Abstract
A series of novel aminomethyl tetrahydrofuranyl (THF)-1β-methylcarbapenems which have excellent broad-spectrum antibacterial activities exhibit modest efficacies against acute lethal infections (3.8 mg/kg of body weight against Escherichia coli and 0.9 mg/kg against Staphylococcus aureus) in mice when they are administered orally. In an effort to improve the efficacies of orally administered drugs through enhanced absorption by making use of a peptide-mediated transport system, several different amino acids were added at the aminomethyl THF side chains of the carbapenem molecules. The resulting peptidic prodrugs with l-amino acids demonstrated improved efficacy after oral administration, while the d forms were less active than the parent molecules. After oral administration increased (3 to 10 times) efficacy was exhibited with the alanine-, valine-, isoleucine-, and phenylalanine-substituted prodrugs against acute lethal infections in mice. Median effective doses (ED50s) of <1 mg/kg against infections caused by S. aureus, E. coli, Enterobacter cloacae, or penicillin-susceptible Streptococcus pneumoniae were obtained after the administration of single oral doses. Several of the peptidic prodrugs were efficacious against Morganella morganii, Serratia marcescens, penicillin-resistant S. pneumoniae, extended-spectrum β-lactamase-producing Klebsiella pneumoniae, and E. coli infections, with ED50s of 1 to 14 mg/kg by oral administration compared with ED50s of 14 to >32 mg/kg for the parent molecules. In general, the parent molecules demonstrated greater efficacy than the prodrugs against these same infections when the drugs were administered by the subcutaneous route. The parent molecule was detectable in the sera of mice after oral administration of the peptidic prodrugs.
The treatment of infectious diseases has become increasingly complex due to changes in both bacterial and patient populations (10, 11, 14). The selection of antibiotic therapy requires consideration of the resistance patterns of the pathogen as well as the route of administration of the antibiotic, the length of hospitalization, and administrative directives. The carbapenem class of antibiotics has been shown to be effective against a broad spectrum of gram-positive and gram-negative bacteria and stable to most known resistance mechanisms. Presently, commercial carbapenems are effective only when they are administered by the parenteral route (3), while a few ester-type carbapenems are being developed for use as oral therapy (5, 16). In addition, many ester-type prodrugs of cephalosporins and tribactams are in use or are under investigation for use as oral treatments (13, 20). For the carbapenem class of antibiotics, an orally active compound that is stable to enzymatic hydrolysis and that has a broad spectrum of antibacterial activity would be an ideal candidate for development as a therapeutic agent. Three novel 1β-methylcarbapenems containing an aminomethylene-substituted tetrahydrofuranylthio side chain were synthesized and were shown to have potent broad-spectrum in vitro activities (19). These three compounds, CL 191,121 (the 2R,3R diastereomer), CL 188,624 (a mixture of the 3R,5S and the 3S,5R diastereomers), and CL 190,294 (a mixture of the 3R,5R and 3S,5S diastereomers), were demonstrated to have efficacy after administration by the oral as well as the subcutaneous routes in mouse models of acute lethal infection. Increased levels in serum and levels of exposure could result in the greater efficacies of these compounds after administration by the oral route. An effort was initiated (7, 8) to prepare synthetic peptidic prodrugs of these aminomethyl tetrahydrofuranyl (THF) carbapenems by the chemical addition of amino acids at the aminomethyl group of each molecule in an attempt to improve the absorption and efficacy through the di- and tripeptide transport system. The present study was performed to determine the efficacies of the resulting peptide prodrugs against acute lethal bacterial infections in mice.
MATERIALS AND METHODS
Antibiotics.
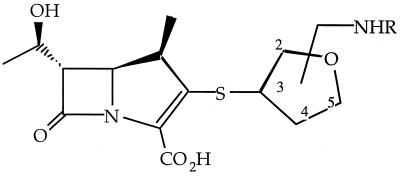
All THF carbapenems and peptidic prodrugs were synthesized at Wyeth-Ayerst Research, Pearl River, N.Y., by a previously described methodology (9). Unless otherwise specified, the peptidic prodrugs were synthesized with l-form amino acids. The compounds synthesized include CL 191,121 (the 3R,2R diastereomer) with an alanine (121-Ala), isoleucine (121-Ile), phenylalanine (121-Phe), or valine (121-Val) substituent; CL 188,624 (a mixture of the 3S,5R and 3R,5S trans diastereomers) with an alanine substituent (624-Ala), and CL 190,294 (a mixture of the 3R,5R and 3S,5S cis diastereomers) with an alanine (294-Ala) substituent (Fig. 1 and Table 1).
