Abstract
Currently, no formal mechanisms or systematic approaches exist to inform developers of new vaccines of the evidence anticipated to facilitate global policy recommendations, before a vaccine candidate approaches regulatory approval at the end of pre-licensure efficacy studies. Consequently, significant delays may result in vaccine introduction and uptake, while post-licensure data are generated to support a definitive policy decision. To address the uncertainties of the evidence-to-recommendation data needs and to mitigate the risk of delays between vaccine recommendation and use, WHO is evaluating the need for and value of a new strategic alignment tool: Evidence Considerations for Vaccine Policy (ECVP). EVCPs aim to fill a critical current gap by providing early (pre-phase 3 study design) information on the anticipated clinical trial and observational data or evidence that could support WHO and/or policy decision making for new vaccines in priority disease areas.
The intent of ECVPs is to inform vaccine developers, funders, and other key stakeholders, facilitating stakeholder alignment in their strategic planning for late stage vaccine development. While ECVPs are envisaged as a tool to support dialogue on evidence needs between regulators and policy makers at the national, regional and global level, development of an ECVP will not preclude or supersede the independent WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) evidence to recommendation (EtR) process that is required for all vaccines seeking WHO policy recommendation.
Tuberculosis (TB) vaccine candidates intended for use in the adolescent and adult target populations comprise a portfolio of priority vaccines in late-stage clinical development. As such, TB vaccines intended for use in this target population provide a ‘test case’ to further develop the ECVP concept, and develop the first WHO ECVP considerations guidance.
Keywords: WHO, Vaccine, Preferred Policy Profile, Evidence Considerations for Vaccine Policy, Data, Policy, Regulators, Policy makers, Clinical research, Implementation, Tuberculosis, Adolescents, Adults, Developers, Country, Regional, Donor, Procurement, Financing
1. Background
A major objective of the World Health Organization (WHO), reaffirmed in the Immunization Agenda 2030 (IA2030) [1], is to promote the development of vaccines with optimal suitability and effectiveness for use in low- and middle-income countries (LMICs), encourage timely national introductions, and ensure rapid and successful implementation, thereby maximizing global vaccine impact.
The pathway from vaccine product concept, through development, licensure and deployment is costly and inherently uncertain for vaccine developers, particularly for vaccines against diseases where the overwhelming burden is in LMICs. Piot et al have characterised a sequence of hurdles that need to be overcome from discovery to vaccine uptake, to achieve sustainable population impact [2]. While there has been significant progress in overcoming the translational gap in recent years (i.e., transitioning a vaccine candidate from bench to early phase clinical testing), many candidates continue to face uncertainty on the path through late stage product development to introduction. This can significantly impact investment in these candidates, particularly those for which a large traditional phase 3 efficacy study is a prerequisite to licensure and those targeted largely, if not solely, for public sector use in LMICs.
Early in vaccine product development, WHO identifies and defines Preferred Product Characteristics (PPCs) for priority vaccines, including those intended for use in LMICs, that articulate preferential product attributes for programmatic use. These PPCs are intended to inform candidate specific target product profiles (TPPs) developed by vaccine developers. As candidates proceed through clinical development, vaccine product developers consult with national regulatory authorities (NRAs) on the data needed for licensure. However, while policy and practice assumptions may underlie PPCs, no mechanism currently exists to make explicit the evidence expected for global policy recommendations, at least until the vaccine approaches regulatory approval, at the end of its efficacy study. Consequently, vaccine candidates that enter phase 3 efficacy testing may face a delay in deployment post-licensure, until a pilot study or post-licensure data to support a policy decision are generated, resulting in significant delays to vaccine introduction and uptake [3].
To address this gap, WHO is developing the concept of a new strategic alignment tool, the Evidence Considerations for Vaccine Policy (referred to as ECVP, and previously referred to as the Preferred Policy Profile, or PPoP). ECVPs aim to provide early (pre-phase 3 study design) information on the anticipated data and evidence that could support WHO policy decision making for new vaccines, in priority disease areas. ECVPs are envisaged to inform vaccine developers and funders of evidence expectations for policy, to enable this to be included in their strategic planning for late stage vaccine development. The ECVP is considered a tool to support dialogue and encourage alignment on evidence needs between regulators, policy makers and the national, regional and global level stakeholders to mutually outline the clinical trial and observational data or evidence needed for policy and program decisions for vaccine candidates, and help mitigate any delay in implementation post-licensure. The ECVP is intended to build upon WHO PPC guidance for vaccines but aims to focus on the data and evidence that are expected to be generated to support decisions on use case and implementation strategy. Furthermore, it will be a tool to facilitate early and ongoing communication between regulators, financing and procurement agencies, national and regional immunization technical advisory groups (NITAGs and RITAGs), country level decision makers, as well as researchers, civil society organisations and community representatives, and technical experts who may be involved in compiling evidence to make recommendations. The purpose of the document is to facilitate a discussion with all relevant stakeholders.
While the ECVP deliberation process and the considerations document itself does not preclude or supersede the independent SAGE evidence to recommendation process [4] that is required for all vaccines seeking WHO policy recommendation, it is intended that development an ECVP will catalyse discussions with SAGE on priority vaccines as they approach efficacy testing.
