Abstract
OBJECTIVE
Obesity and type 2 diabetes are associated with serious adverse health effects, including cancer. Although bariatric surgery has been shown to reduce cancer risk in patients with obesity, the effect of bariatric surgery on cancer risk in patients with obesity and diabetes is less studied. We therefore examined the long-term incidence of cancer after bariatric surgery and usual care in patients with obesity and diabetes in the matched prospective Swedish Obese Subjects (SOS) study.
RESEARCH DESIGN AND METHODS
The SOS study examines long-term outcomes following bariatric surgery or usual care. The current analysis includes 701 patients with obesity and type 2 diabetes at baseline, 393 of whom underwent bariatric surgery and 308 who received conventional obesity treatment. Information on cancer events was obtained from the Swedish National Cancer Register. Median follow-up time was 21.3 years (interquartile range 17.6–24.8 years, maximum 30.7 years).
RESULTS
During follow-up, the incidence rate for first-time cancer was 9.1 per 1,000 person-years (95% CI 7.2–11.5) in patients with obesity and diabetes treated with bariatric surgery and 14.1 per 1,000 person-years (95% CI 11.2–17.7) in patients treated with usual obesity care (adjusted hazard ratio 0.63 [95% CI 0.44–0.89], P = 0.008). Moreover, surgery was associated with reduced cancer incidence in women (0.58 [0.38–0.90], P = 0.016), although the sex-treatment interaction was nonsignificant (P = 0.630). In addition, diabetes remission at the 10-year follow-up was associated with reduced cancer incidence (0.40 [0.22–0.74], P = 0.003).
CONCLUSIONS
These results suggest that bariatric surgery prevents cancer in patients with obesity and diabetes and that durable diabetes remission is associated with reduced cancer risk.
Introduction
Obesity and type 2 diabetes are both associated with increased cancer risk and cancer mortality (1–4). In a study by Pearson-Stuttard et al. (5), an estimated 6% of all cancer cases worldwide in 2012 were attributable to the combined effects of diabetes and overweight/obesity. Additionally, the combination of diabetes and overweight/obesity contributed to as much as 25–40% of all liver cancer, esophageal adenocarcinoma, and endometrial cancer cases. Thus, the global pattern of increasing obesity and diabetes has led to an increased cancer prevalence. The causal link between obesity and 13 specific cancer types, referred to as obesity related, is well established (6). In addition, some of these cancer types (i.e., liver, pancreatic, endometrial, colon and rectal, breast, bladder) have been suggested to be diabetes (mainly type 2) related (7). However, at present, there is no clear consensus regarding which cancers are directly linked to type 2 diabetes (8,9). Obesity is a modifiable risk factor for type 2 diabetes and cancer, and bariatric surgery is the most effective intervention for substantial and sustained weight loss in patients with obesity (10). Furthermore, the beneficial effects of bariatric surgery on type 2 diabetes are well described (11,12), and the majority of patients with diabetes achieve short-term diabetes remission after bariatric surgery. Numerous observational and retrospective cohort studies of patients undergoing bariatric surgery have contributed to the understanding that intentional weight loss leads to reduced cancer risk and mortality in patients with obesity (13–17). However, the effects of bariatric surgery on incident cancer in patients with obesity and diabetes is less studied. To this end, we examined the long-term incidence of cancer after bariatric surgery and usual care in patients with obesity and diabetes in the ongoing, matched, prospective controlled Swedish Obese Subjects (SOS) study.
Research Design and Methods
Study Design and Participants
The ongoing, prospective controlled SOS intervention study enrolled 4,047 individuals with obesity between 1987 and 2001, as previously described (Supplementary Material) (18). In brief, 2,010 individuals chose to undergo surgery, and a contemporaneously matched control group of 2,037 participants with obesity was created on the basis of 18 variables. The surgery and control groups had identical inclusion and exclusion criteria. The inclusion criteria were age 37–60 years and a BMI of ≥34 kg/m2 in men and ≥38 kg/m2 in women before or at the time of the matching examination. The exclusion criteria were established to exclude patients with unacceptable surgical risks. The primary end point of the study was overall mortality (18), and the secondary end points were diabetes (19,20), gallbladder disease (21), and cardiovascular disease (22). The outcome of the current report—cancer incidence in patients with obesity and diabetes—was not a predefined end point. Seven regional ethics review boards in Sweden (Gothenburg, Lund, Linköping, Örebro, Karolinska Institute, Uppsala, Umeå) approved the SOS study protocol, and informed consent was obtained from all participants.
