Abstract
Lactate accumulation in the human gut is linked to a range of deleterious health impacts. However, lactate is consumed and converted to the beneficial short-chain fatty acids butyrate and propionate by indigenous lactate-utilizing bacteria. To better understand the underlying genetic basis for lactate utilization, transcriptomic analyses were performed for two prominent lactate-utilizing species from the human gut, Anaerobutyricum soehngenii and Coprococcus catus , during growth on lactate, hexose sugar or hexose plus lactate. In A. soehngenii L2-7 six genes of the lactate utilization (lct) cluster, including NAD-independent d-lactate dehydrogenase (d-iLDH), were co-ordinately upregulated during growth on equimolar d- and l-lactate (dl-lactate). Upregulated genes included an acyl-CoA dehydrogenase related to butyryl-CoA dehydrogenase, which may play a role in transferring reducing equivalents between reduction of crotonyl-CoA and oxidation of lactate. Genes upregulated in C. catus GD/7 included a six-gene cluster (lap) encoding propionyl CoA-transferase, a putative lactoyl-CoA epimerase, lactoyl-CoA dehydratase and lactate permease, and two unlinked acyl-CoA dehydrogenase genes that are candidates for acryloyl-CoA reductase. A d-iLDH homologue in C. catus is encoded by a separate, partial lct, gene cluster, but not upregulated on lactate. While C. catus converts three mols of dl-lactate via the acrylate pathway to two mols propionate and one mol acetate, some of the acetate can be re-used with additional lactate to produce butyrate. A key regulatory difference is that while glucose partially repressed lct cluster expression in A. soehngenii , there was no repression of lactate-utilization genes by fructose in the non-glucose utilizer C. catus . This suggests that these species could occupy different ecological niches for lactate utilization in the gut, which may be important factors to consider when developing lactate-utilizing bacteria as novel candidate probiotics.
Keywords: Human gut microbiota, lactate-utilizing bacteria, anaerobic metabolism, upregulation by lactate, transcriptomics
Data Summary
Novel draft genomes generated for this study have been made available from GenBank (https://www.ncbi.nlm.nih.gov/bioproject/) under BioProject number PRJNA701799. RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-10136. Further details of additional existing genomic data that were analysed in this project are given in Tables 1 and S2 (available in the online version of this article), and at https://github.com/SheridanPO/Lactate-utilizing-bacteria. Supplementary Material can be found in Figshare: https://doi.org/10.6084/m9.figshare.16510344.v1[1].
Table 1.
New and existing strains used for in vitro cultivation studies in this work, and their corresponding genomes. ‘N/A’ indicates no genome available for this strain, which was used for in vitro work only. The full list of strains used for genomic-based analyses is shown in Table S2
|
Species |
Strain |
Donor |
Genome accession |
Contigs |
CDS no. |
Genome reference |
Strain reference |
|---|---|---|---|---|---|---|---|
|
L2-7 |
1 |
GCA_900209925 |
1 |
3005 |
[71] |
[72] |
|
|
SL6/1/1 |
2 |
498 |
2900 |
This study |
[73] |
||
|
HTF-83D |
3 |
262 |
2809 |
This study |
This study |
||
|
SM6/1 |
2 |
341 |
3202 |
This study |
[73] |
||
|
DSM3353 |
4 |
GCA_900209925 |
175 |
2829 |
Unpublished |
[74] |
|
|
HTF-920 |
7 |
n/a |
n/a |
n/a |
n/a |
This study |
|
|
SS2/1 |
2 |
GCA_000154545 |
34 |
3022 |
Unpublished |
[73] |
|
|
SSC/2 |
2 |
GCA_000210695 |
120 |
3170 |
Unpublished |
[73] |
|
|
DSM14662 |
8 |
GCA_000154305 |
26 |
3460 |
Unpublished |
[72] |
|
|
GD/7 |
9 |
GCA_000210555 |
1 |
3261 |
Unpublished |
[73] |
Impact Statement.
Lactate can be produced as a fermentation by-product by many gut bacteria but has the potential to perturb intestinal microbial communities by lowering luminal pH, and its accumulation has been linked to a range of deleterious health outcomes. Fortunately, in healthy individuals, lactate tends not to accumulate as it is consumed by cross-feeding lactate-utilizing bacteria, which can convert it into the beneficial short-chain fatty acids butyrate and propionate. Lactate-utilizing gut bacteria are therefore promising candidates for potential development as novel probiotics. However, lactate utilizers are taxonomically diverse, and the genetic repertoire that underpins the utilization of lactate by these specialized gut bacteria is not fully understood. In this study, we used transcriptomics to compare gene-expression profiles of Anaerobutyricum soehngenii and Coprococcus catus, two prominent lactate-utilizing species in the human gut, during growth on lactate alone, sugar alone or sugar plus lactate. The results revealed strong upregulation of key, but distinct, gene clusters that appear to be responsible for lactate utilization by these, and other, gut bacterial species. Our results therefore increase mechanistic comprehension of different lactate utilization pathways used by gut bacteria, which may help to inform selection of optimal lactate-utilizing species for development as novel therapeutics against colonic microbiota perturbations.
Introduction
The human large intestinal microbiota is dominated by obligately anaerobic bacteria, whose growth is largely dependent on the supply of complex carbohydrates and proteins available for fermentation. These substrates are mostly fermented by resident gut anaerobes to short-chain fatty acids (SCFAs) and gases, and in the healthy colon the major faecal SCFAs detected are acetate, propionate and butyrate [2]. Lactate is another fermentation product of many anaerobic bacteria that colonize the mammalian gut, sometimes as a major metabolite, as in Lactobacillus and Bifidobacterium spp. [3]. However, as the pKa (around 3.8) for lactic acid is lower than that of other fermentation acids such as acetate (4.76), accumulation of lactate has the potential to dramatically change the gut environment and gut microbiota composition via reduction in prevailing pH [4].
Lactate may therefore help to inhibit the growth of some pathogenic bacteria with poor tolerance for lower pH [5] and a gut microbiota dominated by lactate-producing bacteria is found in many healthy pre-weaned infants [6]. On the other hand, there are many known deleterious impacts of lactate accumulation in the gut. Indeed, the phenomenon of lactic acidosis, driven by excessive production of lactate following fermentation of easily digestible dietary carbohydrates, is well known in ruminants and is a major problem in animal husbandry [7, 8]. Similarly, in humans, accumulation of toxic d-lactate is life-threatening in short bowel syndrome [9] and lactate accumulation is also associated with severe colitis [10]. Additionally, lactate can be utilized as a carbon and energy source by certain intestinal pathogens such as Salmonella [11] and Campylobacter [12], compounding the potential heath detriments of lactate accumulation in the gut.
However, in the colon of healthy adult humans, as well as in the non-acidotic rumen, lactate does not accumulate, due the activities of cross-feeding, lactate-utilizing bacteria [13]. Colonic lactate concentrations are therefore a balance between bacterial production during fermentation and utilization by lactate-utilizing bacteria, which can use lactate to form acetate, propionate or butyrate [14]. Recent research has linked the populations and activities of these lactate-utilizing bacteria with overall fermentation patterns and productivity [15], and a low abundance of commensal lactate utilizers has also been proposed to make gut microbial communities inherently less stable and more prone to lactate-induced perturbations [16]. As such, lactate-utilizing bacteria have been proposed as promising candidates for development as novel probiotics [16–19].
The ability to utilize lactate as an energy source for growth appears to be limited to relatively few bacterial species among the human intestinal microbiota, although these species are taxonomically diverse and can utilize lactate in different ways. Selective isolation on DL lactate-containing media resulted in the recovery of Lachnospiraceae species subsequently identified as Eubacterium hallii (since reclassified as the two species Anaerobutyricum hallii and Anaerobutyricum soehngenii [20]), Anaerostipes hadrus and Anaerostipes caccae [21, 22]. These isolates produce butyrate from lactate, with net consumption of acetate, suggesting that they initially convert lactate into pyruvate, which is then routed into the butyrate pathway [22]. The lactate to pyruvate conversion is energetically unfavourable and recent evidence in Acetobacterium woodii indicates that it is dependent on electron confurcation [23]. Alternative routes for lactate utilization that are known to be important in the rumen [24] result in propionate formation. These include the acrylate pathway found in the Negativicutes species Megasphaera elsdenii and in at least one member of the Lachnospiraceae , Coprococcus catus [25], and the succinate pathway that is found among the Negativicutes in Selenomonas ruminantium and Veillonella spp. [25]. Less advantageously, lactate is also a co-substrate for sulfate-reducing bacteria that use it to form acetate and sulfide, the latter of which may be genotoxic [26].
Here we examine the distribution, organization and regulation of genes involved in lactate utilization among dominant representatives of the human intestinal microbiota, based on new isolates of lactate-utilizing species and on newly available genome sequences. In particular, we identify two gene clusters whose transcription is upregulated during growth with lactate as carbon and energy source. One of these corresponds to the predicted lactate utilization locus, the lct gene cluster, of A. woodii [23] that was recently identified from proteomic analysis in A. soehngenii [27], while the second encodes activities involved in the acrylate pathway of C. catus . This investigation reveals differences in the regulation, phylogenetic distribution and metabolic function of these two clusters, and provides novel insights into the mechanistic basis of different lactate-utilization strategies used by human gut bacteria.
Methods
Isolation of new strains of lactate-utilizing bacteria
New strains of A. soehngenii (HTF-83D) and A. hadrus (HTF-920, HTF-146, HTF-370 and HTF-412) were isolated from human faecal samples by dilution and culturing of single colonies on the anaerobic medium YCFA [28] supplemented with glucose. Taxonomic identification of strains was initially carried out by 16S rRNA gene sequencing and blastn querying each sequence against the NCBI 16S rRNA gene reference database. The new strains that were putatively assigned to the Anaerobutyricum genus based on 16S rRNA gene sequences were definitively classified as either A. hallii or A. soehngenii by comparing the average nucleotide identity (ANI) and average amino acid identity (AAI) of the new isolate draft genomes with genome sequences from the type strains of A. hallii (DSM3353T) and A. soehngenii (L2-7T) using nucmer [29] for ANI and CompareM (https://github.com/dparks1134/CompareM) for AAI (Table S1).