TB vaccine candidates intended for use in the adolescent and adult target populations [5] are in late-stage clinical development and are considered priority vaccines for WHO. The WHO’s Immunization, Vaccine & Biologicals (IVB) department, and the Global TB (GTB) Programme see this as an ideal ‘test case’ to further develop the ECVP concept, and the first ECVP considerations guidance to accelerate their pathway to WHO policy recommendation and use. WHO has developed PPCs for TB vaccines for use in this priority population [6], and to inform TPPs for candidates approaching late-stage clinical development.
This report summarizes the findings from two virtual stakeholder consultations which were held on May 17th and 24th, 2021, to discuss the concept of ECVP’s in general, and their application to TB vaccines for adults and adolescents, in particular.
2. Objectives and format of the stakeholder consultations
WHO convened country, regional and global stakeholders, with vaccine product, policy, financing, implementation, community engagement, and TB vaccine development expertise. The intent of this consultation was to i) examine the perception of a ‘guidance gap’ between WHO PPCs and scientific advice from NRAs, and review by policy bodies such as WHO’s SAGE, and ii) whether earlier engagement/guidance on data and evidence that may inform policy would help to de-risk investment in late stage product development for vaccines in general, and TB vaccines in particular. A draft framework for a generic ECVP was introduced to facilitate the discussion, which was contextualized in the notion of a TB vaccine ECVP as a test case.
The first meeting consisted of a series of presentations from global experts (section 3); while the second meeting comprised a series of round tables with relevant stakeholders, namely vaccine developers/manufacturers, regulatory agencies, country and regional representatives and donor/procurement agencies (section 4). After the meeting, a revised draft generic ECVP framework was shared with the meeting attendees for additional input to clarify the benefit of ECVPs and confirm whether there is a guidance gap they could address, and to advise on whether the proposed attributes within the generic ECVP framework are appropriate.
3. Summary of presentations
In this section, we summarise the presentations that framed the discussion for the round tables.
3.1. Lessons learned from other vaccines (RTS,S and COVID-19)
Despite malaria prevention being a high global health priority, the first efficacious malaria vaccine endured a protracted journey through regulatory, policy and financing pathways. This contrasts with the accelerated timelines seen in 2020–2021 in emergency authorization processes for COVID-19 vaccines. The lessons learned from both the RTS,S (malaria) and COVID-19 vaccine development and implementation experiences were reviewed by Dr David Kaslow (PATH) and Dr Alejandro Cravioto (National Autonomous University of Mexico), to identify which of these could be applied to a future ECVP mechanism and proactively anticipate what is needed to expedite regulatory, policy and introduction decision making for TB vaccines.
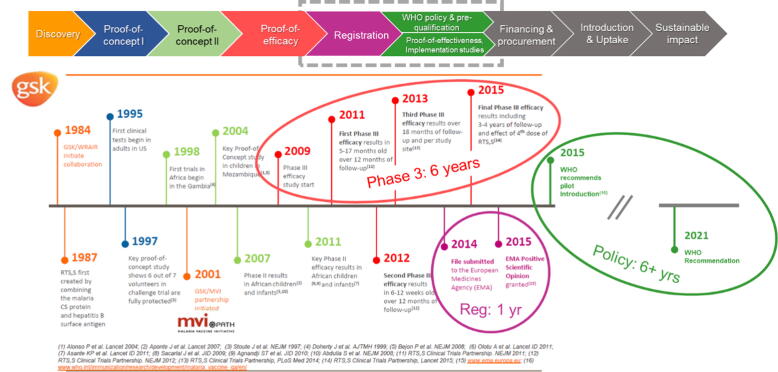
In 2019, malaria caused 229 million cases worldwide, with 409,000 deaths, 67% (274,000) of which were in children under 5 years of age, living in the world’s poorest countries. [7] The world’s first malaria vaccine RTS,S (Mosquirix®*) has taken over 35 years to evolve from concept to the point of consideration for global policy recommendation by WHO (approved in October 2021). This journey encompassed a six-year phase 3 efficacy programme that included a four year follow up, a regulatory assessment through the Article 58 procedure of the European Medicines Agency (EMA), and since 2015, a rigorous six-year extended policy review process (Fig. 1).
Fig. 1.
Timelines for the malaria vaccine RTS,S (Mosquirix) from concept to the point of consideration for global policy recommendation (over 35 years).