Data Collection and Definitions
Baseline characteristics were obtained from clinical examination, questionnaires, and centralized blood biochemistry. Screen-detected diabetes at the baseline examination without previous diagnosis and established diabetes diagnosis before study inclusion were defined as HbA1c ≥48 mmol/mol (≥6.5%), fasting blood glucose of ≥6.1 mmol/L (corresponding to a fasting plasma glucose of ≥7 mmol/L), or diabetes medication use (insulin, oral antidiabetic drugs, or both). Diabetes remission was defined as HbA1c <48 mmol/mol or fasting blood glucose <6.1 mmol/L (plasma glucose <7.0 mmol/L) without receipt of diabetes medication. From 1987 through 2009, fasting glucose concentrations were measured in venous whole blood. After 2009, venous fasting plasma glucose was measured, and the concentrations were converted to those for blood glucose (19). The study was initiated before repeated measurements were routinely used for the diagnosis of diabetes; therefore, single determinations of fasting glucose and HbA1c were used. Baseline alcohol intake was calculated from dietary questionnaires, as previously described (23). Smoking was defined as a positive answer to the question, “Do you smoke daily?” Data on cancer incidence, death, and emigration were obtained by cross-checking social security numbers from the SOS database with the Swedish National Cancer Registry, the Cause of Death Registry, and the Registry of the Total Population. The Swedish National Cancer Registry has >95% coverage for all malignant tumors of which 99% are morphologically verified (24). ICD codes registered for patients in the SOS study are listed in Supplementary Table 1. The cutoff date for the current report was 31 December 2018.
Statistical Analyses
Mean values and SDs or 95% CIs were used to describe the baseline characteristics and changes over time. Baseline characteristics between treatment groups were compared with t tests for continuous variables and Fisher exact test for dichotomous variables. Time of progression to first cancer after inclusion was compared between treatment groups using Kaplan-Meier estimates of cumulative incidence rates. Additionally, hazard ratios (HRs) from a Cox proportional hazards model with a single covariate for treatment group were calculated. To assess the baseline differences between the surgery and control groups, analyses were adjusted for the following predefined risk factors: sex, age, serum insulin, abdominal sagittal diameter (25), alcohol, education, and smoking status. In sensitivity analyses, patients with preinclusion events were excluded, cancer cases that occurred during the first 3 years of the intervention were excluded to account for undetected prevalent cancer at baseline, and deaths unrelated to cancer were treated as competing events in a competing risks regression analysis.
In the interaction analysis, the incidence rates were calculated in subgroups defined by risk factors at baseline. The association of bariatric surgery with the incidence of cancer events was tested by including the corresponding interaction term (i.e., the product of type of treatment [surgery or control] and corresponding continuous variable) in the regression model.
The expected number of surgeries needed to prevent one cancer event over 10 years (number needed to treat) was calculated in various subgroups as the reciprocal of the absolute risk difference between individuals in the surgery and control groups. A per-protocol approach was used in all analyses; thus, all participants were included in their original study group until any bariatric surgery was performed in the control group or there was a change in or removal of the bariatric surgical procedure in the surgery group, after which they were censored from the analysis.
All P values are two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were done using the Stata 15.1 software.
Results
Baseline Characteristics, Weight Changes, and Remission Rates During Follow-up
All participants with diabetes at baseline were included in the analysis. Characteristics of the 393 surgically treated participants and the 308 control participants are shown in Table 1. The surgery group was, on average, younger than the control group; had a higher BMI, more central fat, and higher serum insulin; and were less likely to have a university education. No differences in other baseline parameters, including baseline alcohol intake, smoking status, diabetes medication use, and cancer before inclusion, were observed. The majority of the participants in the surgery and control groups (60.1% and 59.1%, respectively) were women.
Table 1.