Bacterial strains, growth conditions and genomes
The bacterial strains and genomes used in this work, including both new and previously isolated strains, are described in Tables 1 and S2. Routine culturing of bacterial strains was in anaerobic M2GSC medium [30] in 7.5 ml aliquots in Hungate tubes, sealed with butyl rubber septa (Bellco Glass). Growth experiments were carried out in basal YCFA medium [28] supplemented with 35 mM lactate and/or 11 mM glucose (or fructose for C. catus since this species is incapable of growing on glucose as sole carbon source). All cultures were incubated anaerobically without agitation at 37 °C, using the anaerobic methods described previously by Bryant [31].
DNA isolation, genome sequencing and annotation
Genomic DNA was extracted with the Ultraclean Microbial DNA isolation kit (MoBio Laboratories, Carlsbad, CA, USA). The DNA concentration and purity were measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and the Qubit double-stranded DNA (dsDNA) HS and BR assay kits (Life Technologies, Carlsbad, CA, USA). One nanogram of bacterial DNA was used for library preparation. The DNA library was prepared using the Nextera XT library preparation kit with the Nextera XT v2 index kit (Illumina, San Diego, CA, USA). The library fragment length was aimed at fragments with a median size of 575 bases and was assessed with the Genomic DNA ScreenTape assay with the 2200 Tape-Station system (Agilent Technologies, Waldbronn, Germany). Subsequently, the library was sequenced on an Illumina MiSeq sequencer, using a 2×250 (500v2) cartridge, with the MiSeq reagent kit v2 generating 250 bp paired-end reads, (Illumina, San Diego, CA, USA). MiSeq data were processed with MiSeq control software v2.4.0.4 and MiSeq Reporter v2.4 (Illumina, San Diego, CA, USA). The resulting fastq files were filtered using TrimGalore v0.4.0 (https://github.com/FelixKrueger/TrimGalore), removing one nucleotide off the 3′ end (--trim1) and removing both pairs of the paired end reads if one read did not pass filtering (--paired). Filtered fastq files were assembled using the IDBA_UD assembler v1.1.3 [32] using standard settings. Newly generated genome sequences are available from GenBank (BioProject PRJNA701799), with individual accession numbers shown in Tables 1 and S2. Additional sequences were retrieved from GenBank (accession numbers in Table S2). New genome sequences were annotated using Prokka v1.11 [33] and publicly available sequences were reannotated in the same manner to ensure uniformity between annotation methods. Genome completeness and contamination was estimated using CheckM v1.1.3 [34] (Table S2).
Functional annotation of lactate-utilization loci and phylogenetic analyses
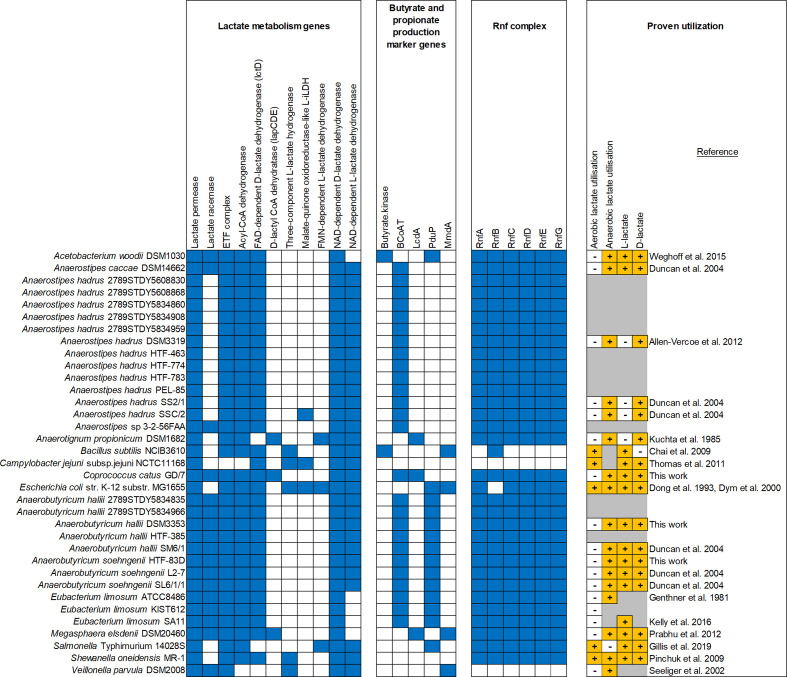
Lactate-utilization loci were searched for using the hidden Markov model (HMM) profiles of conserved domains found in three-component l-lactate hydrogenase (PF02754, PF02589, PF13183, PF11870), FAD-dependent d-lactate dehydrogenase (PF01565, PF02913, PF09330, PF12838, PF02754), FMN-dependent l-lactate dehydrogenase (PF01070, PF00173), NAD-dependent d-lactate dehydrogenase (PF00389, PF02826), NAD-dependent l-lactate dehydrogenase (PF02866, PF00056), lactate permease (PF02652), lactate racemase (PF09861) and ETF (PF00766, PF01012) using hmmsearch in HMMER3 [35]. Butyrate and propionate producing pathways were detected by blastP against UniProt [36] and KEGG [37] with a threshold of 60% identity using the marker genes butyrate kinase and butyryl-CoA:acetate CoA-transferase as indicators of butyrate production, and propanediol utilization protein (PduP), lactoyl CoA dehydratase alpha-subunit (LcdA) and methyl-malonyl-CoA decarboxylase alpha-subunit (MmdA) as indicators of propionate production through the propanediol, acrylate and succinate pathways, respectively [25]. Rnf complexes were detected by querying against amino acid sequences of the experimentally proven Rnf complexes of Clostridium ljungdahlii and A. woodii [38, 39].
All genes containing the lactate permease Pfam [40] domain PF02652 in UniProt (June 2020) [36] were downloaded and clustered into groups of >70 % similarity using CD-HIT v4.8.1 [41]. Representatives of each cluster were aligned using MAFFT l-INS-I v7.407 [42]. The acyl-CoA dehydrogenase in the lct cluster (L2-7_01909) was used as a blastP query against C. catus GD/7 and the A. hadrus genomes, homologues were aligned using MAFFT l-INS-i [42] and a HMM profile was created from the alignment using hmmbuild in HMMER3 [35]. This profile was then queried against the genomes of known lactate utilizers using hmmsearch (-T 80) to detect divergent members of the acyl-CoA dehydrogenase protein superfamily. Matching sequences were aligned using MAFFT l-INS-i v7.407 [42] and the alignment was manually inspected for likely mis-annotations (none were found). For both the lactate permease and acyl-CoA dehydrogenase alignments, spurious sequences and poorly aligned regions were removed with trimal v1.4.1 (automated1, resoverlap 0.55 and seqoverlap 60) [43] and maximum-likelihood trees were constructed with IQ-TREE v1.6.11 [44] using the best fitting protein model predicted in ModelFinder (from IQ-TREE v1.6.11) [45]. Branch supports were computed using the SH-aLRT test [46] for the lactate permease tree and ultrafast bootstraps for the acyl-CoA dehydrogenase tree. Both trees were rooted using minimal ancestor deviation [47]. The tree figures were generated using iTOL [48].
Differential gene-expression analysis
Fresh YCFA broth supplemented with 11 mM monosaccharide (glucose for A. soehngenii and fructose for C. catus ), 35 mM lactate or both 11 mM monosaccharide and 35 mM lactate were inoculated 1:75 with an overnight grown culture of either A. soehngenii L2-7 or C. catus GD/7. These cultures were grown to OD650 0.3 (all cultures were in exponential growth phase at this OD650 reading) before cultures were centrifuged to cell pellets (5 min, 1200 g , 20 °C). The supernatant was transferred to a new tube for SCFA and monosaccharide analysis, and the cell pellet was resuspended in 500 µl of RNAlater (Invitrogen), left at room temperature for 5 min and frozen at −70 °C. Total RNA was extracted using the RNeasy PowerMicrobiome Kit (QIAGEN), following the manufacturer’s instructions. This included a bead beating step for lysing Gram-positive cell walls. Ribosomal RNA was depleted using the Illumina Ribo-Zero rRNA removal kit. Libraries were prepared using the Illumina TruSeq stranded mRNA library kit and sequenced at the Centre for Genome-Enabled Biology and Medicine (CGEBM) at The University of Aberdeen using a High Output 1×75 kit on the Illumina NextSeq platform producing 75 bp single end reads. Reads were combined from two runs producing between 14 237 482 and 16 008 520 reads per sample. These transcriptomic datasets are available from the ArrayExpress database at EMBL-EBI under accession number E-MTAB-10136. Quality of raw reads was assessed using FastQC v0.11.3 [49] and sequences were filtered using TrimGalore v0.4.0. Between 14 141 677 and 15 921 037 sequences remained per sample, which, on average, was 99% of the raw reads. The appropriate reference genomes for A. soehngenii L2-7 and C. catus GD/7 (Table S2) were indexed in preparation for alignment using the HISAT2 build function (version 2.1.0) [50]. Filtered reads were then aligned to the appropriate reference genome using HISAT2 v2.1.0 [50] with default settings. Alignments were then converted to BAM format and sorted using SAMtools v1.2 [51]. Reads aligned to gene regions were counted using featureCounts (subread package version 5.0-pl) [52] with settings to split multi-mapping reads as a fraction between aligned regions. Genes with significant differential expression (FDR<0.05) between conditions were identified using edgeR v3.16.5 [53], utilizing the GLM function, as more than two growth conditions were used in this study.