FIG. 1.
Structures of THF carbapenems and peptidic prodrugs.
TABLE 1.
Stereochemistries and substituents of the compounds testeda
| Compound | Stereochemistry | R |
|---|---|---|
| CL 191,121 | 3R,2R | H, alanine, isoleucine, phenylalanine, valine |
| CL 188,624 | 3S,5R and 3R,5S (trans) | H, alanine |
| CL 190,294 | 3R,5R and 3S,5S (cis) | H, alanine |
See Fig. 1.
Bacterial strains.
The bacterial strains used in this study represent standard laboratory strains and clinical isolates of Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Enterobacter cloacae, Serratia marcescens, Staphylococcus aureus, and Streptococcus pneumoniae adapted to cause infection in a murine systemic infection model. All strains were stored frozen at −70°C.
Murine systemic infection model.
The therapeutic efficacies of the peptidic prodrugs were evaluated in a murine model of bacterial infection. Female CD-1 mice (Charles River Laboratories) that weighed 20 ± 2 g each were challenged by intraperitoneal injection of sufficient bacterial cells from a 5-h broth culture suspended in either Trypticase soy broth or 5% hog gastric mucin to kill nontreated control mice within 24 to 48 h. Twofold serially diluted doses of the antibacterial agents in 0.2% aqueous agar were administered subcutaneously or orally 0.5 h after infection. In each test, five mice were treated with each dose. The 7-day survival ratios from three separate tests were pooled for estimation of the median effective dose (ED50) by a computerized program for probit analysis (2).
Pharmacokinetics.
Female CD-1 mice (Charles River Laboratories) that weighed 20 ± 2 g were administered 20 mg of each compound per kg of body weight in 0.5% methylcellulose by oral gavage. Mice were killed by CO2 inhalation, and blood from three mice obtained at each time point (0.25, 0.5, 1, 2, 4, 6, and 24 h after dosing) was pooled for analysis of the levels in serum. The levels in serum were determined by a microbiological assay with Bacillus subtilis grown on antibiotic medium 1 (Difco Laboratories, Detroit, Mich.) as the indicator organism. The levels in serum and compound identities were confirmed by high-pressure liquid chromatographic (HPLC) analysis. The HPLC assay used a Phenomenex Ultracarb 5-μm-particle-size column with an 85% 0.005 M tetraammonium dihydrogenphosphate 15% acetonitrile mobile phase. The detector was set at 299 nm, and the flow rate was 1 ml/min. The limit of detection was 0.125 μg/ml by bioassay and 0.05 μg/ml by the HPLC method.
RESULTS
The protective effects of three THF carbapenem isomers (CL 191,121, CL 188,624, and CL 190,294) against an acute lethal infection caused by S. aureus were compared with those of their corresponding alanine peptidic prodrugs (Table 2). CL 191,121 was the most active of the parent carbapenems by both the oral and the subcutaneous routes of administration. The addition of an alanine moiety to the aminomethyl THF side chain of CL 191,121 resulted in an increase in efficacy after oral administration, with the ED50 reduced from 0.9 to 0.11 mg/kg, while the comparable efficacies after administration by the subcutaneous route were maintained. The alanine prodrugs of the other two carbapenem isomers, CL 190,294 and CL 188,624, administered by either route did not exhibit increased efficacy in this infection model.
TABLE 2.
In vivo efficacies of THF carbapenems and peptidic prodrugs against an acute lethal infection with S. aureus Smitha
| Compound | ED50 (mg/kg [95% confidence limits])
|
MIC (μg/ml) | |
|---|---|---|---|
| Oral route | Subcutaneous route | ||
| CL 191,121 | 0.9 (0.75–1.2) | 0.04 (0.04–0.06) | ≤0.03 |
| 121-Ala | 0.11 (0.09–0.14) | 0.05 (0.04–0.06) | |
| CL 188,624 | 1.6 (1.4–2.4) | 0.09 (0.07–0.12) | ≤0.06 |
| 624-Ala | 0.82–1.6 | 0.05–0.09 | |
| CL 190,294 | 1.2 (1.1–1.6) | 0.06 (0.04–0.07) | ≤0.06 |
| 294-Ala | 0.82–1.6 | 0.09–0.2 | |
The challenge dose was 4.2 × 105 CFU/mouse.