Baseline characteristics and remission rates during follow-up for SOS study participants with obesity and diabetes
| Characteristic | Surgery (n = 393)a | Control (n = 308) | P |
|---|---|---|---|
| Baseline | |||
| Women | 236 (60.1) | 182 (59.1) | 0.816 |
| Age (years) | 48.6 (6.0) | 50.5 (6.3) | <0.001 |
| BMI (kg/m2) | 42.4 (4.9) | 40.1 (4.7) | <0.001 |
| Sagittal diameter (cm) | 30.0 (3.7) | 28.6 (3.5) | <0.001 |
| Blood glucose (mmol/L) | 8.2 (2.7) | 8.2 (2.7) | 0.805 |
| HbA1c | |||
| % | 7.8 (1.5) | 7.7 (1.4) | 0.415 |
| mmol/mol | 61.6 (16.7) | 60.5 (15.8) | 0.415 |
| Serum insulin (pmol/L) | 170.0 (113.1) | 151.2 (101.9) | 0.022 |
| Alcohol consumption (g/day) | 5.0 (7.7) | 5.3 (8.2) | 0.589 |
| Daily smoking | 100 (25.5) | 67 (22.1) | 0.325 |
| Cancer before inclusion | 21 (5.3) | 17 (5.5) | 1.000 |
| Year of inclusionb | 1994.0 (3.5) | 1994.1 (3.6) | 0.519 |
| Diabetes medication | 164 (41.7) | 139 (45.1) | 0.398 |
| University education | 46 (11.7) | 54 (17.5) | 0.030 |
| Follow-up | |||
| 2-year remission | 229 (69.8) | 39 (15.9) | <0.001 |
| 10-year remission | 91 (34.1) | 11 (6.5) | <0.001 |
Data are mean (SD) except where n (%) is noted.
Type of surgery: vertical banded gastroplasty (n = 256, 65.1%), nonadjustable or adjustable banding (n = 69, 17.6%), and gastric bypass (n = 68, 17.3%).
Patients were included between 1987 and 2001.
Weight changes over 20 years in surgery and control participants with baseline diabetes are shown in Supplementary Fig. 1. The average weight loss was 27.5 kg and 22.7 kg in surgery participants and 3.2 kg and 4.8 kg in control participants over 2 and 10 years, respectively. The diabetes remission rate in surgery participants was 69.8% at 2 years and 34.1% at 10 years (Table 1). On the date of analysis, the follow-up time was up to 30.7 years, with a median of 21.3 years (interquartile range 17.6–24.8 years).
Incidence of Overall Cancer During Follow-up
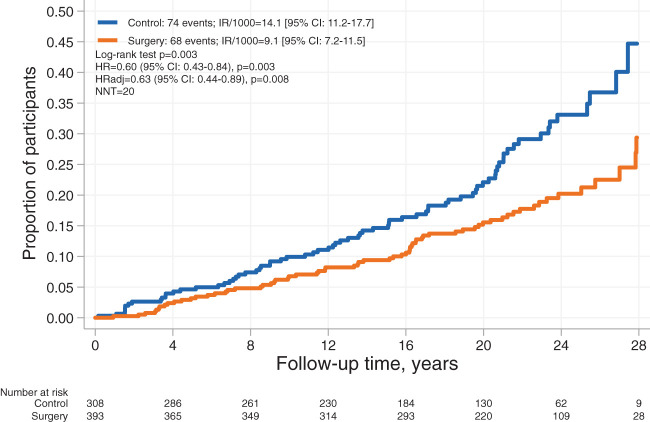
Of the 142 first-time cancers (Supplementary Table 2) recorded during the follow-up period, 74 occurred in the control group and 68 in the surgery group, and the unadjusted HR with surgery was 0.60 (95% CI 0.43–0.84, P = 0.003) (Fig. 1). After multivariable adjustments, the adjusted HR (HRadj) with surgery was 0.63 (95% CI 0.44–0.89, P = 0.008). In sensitivity analyses, the exclusion of 11 cancers that occurred during the first 3 years or the exclusion of 12 patients with preinclusion events yielded similar results (HRadj 0.70 [0.49–1.00, P = 0.048] and 0.66 [0.46–0.96, P = 0.027], respectively). Furthermore, treating deaths unrelated to cancer as competing events in a competing risks regression model also yielded a similar risk estimate (Sub-HRadj 0.68 [0.48–0.96], P = 0.027).
Figure 1.
Kaplan-Meier estimates of cumulative incidence of first-time cancer events in control and surgery participants with obesity and diabetes. Per-protocol analysis adjusted for age, sex, education, alcohol consumption, smoking, sagittal diameter, and serum insulin. IR/1000, incidence rate per 1,000 person-years; NNT, number needed to treat.