Short-chain fatty acid, monosaccharide and alcohol analysis
SCFA concentrations were measured by gas chromatography (GC) as described previously [54]. In brief, following derivatization of the samples using N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide, the samples were analysed using a Hewlett Packard gas chromatograph (GC) fitted with a silica capillary column using helium as the carrier gas. l-lactate concentration was assessed by Lactate Reagent (Trinity biotech) using a Konelab 30 chemistry analyser. d-lactate was calculated as the difference between total lactate and l-lactate. Methanol, ethanol, propanol, butanol and pentanol concentrations were also measured by GC using a ZB WAX column. Glucose and fructose concentration were assessed using the glucose hexokinase and fructose hexokinase assays from Konelab, Clinical Diagnostics Finland.
Results
Lactate utilization in human colonic bacteria
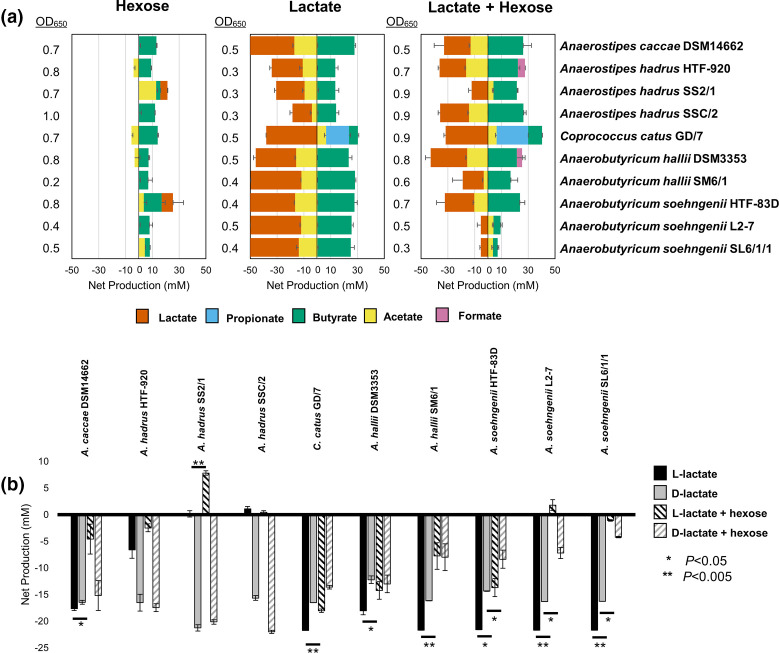
Some anaerobic colonic bacteria are capable of utilizing lactate as a carbon and energy source. This process can generate the SCFAs butyrate, propionate or acetate depending on the species [22, 25]. Previous studies have been based on only a small number of available strains, but the isolation and genome sequencing here of new strains of lactate-utilizing species allowed for more in-depth analysis. Each strain was grown in culture provided with glucose (or fructose in the case of C. catus GD/7), dl-lactate, or a combination of the two as energy sources (Fig. 1). All nine strains identified as either Anaerostipes or Anaerobutyricum spp. showed the ability to consume lactate and acetate with production mainly of butyrate, as previously reported [22]. This contrasts with production mainly of propionate from lactate by C. catus (Fig. 1). The A. hadrus strains tested showed little or no ability to utilize l-lactate, whereas the other species utilized both isomers (Fig. 1b). Lactate utilization was repressed to varying extents by the presence of hexose sugar in the growth medium for the different Anaerobutyricum and Anaerostipes strains, with utilization of both lactate stereoisomers particularly repressed by the presence of glucose for A. soehngenii strains L2-7 and SL6/1/1, but no repression of d-lactate utilization in A. hadrus strains (Fig. 1b). Interestingly, although propionate was the main SCFA produced by C. catus GD/7, when grown on lactate some butyrate and acetate production was also observed, indicating the presence of these two SCFAs as intermediate- or end-products of lactate utilization in this organism.
Fig. 1.
Fermentation profiles of lactate-utilizing bacteria grown on sugar, on lactate, or a combination of both. (a) Net production (to the right of the figures), or consumption (to the left of the figures) of various short-chain fatty acids by lactate-utilizing bacteria grown on either hexose sugar alone, lactate alone or a combination of both. Cultures were incubated anaerobically for 24 h in media supplemented with 11 mM glucose, 35 mM lactate or both. Glucose was substituted with 11 mM fructose for C. catus GD/7. Iso-butyrate, valerate, iso-valerate, and succinate were not detected in any of the samples. (b) Net consumption or production of l- or d-lactate by the tested strains, with or without simultaneous exposure to hexose sugars in the culture medium. Error bars are the sem of three biological replicates. There was no remaining glucose or fructose in any culture after 24 h, with the exception of A. soehngenii L2-7 (2 mM, SEM 2.0) and SL6/1/1 (6 mM, SEM 3.2) grown with a combination of glucose and lactate. Expanded data are shown in Table S3.
Changes in gene expression during growth on dl-lactate
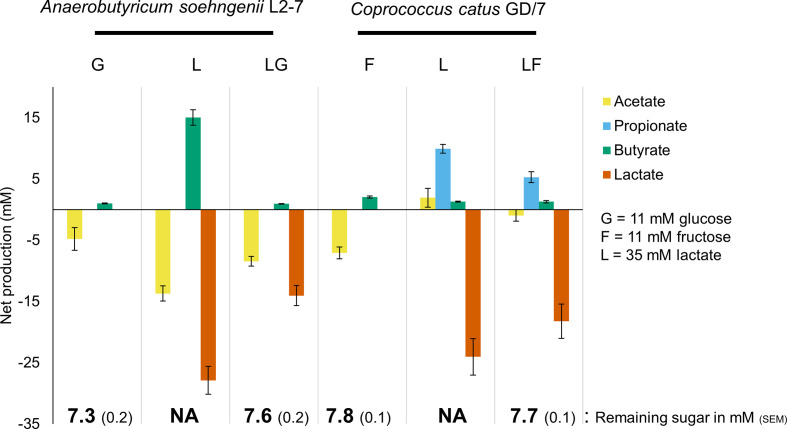
To investigate the genetic control and regulation of lactate utilization, transcriptomic analyses were performed on two prevalent lactate-utilizing species from the human gut, A. soehngenii and C. catus . This involved assessing the differential expression of genes during growth on a hexose sugar (glucose for A. soehngenii and fructose for C. catus ), on dl-lactate or on a combination of sugar plus dl-lactate (Fig. S1). Growth was carefully monitored in each of eight replicate experiments for each strain to ensure sampling during exponential growth phase (all cells harvested at OD650=0.3 in order to keep this consistent between experiments and strains). RNA was extracted from cell pellets and the culture supernatants were analysed for their SCFA profiles and remaining hexose (Fig. 2). In agreement with the results shown in Fig. 1, in the absence of glucose A. soehngenii L2-7 had utilized much of the lactate at the time of sampling, but lactate utilization was significantly repressed in the presence of glucose (P<0.001). In the absence of hexose sugar, C. catus GD/7 had also utilized much of the lactate, but utilization was slightly lower in the presence of fructose (P<0.01). Sugar was still present at 65–70% of the initial concentration in the growth media supplemented with it (Fig. 2, Table S4), indicating there was potential for repression of lactate utilization at the point of RNA harvesting.
Fig. 2.
Fermentation end products of A. soehngenii L2-7 and C. catus GD/7 during growth on lactate, a monosaccharide alone or on both in combination. Cultures were harvested for RNA extraction at an OD650 reading of 0.3. Iso-butyrate, valerate, iso-valerate and succinate were not detected in any of the samples. Error bars are the sem of eight biological replicates.
SCFA production levels were generally as expected, except for a relatively low concentration of butyrate in the A. soehngenii cultures incubated with lactate plus glucose compared to those from lactate growth alone (Fig. 2). Although speculative, we have two possible alternative explanations for this finding. It may be that more of the carbon went into cell mass, or was incorporated into other fermentation or intermediate precursor metabolites not measured here (such as CO2 or propanediol) when both carbon sources were present.
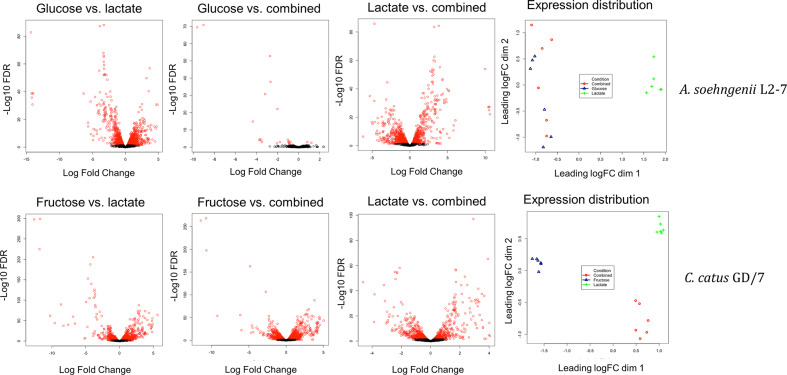
Transcriptomics sequencing was carried out on RNA extractions from six of the replicates per strain (Fig. S1). The results from the experiments with A. soehngenii L2-7 showed differences in overall transcriptomic profile were observed during growth on lactate compared to glucose alone or a combination of glucose and lactate (Fig. 3). Conversely, little overall difference in transcriptomic profile was observed between glucose and the combination of glucose and lactate, with only 25 differentially expressed genes as opposed to 1427 between glucose and lactate as a sole carbon source. In C. catus GD/7, the overall transcriptomic profile was again strongly influenced by the growth substrate (Fig. 3). In C. catus GD/7, however, and in contrast to A. soehngenii L2-7, lactate-induced genes were not simply repressed by the presence of monosaccharide (fructose), with all three transcriptome profiles differing from each other.
Fig. 3.
Volcano plots of log fold gene-expression change vs. –log10 of the false discovery rate (FDR) value and multidimensional scaling plots showing the gene-expression distribution of samples during growth on lactate, monosaccharide or a combination of the two. Genes with a significant FDR value <0.05 in the volcano plots are highlighted in red. A complementary numerical overview is given in Table S5. ‘Combined’ indicates cultures incubated with both lactate and hexose.