To determine the effect of amino acid stereochemistry on in vivo efficacy, both the l and the d forms of alanine and phenylalanine peptidic derivatives of compound CL 191,121 were prepared and tested against acute lethal infections caused by S. aureus and E. coli 311, respectively. Against the S. aureus infection the d-alanine derivative of CL 191,121 was much less active than the l-alanine derivative of CL 191,121 when they were given by the oral route, with ED50s of >8.0 and 0.11 mg/kg, respectively (Table 3). In fact, the d-form peptidic prodrugs were less efficacious than the parent molecule against both the S. aureus and the E. coli infections when the drugs were administered by the oral route. Against this same infection the 121-d-Ala compound was also 10-fold less active than both the 121-l-Ala form of CL 191,121 and the parent CL 191,121 molecule by subcutaneous administration. Against an E. coli 311 infection 121-l-Phe was threefold more active than the parent CL 191,121 when the drugs were administered orally, while 121-d-Phe was 10-fold less active (ED50s, 1.1, 3.6, and 33.7 mg/kg, respectively). The 121-d-Phe compound was also more than 30 times less efficacious than both CL 191,121 and 121-l-Phe when the drugs were administered subcutaneously.
TABLE 3.
In vivo efficacies of a THF carbapenem and its d versus l amino acid prodrugs against acute lethal infections in mice
| Organism | Challenge dose (CFU/mouse) | Compound | R | ED50 (mg/kg [95% confidence limits])
|
MIC (μg/ml) | |
|---|---|---|---|---|---|---|
| Oral route | Subcutaneous route | |||||
| S. aureus Smith | 4.2 × 105 | 121-l-Ala | l-Alanine | 0.11 (0.09–0.14) | 0.05 (0.04–0.06) | |
| 121-d-Ala | d-Alanine | >8.0 | 0.5–1 | |||
| CL 191,121 | H | 0.90 (0.75–1.2) | 0.05 (0.04–0.06) | ≤0.03 | ||
| E. coli 311 | 2.4 × 106 | 121-l-Phe | l-Phenylalanine | 1.1 (0.7–1.2) | 0.48 (0.37–0.53) | |
| 121-d-Phe | d-Phenylalanine | 33.7 (20–60) | 19.2 (11–36) | |||
| CL 191,121 | H | 3.6 (2.6–4.0) | 0.4 (0.25–0.48) | ≤0.03 | ||
In addition to the alanine and phenyalanine peptidic prodrugs, the valine and isoleucine peptidic prodrugs of CL 191,121 were synthesized. The efficacies of these prodrugs against acute lethal murine infections caused by S. aureus, penicillin-susceptible and -resistant S. pneumoniae, E. coli, K. pneumoniae, M. morganii, E. cloacae, or S. marcescens in mice were compared to that of the parent CL 191,121 molecule (Tables 4 and 5). Against S. aureus Smith, 121-Ala, 121-Val, and 121-Ile were five to eight times more efficacious when they were administered by the oral route (ED50s, 0.11 to 0.16 mg/kg), while 121-Phe was approximately twofold more efficacious than CL 191,121 (Table 4). When these compounds were given subcutaneously, CL 191,121 was the most efficacious, followed by the 121-Ala, 121-Phe, 121-Val, and 121-Ile peptidic prodrugs, with ED50s of 0.04, 0.05, 0.08, 0.10, and 0.23 mg/kg, respectively. When administered orally, the three peptidic prodrugs 121-Ala, 121-Val, and 121-Ile were 10 to 20 times more active than CL 191,121 against an infection caused by a penicillin-susceptible strain of S. pneumoniae. The ED50s of these compounds ranged between 0.06 and 0.13 mg/kg, whereas the ED50 of the parent compound was 1.4 mg/kg. Orally administered compound 121-Val (ED50, 1.0 mg/kg) was 20 times more efficacious than CL 191,121 (ED50, 21 mg/kg) against a penicillin-resistant isolate of S. pneumoniae. Compounds 121-Ala and 121-Ile were both approximately 10 times more active than the parent, with ED50s of 1.9 and 1.8 mg/kg, respectively. When given subcutaneously, CL 191,121 and 121-Ala demonstrated comparable efficacies against both the penicillin-susceptible and penicillin-resistant S. pneumoniae isolates, while 121-Val and 121-Ile were two to six times less efficacious.
TABLE 4.