Next, we analyzed overall cancer incidence separately in women (86 events) and men (56 events). Cancer incidence in women was significantly lower in the surgery group compared with the control group both in unadjusted and adjusted analyses (HR 0.57 [95% CI 0.38–0.88, P = 0.010], HRadj 0.58 [0.38–0.90, P = 0.016]). No association with surgery was detected in men (HRadj 0.79 [0.46–1.38], P = 0.413) (Supplementary Fig. 2), although a difference in treatment effect between men and women could not be detected (interaction P = 0.630). Moreover, there were no risk factor-treatment interactions with respect to baseline age, sagittal diameter, measures of glycemic control, education, alcohol consumption, or smoking status (Supplementary Table 3).
In both univariable and multivariable Cox regression models, surgical treatment and younger age were associated with a lower risk of overall cancer, whereas no association was found for sex, alcohol consumption, daily smoking, sagittal diameter, serum insulin, or education (Table 2). When the multivariable analysis was stratified by sex, age remained an independent predictor in both men (P < 0.001) and women (P = 0.003). In addition, abdominal sagittal diameter emerged as an independent predictor in women (P = 0.027) but not in men (P = 0.897) (Supplementary Table 4).
Table 2.
Univariable and multivariable Cox proportional hazards models for incident cancer
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Surgery (yes vs. no) | 0.60 (0.43–0.84) | 0.003 | 0.63 (0.44–0.89) | 0.008 |
| Age (years) | 1.07 (1.04–1.10) | <0.001 | 1.07 (1.04–1.10) | <0.001 |
| Sex (men vs. women) | 1.04 (0.75–1.46) | 0.801 | 0.89 (0.61–1.31) | 0.558 |
| Alcohol consumption (g/day) | 1.01 (0.98–1.03) | 0.560 | 1.01 (0.98–1.03) | 0.664 |
| Daily smoking (yes vs. no) | 1.28 (0.88–1.86) | 0.204 | 1.46 (0.99–2.15) | 0.057 |
| Sagittal diameter (cm) | 1.03 (0.98–1.08) | 0.230 | 1.05 (1.00–1.10) | 0.075 |
| Serum insulin (pmol/L) | 1.00 (0.99–1.01) | 0.506 | 1.01 (1.00–1.01) | 0.154 |
| University education | 0.95 (0.59–1.53) | 0.834 | 0.89 (0.56–1.42) | 0.625 |
HRs are expressed as “vs.” for dichotomous variables and per unit for continuous variables. Men coded as 1 and women as 0. Yes coded as 1 and no as 0.
Next, we investigated whether degree of weight loss after surgery was associated with cancer development. Within the surgery group, no statistically significant association between weight loss and risk of cancer could be demonstrated (P = 0.092). However, when comparing surgery participants with greatest loss of body weight during the 1st year of follow-up with those with the least weight loss in a somewhat underpowered subanalysis, there was a trend toward lower risk for future cancer after greater weight loss (comparing weight loss tertiles with greatest to smallest weight loss, HRadj 0.58 [95% CI 0.30–1.12], P = 0.102) (Supplementary Table 5).
Cancer Events by Baseline Diabetes Duration
To analyze whether diabetes duration at baseline was associated with cancer incidence, additional analyses were performed. When testing associations between bariatric surgery and cancer incidence in subgroups of participants with screen-detected diabetes or established diabetes separately, similar results were obtained in both groups (HRadj 0.63 [95% CI 0.39–1.03, P = 0.066] and 0.64 [0.39–1.03, P = 0.068], respectively) (Supplementary Fig. 3).
Cancer Events by 10-Year Remission Status
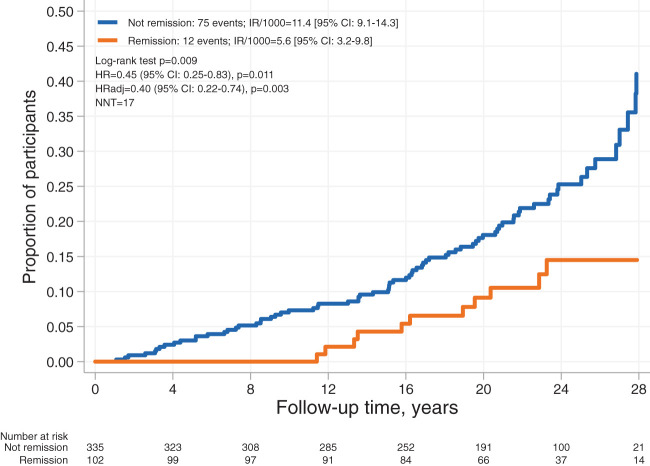
We also analyzed whether diabetes remission status during follow-up was associated with cancer incidence. This analysis showed that diabetes remission at the 10-year follow-up was associated with reduced cancer incidence in both unadjusted and adjusted analyses (HR 0.45 [95% CI 0.25–0.83, P = 0.011] and HRadj 0.40 [0.22–0.74, P = 0.003]) in surgery and control participants combined (Fig. 2). The exclusion of 10 patients with preinclusion events in a sensitivity analysis yielded similar results (HR 0.42 [0.22–0.81], P = 0.010).