* Registered owner is GSK
The global and country-level financing review stage is yet to come. The protracted regulatory-policy-financing pathway for this vaccine, for which there is an urgent public health need and clear demand from endemic countries, is frequently cited as the basis for needing earlier and improved coherence between the evidence expectations for regulatory authorities, global policy makers, and public financing entities to avoid what is referred to as the vaccine implementation gap. [8]
The era of COVID-19 vaccine development, policy making, and introduction has brought new paradigms to global partnerships, novel financing mechanisms, risk assessment, and unprecedented timelines to regulatory approval and deployment, as national regulatory authorities and global policy makers worked together. [9], [10]
The key points included:
-
•
There were misalignments between regulators and policy makers on the interpretation of evidence related to RTS,S, and assessment of benefits and risks of the malaria vaccine. This occurred in spite of a technology roadmap with strong global consensus (first published in 2006 and updated in 2013 [11]), an agreed WHO PPC for malaria vaccines [12], and robust oversight from a Joint Technical Expert Group (JTEG) that reported to both the WHO Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC). [13]
-
•
EMA offered a positive regulatory opinion in 2015, based on the indication of active immunisation of children aged 6 weeks up to 17 months against malaria caused by Plasmodium falciparum and against hepatitis B. [14]
-
•
However, WHO SAGE and MPAC did not recommend use of the vaccine in 6–12-week-old infants, and requested additional data in 5–17-month olds. [15]
-
•
There were critical evidence-to-recommendation gaps identified during the post-licensure review of RTS,S; for example the absence of real-world evidence of the feasibility of delivering all 4 doses within the schedule, including one within the second year of life, resulting in uncertainties about the acceptability, implementation, and cost-effectiveness of the vaccine. SAGE also requested evidence on mortality to assess the public health impact of the vaccine, a parameter infeasible to measure in the artificial context of intensive monitoring and idealized standard of care and case management in a phase 3 clinical efficacy study. This evidence gap resulted in a delay of several years, and substantial additional investment, between definitive regulatory and the anticipated policy decisions.
-
•
Changes in the epidemiologic landscape, the environment of other non-vaccine interventions, or the attainment of an efficacy threshold by a candidate vaccine can lead to revisions in target vaccine efficacy thresholds. One example of a change due to the attainment of an efficacy threshold was the initial target of a malaria vaccine that could achieve at least 50% protective efficacy against severe disease and death and last longer than on year. This goal was stipulated in the first 2006 malaria vaccine roadmap, and was partially met with a 50% reduction in severe malaria by the phase III efficacy study (2013). In drafting the updated malaria roadmap in 2013 and the PPC in 2014, developed by WHO and collaborators, the efficacy goal was changed to a 75% reduction in clinical malaria during 2 years, with a booster dose - to inform targets for next-generation malaria vaccines. Scenario planning and sensitivity analyses of efficacy outcomes could help to identify key assumptions driving favourable regulatory, policy, and financing decisions, and the ‘tipping points’ with respect to vaccine efficacy, safety profile, impact, and potential effectiveness.
-
•
New platform technologies, e.g., mRNA, and innovative regulatory approaches, e.g., conditional marketing authorization [16], [17] (CMA), should be leveraged to accelerate development of TB vaccines, but the phase 3 studies should be designed to address the needs of both regulators and policy makers. Assessment of vaccine effectiveness and pharmacovigilance has been shown to be feasible, while the vaccine is in use, in the case of COVID-19 vaccines for which vaccine efficacy had already been demonstrated in placebo controlled clinical endpoint efficacy trials. This approach enables faster access to an urgently needed vaccine, whilst also improving the investment case for vaccine manufacturers – providing there are financing and procurement commitments in place.
-
•
A strategic alignment tool - and process - analogous to the stakeholder development of WHO PPCs or target product profiles to delineate expectations of vaccine attributes, is needed to mitigate the widening evidence gap between what is needed for registration and what is needed for policy recommendations. This key stakeholder alignment tool should articulate expectations for policy parameters such as feasibility of delivery, resource utilisation, impact on equity, end-user acceptability to beneficiaries, affected communities, and national programs, etc, before phase 3 study design. This will be particularly important as more candidates on novel platforms, for example mRNA, for which there is currently limited field experience, approach licensure, policy, and financing reviews. Historically, evidence consideration by SAGE comes late in phase 3, but the ECVP mechanism would facilitate earlier, proactive engagement and alignment.
3.2. The urgent need for TB vaccines in the context of TB preventative strategies
Dr Matteo Zignol (WHO) reviewed the urgent need for TB vaccines. TB remains one of the leading infectious killers worldwide and the leading cause of death among people living with HIV (PLHIV). Despite it being a preventable, treatable and curable disease, every year it claims more than a million lives and affects 10 million people [18], with enormous impacts on families and communities. In 2020, the impact of the COVID-19 pandemic on the health workforce, reduced access to health facilities due to lockdowns and the reallocation of financial and human resources from TB services, have resulted in reduced access to TB services and in further transmission of TB, with a drop in TB case notification of around 20% compared to 2019, worldwide. TB is an emergency.
Achieving the End TB Strategy targets of 2035 requires that new vaccines advance from the research pipeline into large scale programmatic use, especially in the latter decade of the Strategy. This includes both pre- and post-exposure vaccines, which represent the most important tools to End TB. Efforts should be made to explore ways in which data can be generated for people living with HIV (PLHIV), pregnant women, children, diabetics, immunocompromised people, and how findings from non-vulnerable groups can be translated to vulnerable groups.
Finally, TB vaccine recommendations and policies for use should be closely linked to current and prospective policies on diagnosis, and treatment of TB infection, and disease, and should be developed with engagement of all stakeholders, including Member States, patients and civil society representatives and technical agencies.