In vivo efficacies of 1β-methyl THF carbapenem and peptidic prodrugs against acute lethal infections caused by gram-positive bacteria in mice
| Compound | Route | ED50 (mg/kg [95% confidence limits])a
|
||
|---|---|---|---|---|
| S. aureus Smith | S. pneumoniae 6301 (penicillin susceptible) | S. pneumoniae 1894 (penicillin resistant) | ||
| CL 191,121 | Oral | 0.90 (0.75–1.2) | 1.4 (1.1–1.8) | 21 (21–35) |
| Subcutaneous | 0.04 (0.039–0.06) | 0.04 (0.03–0.05) | 0.53 (0.50–0.79) | |
| 121-Ala | Oral | 0.11 (0.09–0.14) | 0.13 (0.11–0.16) | 1.9 (1.4–2.2) |
| Subcutaneous | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) | 0.5 (0.35–0.57) | |
| 121-Val | Oral | 0.14 (0.1–0.16) | 0.08 (0.05–0.09) | 1.0 (0.85–1.2) |
| Subcutaneous | 0.10 (0.08–0.12) | 0.09 (0.07–0.11) | 1.1 (0.92–1.3) | |
| 121-Ile | Oral | 0.16 (0.13–0.35) | 0.06 (0.05–0.08) | 1.8 (1.4–2.2) |
| Subcutaneous | 0.23 (0.22–0.29) | 0.17 (0.14–0.26) | 3.3 (2.5–4.5) | |
| 121-Phe | Oral | 0.40 (0.26–0.60) | NT | NT |
| Subcutaneous | 0.08 (0.06–0.13) | NT | NT | |
Challenge doses were 4.2 × 105, 3 × 101, and 2 × 101 CFU/mouse for S. aureus Smith, S. pneumoniae 6301, and S. pneumoniae 1894, respectively. The CL 191,121 MICs for the three strains were ≤0.03, ≤0.015, and 0.5 μg/ml, respectively. NT, not tested.
TABLE 5.
In vivo efficacies of 1β-methyl THF carbapenem and peptidic prodrugs against acute lethal infections caused by gram-negative bacteria in mice
| Compound | Route | ED50 (mg/kg [95% confidence limits])
|
||||
|---|---|---|---|---|---|---|
| E. coli 311 | K. pneumoniae AD | M. morganii 84-11 | E. cloacae 84-29 | S. marcescens F35 | ||
| CL 191,121 | Oral | 3.6 (2.1–4.2) | 14.3 (9.6–22) | 14 (11–18) | 2.0 (1.6–2.6) | 14 (11–19) |
| Subcutaneous | 0.4 (0.30–0.47) | 2.6 (1.9–3.6) | 1.6 (1.3–2.0) | 0.22 (0.12–0.28) | 1.7 (1.4–2.2) | |
| 121-Ala | Oral | 0.55 (0.44–0.67) | 1.4 (1.0–1.9) | 2.1 (1.7–2.6) | 0.38 (0.29–0.47) | 2.0 (1.8–3.3) |
| Subcutaneous | 0.28 (0.22–0.33) | 2.6 (1.9–3.7) | 1.4 (1.1–1.8) | 0.37 (0.28–0.46) | 0.74 (0.7–1.1) | |
| 121-Val | Oral | 0.31 (0.25–0.38) | 1.5 (1.2–1.9) | 1.8 (1.3–2.3) | 0.27 (0.15–0.27) | 1.8 (1.3–2.4) |
| Subcutaneous | 0.29 (0.24–0.36) | 2.9 (2.2–3.8) | 2.0 (1.5–2.6) | 0.45 (0.29–0.50) | 2.7 (1.9–3.8) | |
| 121-Ile | Oral | 0.52 (0.39–0.61) | 1.4 (1.1–1.7) | 4.6 (3.6–5.7) | 0.39 (0.30–0.48) | NT |
| Subcutaneous | 1.1 (0.89–1.5) | 2.8 (2.2–3.6) | 9.0 (7.2–13) | 1.1 (0.82–1.4) | NT | |
| 121-Phe | Oral | 1.1 (0.7–1.2) | NT | NT | NT | NT |
| Subcutaneous | 0.48 (0.37–0.53) | NT | NT | NT | NT | |
The challenge doses were 2.4 × 106, 7.0 × 103, 1.5 × 106, 2.8 × 106, and 3.5 × 103 CFU/mouse for the five strains, respectively. The CL 191,121 MICs for the five strains were ≤0.03, ≤0.03, 1.0, 0.06, and 0.12 μg/ml, respectively. NT, not tested.