In A. soehngenii L2-7, 11 transcripts showed >5 log2-fold increase in expression during growth on lactate alone compared with growth on glucose alone, and increases were lower when glucose was present alongside lactate (Table 2). Six transcripts (L2-7_01905–01910) that showed the highest coordinate upregulation (log2 >14) were encoded by the lct gene cluster that includes an NAD-independent lactate dehydrogenase (iLDH), lactate permease, ETF electron transfer flavoprotein (alpha and beta subunits), lactate racemase and an acyl-CoA dehydrogenase (Table 2). The linked regulatory gene lctA showed a lower amplitude of induced expression. A second cluster of three genes that showed the same behaviour, also at lower amplitude, included an autoinducer, RNA polymerase sigma factor and HTH regulatory protein. Two further transcripts, from a second lactate racemase and closely linked aquaporin genes, were also highly induced by lactate, but were not repressed by glucose. The components of the Rnf complex, which couples the oxidation of reduced ferredoxin to the reduction of NAD [38], were highly induced by lactate and repressed by glucose, as were the components of the Hnd complex, which catalyses the reduction of NADP in the presence of molecular H2 to yield NADPH [55, 56].
Table 2.
Selected genes with high expression differences during growth on glucose (G), fructose (F), lactate (L) or a combination of substrates (LG/LF). All transcripts are listed that exhibited >5 log2-fold changes in expression, together with expression changes for linked genes within the same gene cluster. Changes of less than fivefold are listed for the Rnf and Hnd genes clusters in A soehngenii because of their metabolic relevance. All listed genes have an FDR value of <0.05. NoDiff=No significant difference in expression. Expanded to all genes in Tables S6–S11
|
log2-fold change |
||||
|
A. soehngenii L2-7 |
|
G v L |
G v LG |
L v LG |
|
L2-7_01905 |
Lactate permease (lctE) |
−14.3 |
−4.4 |
10.0 |
|
L2-7_01906 |
d-iLDH (lctD) |
−14.2 |
−3.7 |
10.5 |
|
L2-7_01907 |
ETF-B (lctB) |
−14.1 |
−3.5 |
10.6 |
|
L2-7_01908 |
ETF-A (lctC) |
−14.2 |
−3.7 |
10.5 |
|
L2-7_01909 |
Acyl-CoA dehydrogenase (lctG) |
−14.1 |
−3.7 |
10.4 |
|
L2-7_01910 |
Lactate racemase (lctF) |
−14.0 |
−3.5 |
10.5 |
|
L2-7_01911 |
LutR transcriptional regulator (lctA) |
−3.0 |
NoDiff |
2.5 |
|
L2-7_02157 |
Aquaporin family protein |
−6.3 |
−9.6 |
−3.3 |
|
L2-7_02158 |
Racemase |
−5.3 |
−9.0 |
−3.7 |
|
L2-7_02594 |
Putative cyclic lactone autoinducer peptide |
−5.2 |
NoDiff |
5.8 |
|
L2-7_02595 |
Hypothetical protein |
−4.9 |
NoDiff |
4.9 |
|
L2-7_02596 |
RNA polymerase subunit sigma-70 |
−5.7 |
NoDiff |
6.0 |
|
L2-7_02597 |
Helix-turn-helix domain-containing protein |
−5.7 |
NoDiff |
5.8 |
|
L2-7_00821 |
Hnd complex, subunit A |
−4.33 |
NoDiff |
5.12 |
|
L2-7_00822 |
Hnd complex, subunit C |
−4.07 |
NoDiff |
4.56 |
|
L2-7_00823 |
Hnd complex, subunit D |
−3.98 |
NoDiff |
4.38 |
|
L2-7_00377 |
Rnf complex, subunit C |
−2.90 |
NoDiff |
2.91 |
|
L2-7_00378 |
Rnf complex, subunit D |
−3.08 |
NoDiff |
2.98 |
|
L2-7_00379 |
Rnf complex, subunit G |
−3.20 |
NoDiff |
3.13 |
|
L2-7_00380 |
Rnf complex, subunit E |
−3.33 |
NoDiff |
3.25 |
|
L2-7_00381 |
Rnf complex, subunit A |
−3.27 |
NoDiff |
3.22 |
|
L2-7_00382 |
Rnf complex, subunit B |
−3.24 |
NoDiff |
3.15 |
|
log2-fold change |
||||
|
C. catus GD/7 |
|
F v L |
F v LF |
L v LF |
|
GD-7_01094 |
Propionyl CoA transferase (lapA) |
−10.3 |
−9.2 |
1 |
|
GD-7_01095 |
Lactoyl CoA epimerase (lapB) |
−11.8 |
−10.7 |
1.1 |
|
GD-7_01096 |
Lactoyl CoA dehydratase subunit (lapC) |
−12.6 |
−11.4 |
1.2 |
|
GD-7_01097 |
Lactoyl CoA dehydratase subunit (lapD) |
−13.1 |
−11.9 |
1.2 |
|
GD-7_01098 |
Lactoyl CoA dehydratase subunit (lapE) |
−13 |
−11.9 |
1.2 |
|
GD-7_01099 |
Lactate permease (lapF) |
−13 |
−11.9 |
1.2 |
|
GD-7_00065 |
3-hydroxybutyryl-CoA dehydratase |
−8.6 |
−6.2 |
2.5 |
|
GD-7_00066 |
H+/gluconate symporter and related permeases |
−6.9 |
−4.6 |
2.3 |
|
GD-7_00760 |
Amidohydrolase family protein |
−7.6 |
−4.4 |
3.2 |
|
GD-7_00761 |
MFS transporter |
−8.3 |
−4.7 |
3.6 |
|
GD-7_01087 |
Acyl-CoA dehydrogenase |
−9.6 |
-6 |
3.6 |
|
GD-7_01088 |
ABC transporter substrate-binding protein |
−6.6 |
−3.5 |
3.1 |
|
GD-7_01551 |
Acyl-CoA dehydrogenase |
−11.7 |
−10.8 |
1 |
In C. catus GD/7, seven genes showed >10 log2 fold increased expression during growth on lactate alone compared with fructose alone (Table 2). Six of these were encoded by a cluster of linked genes, but this cluster showed no sequence similarity with the lct cluster of A. soehngenii described above and comprised genes (GD-7_01094–01099) encoding three components of lactoyl-CoA dehydratase, propionyl-CoA transferase, a methyl-malonyl-CoA epimerase homologue and a lactate permease (Table 2). These genes encode most of the activities required for the acrylate pathway to convert lactate into propionate, consistent with previous evidence using isotopically labelled lactate showing that this bacterium uses the acrylate pathway for lactate utilization [25]. This locus is thus a cluster encoding lactate utilization via the acrylate pathway (lap). It seems likely that the methyl-malonyl-CoA epimerase homologue (lapB) functions as a lactoyl-CoA epimerase, catalysing the reversible conversion of l-lactoyl-CoA to d-lactoyl-CoA. The upregulated lactate permease gene was one of four encoded at separate locations in the C. catus GD/7 genome (Table S12). These six genes, together with two unlinked genes (GD-7_01551, GD-7_01087) encoding acyl-CoA dehydrogenases, were also highly upregulated on lactate when fructose was present. The two acyl-CoA dehydrogenases are candidates for propionyl-CoA dehydrogenase (acrylate reductase) activity.
Wider occurrence of lactate-utilization gene clusters in the genomes of human colonic bacteria
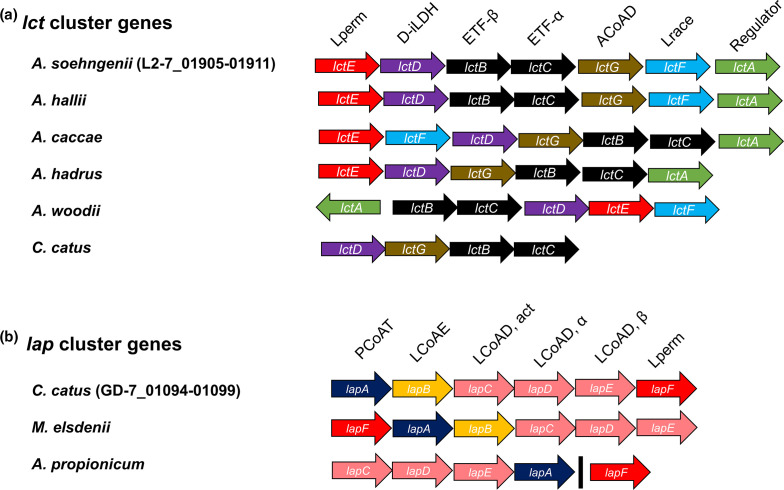
Homologues of the putative lactate utilization genes identified in A. soehngenii L2-7 and C. catus GD/7 were also detected in other known lactate-utilizing bacteria from the human colonic microbiota and other environments (Fig. 4a, b, Table S13 and S14). Homologues of the upregulated lct genes from A. soehngenii L2-7 were present in the two species of Anaerobutyricum , Anaerostipes caccae , Eubacterium limosum , C. catus and Megasphaera elsdenii (Table S13), although as noted above they were upregulated only moderately in C. catus (Table S9). The genome for A. hadrus DSM3319 lacked the racemase gene, consistent with the observation that it consumed only the D isomer of lactate (Fig. 1b). The lct homologues are also present in the Acetobacterium woodii gene cluster, but this cluster does not contain the putative acyl-CoA dehydrogenase (ACoAD) gene lctG.
Fig. 4.
Organization of lactate utilization loci observed in (a) A. soehngenii and (b) C. catus and other previously described lactate-utilizing bacteria. The black line in the A. propionicum lap cluster indicates that lapF gene is present in a different region of the genome. Lactate permease (Lperm), NAD-independent d-lactate dehydrogenase (d-iLDH), electron transfer flavoprotein (ETFα and β), acyl-CoA dehydrogenase (ACoAD), lactate racemase (Lrace), lactate operon transcriptional regulator (Regulator) propionyl-CoA transferase (PCoAT), lactoyl CoA epimerase (LCoAE), lactoyl-CoA transferase (LCoAD, act, α and β). Expanded to more lactate-utilizing bacteria genomes in Tables S13 and S14.