3.3. Phase 3 TB vaccine clinical trial designs will need enhanced regulatory and policy strategy considerations beyond safety and efficacy
The phase 3 trial design and regulatory considerations for a TB vaccine indicated for use in adolescents and adults were reviewed by Dr Taryn Rogalski-Salter and Dr Alexander Schmidt (Bill & Melinda Gates Medical Research Institute). As with any vaccine, the licensure package for a new TB vaccine will need to include sufficient evidence of safety and efficacy in the target population, i.e., the benefit/risk assessment must conclude that benefits of vaccination outweigh risks, and the quality of the vaccine is assured. With TB being a leading cause of death from infectious diseases in many LMICs, delays in registration and implementation will come at a particularly high cost in terms of excess morbidity and mortality. This pressing burden of disease needs to be considered by regulators and policy makers to determine the most expeditious route to first and subsequently global registration, and to define which data are required for CMA, licensure and recommendation for use. Based on lessons learned from COVID-19 vaccine development, strategies could include CMA, WHO’s emergency use listing (EUL) [19], or other benefit/risk-based introduction. This would enable generation of effectiveness data while in use, to support an eventual global policy recommendation.
From an implementation perspective, it is important that vaccine efficacy (VE) data be generated for populations in high burden settings, regardless of previous infection with Mycobacterium tuberculosis (Mtb), because systematic screening for Mtb infection is currently neither feasible nor affordable in LMICs (it is estimated that one-fourth of the world's population has latent TB) [20]. The phase 3 TB VE trial will need to address scientific and logistical challenges, and the design includes several variables. For prevention of disease (PoD) VE trials, the definition of the primary endpoint is critical. In the M72/AS01E vaccine Phase 2b trial, sensitivity analyses indicated that the more stringent requirement for laboratory confirmation of pulmonary TB was associated with a higher VE point estimate (68% vs 50%) and a slightly lower observed incidence rate (IR) (0.5% vs 0.6%). However, the confidence intervals (CIs) for both VE and IR were wide and overlapping. [21]
Clinical trial simulations help us understand some of the scientific challenges in demonstrating PoD: the observed VE and the required lower bound (LB) of the 95% CI determine the number of disease events (e.g., laboratory-confirmed pulmonary TB) needed to demonstrate VE. The number of events needed for analysis is very sensitive to both VE and the LB. The lower the VE, the more cases are needed to demonstrate that the LB exceeds zero. For example, a simulation exercise conducted at Gates MRI indicates that 110, 90 and 70 events are needed with a VE of 50%, 55%, and 60%, respectively, to have > 90% power to demonstrate VE with a 95% CI LB. Raising the required 95% CI LB leads to a significant increase in the number of events needed to infer efficacy. For example, if VE = 50%, 110 events are needed for 90% power to observe a 95% CI LB > 0, but>240 events are needed to observe a 95% CI LB > 20%. The period to observe the number of events needed to infer efficacy with a chosen LB depends on a) the overall IR in the trial population, b) the number of trial participants, c) the time to full enrolment, and d) the duration of follow up. Since TB incidence is highly heterogeneous within most high burden countries, and even within health districts, it is critically important to select clinical trial sites with very high IRs to accelerate accrual of events. However, incidence is often not known at site level and site-level epidemiological studies are needed to optimize site selection.
The number of sites needed to enrol a large (e.g., 18,000 participants) Phase 3 trial is substantial (e.g., >50), and clinical trials capacity does not always exist in TB hotspots as incidence is typically highest in the poorest and lowest-resourced communities in LMICs. Clinical research capacity would need to be built at these TB hotspots or sites with lower incidence used. Clinical trial simulations suggest that if it took 3 years to enrol 16,000 participants in TB hotspots with an average TB IR of 0.5% per person-year of follow-up, there would be a 90% probability to observe 70 TB events within 3.5 years, 140 events in 5.5 years and 200 events within 7 years of the start of enrolment. The length of time to accumulate the necessary TB events to measure efficacy of vaccine candidates is misaligned with the goal of accelerating the timelines for availability of a TB vaccine to help end the epidemic of TB. A lesson learned from COVID-19 vaccine development is that large trials that enrol quickly conducted in areas with high disease incidence will deliver results much earlier and generate the evidence to support licensure/registration much more rapidly.
For a TB vaccine to be authorized / licensed and recommended, safety and efficacy should ideally be demonstrated in a general population, however the risk of progression to TB differs greatly amongst population subsets. Since Mtb infection is a prerequisite for developing TB, most of the TB cases in a VE trial will be observed amongst participants who were already infected with Mtb, as detected by an Interferon Gamma Release Assay (IGRA). Very few cases of active TB will be observed amongst baseline IGRA-negatives. People living with HIV, or other pre-existing conditions (e.g., diabetes mellitus) who are at increased risk for developing TB should be included in Phase 3 VE trials to gather safety data and descriptive efficacy data in these sub-populations. However, the number of TB cases win these subsets will likely not be sufficient to demonstrate statistically significant VE. At the time of licensure, data on concomitant use with other vaccines, use in pregnancy or in (non-HIV) immunocompromised people may be very limited, if available at all.
With these considerations in mind, evidence requirements for the regulatory strategy, whether that be an initial conditional use or full licensure, and for subsequent policy recommendation and financing, need to be considered carefully and ideally prior to the start of Phase 3 trials to align on data needed for decision-making. More stringent requirements will take additional time – often years – and significantly more cost, to generate. Benefits and risks of vaccination (versus no new intervention) need to be evaluated in terms of labelling indications at the point of initial vs later licensure and post-marketing activities, including pharmacovigilance. Since the TB vaccine will ultimately be produced by a vaccine manufacturer/s entity with manufacturing capabilities and capacity to support a global market, it will also be important to engage the commercialization partner/s in these discussions related to data and evidence for regulatory approval and policy development.