These same prodrugs were tested for their efficacies against infections caused by gram-negative bacterial isolates (Table 5). By the oral route of administration the alanine and valine derivatives of CL 191,121 were the most efficacious of the compounds tested against infections caused by E. coli, K. pneumoniae, M. morganii, E. cloacae, and S. marcescens. By the oral route of administration the two peptidic prodrugs were more efficacious than the parent CL 191,121 against these infections, with the ED50s of 121-Ala and 121-Val ranging from 0.27 to 2.1 mg/kg, whereas the ED50s of CL 191,121 ranged between 2 and 14.3 mg/kg. The efficacy of compound 121-Ile was equivalent to those of 121-Ala and 121-Val against the E. coli, K. pneumoniae, and E. cloacae infections, but it was twofold less active than 121-Ala and 121-Val against the M. morganii infection. Compound 121-Phe was two to three times less efficacious than the other prodrug compounds against the E. coli infection. In general, 121-Ala and 121-Val demonstrated efficacies comparable to that of CL 191,121 when the drugs were administered subcutaneously.
The peptidic prodrugs 121-Ala and 121-Val were also efficacious against infections caused by E. coli and K. pneumoniae isolates expressing extended-spectrum β-lactamases (ESBL) (Table 6). The compound 121-Val was efficacious against two E. coli isolates expressing either TEM-1 plus TEM-12 or TEM-1 plus SHV-7 (ED50s, 0.92 and 0.15 mg/kg, respectively). These ED50s represent 10-fold decreases in the ED50 compared with that of the parent molecule. Peptidic derivatives 121-Ala and 121-Val were also efficacious against ESBL-producing K. pneumoniae strains, with ED50s after administration by the oral route of 14.5 and 12.2, 3.3 and 1.8, and 5.3 and 4.4 mg/kg against strains expressing TEM-10 plus TEM-12, TEM-10 plus SHV-1, and TEM-20, respectively.
TABLE 6.
In vivo efficacies of 1β-methyl THF carbapenem and peptidic prodrugs against acute lethal infections caused by gram-negative ESBL-producing bacteria in mice
| Compound | Route | ED50 (mg/kg [95% confidence limits])
|
||||
|---|---|---|---|---|---|---|
| E. coli 1520 (TEM-1 + TEM-12) | E. coli 2344 (TEM-1 + SHV-7) | K. pneumoniae 1605 (TEM-10 + TEM-12) | K. pneumoniae 2010 (TEM-10 + SHV-1) | K. pneumoniae 1514 (TEM-20) | ||
| CL 191,121 | Oral | 8.9 (6.8–11.7) | 1.3 (1.0–1.6) | 42 (32–55) | 20 (15–29) | 38 (30–48) |
| Subcutaneous | 1.3 (1.0–1.7) | 0.03 (0.02–0.04) | 3.5 (2.7–4.4) | 1.1 (0.78–1.3) | 2.6 (2.1–3.3) | |
| 121-Ala | Oral | NT | 0.21 (0.17–0.26) | 14.5 (11–18) | 3.3 (3.1–4.2) | 5.3 (4.0–6.5) |
| Subcutaneous | NT | 0.06 (0.04–0.07) | 4.3 (3.2–5.3) | 0.9 (0.6–1.2) | 2.1 (1.7–2.6) | |
| 121-Val | Oral | 0.92 (0.69–1.2) | 0.15 (0.12–0.19) | 12.2 (9.4–15) | 1.8 (1.3–2.4) | 4.4 (3.4–5.3) |
| Subcutaneous | 2.9 (2.2–3.9) | 0.08 (0.07–0.11) | 9.6 (7.2–13) | 1.7 (1.2–2.2) | 3.9 (3.0–4.8) | |
The challenge doses were 3.1 × 107, 2.7 × 105, 2.0 × 106, 4.4 × 105, and 2.3 × 106 CFU/mouse for the five strains, respectively. The CL 191,121 MICs for the five strains were ≤0.03, ≤0.03, 0.12, 0.12, and 0.25 μg/ml, respectively. NT, not tested.
