.
- 6.Clark A., Tate J., Parashar U., Jit M., Hasso-Agopsowicz M., Henschke N., et al. Mortality reduction benefits and intussusception risks of rotavirus vaccination in 135 low-income and middle-income countries: a modelling analysis of current and alternative schedules. Lancet Global Health. 2019;7(11):e1541–e1552. doi: 10.1016/S2214-109X(19)30412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman H., Henschke N., Hungerford D., Pitan F., Ndwandwe D., Cunliffe N., et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2021;11 doi: 10.1002/14651858.CD008521.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M., et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3(1) doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization WH. Strategic Advisory Group of Experts on Immunization (SAGE) - October 2020; 2020 [Available from: https://www.who.int/news-room/events/detail/2020/10/05/default-calendar/sage_meeting_october_2020.
- 10.IGME) UNI-aGfCMEU. Levels & Trends in Child Mortality: Report 2019, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York; 2019.
- 11.Tate J.E., Parashar U.D. Approaches to monitoring intussusception following rotavirus vaccination. Expert Opinion Drug Safety. 2019;18(1):21–27. doi: 10.1080/14740338.2019.1561857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 13.Zaman K., Anh D.D., Victor J.C., Shin S., Yunus M.d., Dallas M.J., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 14.Colgate E.R., Haque R., Dickson D.M., Carmolli M.P., Mychaleckyj J.C., Nayak U., et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin Infect Dis. 2016;63(5):634–641. doi: 10.1093/cid/ciw346. [DOI] [PubMed] [Google Scholar]
- 15.Madhi S.A., Cunliffe N.A., Steele D., Witte D., Kirsten M., Louw C., et al. Effect of Human Rotavirus Vaccine on Severe Diarrhea in African Infants. N Engl J Med. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 16.Steele A.D., Reynders J., Scholtz F., Bos P., de Beer M.C., Tumbo J., et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202(S1):S93–S100. doi: 10.1086/653550. [DOI] [PubMed] [Google Scholar]
- 17.Zaman K., Sack D.A., Neuzil K.M., Yunus M., Moulton L.H., Sugimoto J.D., et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLoS Med. 2017;14(4):e1002282. doi: 10.1371/journal.pmed.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari N., Rongsen-Chandola T., Bavdekar A., John J., Antony K., Taneja S., et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. The Lancet. 2014;383(9935):2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isanaka S., Langendorf C., McNeal M.M., Meyer N., Plikaytis B., Garba S., et al. Rotavirus vaccine efficacy up to 2 years of age and against diverse circulating rotavirus strains in Niger: Extended follow-up of a randomized controlled trial. PLoS Med. 2021 Jul 2;18(7):e1003655. doi: 10.1371/journal.pmed.1003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni P.S., Desai S., Tewari T., Kawade A., Goyal N., Garg B.S., et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35(45):6228–6237. doi: 10.1016/j.vaccine.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feikin D.R., Laserson K.F., Ojwando J., Nyambane G., Ssempijja V., Audi A., et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine. 2012;30:A52–A60. doi: 10.1016/j.vaccine.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Sow S.O., Tapia M., Haidara F.C., Ciarlet M., Diallo F., Kodio M., et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine. 2012;30:A71–A78. doi: 10.1016/j.vaccine.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 23.Tapia M.D., Armah G., Breiman R.F., Dallas M.J., Lewis K.D.C., Sow S.O., et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub-Saharan Africa. Vaccine. 2012;30:A79–A85. doi: 10.1016/j.vaccine.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Steele A.D., De Vos B., Tumbo J., Reynders J., Scholtz F., Bos P., et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28(39):6542–6548. doi: 10.1016/j.vaccine.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari N., Sharma P., Glass R.I., Ray P., Greenberg H., Taneja S., et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine. 2006;24(31-32):5817–5823. doi: 10.1016/j.vaccine.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Bhandari N., Sharma P., Taneja S., Kumar T., Rongsen‐Chandola T., Appaiahgari M., et al. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200(3):421–429. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- 27.Chandola T.R., Taneja S., Goyal N., Antony K., Bhatia K., More D., et al. ROTAVAC® does not interfere with the immune response to childhood vaccines in Indian infants: A randomized placebo controlled trial. Heliyon. 2017;3(5):e00302. doi: 10.1016/j.heliyon.2017.e00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari N., Antony K., Balraj V., Rongsen-Chandola T., Kumar T., Sinha B., et al. Assessment of risk of intussusception after pilot rollout of rotavirus vaccine in the Indian public health system. Vaccine. 2020;38(33):5241–5248. doi: 10.1016/j.vaccine.2020.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groome M.J., Tate J.E., Arnold M., Chitnis M., Cox S., de Vos C., et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clin Infect Dis. 2020;70(8):1606–1612. doi: 10.1093/cid/ciz431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy S.N., Nair N.P., Tate J.E., Thiyagarajan V., Giri S., Praharaj I., et al. Intussusception after rotavirus vaccine introduction in India. N Engl J Med. 2020;383(20):1932–1940. doi: 10.1056/NEJMoa2002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tate J.E., Mwenda J.M., Armah G., Jani B., Omore R., Ademe A., et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378(16):1521–1528. doi: 10.1056/NEJMoa1713909. [DOI] [PubMed] [Google Scholar]
- 32.Pitzer V.E., Bennett A., Bar-Zeev N., Jere K.C., Lopman B.A., Lewnard J.A., et al. Evaluating strategies to improve rotavirus vaccine impact during the second year of life in Malawi. Sci Transl Med. 2019;11(505) doi: 10.1126/scitranslmed.aav6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson A., Robinson K., Vallée-Tourangeau G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine. 2016;34(8):1018–1024. doi: 10.1016/j.vaccine.2015.11.065. [DOI] [PubMed] [Google Scholar]