The demand for and uptake of TB vaccines in target markets is uncertain, presenting an investment risk to late-stage manufacturing partners. Incentives, guarantees or financial commitments may help to alleviate this risk. Given these considerations inherent in accelerated programs, early and continued engagement with stakeholders, including LMIC NRAs, NITAGs, RITAGs, SAGE, Gavi, the Vaccine Alliance WHO and others will be key to align on expectations for data, share information on the development status and plans such as design of the phase 3 study, and allow review of data as they become available. The ECVP aims to address these challenges by outlining the anticipated data and evidence (including measures of efficacy and duration of protection (DoP) that would be required at the time of conditional approval and/or licensure, depending on the vaccine), that could support WHO policy decision making for these urgently required new vaccines.
3.4. Modelling the potential health and economic impact of adolescent/adult TB vaccines
A summary of the evidence on the potential health, socio-economic, and wider impact of adolescent/adult TB vaccines was presented by Prof. Richard White (London School of Hygiene & Tropical Medicine). The global TB vaccine modelling literature suggests that globally, PoD vaccines in IGRA-positive populations would provide faster and greater impact than prevention of infection (PoI) vaccines, but the impact of PoI vaccines increases in higher transmission settings, e.g. India and South Africa (SA). [5], [22], [23], [24] Modelling also suggested in LMICs, as little as 5 years duration of protection may be cost effective if targeted at adolescents and adults with 10-yearly mass campaigns; and 50% VE, duration of protection around five years in China, four years in SA and three years in India could lead to ∼ 25% reduction in TB incidence in 2050. [5], [23] In LMICs, as low as 20% VE could be cost effective if delivered to adolescents/adults. In LMICs, adolescent and adult vaccination may deliver greater and faster impact than infant vaccination. Children are important, and to reduce TB in 0–4-year olds, vaccination of adolescents/adults may be more effective than vaccinating neonates directly. In ageing, reactivation driven epidemics, such as China, vaccines suitable for latently infected older adults (>60 years) may provide greater impact than adolescent vaccination. [25]
The implications of this modelling evidence for vaccine development were summarized.
-
•
With respect to priority target populations, if maximum population-level impact by 2050 is the goal, development of vaccines for adolescents/adults should be prioritized. Further, in populations like China the inclusion of older adults (at least 60–64 years) in clinical trials should be considered.
-
•
Post-infection populations should be recruited in all settings, but pre-infection populations should also be recruited in higher transmission settings (e.g. India and SA), and if feasible, trials should be powered to assess efficacy in both populations
-
•
HIV-positive populations should be included in the phase 3 efficacy study
-
•
In all settings, PoD endpoints would be useful for demonstrating future impact, however, in higher transmission settings (like India and SA) PoI endpoints could be used, especially as proof of concept
-
•
It would be desirable to assess feasibility of designing trials to detect lower VEs because of the anticipated cost effectiveness of vaccines with lower VEs
-
•
Studies would benefit from extended follow up to five plus years, but vaccines with shorter duration of protection may be impactful and cost-effective.
The results from two recent modelling studies were also shared. The first study explored the impact of TB vaccines on multidrug and rifampicin-resistant TB (MDR/RR-TB) and showed that new TB vaccines could substantially reduce MDR/ RR-TB, and avert second -line therapy, and they may be cost effective, depending on the vaccine characteristics and setting. [26] The second recent study estimated the potential M72/AS01E vaccine cost effectiveness in SA and India, and suggested that an M72-like vaccine could be cost effective in both settings, depending on eventual vaccine characteristics, price, delivery costs, and duration of protection.
There are forthcoming country interviewee data on feasibility of M72/AS01E vaccine or BCG revaccination implementation strategies in SA, India and China and modelling evidence from a ‘Full Value Assessment of TB Vaccines’ based on the WHO PPCs, and M72/AS01E and BCG revaccination-specific modelling evidence in SA and India. These modelling results will inform the guidance developed within the ECVP.
3.5. Considerations for financing a TB vaccine for adolescents and adults
Deepali Patel (Gavi) reviewed the considerations for financing a TB vaccine for use in Gavi- eligible countries. Gavi’s vaccine investment strategy (VIS) is undertaken every 5 years [27] (the next one is anticipated in 2023–2024) to evaluate new opportunities for investment in vaccines and other immunisation products that may be licensed and positioned for WHO prequalification within 5 years of the decision. Gavi identifies and reviews the latest evidence for each candidate investment along a number of criteria including health and economic impact, value for money, and equity. The process is consultative with partners and external stakeholders to develop the recommendations.
There are considerable knowledge gaps for VIS decision making that have been seen in the past years.
For TB vaccines, VIS assessment would include identification of a candidate sufficiently far along in clinical development to form the profile of an investment, an assessment of that candidate against the criteria in the VIS evaluation framework, comparison to other vaccine candidates up for consideration at that time, other financiers of a TB vaccine, and how non-infant TB vaccines fit into the broader TB control programs.
The evaluation framework criteria are expected to remain largely the same in the next VIS cycle as the previous, with updates dependent on how analytics and data have broadly evolved across vaccine candidates, e.g., impact of the vaccine on antimicrobial resistance (AMR). If Gavi were to approve a TB vaccine investment, only countries under Gavi’s eligibility threshold qualify for Gavi support. Given the burden of TB in non-Gavi countries, immunisation funders would have to consider how access to novel TB vaccines for these countries could also be realised.