Figure 2.
Kaplan-Meier estimates of cumulative incidence of first-time cancer events stratified by 10-year remission status in surgery and control participants combined. Adjusted for age, sex, education, alcohol consumption, smoking, sagittal diameter, and serum insulin. IR/1000, incidence rate per 1,000 person-years; NNT, number needed to treat.
Incidence of Obesity-Related Cancer During Follow-up
We also specifically analyzed the combined incidence of the 13 obesity-related cancer types (6) (Supplementary Table 1). During the follow-up period, 42 events occurred in the control group and 42 in the surgery group, and there was no association between bariatric surgery and incidence of obesity-related cancer in men and women combined (HR 0.74 [95% CI 0.48–1.13], P = 0.158) (Supplementary Table 6). Only age was associated with risk of obesity-associated cancer both in univariable and multivariable analyses.
When incidence of obesity-related cancer was analyzed separately in women (56 events) and men (28 events), we found that bariatric surgery was associated with a reduced incidence of obesity-related cancer in unadjusted analyses in women (HR 0.58 [95% CI 0.35–0.98], P = 0.040) but did not reach significance after multivariable adjustments (HRadj 0.64 [0.38–1.09], P = 0.101) (Supplementary Fig. 4). Similar to overall cancer, no association with surgery was detected in men (HR 1.09 [0.52–2.29], P = 0.828), and there was no detectable difference in treatment effect between men and women (interaction P = 0.166).
Conclusions
In this prospective controlled intervention study with up to 31 years of follow-up, we found that bariatric surgery was associated with a decreased risk of cancer in patients with obesity and diabetes. This finding is in line with previous studies that have evaluated the effect of bariatric surgery on cancer risk in patients with obesity (13–17). Moreover, for the first time, we show that durable diabetes remission in patients with obesity and diabetes is associated with a drastically reduced risk of cancer development long term.
Remission of type 2 diabetes is often achieved after bariatric surgery–induced weight loss. We and others have previously reported that patients with a short diabetes duration at the time of surgical treatment have a higher chance for diabetes remission after surgery and a lower risk of diabetes complications long term (20,26). However, the association between bariatric surgery and cancer risk was similar in groups of patients with short or long diabetes duration. On the other hand, we observed that durable diabetes remission was associated with a 60% reduction of cancer risk. Patients who are in remission no longer require antidiabetic treatment (including insulin) and have lower endogenous insulin levels (27). Thus, this finding supports the role of insulin as a reversible cancer risk factor. Moreover, the observed cancer risk reduction in the current report adds to other health benefits of bariatric surgery in patients with obesity and diabetes, such as reduced risk of microvascular complications, myocardial infarction, and mortality (28–30).
In the overall analysis, during follow-up we found a 37% reduced risk of incident cancer after bariatric surgery in patients with obesity and diabetes. This may be compared with the meta-estimate from six observational studies displaying a 45% cancer risk reduction with bariatric surgery in patients with obesity, irrespective of diabetes status (17). When performing analyses stratified by sex, bariatric surgery was associated with a reduced overall risk of cancer only in women, although we could not detect a difference in treatment effect between men and women (sex-treatment interaction nonsignificant). Nonetheless, it has been shown repeatedly that cancer risk reduction in patients with obesity is more marked among women than men (13–15,17). Possible explanations for the association with surgery in women may be that cancer rates are higher in women with diabetes than in men with diabetes (9) and that common cancer types associated with obesity are female specific (6). These assumptions are supported by our current results where the risk reduction for obesity-related cancers in women was even more pronounced than the overall cancer risk reduction in women. Furthermore, both estrogen and insulin are cancer risk factors that are increased in obesity, each with the capacity to regulate the secretion and signaling pathway of the other (31), and bariatric surgery has been shown to reduce circulating levels of estrogen as well as other cancer-associated biomarkers (32–34). Moreover, insulin may increase the fraction of bioactive estrogen by suppression of sex hormone–binding globulin and thereby contribute to the promotion of estrogen-dependent breast and endometrial cancer (31,35). Indeed, we previously examined the association of bariatric surgery with incidence of female-specific cancer events irrespective of diabetes status in the SOS study and found that bariatric surgery was associated with a greater cancer risk reduction in women with hyperinsulinemia at baseline (36). In patients with diabetes however, insulin levels are harder to interpret, and in the current study, the cancer risk reduction with surgery was comparable, regardless of baseline insulin levels.