Analysis of five other publicly available C. catus genomes confirmed the presence of highly similar lap cluster genes in all other tested isolates of this species (Table S14). Homologs of the six clustered lap cluster genes were also detected in Megasphaera elsdenii , another species known to use the acrylate pathway for lactate utilization, although the percentage identity was very low for the lactate permease gene (Table S14). Five of the six lap cluster genes, were detected in Anaerotignum propionicum , excluding lapB. This supports the prediction of lapB as a lactoyl-CoA epimerase, as Anaerotignum propionicum cannot utilize l-lactate [57].
In most butyrate-producing Firmicutes species from the human colon, most or all of the genes encoding the central butyrate pathway are present in a single gene cluster, although the butyryl-CoA acetate:CoA-transferase is separately encoded [58]. This was found to be true for A. soehngenii but not for C. catus GD/7, where the key functions are encoded in at least three separate locations in the genome (Table S15). While the crotonase and beta-hydroxybutyrate dehydrogenase genes are linked in C. catus GD/7, two candidate thiolase genes are unlinked. Meanwhile, the butyryl-CoA dehydrogenase (BCD) in the C. catus GD/7 genome (GD-7_01569) is situated downstream of the iLDH (GD-7_01568) and upstream of two ETF genes (GD-7_01570, 01571) (Fig. 4a, Table S15). These appear to be homologous with the lctBCFG genes of A. soehngenii , although these lct genes were not strongly upregulated by lactate in C. catus.
Of the lactate-utilizing bacteria analysed, only Veillonella parvula , Bacillus subtilis and Campylobacter jejuni appear to lack the Rnf complex, and all genomes except C. jejuni and Shewanella oneidensis encoded genes indicative of butyrate and/or propionate production (Fig. 5). For bacteria that did not encode l-iLDHs, the lack of a lactate racemase corresponded with an inability to utilize l-lactate. As described earlier, the lct and lap clusters use different mechanisms of lactate utilization and are present in a different range of organisms. Additionally, Shewanella oneidensis uses a third mechanism of lactate utilization, involving a very different lactate dehydrogenase [59]. Homologues of this three-component l-lactate dehydrogenase are present in the genomes of B. subtilis , C. jejuni , E. coli and V. parvula (Fig. 5). Other types of NAD-independent l- and d-lactate dehydrogenases have also been found in organisms from all three domains of life [60]. Thus, there appear to be several divergent paradigms of lactate utilization in bacteria. However, all of these mechanisms appear to require members of the lactate permease superfamily and most possess some member of the acyl-CoA dehydrogenase superfamily. Therefore, the diversity of these proteins warranted detailed further investigation.
Fig. 5.
Distribution of lactate utilization and associated genes in lactate-utilizing bacteria. Presence (blue) and absence (white) of genes was determined as described in Methods. The ability (+) or inabilty (-) of selected strains to utilize different isomers of lactate during aerobic or anaerobic growth was obtained from the literature [12, 21–23, 57, 59, 67, 75–81]. Certain details could not be recovered for some strains (marked in grey). Full names of genes indicated by short-hand gene nomenclature are as follows: Butyryl-CoA:acetate CoA-transferase (BCoAT), lactoyl-CoA dehydratase, alpha subunit (LcdA), propanediol utilization CoA-dependent propionaldehyde dehydrogenase (PduP), methyl-malonyl-CoA decarboxylase, alpha-subunit (MmdA) and Rnf complex subunits (RnfA-G).
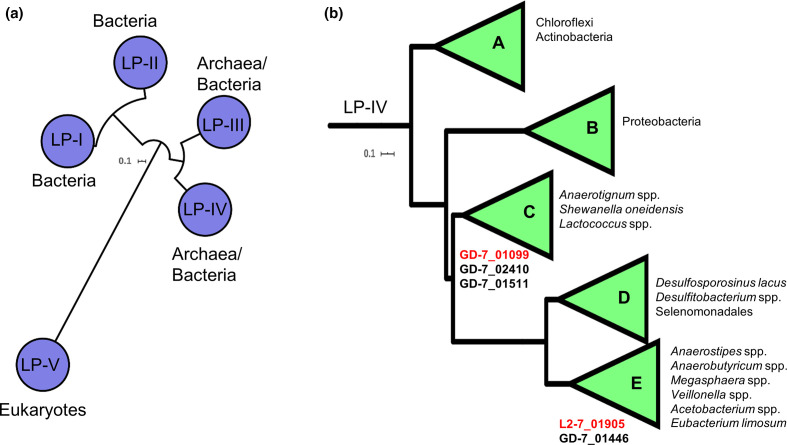
Phylogeny of lactate permease superfamilies
Phylogenetic analysis of 17 653 lactate permeases from bacteria, archaea and eukaryotes revealed five families of lactate permease: LP-I and LP-II, which were present exclusively in bacteria; LP-III and LP-IV, which were present in bacteria and archaea; and LP-V, which was present exclusively in eukaryotes (Fig. 6a). The lactate permeases identified in the transcriptomics work presented here belong to LP-IV, which was further divided into five subfamilies (A–E). Three of the four lactate permease genes in the C. catus GD/7 genome, including the copy upregulated on lactate, clustered with the Anaerotignum and S. oneidensis lactate permeases in LP-IV-C (Fig. 6b). The fourth clustered with the lactate-induced A. soehngenii lactate permease in LP-IV-E. This subfamily notably also contains the lactate permeases of Anaerostipes spp., Megasphaera spp., Veillonella spp., Acetobacterium spp. and E. limosum .
Fig. 6.
Phylogeny of lactate permease families. (a) Families of lactate permease, and (b) subfamilies of LP-IV. The conserved lactate permease domain PF02652 was detected in 17 653 protein sequences from Uniprot (June 2020). These proteins were formed into 1627 clusters of 70% identity. A maximum-likelihood tree was constructed from the representative sequences of these clusters using the LG+F+R8 model. All branches shown have >70 % support of 1000 SH-alrt replicates. Proteins were clustered into protein families and subfamilies using the average branch length distances to the leaves in the phylogenetic tree. The tree was rooted using minimal ancestor deviation. Lactate permeases of C. catus GD/7 and A. soehngenii L2-7 are listed underneath the subfamilies of which they belong to. The upregulated lactate permeases detected in the preceding transcriptomics work in this study are indicated in red font. Representative genes of each family of lactate permease and each subfamily of LP-IV are listed in Table S16 and Table S17, respectively.
Lactate permeases are also notably absent from the genomes of the gut bacteria Eubacterium rectale A1-86, Roseburia intestinalis L1-82, Roseburia hominis A2-183, Coprococcus sp. L2-50 and Faecalibacterium praunsnitzii A2-165, which do not utilize lactate [22], thus indicating a role for lactate permease as a marker gene of lactate utilization, despite disparate mechanisms of lactate oxidation.
Phylogeny of Acyl-CoA dehydrogenase superfamilies
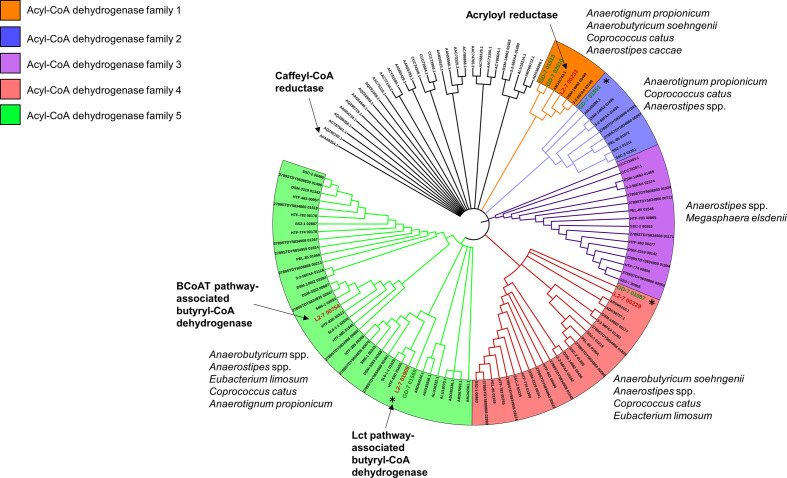
The acyl-CoA dehydrogenase in the lct cluster (L2-7_01909) and its homologues in the C. catus and A. hadrus genomes were used to create a HMM profile to detect divergent members of this protein superfamily in the genomes of other lactate-utilizing bacteria. These genes were found to be ubiquitous in lactate-utilizing bacteria, with many genomes possessing more than one copy (Table 3). Analysis of these genes revealed that they can be divided into a multitude of clades (Fig. 7), many of which, such as the experimentally proven [61] caffeyl-CoA reductase of A. woodii, are the only representatives of their clade in our dataset. One clade (acyl-CoA dehydrogenase family 5) contained the butyryl-CoA dehydrogenase present in the gene cluster responsible for butyrate production from carbohydrates [58]. This clade also contained the lactate induced lct cluster acyl-CoA dehydrogenase, indicating that it too is a butyryl-CoA dehydrogenase. The highly lactate-induced acyl-CoA dehydrogenases of C. catus (GD-7_01551 and GD-7_01087) form separate clades (acyl-CoA dehydrogenase families 2 and 4, respectively) with genes from several other species. No genes of these clades have been experimentally characterized, complicating functional prediction. Additionally, genes from C. catus , A. soehngenii and A. caccae form a clade (family 1) with the experimentally proven [62] acryloyl reductase of Anaerotignum propionicum .
Table 3.