The draft ECVP framework which has been developed incorporates the key evaluation criteria and indicators used by Gavi for their VIS evidence review and ranking for the vaccines. The ECVP will help to fill the knowledge gaps for vaccine financing decision making, including for TB vaccines.
4. Roundtables
Four roundtables with vaccine developers, regulators, country and regional representatives and donor and procurement agencies were convened to discuss their perspectives on whether and how a ECVP, if it existed, would inform their decision making. The key challenges and the potential benefits of the ECVP highlighted in these discussions are mentioned in Table 1.
Table 1.
Key discussion points of the Roundtables.
| Stakeholder type | Key Challenges and Considerations | Perceived benefits of the ECVP and how it could address challenges identified |
|---|---|---|
| Vaccine Developers and Manufacturersrepresenting both the developing countries vaccine manufacturers network (Rajinder Suri, DCVMN) and industrialized countries pharmaceutical companies (Ugur Sahin, BioNTech) | Key gap between the information in the WHO PPC guidance and what is considered to inform policy recommendations include:
|
|
RegulatorsThe regulators included:
|
Context: Dr Boitumelo Semete provided context to the regulatory environment in SA and the urgent need for new TB vaccines, to frame the discussion:
|
|
|
|
|
Country and Regional level representativesThis panel included:
|
Context: Prof. Shabir Mahdi opened this round table by describing the policy decision making process in South Africa, with respect to new TB vaccines:
|
|
|
|
|
Donor and Procurement agenciesThe panel included representatives from:
|
|
|
5. Survey on the potential value and content of a ECVP
Following the two meetings, a survey was circulated to the meeting participants to determine their understanding of the ECVP purpose, assess whether the ECVP would address a guidance gap and request their initial feedback on the generic ECVP draft framework.
The survey questions focused on the:
-
•
Objectives of the ECVP, the target audience and the preferred product development stage for generation of the data/evidence;
-
•
Clarity regarding how the ECVP relates to the SAGE evidence to recommendation process;
-
•
Clarity regarding how the ECVP relates to the WHO PPC guidance;
-
•
Value of the ECVP framework, i.e. does it address a guidance gap;
-
•
Rationale for choosing TB vaccines for adults and adolescents as a test case for development of the first ECVP
There was broad agreement from the meeting participants that most of these points were well described in the meetings, and in the draft ECVP framework. However, many respondents did seek further clarity on how the ECVP framework would prepare the pathway for earlier, formal SAGE engagement, for example in establishing a SAGE vaccine working group, or whether SAGE would review the ECVP at the draft stage and provide input. Earlier SAGE engagement in evaluating pipeline vaccines, as well as involvement in considering data needs for policy is indeed the objective of the ECVP. Assessing the feasibility of, and testing the mechanism for this earlier engagement is the purpose of the first TB vaccine ECVP that has been proposed.
Feedback received on the draft generic ECVP framework included the need to:
-
•
Specify that those aspects related to manufacturing and quality are more pertinent to the regulatory dossier and are outside the scope of this early policy consideration framework;
-
•
Clarify that for the examples of specific populations listed, some might be prioritized for a particular vaccine or country/region (context), if distinct from the target population;
-
•
Clarify that resource use for vaccine implementation should include vaccine cost, operational costs, and implementation costs;
-
•
Consider that engagement timelines should be considered globally and by individual countries for their planning purposes.
With respect to the TB test case specifically, specific consideration of aspects related to ensuring vaccine access, such as market shaping and potential procurement mechanisms, will be addressed in a ‘late stage’ TB vaccine roadmap that is currently under development, intended to chart the path to commercialization and implementation of TB vaccines in high burden countries.
The feedback was incorporated in the draft ECVP framework. A WHO ECVP Working group has been set up to finalize the ECVP framework and prepare the TB vaccine test case. The draft ECVP framework can be accessed at WHO Evidence Considerations for Vaccine Policy Development (ECVP).
6. Conclusion and next steps
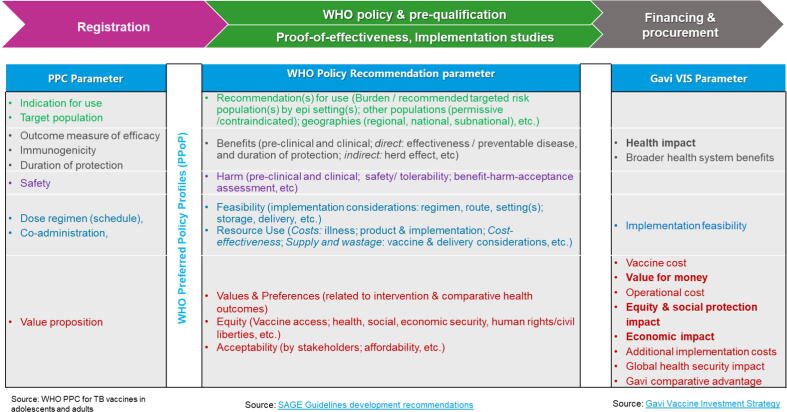
The meeting attendees felt that there is currently a significant guidance gap between the WHO PPCs and NRA advice, and review of vaccines by policy bodies such as WHO’s SAGE. Fig. 2 highlights the key parameters required for registration, policy and financing, and how these evolve across the different stages. The ECVP guidance would help to articulate priorities and evidence needs for clinical research and development, implementation and programmatic feasibility. This guidance would enable proactive generation of data in the Phase 3 trials to inform regulatory, policy, financing and implementation considerations for vaccines.