Studies also have suggested a possible association between the magnitude of weight loss and cancer risk reduction following both lifestyle and bariatric surgery interventions (37–39). We observed a similar, but not significant trend where patients in the highest weight loss tertile (average −44.8 kg) had a somewhat lower risk of cancer compared with the lowest weight loss tertile (average −14.9 kg). In the randomized Look AHEAD (Action for Health in Diabetes) trial, intensive lifestyle intervention led to an average weight loss of 8.7 kg at 1 year and 6.8 kg at 12 years, and there was a tendency toward a reduced risk of obesity-related cancer in individuals with overweight/obesity and type 2 diabetes (38). In light of these data, it may be speculated that interventions resulting in greater weight loss should be recommended in patients with obesity and diabetes.
Also of note, the link between diabetes and cancer appears to be bidirectional. For example, it has been reported that cancer survivors have a higher risk of developing type 2 diabetes (40). Furthermore, treatments ensuring glycemic control and weight loss regimens seem important not only for reducing the risk of cancer but also for improving prognosis in cancer survivors, as illustrated by results from the Reach for Health Study (41). In the Reach for Health Study, the effects of weight loss and metformin treatment on type 2 diabetes–related biomarkers in postmenopausal breast cancer survivors with overweight/obesity were analyzed. It was demonstrated that both treatments advantageously affect concentrations of insulin and sex hormone–binding globulin, and the combined effects of weight loss and metformin treatments were found to be additive (41). The complexity of these important findings points to the urgent need for additional research in this area.
Major strengths of the SOS study are the very long follow-up and the prospective study design, examinations to determine diabetes status, and the possibility of acquiring information from comprehensive national registers. Several limitations also are noted. First, the SOS study was initiated before repeated measurements were routinely used for the diagnosis of type 2 diabetes; thus, diabetes diagnosis was based on measurements at a single time point and/or use of diabetes medication. Second, the majority of the participants in the surgery group had undergone vertical banded gastroplasty or banding, procedures rarely used today. Third, because of high postoperative mortality after bariatric surgery at the time when the SOS study was started, the study could not be randomized for ethical reasons. Fourth, cancer incidence was not a predefined end point in the SOS study and, thus, SOS was not designed to address the current research question or powered for subgroup/sex-stratified analyses as was performed here; hence, all the current study analyses are explorative. Finally, because of the high probability of patients undergoing treatment changes during the long follow-up period, we were unable to examine the potential impact of various diabetes medications on cancer risk, and it should also be noted that because of lack of consensus regarding cancers directly associated with type 2 diabetes, independent from obesity, we refrained from performing such analyses (8,9).
In conclusion, with increasing rates of obesity and diabetes worldwide, a greater emphasis on cancer prevention strategies is needed. Our results suggest that bariatric surgery may greatly reduce the risk of cancer among patients with obesity and diabetes. Moreover, durable diabetes remission seems imperative for cancer prevention in patients with obesity and diabetes.
Article Information
Acknowledgments. The authors thank the staff members at 480 primary health care centers and 25 surgical departments in Sweden who participated in the SOS study. The authors also thank Christina Torefalk and Björn Henning (both from Institute of Medicine, University of Gothenburg) for administrative support.
Funding. The research reported in this article was supported by the Swedish state under an agreement between the Swedish government and the county councils (the ALF agreement [ALFGBG-717881 and ALFGBG-717891]), the Swedish Research Council (2017-01707), the Novo Nordisk Foundation (19OC0057184), the Swedish Heart-Lung Foundation (20180410), and the Swedish Diabetes Foundation (2019-417).