Acyl-CoA dehydrogenase families of lactate-utilizing bacteria. These enzymes are ubiquitous in lactate-utilizing bacteria, with some genomes possessing multiple genes. Many of these genes form five distinct gene families (Fig. 7). The number of genes of a given family that were upregulated during growth on lactate in A. soehngenii L2-7 and C. catus GD/7 is shown in parentheses. Black slashes indicate differences in gene copy number between queried genomes of the same species. Expanded to include more lactate-utilizing bacteria genomes in Table S18
|
Acyl-CoA dehydrogenase families |
||||||
|---|---|---|---|---|---|---|
|
Species |
1 |
2 |
3 |
4 |
5 |
No. of genomes queried |
|
2 |
1 (1) |
0 |
1 |
1 (1) |
1 |
|
|
0/1 |
0 |
0 |
0/1 |
2 (1) |
3 |
|
|
0 |
0 |
0 |
0 |
2 |
5 |
|
|
1 |
1 |
1 |
2 |
1 |
1 |
|
|
Anaerostipes sp. 3-2-56FAA |
1 |
1 |
1 |
2 |
1 |
1 |
|
0 |
0/1 |
1 |
0/1/2 |
1 |
12 |
|
|
0 |
0 |
0 |
0/1 |
0/2 |
3 |
|
|
0 |
0 |
2 |
0 |
0 |
1 |
|
|
0 |
0 |
0 |
1 |
0 |
1 |
|
|
1 |
1 |
0 |
0 |
1 |
1 |
|
Fig. 7.
Phylogeny of Acyl-CoA dehydrogenase genes present in lactate-utilizing bacteria. Branch validation was performed using 1000 ultrafast bootstrap replicates and a hill-climbing nearest-neighbour interchange (NNI) search was performed to reduce the risk of overestimating branch supports. Branches with less than 90% UFBoot support were collapsed. Genes labelled ‘Caffeyl-CoA reductase’ and ‘Acryloyl reductase’ were experimentally verified by Bertsch et al. [61] and Hetzel et al. [62], respectively, and the gene labelled ‘Butyryl-CoA dehydrogenase’ is present in the butyrate production loci as described previously [58]. This maximum-likelihood tree was reconstructed using the LG+G4 model. The tree was rooted using minimal ancestor deviation. Anaerobutyricum soehngenii L2-7 and Coprococcus catus GD/7 genes are represented in red and green font, respectively. Asterisks indicate genes that were highly upregulated by lactate in the transcriptomics work presented in this study.
A. soehngenii L2-7 and C. catus GD/7 possess four and five acyl-CoA dehydrogenases, respectively (Fig. 7, Table 3). Two of the A. soehngenii L2-7 gene products group with butyryl-CoA dehydrogenase (BCD) enzymes, including the BCD in the central butyrate pathway (L2-7_00754) and the upregulated lctG product (L2-7_01919). This strongly suggests that the lactate-induced lctG gene product is an alternative BCD enzyme that links reduction of crotonyl-CoA to the oxidation of lactate when lactate is being utilized.
Discussion
Lactate can be utilized by many aerotolerant bacteria through its conversion to pyruvate via d- or l-lactate oxidases [60, 63]. This reaction is not available to obligate anaerobes however, and different mechanisms are therefore required in the absence of oxygen. As noted previously, anaerobic conversion of lactate to pyruvate is energetically unfavourable [23] and this ability appears to have a limited phylogenetic distribution. Here, we have identified two different six-gene clusters (lct and lap) whose transcriptional expression is highly upregulated during growth on lactate as the sole added carbon and energy source. These two gene clusters correspond to two entirely different mechanisms for lactate utilization. The upregulated cluster in A. soehngenii corresponds to five genes of the lct gene cluster identified previously in Acetobacterium woodii , which is an acetogen that has been shown to convert lactate to pyruvate via a mechanism involving electron confurcation [23]. Products of this lct gene cluster were recently detected by proteomic analysis [27], and the cluster reported in the related lactate-utilizing species A. hallii, A. rhamnosivorans and Anaerostipes caccae. Five of the six clustered genes are also present in Anaerostipes hadrus, but all strains of this species with sequenced genomes examined here lacked the lactate racemase (lctF), thus explaining why they can only utilize d-lactate.
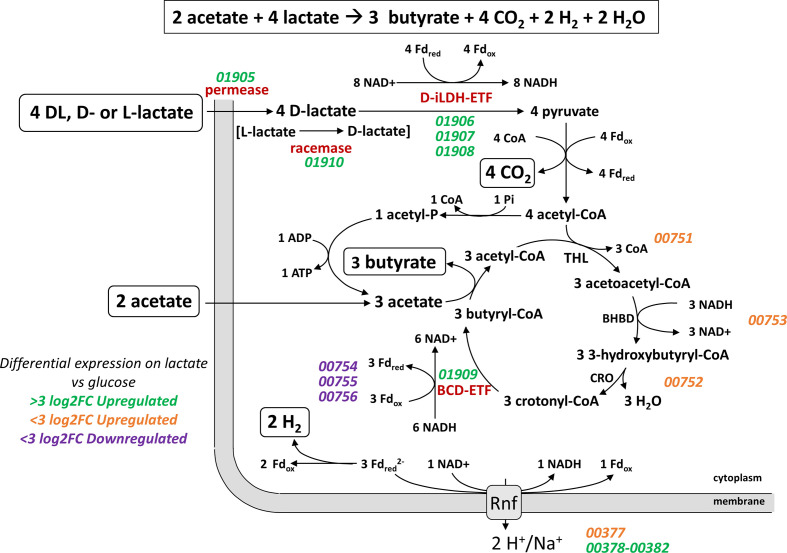
Our analysis confirms that the lct gene cluster is present in the newly sequenced strains of A. soehngenii , A. hallii and A. hadrus described here. Notably, however, the lct cluster in all these strains encodes an acyl-CoA dehydrogenase specified by the lctG gene that is lacking from the A. woodii cluster [64]. Since the lctG gene product is related to, but distinct from, the BCD gene encoded by the central butyrate pathway cluster (L2-7_00751-00756) [58], it seems likely that it is an alternative butyryl-CoA dehydrogenase (BCD) that acts to reduce crotonyl-CoA when lactate is the growth substrate (Fig. 8). While the conversion of pyruvate to acetyl-CoA can produce enough reduced ferredoxin to balance the oxidation of lactate via the d-iLDH/ETF complex [65], redox balance and net energy production are also linked to the operation of the butyrate cycle (Fig. 8). The upregulation by lactate of an orthologous enzyme catalysing the BCD reaction, encoded by lctG, may therefore play a critical role. The exact relationships between the co-ordinately upregulated iLDH, BCD and ETF proteins are not known for A soehngenii , although it has been suggested that in C. butyricum the LDH-ETF and BCD-ETF complexes act separately [66].
Fig. 8.
Proposed mechanisms of butyrate formation from lactate in A. soehngenii L2-7. Thiolase (THL), β-hydroxybutyryl-CoA dehydrogenase (BHBD), crotonase (CRO), NAD-independent d-lactate dehydrogenase (d-iLDH), electron transfer flavoprotein (ETF), ferredoxin (Fd), butyryl-CoA dehydrogenase (BCD), adenosine triphosphate (ATP), adenosine diphosphate (ADP), oxidized nicotinamide adenine dinucleotide (NAD+), reduced nicotinamide adenine dinucleotide (NADH), inorganic phosphate (Pi), and Coenzyme A (CoA). Locus tags are in italics and are coloured based on log2 fold change of their expression during growth on lactate versus glucose.
It has been suggested that the molecular hydrogen producing-Hnd complex is involved in balancing redox states during lactate oxidation in Desulfovibrio species [56], and its induction during growth of A. soehngenii on lactate indicates a likely mechanism for the lactate oxidation-coupled hydrogen production observed in this species [22]. However, resolution of these mechanisms will require further detailed biochemical investigation of the enzymes’ activity and ETF binding of the LctG protein. It would be of particular interest to carry out enzymatic studies to establish that the lctG product does indeed have butyryl-CoA dehydrogenase activity. In addition, it would be interesting to carry out affinity studies comparing the binding preferences of the LctG enzyme and its BCD homologue for the two pairs of ETFAB proteins that are encoded by the lct cluster and by the central butyrate pathway genes.
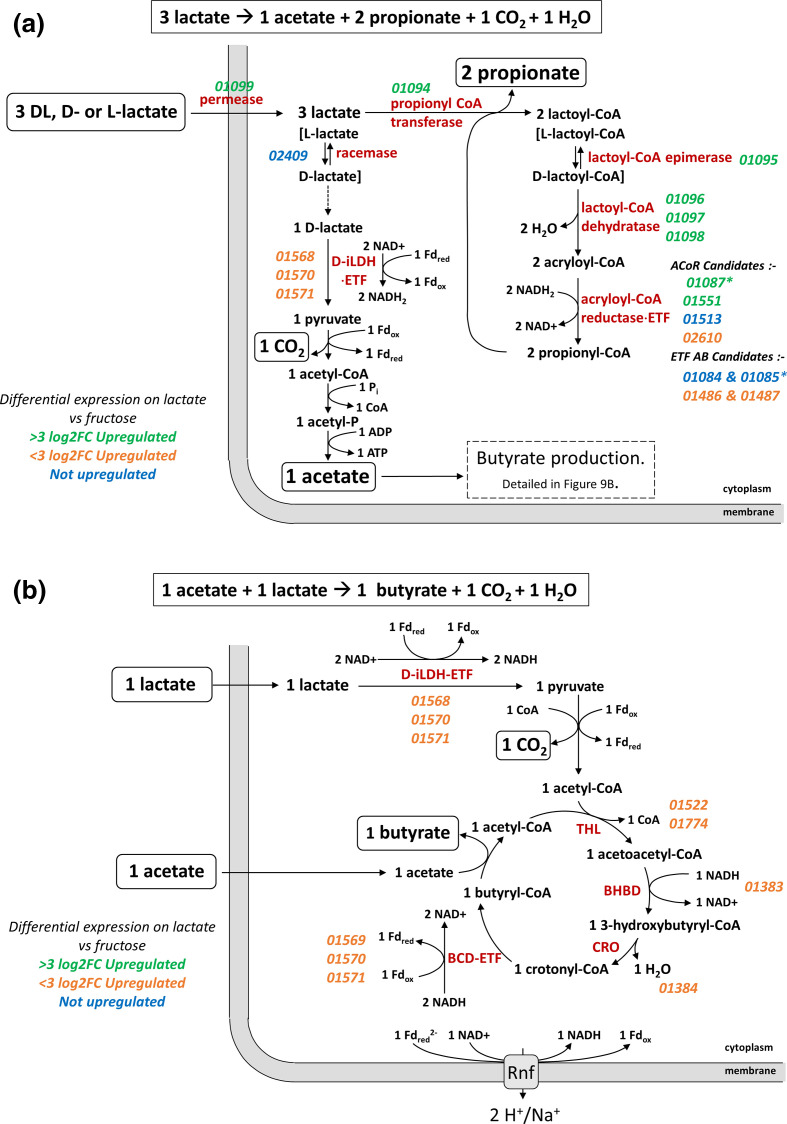
The lap cluster was identified in C. catus GD/7. This cluster has no equivalent in A. soehngenii and codes for enzymes required for the acrylate pathway for the conversion of lactate to propionate, notably the three subunits of lactoyl-CoA dehydratase, propionyl-CoA transferase and a presumptive lactoyl-CoA epimerase (Fig. 9). Although lactate permeases are present in both the lap and lct clusters, their sequences are only distantly related to each other. In addition, it seems likely that one of the two unlinked acyl-CoA dehydrogenases that were also upregulated by lactate must correspond to propionyl-CoA dehydrogenase that, along with ETF proteins, carries out the acryloyl-CoA reductase reaction [62].