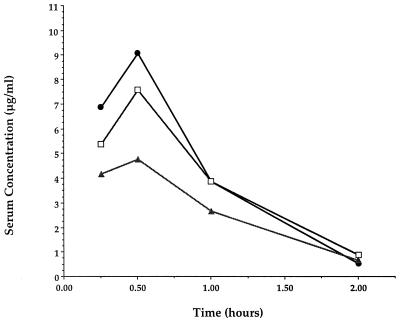
The increased oral efficacy obtained by the addition of the amino acid moiety to the aminomethyl side chains of these THF carbapenems can be attributed to increased levels of the circulating parent carbapenem in serum (Fig. 2). Peak levels of 4.8, 7.4, and 9.2 μg of the parent molecule CL 191,121 per ml were observed in the sera of mice given oral doses of 20 mg of 121-Ala, 121-Val, and 121-Ile per kg, respectively. No detectable circulating levels of the peptidic carbapenems were observed with the dose tested. The levels in serum were below the limit of detection at all time points after the oral administration of an equivalent dose of CL 191,121.
FIG. 2.
Levels of the parent THF carbapenem (CL 191,121) in serum determined by HPLC after oral administration of peptidic prodrugs to mice at 20 mg/kg. No drug was detected in serum after administration of the parent compound. ●, 121-Ile; □, 121-Val; ▴, 121-Ala.
DISCUSSION
The aminomethyl THF-1β-methylcarbapenems have potent in vitro activities against a broad spectrum of gram-positive and gram-negative bacteria (19). The compounds themselves possess excellent efficacies when administered subcutaneously and moderate efficacies when administered orally against a variety of murine infections caused by both gram-positive and gram-negative organisms without the need for the coadministration of a dehydropeptidase inhibitor. The levels of the parent THF compounds in serum were undetectable after oral administration. The observed low level of absorption of these molecules after oral administration correlates with the much improved efficacy noted when the compounds were dosed subcutaneously. Amino acid prodrugs of the THF carbapenems were synthesized with the aim of increasing the level of absorption through the use of an active transport system such as the di- or tripeptide transport system (7). The therapeutic efficacies of the peptidic prodrugs of optically pure CL 191,121 (3R,2R) after oral administration were 5 to 10 times greater than that of the parent molecule alone. The alanine peptidic derivatives of the 3,5-cis and -trans racemic mixtures (CL 190,294 and CL 188,624, respectively) did not demonstrate this same improved efficacy over the efficacies of the parent molecules. The use of a specific transport system is suggested by the increased efficacies of the CL 191,121 derivatives with the l-form amino acids of alanine and phenylalanine after oral administration but the decreased activities of the d-form amino acids. The novel peptidic prodrugs of this THF carbapenem demonstrated efficacies comparable to those of other investigational oral carbapenems (5, 18), ester prodrugs of active carbapenems (16), esters of tribactam antibiotics (15, 20), the orally bioavailable oxazolidinones (4) and ketolides (1), and ester-type cephalosporins (6, 13, 17) against infections caused by both gram-negative and gram-positive isolates. The peptidic prodrugs of these novel aminomethyl THF-1β-methylcarbapenems are promising new agents for the treatment of infections caused by a variety of gram-positive and gram-negative bacteria. In addition, unlike the oral cephalosporins, these peptidic prodrugs have excellent potential for use as therapy against penicillin-resistant S. pneumoniae and ESBL-producing gram-negative bacteria. The incidence of both of these groups of organisms is increasing in hospitals and tertiary-care facilities. The use of an oral carbapenem with the efficacy against experimental infections observed in the present study has the potential to allow a switch from intravenous to oral therapy and earlier hospital discharge.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In vivo antibacterial activity of RU 004, a novel ketolide highly active against respiratory pathogens, abstr. F171; p. 143. [Google Scholar]
- 2.Finney D. Probit analysis. 3rd ed. London, United Kingdom: Cambridge University Press; 1971. [Google Scholar]
- 3.Fish D, Singletary T. Meropenem, a new carbapenem antibiotic. Pharmacotherapy. 1997;17:644–699. [PubMed] [Google Scholar]