Fig. 2.
Key parameters considered in vaccine registration, WHO policy consideration and PQ and vaccine financing and procurement.
Development of ECVPs for vaccines approaching late stage development will help in the alignment of the data needs between regulators and policy makers, and facilitate engagement of the key stakeholders in an organised fashion, to increase predictability of vaccine development programs and de-risk investment. The outcome would be to accelerate the development, planning, financing, introduction and access (including equitable access) to vaccines, especially in LMICs. Using TB vaccines for adults and adolescents as a ECVP test case has considerable merit, given the development stage of candidates and the urgent public health need for these vaccines.
The ECVP considerations should be a living document and will be non-binding, so as to not inhibit innovation in new vaccine development and delivery, as well as to avoid interference with the formal SAGE Evidence-to-Recommendation process. The ECVP may need to be updated based on significant changes in the disease epidemiology, vaccine pipeline development, use of other TB interventions, etc. The TB ECVP test case aims to assess how this novel process and the development of early policy considerations can foster SAGE engagement ideally well before phase 3 design, to meet anticipated expectations for policy recommendation, whilst preserving the mandate of SAGE in this regard.
As a next step, it was recommended that a WHO ECVP WG be set up which would finalise the draft generic ECVP framework and then work to develop a ECVP for TB vaccines for use in adults and adolescents.
In parallel, a roadmap for the ‘late stage’ development of TB vaccines is being prepared that will address aspects such as commercial manufacturing, financing, access, and other considerations which will not be covered by the ECVP. Development of a ECVP for new TB vaccines will be one of the activities included in this ‘late stage’ TB vaccine roadmap.
7. Disclaimer
All authors have participated in the article preparation, including the conception and design underlying the articles messages, as well as, during drafting and revising the content of the article. None of the authors have expressed any conflict of interest. The authors alone are responsible for the views expressed in the article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: TRS and AS are employees of the Bill & Melinda Gates Medical Research Institute and are involved in developing a late-stage TB vaccine. The other co-authors have no COIs to declare.
Acknowledgments
Acknowledgements
The World Health Organisation gratefully acknowledges the participation of all presenters and round table panellists in this meeting, as well as the meeting participants, many of whom responded to the ECVP survey. Sincere thanks to Prof. Ugur Sahin (BioNTech, Mainz, Germany) and Dr Rajinder Suri (Developing Countries Vaccine Manufacturing Network, Nyon, Switzerland) for their participation in the roundtable and for review of this manuscript. The authors also thank Jean-Pierre Amorij (UNICEF) and Askar Yedilbayev (WHO Regional Office for Europe) for their review of this manuscript.
Funding
WHO’s work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [grant INV-003658].
References
- 1.Immunisation Agenda 2030. Accessed on October 12, s2021 at https://www.immunizationagenda2030.org/.
- 2.Piot P., Larson H.J., O’Brien K.L., N’kengasong J., Ng E., Sow S., et al. Immunization: vital progress, unfinished agenda. Nature. 2019;575(7781):119–129. doi: 10.1038/s41586-019-1656-7. [DOI] [PubMed] [Google Scholar]
- 3.THE YELLOW HOUSE: Vaccine equity: a comparison of new vaccine introductions with all the talk about vaccine equity, how long does it normally take for a new vaccine to be available? Accessed on 14 June, 2021 at https://www.dropbox.com/s/r3u1fz88rewho0w/Vaccine%20Equity%20-%20a%20comparison%20of%20new%20vaccine%20introductions%20-%20TYH%2029%20April%202021.pdf?dl=0.
- 4.WHO Guidance for the development of evidence-based vaccine-related recommendations. Accessed on 3 August 2021 at https://www.who.int/publications/m/item/guidance-for-the-development-of-evidence-based-vaccine-related-recommendations.
- 5.Knight G.M., Griffiths U.K., Sumner T., Laurence Y.V., Gheorghe A., Vassall A., et al. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A. 2014;111(43):15520–15525. doi: 10.1073/pnas.1404386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Preferred Product Characteristics for New Tuberculosis Vaccines. Accessed on June 12, 2021 at http://apps.who.int/iris/bitstream/handle/10665/273089/WHO-IVB-18.06-eng.pdf?ua=1.
- 7.WHO World malaria report 2019: Accessed on June 14, 2021 at https://www.who.int/publications/i/item/9789241565721.