Fig. 9.
Proposed mechanisms of (a) propionate and acetate formation from lactate and (b) butyrate formation from acetate and lactate in C. catus GD/7. NAD-independent d-lactate dehydrogenase (d-iLDH), electron transfer flavoprotein (ETF), ferredoxin (Fd), thiolase (THL), β-hydroxybutyryl-CoA dehydrogenase (BHBD), crotonase (CRO), butyryl-CoA dehydrogenase (BCD), adenosine triphosphate (ATP), adenosine diphosphate (ADP), oxidized nicotinamide adenine dinucleotide (NAD+), reduced nicotinamide adenine dinucleotide (NADH), inorganic phosphate (Pi), and Coenzyme A (CoA). Locus tags are in italics and are coloured based on log2 fold change of their expression during growth on lactate versus fructose. Asterisks indicate most likely candidate genes.
Operation of the acrylate pathway is normally assumed to result in the conversion of three mol lactate to two mol propionate and one mol of acetate (Fig. 9a) [3]. The acetate arises through conversion of lactate to pyruvate via iLDH, with the reducing equivalents balancing those needed to reduce two acrylate to two propionate. Our results however indicate the formation of some butyrate, in addition to propionate, during growth on lactate. This can be explained readily if we assume that some of the acetate produced is converted along with additional lactate to butyrate (Fig. 9b), as happens in A. soehngenii . The finding that the iLDH gene is linked to BCD and ETF genes in C. catus GD/7 appears consistent with this proposal. An unexplained feature is the 3-OH butyryl-CoA dehydratase transcript (GD-7_00065) that was upregulated by lactate, although this might possibly be an orthologue of the second β-hydroxybutyryl-CoA dehydrogenase gene (GD-7_01384) that is linked to crotonase in C. catus GD/7.
Homologues of lap genes were detected in a few other bacterial species, but notably were present in Megasphaera elsdenii, which is also known to use the acrylate pathway for lactate utilization. Indeed, M. elsdenii is known to convert lactate to butyrate under conditions of carbon-limited growth [67]. It is not known whether the same applies in C. catus. M. elsdenii also encodes activities involved in the formation of propionyl-CoA from pyruvate via malate and succinate [68]. However, the gene encoding for methyl-malonyl-CoA decarboxylase alpha-subunit (mmdA), an indicator of propionate production through the succinate pathway, was not present in the genome of C. catus GD/7 (Fig. 5), indicating that C. catus does not utilize this pathway.
Two other anaerobic lactate-utilizing species, Veillonella parvula and Anaerotignum propionicum, showed high levels of sequence identity with several genes of the lct cluster, but lacked a close homologue of the d-iLDH. The latter species however also carries homologues for most of the lap cluster and utilizes lactate via the acrylate pathway [69], whereas V. parvula encodes a three-component l-lactate dehydrogenase, homologous to that of S. oneidensis (Fig. 5), and uses the ‘randomizing’ succinate pathway rather than the acrylate pathway in producing propionate [3].
To summarize, we have shown that two distinct routes for anaerobic lactate fermentation in obligate anaerobes, via pyruvate to butyrate and via acrylate to propionate, both involve major transcriptional upregulation of the key genes involved (the lct cluster in A. soehngenii and the lap cluster in C. catus ) in response to lactate. There was however a clear difference between the two strains in the impact of hexose sugars on gene expression. Whereas A. soehngenii lct expression was subject to partial repression by glucose, this was not the case for C. catus acrylate genes with fructose. We should note, however, that present and previous results detected no glucose repression in strains of A. hadrus (referred to as ‘A. coli’ SS2/1 in Muñoz-Tamayo et al. [70]), which, like A. soehngenii , also possess the lct cluster and produce butyrate from d-lactate. The lack of repression of lactate utilization genes by hexose in species such as C. catus and A. hadrus may give them particularly important roles in controlling lactate concentrations within gut communities under conditions when there are significant concentrations of free sugars. For example, in the rumen, C. catus and Megasphaera elsdenii have been implicated in differences in animal productivity that are associated with lactate utilization on diets high in readily fermentable carbohydrates [15]. In the human colon monosaccharides are generally assumed to be present at very low concentrations, with non-digestible polysaccharides and oligosaccharides being the main available energy sources for bacterial growth. Duncan et al. [22] showed that in co-cultures between Bifidobacterium adolescentis and A. soehngenii L2-7 (then named ‘ Eubacterium hallii ’) growing on starch, lactate produced from starch by B. adolescentis was converted completely into butyrate. This suggests that bacteria such as A. soehngenii can play a very significant role in preventing lactate accumulation where polysaccharides such as resistant starch are also available as energy sources. A more detailed, quantitative, investigation into the impact of different concentrations of hexoses, oligosaccharides and polysaccharides on the lactate utilization rates in a range of dominant gut lactate-utilizing bacteria, preferably done in anaerobic continuous culture, would clearly be desirable in the future. Better understanding of the regulatory responses to these alternative carbon sources may be relevant when selecting strains of lactate-utilizing bacteria intended for use as therapeutic probiotics with the aim of reducing intestinal lactate concentrations and enhancing the stability of the microbial community.
Supplementary Data
Funding information
P.L., S.H.D., H.J.F., A.W.W. and the Rowett Institute received core financial support from the Scottish Government Rural and Environmental Sciences and Analytical Services (SG-RESAS). The work was also partly funded by Chr. Hansen, a global bioscience company that develops natural ingredient solutions for the food, nutritional, pharmaceutical and agricultural industries, who supported P.O.S. and E.T. Chr. Hansen exerted no influence on results obtained and presented in this manuscript. This also does not alter our adherence to Microbial Genomics’ policies on sharing data and materials.
Acknowledgements
The authors would like to thank Donna Henderson (Rowett Institute, University of Aberdeen) for carrying out gas chromatography analysis of short-chain fatty acids, and also thank the Centre for Genome-Enabled Biology and Medicine (CGEBM) at the University of Aberdeen for carrying out the Illumina-based transcriptomics sequencing. We would also like to acknowledge the support of the Maxwell computer cluster funded by the University of Aberdeen.
Author contributions
P.O.S. carried out laboratory and in silico experiments/data analysis. P.L., S.H.D., H.J.F. and A.W.W. also contributed to experimental design, data interpretation and visualization. E.T. and H.J.M. generated essential strain data. S.S. contributed to formal analysis and visualization. P.O.S., P.L., S.H.D., H.J.F. and A.W.W. wrote the manuscript. All co-authors commented on advanced drafts of the manuscript, and contributed edits to the final draft.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
All supporting data, code and protocols have been provided within the article or through supplementary data files. Eighteen supplementary tables and one supplementary figure are available with the online version of this article.