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Duality of Interest. L.M.S.C. has received consulting fees from Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.S., L.M.S.C., P.-A.S., J.C.A.-A., F.K., P.J., M.P., and M.T. contributed to the acquisition and interpretation of data, critically reviewed the report, contributed to the revision, and approved the final version of the manuscript. K.S., L.M.S.C., M.P., and M.T. designed the study. K.S. and M.T. wrote the manuscript with contributions from all authors. M.P. designed and conducted the statistical analyses. K.S. and M.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01479452, clinicaltrials.gov.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16903630.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. Bjornsdottir HH, Rawshani A, Rawshani A, et al. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci Rep 2020;10:17376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 3. Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects study. N Engl J Med 2020;383:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou XH, Qiao Q, Zethelius B, et al.; DECODE Study Group . Diabetes, prediabetes and cancer mortality. Diabetologia 2010;53:1867–1876 [DOI] [PubMed] [Google Scholar]
- 5. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018;6:e6–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F; International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ling S, Brown K, Miksza JK, et al. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care 2020;43:2313–2322 [DOI] [PubMed] [Google Scholar]
- 9. Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia 2018;61:2140–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 11. Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-yearfollow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021;397:293–304 [DOI] [PubMed] [Google Scholar]
- 12. Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg 2019;269:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sjöström L, Gummesson A, Sjöström CD, et al.; Swedish Obese Subjects Study . Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–662 [DOI] [PubMed] [Google Scholar]
- 16. Taube M, Peltonen M, Sjöholm K, et al. Association of bariatric surgery with skin cancer incidence in adults with obesity: a nonrandomized controlled trial. JAMA Dermatol 2020;156:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc 2013;27:4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sjöström L, Narbro K, Sjöström CD, et al.; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 19. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 20. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 21. Anveden Å, Peltonen M, Näslund I, Torgerson J, Carlsson LMS. Long-term incidence of gallstone disease after bariatric surgery: results from the nonrandomized controlled Swedish Obese Subjects study. Surg Obes Relat Dis 2020;16:1474–1482 [DOI] [PubMed] [Google Scholar]
- 22. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 23. Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish Obese Subjects study. Obesity (Silver Spring) 2013;21:2444–2451 [DOI] [PubMed] [Google Scholar]
- 24. Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27–33 [DOI] [PubMed] [Google Scholar]
- 25. Neeland IJ, Ross R, Després JP, et al.; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity . Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–725 [DOI] [PubMed] [Google Scholar]
- 26. Coleman KJ, Haneuse S, Johnson E, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care 2016;39:1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sjöström L, Lindroos AK, Peltonen M, et al.; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 28. Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg 2017;27:2724–2732 [DOI] [PubMed] [Google Scholar]
- 30. Carlsson LMS, Sjöholm K, Karlsson C, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2017;5:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castagneto-Gissey L, Casella-Mariolo J, Casella G, Mingrone G. Obesity surgery and cancer: what are the unanswered questions? Front Endocrinol (Lausanne) 2020;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farey JE, Fisher OM, Levert-Mignon AJ, Forner PM, Lord RV. Decreased levels of circulating cancer-associated protein biomarkers following bariatric surgery. Obes Surg 2017;27:578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khosravi-Largani M, Nojomi M, Aghili R, et al. Evaluation of all types of metabolic bariatric surgery and its consequences: a systematic review and meta-analysis. Obes Surg 2019;29:651–690 [DOI] [PubMed] [Google Scholar]
- 34. Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14:1940–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer 2012;19:R225–R241 [DOI] [PubMed] [Google Scholar]
- 36. Anveden Å, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects study. Gynecol Oncol 2017;145:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunsinger MA, Wood GC, Still C, et al. Maximizing weight loss after Roux-en-Y gastric bypass may decrease risk of incident organ cancer. Obes Surg 2016;26:2856–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeh HC, Bantle JP, Cassidy-Begay M, et al.; Look AHEAD Research Group . Intensive weight loss intervention and cancer risk in adults with type 2 diabetes: analysis of the Look AHEAD randomized clinical trial. Obesity (Silver Spring) 2020;28:1678–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stroud AM, Dewey EN, Husain FA, et al. Association between weight loss and serum biomarkers with risk of incident cancer in the Longitudinal Assessment of Bariatric Surgery cohort. Surg Obes Relat Dis 2020;16:1086–1094 [DOI] [PubMed] [Google Scholar]
- 40. Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev 2020;41:bnz014. [DOI] [PubMed] [Google Scholar]
- 41. Patterson RE, Marinac CR, Sears DD, et al. The Effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. J Natl Cancer Inst 2018;110:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]