- 8.O'Brien K.L., Binka F., Marsh K., Abramson J.S. Mind the gap: jumping from vaccine licensure to routine use. Lancet. 2016 May 7;387(10031):1887–1889. doi: 10.1016/S0140-6736(16)30394-4. PMID: 27203633. [DOI] [PubMed] [Google Scholar]
- 9.Black S., Bloom D.E., Kaslow D.C., Pecetta S., Rappuoli R. Transforming vaccine development. Semin Immunol. 2020;50:101413. doi: 10.1016/j.smim.2020.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rappuoli R., De Gregorio E., Del Giudice G., Phogat S., Pecetta S., et al. Vaccinology in the post-COVID-19 era. Proc Natl Acad Sci U S A. 2021;118(3) doi: 10.1073/pnas.2020368118. PMID: 33431690; PMCID: PMC7826410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malaria vaccine roadmap, 2013. Accessed on June 14, 2021 at https://www.malariavaccine.org/sites/mvi/files/content/page/files/TRM_update_nov13.pdf.
- 12.WHO Preferred Product Characteristics (PPC) for Malaria Vaccines, 2014. Accessed on June 14, 2021 at https://apps.who.int/iris/bitstream/handle/10665/149822/WHO_IVB_14.09_eng.pdf?sequence=1&isAllowed=y.
- 13.Background paper of the TRS,S/AS01 malaria vaccine, Sept 2015. Accessed on June 14, 2021 at https://www.who.int/immunization/sage/meetings/2015/october/1_Final_malaria_vaccine_background_paper_v2015_09_30.pdf.
- 14.First malaria vaccine receives positive scientific opinion from EMA. Press release 24/07/2015. Accessed on June 15, 2021 at https://www.ema.europa.eu/en/news/first-malaria-vaccine-receives-positive-scientific-opinion-ema\13.
- 15.World Health Organization Malaria vaccine: WHO position paper, January 2016 - Recommendations. Vaccine. 2018 Jun 14;36(25):3576–3577. doi: 10.1016/j.vaccine.2016.10.047. PMID: 28385607. [DOI] [PubMed] [Google Scholar]
- 16.EMA. Conditional marketing authorisation. Accessed on June 25, 2021 at https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation.
- 17.Cavaleri M., Enzmann H., Straus S., Cooke E. The European Medicines Agency's EU conditional marketing authorisations for COVID-19 vaccines. Lancet. 2021;397(10272):355–357. doi: 10.1016/S0140-6736(21)00085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuberculosis. WHO. Accessed n on July 28, 2021 at https://www.who.int/news-room/fact-sheets/detail/tuberculosis#:~:text=Worldwide%2C%20TB%20is%20one%20of,women%20and%201.2%20million%20children.
- 19.WHO. Emergency use listing procedure, 14 Dec 2020. Accessed on June 16, 2021 at https://www.who.int/publications/m/item/emergency-use-listing-procedure.
- 20.Cohen A., Mathiasen V.D., Schön T., Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54(3):1900655. doi: 10.1183/13993003.00655-201910.1183/13993003.00655-2019.Supp110.1183/13993003.00655-2019.Shareable1. [DOI] [PubMed] [Google Scholar]
- 21.Tait D.R., Hatherill M., Van Der Meeren O., Ginsberg A.M., Van Brakel E., et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med. 2019;381(25):2429–2439. doi: 10.1056/NEJMoa1909953. PMID: 31661198. [DOI] [PubMed] [Google Scholar]
- 22.Weerasuriya C.K., Clark R.A., White R.G., Harris R.C. New tuberculosis vaccines: advances in clinical development and modelling. J Intern Med. 2020;288(6):661–681. doi: 10.1111/joim.13197. PMID: 33128834. [DOI] [PubMed] [Google Scholar]
- 23.Harris R.C., Sumner T., Knight G.M., Zhang H., White R.G. Potential impact of tuberculosis vaccines in China. South Africa, and India. Sci Transl Med. 2020;12(564) doi: 10.1126/scitranslmed.aax4607. [DOI] [PubMed] [Google Scholar]
- 24.Harris R.C., Sumner T., Knight G.M., White R.G. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum Vaccin Immunother. 2016;12(11):2813–2832. doi: 10.1080/21645515.2016.1205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris R.C., Sumner T., Knight G.M., Evans T., Cardenas V., Chen C., et al. Age-targeted tuberculosis vaccination in China and implications for vaccine development: a modelling study. Lancet Glob Health. 2019;7(2):e209–e218. doi: 10.1016/S2214-109X(18)30452-2. [DOI] [PubMed] [Google Scholar]
- 26.Weerasuriya C.K., Harris R.C., McQuaid C.F., Bozzani F., Ruan Y., et al. The epidemiologic impact and cost-effectiveness of new tuberculosis vaccines on multidrug-resistant tuberculosis in India and China. BMC Med. 2021;19(1):60. doi: 10.1186/s12916-021-01932-7. PMID: 33632218; PMCID: PMC7908776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavi. Vaccine investment strategy. Accessed on 8 July 2021 at https://www.gavi.org/our-alliance/strategy/vaccine-investment-strategy.
- 28.WHO, Good Manufacturing Practices. Accessed on June 30, 2021 at https://www.who.int/teams/health-product-and-policy-standards/standards-and-specifications/gmp.
- 29.EMA. Medicines for use outside the European Union. Accessed on June 16, 2021 at https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/medicines-use-outside-european-union.
- 30.EMA. Health technology assessment bodies. Accessed on June 30, 2021 at https://www.ema.europa.eu/en/partners-networks/health-technology-assessment-bodies.
- 31.UNICEF. Scaling vaccine procurement. Accessed on 8 July 2021 at https://www.unicef.org/supply/stories/scaling-vaccine-procurement.