References
- 1.Sheridan PO, Louis P, Tsompanidou E, Shaw S, Harmsen HJ, et al. Figshare. 2022 doi: 10.6084/m9.figshare.16510344.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 3.Gottschalk G. Bacterial Metabolism. Verlag: New York, Heidelberg, Berlin: Springer; 1979. [Google Scholar]
- 4.Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11:2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 5.McWilliam Leitch EC, Stewart CS. Susceptibility of Escherichia coli O157 and non-O157 isolates to lactate. Lett Appl Microbiol. 2002;35:176–180. doi: 10.1046/j.1472-765x.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slyter LL. Influence of acidosis on rumen function. J Anim Sci. 1976;43:910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- 8.Russell JR, Hino T. Regulation of lactate production in Streptococcus bovis: A spiraling effect that contributes to rumen acidosis. J Dairy Sci. 1985;68:1712–1721. doi: 10.3168/jds.s0022-0302(85)81017-1. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, Bando Y, Kurihara H, Satomi K, Nonoyama K, et al. Fecal microflora in a patient with short-bowel syndrome and identification of dominant lactobacilli. J Clin Microbiol. 1997;35:3181–3185. doi: 10.1128/jcm.35.12.3181-3185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernia P, Caprilli R, Latella G, Barbetti F, Magliocca FM, et al. Fecal lactate and ulcerative colitis. Gastroenterology. 1988;95:1564–1568. doi: 10.1016/s0016-5085(88)80078-7. [DOI] [PubMed] [Google Scholar]
- 11.Gillis CC, Hughes ER, Spiga L, Winter MG, Zhu W, et al. Dysbiosis-associated change in host metabolism generates lactate to support salmonella growth. Cell Host & Microbe. 2018;23:54–64. doi: 10.1016/j.chom.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas MT, Shepherd M, Poole RK, van Vliet AHM, Kelly DJ, et al. Two respiratory enzyme systems in Campylobacter jejuni NCTC 11168 contribute to growth on L-lactate. Environ Microbiol. 2011;13:48–61. doi: 10.1111/j.1462-2920.2010.02307.x. [DOI] [PubMed] [Google Scholar]
- 13.Counotte GH, Lankhorst A, Prins RA. Role of DL-lactic acid as an intermediate in rumen metabolism of dairy cows. J Anim Sci. 1983;56:1222–1235. doi: 10.2527/jas1983.5651222x. [DOI] [PubMed] [Google Scholar]
- 14.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72:3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shabat SKB, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016;10:2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SP, Rubio LA, Duncan SH, Donachie GE, Holtrop G, et al. Pivotal Roles for pH, lactate, and lactate-utilizing bacteria in the stability of a human colonic microbial ecosystem. mSystems. 2020;5:e00645-20. doi: 10.1128/mSystems.00645-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Immerseel F, Ducatelle R, De Vos M, Boon N, Van De Wiele T, et al. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol. 2010;59:141–143. doi: 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- 18.Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8:e001319. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilijamse PW, Hartstra AV, Levin E, Wortelboer K, Serlie MJ, et al. Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose-response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes. 2020;6:16. doi: 10.1038/s41522-020-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shetty SA, Zuffa S, Bui TPN, Aalvink S, Smidt H, et al. Reclassification of Eubacterium hallii as Anaerobutyricum hallii gen. nov., comb. nov., and description of Anaerobutyricum soehngenii sp. nov., a butyrate and propionate-producing bacterium from infant faeces. Int J Syst Evol Microbiol. 2018;68:3741–3746. doi: 10.1099/ijsem.0.003041. [DOI] [PubMed] [Google Scholar]
- 21.Allen-Vercoe E, Daigneault M, White A, Panaccione R, Duncan SH, et al. Anaerostipes hadrus comb. nov., a dominant species within the human colonic microbiota; reclassification of Eubacterium hadrum Moore et al . Anaerobe. 1976;18:523–529. doi: 10.1016/j.anaerobe.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weghoff MC, Bertsch J, Müller V. A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ Microbiol. 2015;17:670–677. doi: 10.1111/1462-2920.12493. [DOI] [PubMed] [Google Scholar]
- 24.Counotte GH, Prins RA, Janssen RH, Debie MJ. Role of Megasphaera elsdenii in the Fermentation of dl-[2-C]lactate in the Rumen of Dairy Cattle. Appl Environ Microbiol. 1981;42:649–655. doi: 10.1128/aem.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquet P, Duncan SH, Chassard C, Bernalier-Donadille A, Flint HJ. Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol Lett. 2009;299:128–134. doi: 10.1111/j.1574-6968.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 27.Shetty SA, Boeren S, Bui TPN, Smidt H, de Vos WM. Unravelling lactate‐acetate and sugar conversion into butyrate by intestinal anaerobutyricum and anaerostipes species by comparative proteogenomics. Environ Microbiol. 2020;22:4863–4875. doi: 10.1111/1462-2920.15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Siles M, Khan TM, Duncan SH, Harmsen HJM, Garcia-Gil LJ, et al. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl Environ Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki K, Martin JC, Marinsek-Logar R, Flint HJ. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B(1)4. Anaerobe. 1997;3:373–381. doi: 10.1006/anae.1997.0125. [DOI] [PubMed] [Google Scholar]
- 31.Bryant MP. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 32.Peng Y, Leung HCM, Yiu SM, Chin FYL. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–1428. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 33.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 34.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 36.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–80. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay PL, Zhang T, Dar SA, Leang C, Lovley DR. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio. 2012;4:e00406–12. doi: 10.1128/mBio.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biegel E, Schmidt S, Müller V. Genetic, immunological and biochemical evidence for a Rnf complex in the acetogen Acetobacterium woodii . Environ Microbiol. 2009;11:1438–1443. doi: 10.1111/j.1462-2920.2009.01871.x. [DOI] [PubMed] [Google Scholar]
- 40.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 43.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 47.Tria FDK, Landan G, Dagan T. Phylogenetic rooting using minimal ancestor deviation. Nat Ecol Evol. 2017;1:1–7. doi: 10.1038/s41559-017-0193. [DOI] [PubMed] [Google Scholar]
- 48.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 49.Andrews S. UK: Babraham Institute; 2010. https://www.bioinformatics babraham ac uk/projects/fastqc/ [Google Scholar]
- 50.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 53.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson AJ, Calder AG, Stewart CS, Smith A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett Appl Microbiol. 1989;9:5–8. doi: 10.1111/j.1472-765X.1989.tb00278.x. [DOI] [Google Scholar]
- 55.de Luca G, de Philip P, Rousset M, Belaich JP, Dermoun Z. The NADP-reducing hydrogenase of Desulfovibrio fructosovorans: evidence for a native complex with hydrogen-dependent methyl-viologen-reducing activity. Biochem Biophys Res Commun. 1998;248:591–596. doi: 10.1006/bbrc.1998.9022. [DOI] [PubMed] [Google Scholar]
- 56.Malki S, Saimmaime I, De Luca G, Rousset M, Dermoun Z, et al. Characterization of an operon encoding an NADP-reducing hydrogenase in Desulfovibrio fructosovorans . J Bacteriol. 1995;177:2628–2636. doi: 10.1128/jb.177.10.2628-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuchta RD, Abeles RH. Lactate reduction in Clostridium propionicum. Purification and properties of lactyl-CoA dehydratase. J Biol Chem. 1985;260:13181–13189. doi: 10.1016/S0021-9258(17)38854-3. [DOI] [PubMed] [Google Scholar]
- 58.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 59.Pinchuk GE, Rodionov DA, Yang C, Li X, Osterman AL, et al. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proc Natl Acad Sci U S A. 2009;106:2874–2879. doi: 10.1073/pnas.0806798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang T, Gao C, Ma C, Xu P. Microbial lactate utilization: enzymes, pathogenesis, and regulation. Trends Microbiol. 2014;22:589–599. doi: 10.1016/j.tim.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Bertsch J, Parthasarathy A, Buckel W, Müller V. An electron-bifurcating caffeyl-CoA reductase. J Biol Chem. 2013;288:11304–11311. doi: 10.1074/jbc.M112.444919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hetzel M, Brock M, Selmer T, Pierik AJ, Golding BT, et al. Acryloyl-CoA reductase from Clostridium propionicum. An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur J Biochem. 2003;270:902–910. doi: 10.1046/j.1432-1033.2003.03450.x. [DOI] [PubMed] [Google Scholar]
- 63.Gibello A, Collins MD, Domínguez L, Fernández-Garayzábal JF, Richardson PT. Cloning and analysis of the L-lactate utilization genes from Streptococcus iniae . Appl Environ Microbiol. 1999;65:4346–4350. doi: 10.1128/AEM.65.10.4346-4350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoelmerich MC, Katsyv A, Sung W, Mijic V, Wiechmann A, et al. Regulation of lactate metabolism in the acetogenic bacterium Acetobacterium woodii . Environ Microbiol. 2018;20:4587–4595. doi: 10.1111/1462-2920.14412. [DOI] [PubMed] [Google Scholar]
- 65.Müller V, Chowdhury NP, Basen M. Electron bifurcation: a long-hidden energy-coupling mechanism. Annu Rev Microbiol. 2018;72:331–353. doi: 10.1146/annurev-micro-090816-093440. [DOI] [PubMed] [Google Scholar]
- 66.Detman A, Mielecki D, Chojnacka A, Salamon A, Błaszczyk MK, et al. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb Cell Fact. 2019;18:36. doi: 10.1186/s12934-019-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prabhu R, Altman E, Eiteman MA. Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady-state conditions. Appl Environ Microbiol. 2012;78:8564–8570. doi: 10.1128/AEM.02443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Shen Y, Wang C, Ding L, Zhao F, et al. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front Microbiol. 2019;10:162. doi: 10.3389/fmicb.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johns AT. The mechanism of propionic acid formation by Clostridium propionicum . J Gen Microbiol. 1952;6:123–127. doi: 10.1099/00221287-6-1-2-123. [DOI] [PubMed] [Google Scholar]
- 70.Muñoz-Tamayo R, Laroche B, Walter E, Doré J, Duncan SH, et al. Kinetic modelling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol Ecol. 2011;76:615–624. doi: 10.1111/j.1574-6941.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 71.Shetty SA, Ritari J, Paulin L, Smidt H, De Vos WM. Complete genome sequence of Eubacterium hallii strain L2-7. Genome Announc. 2017;5:e01167-17. doi: 10.1128/genomeA.01167-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–1661. doi: 10.1128/AEM.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, et al. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holdeman LV, Moore WEC. New genus, Coprococcus, twelve new species, and emended descriptions of four previouly described species of bacteria from human feces. Int J Syst Bacteriol. 1975;25:98. doi: 10.1099/00207713-25-1-98a. [DOI] [Google Scholar]
- 75.Chai Y, Kolter R, Losick R. A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation. J Bacteriol. 2009;191:2423–2430. doi: 10.1128/JB.01464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong JM, Taylor JS, Latour DJ, Iuchi S, Lin EC. Three overlapping lct genes involved in L-lactate utilization by Escherichia coli . J Bacteriol. 1993;175:6671–6678. doi: 10.1128/jb.175.20.6671-6678.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dym O, Pratt EA, Ho C, Eisenberg D. The crystal structure of D-lactate dehydrogenase, a peripheral membrane respiratory enzyme. Proc Natl Acad Sci U S A. 2000;97:9413–9418. doi: 10.1073/pnas.97.17.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Genthner BR, Davis CL, Bryant MP. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981;42:12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly WJ, Henderson G, Pacheco DM, Li D, Reilly K, et al. The complete genome sequence of Eubacterium limosum SA11, a metabolically versatile rumen acetogen. Stand in Genomic Sci. 2016;11:26. doi: 10.1186/s40793-016-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillis CC, Winter MG, Chanin RB, Zhu W, Spiga L, et al. Host-derived metabolites modulate transcription of salmonella genes involved in l-lactate utilization during gut colonization. Infect Immun. 2019;87:e00773-18. doi: 10.1128/IAI.00773-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seeliger S, Janssen PH, Schink B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol Lett. 2002;211:65–70. doi: 10.1111/j.1574-6968.2002.tb11204.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.