- 4.Ford C, Hamel J, Wilson D, Moerman J, Stapert D, Yancey R, Hutchinson D, Barbachyn M, Brickner S. In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob Agents Chemother. 1996;40:1508–1513. doi: 10.1128/aac.40.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuoka T, Ohya S, Utsui Y, Domon H, Takenouchi T, Koga T, Masuda N, Kawada H, Kakuta M, Kubota M, Ishii C, Sakagawa E, Harasaki T, Hirasawa A, Abe T, Yasuda H, Iwata M, Kuwahara S. In vitro and in vivo antibacterial activities of CS-834, a novel oral carbapenem. Antimicrob Agents Chemother. 1997;41:2652–2663. doi: 10.1128/aac.41.12.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klugman K, Goldstein F, Kohno S, Baquero F. The role of fourth-generation cephalosporins in the treatment of infections caused by penicillin-resistant streptococci. Clin Microbiol Infect. 1997;3:S48–S60. [Google Scholar]
- 7.Lin Y-I, Bitha P, Sakya S, Li Z, Lang S, Yang Y, Bhachech N, Weiss W, Petersen P, Jacobus N, Bush K, Testa R. Peptidic prodrugs of novel aminomethyl-THF 1β-methylcarbapenems. Bioorg Med Chem Lett. 1997;7:1665–1670. [Google Scholar]
- 8.Lin Y-I, Bitha P, Sakya S, Li Z, Strohmeyer T, Lang S, Yang Y, Bhachech N, Weiss W, Petersen P, Jacobus N, Bush K, Testa R. Mono and bis double ester prodrugs of novel aminomethyl-THF 1β-methylcarbapenems. Bioorg Med Chem Lett. 1997;7:1811–1816. [Google Scholar]
- 9.Lin Y-I, Bitha P, Sakya S, Strohmeyer T, Li Z, Lee V, Lang S, Yang Y, Bhachech N, Weiss W, Petersen P, Jacobus N, Bush K, Testa R, Tally F. Synthesis and structure activity relationships of novel THF 1β methylcarbapenems. Bioorg Med Chem Lett. 1997;7:1671–1676. [Google Scholar]
- 10.Murray B. New aspects of antimicrobial resistance and the resulting therapeutic dilemmas. J Infect Dis. 1991;163:1185–1194. [PubMed] [Google Scholar]
- 11.Murray B. Problems and dilemmas of antimicrobial resistance. Pharmacotherapy. 1992;12(6 Pt 2):86S–93S. [PubMed] [Google Scholar]
- 12.Nagano R, Shibata K, Naito T, Fuse A, Asano K, Hashizume T, Nakagawa S. Therapeutic efficacy of BO-3482, a novel dithiocarbamate carbapenem, in mice infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2278–2281. doi: 10.1128/aac.41.10.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry C, Brogden R. Cefuroxime axetil. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1996;52:125–158. doi: 10.2165/00003495-199652010-00009. [DOI] [PubMed] [Google Scholar]
- 14.Shlaes D, Binczewski B, Rice L. Emerging antimicrobial resistance and the immunocompromised host. Clin Infect Dis. 1993;17(Suppl. 2):S527–S536. doi: 10.1093/clinids/17.supplement_2.s527. [DOI] [PubMed] [Google Scholar]
- 15.Spangler S, Lin G, Jacobs M, Applebaum P. Postantibiotic effect of sanfetrinem compared with those of six other agents against 12 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1997;41:2173–2176. doi: 10.1128/aac.41.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Hohmura M, Nishi T, Sato K, Hayakawa I. Antimicrobial activity of DU-6681a, a parent compound of novel oral carbapenem DZ-2640. Antimicrob Agents Chemother. 1997;41:1260–1268. doi: 10.1128/aac.41.6.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji M, Ishi Y, Ohno A, Miyazaki S, Yamaguchi K. In vitro and in vivo antibacterial activity of S-1090, a new oral cephalosporin. Antimicrob Agents Chemother. 1995;39:2544–2551. doi: 10.1128/aac.39.11.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji M, Ishi Y, Ohno A, Miyazaki S, Yamaguchi K. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob Agents Chemother. 1998;42:94–99. doi: 10.1128/aac.42.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss W, Yang Y, Petersen P, Shelofsky A, Bush K, Jacobus N, Bitha P, Lin Y-I, Testa R, Tally F. Programs and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. In vitro antibacterial activity and β-lactamase stability of novel THF carbapenems, abstr. F74; p. 128. [Google Scholar]
- 20.Wise R, Andrews J, Ros L, Child J, Mortiboy D. A study to determine the pharmacokinetics and inflammatory fluid penetration of two doses of a solid formulation of the hexetil prodrug of a trinem, sanfetrinem (GV 104326) Antimicrob Agents Chemother. 1997;41:1761–1764. doi: 10.1128/aac.41.8.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]