Abstract
OBJECTIVE
Type 1 diabetes is described to have an acute onset, but autoantibodies can appear several years preceding diagnosis. This suggests a long preclinical phase, which may also include metabolic parameters. Here we assessed whether elevations in glycemic, lipid, and other metabolic biomarkers were associated with future type 1 diabetes risk in adults.
RESEARCH DESIGN AND METHODS
We studied 591,239 individuals from the Swedish AMORIS cohort followed from 1985–1996 to 2012. Through linkage to national patient, diabetes, and prescription registers, we identified incident type 1 diabetes. Using Cox regression models, we estimated hazard ratios for biomarkers at baseline and incident type 1 diabetes. We additionally assessed trajectories of biomarkers during the 25 years before type 1 diabetes diagnosis in a nested case-control design.
RESULTS
We identified 1,122 type 1 diabetes cases during follow-up (average age of patient at diagnosis: 53.3 years). The biomarkers glucose, fructosamine, triglycerides, the ratio of apolipoprotein (apo)B to apoA-I, uric acid, alkaline phosphatase, and BMI were positively associated with type 1 diabetes risk. Higher apoA-I was associated with lower type 1 diabetes incidence. Already 15 years before diagnosis, type 1 diabetes cases had higher mean glucose, fructosamine, triglycerides, and uric acid levels compared with control subjects.
CONCLUSIONS
Alterations in biomarker levels related to glycemia, lipid metabolism, and inflammation are associated with clinically diagnosed type 1 diabetes risk, and these may be elevated many years preceding diagnosis.
Introduction
Type 1 diabetes accounts for 99% of all diabetes in children and adolescents but can develop at any age. Notably, a recent study based on data from UK biobank estimated that 42% of all cases are diagnosed among patients between ages 30 and 60 years (1). Research regarding the pathogenesis of type 1 diabetes with adult onset is limited, but it has been shown that adult patients often present with only one autoantibody, whereas children tend to be positive for multiple autoantibodies. Patients with onset in adulthood are also described to have less severe decline in insulin secretion and a longer asymptomatic period compared with children and adolescents (2,3). Furthermore, it has been proposed that insulin resistance contributes to the development of autoimmune diabetes in adults, especially for the subgroup with latent autoimmune diabetes in adults (4,5).
Type 2 diabetes is known to develop over a long period of time through a prediabetes stage with elevated blood glucose levels. In line with this, we previously found that individuals who developed type 2 diabetes differed from those who did not in terms of several glycemia- and lipid-related biomarkers up to 25 years prior to diagnosis (6). Type 1 diabetes, on the other hand, is described to have an acute onset, and symptoms can develop quickly over a few weeks or even days. However, the first autoantibody indicating an autoimmune reaction typically occurs several years prior to manifestation of type 1 diabetes, suggesting a long preclinical phase (7). Similarly, it has been shown that adults who develop diabetes can have autoantibodies 10 years prior to diagnosis (8–10).
It is possible that other metabolic processes are operating in parallel, or perhaps even prior, to appearance of autoantibodies. Metabolic alterations, including lipid species and amino acids, have indeed been found in asymptomatic children who later develop type 1 diabetes (11,12). Corresponding studies in adults are, however, lacking. The aim of the current study was to describe alterations in biomarkers reflecting glycemia and lipid metabolism, as well as inflammatory markers, and the risk of adult-onset type 1 diabetes and to assess their temporal relationship with the development of type 1 diabetes. This knowledge could increase our understanding of metabolic changes that occur during the development of type 1 diabetes in adults.
Research Design and Methods
Study Population
The Swedish AMORIS (Apolipoprotein-related MOrtality RISk) cohort has previously been described (13). In brief, this longitudinal cohort consists of 812,073 individuals who predominantly lived in Stockholm, Sweden, and who underwent health examinations through either occupational health checkups or outpatient visits between 1985 and 1996 (baseline period). The AMORIS cohort included ∼35% of people living in the greater Stockholm area at the inclusion time, and sociodemographic characteristics were comparable with the general working population in Stockholm (13), whereas the mortality rate was lower than for the general population as a whole (6). Eligible for the current study were all individuals with at least one biomarker measurement of interest in the baseline period (n = 623,152). We excluded all individuals with a documented diagnosis of diabetes at baseline in any of the registers described below, or suspicion of diabetes at baseline (glucose >7.8 mmol/L or fructosamine >2.3 mmol/L or HbA1c >6.4%) (n = 31,913), resulting in an analytical sample of 591,239 individuals. Using the unique Swedish personal identity number, study participants were followed until 31 December 2011 by linkage to the Swedish Cause of Death Register, the National Patient Register (PR), the National Diabetes Registry (NDR), and the Prescribed Drug Register (PDR). Country of birth and occupational status were ascertained from the Swedish Censuses from 1970 to 1990—whichever was closest to the first measurement. Gainfully employed individuals were classified as manual and lower nonmanual workers or higher nonmanual workers, representing low and high socioeconomic status, respectively.
This study complied with the Declaration of Helsinki and was approved by the regional ethics committee at Karolinska Institutet, Stockholm, Sweden (registration no. 2010/1:7).
Biomarkers
All laboratory analyses were performed on fresh blood samples at the accredited CALAB laboratory (Stockholm, Sweden) using standardized, automated, and well-documented methodologies (6,13,14). In the current study, we included information on date of health examination (blood sampling), fasting status, and blood levels regarding glucose, fructosamine, triglycerides, total cholesterol, apolipoprotein (apo)A-I, and apoB and the apoB–to–apoA-I ratio. In addition, we included information from haptoglobin, uric acid, albumin, C-reactive protein, leukocyte count, urea, γ-glutamyl transferase, and alkaline phosphatase. The majority of these biomarkers were part of a standard analysis package, which represented regular health screenings (13), and have previously been studied in relation to type 2 diabetes (6). Furthermore, we obtained information on BMI from health examinations for a proportion of the study population (25%). Information on HbA1c was only available for 13,811 (2.3%) of the cohort population and could not be used in the current study. Fructosamine was available for most study participants, has been shown to correlate well with HbA1c in subjects with type 1 diabetes, and does not require fasting samples (15).
Identification of Incident Type 1 Diabetes
For identification of incident type 1 diabetes cases, we used information from NDR (16), PR (17), and PDR (18). In total, 2,534 individuals had a record of type 1 diabetes in NDR or PR during follow-up. Individuals with a type 2 diabetes record at any time, in either register, were not included. To additionally exclude any potentially misclassified type 2 diabetes cases, we specified that patients with type 1 diabetes have an age of diagnosis of <30 years, a record of insulin prescription, or a combination of these (n = 1,122) (Supplementary Fig. 1). Further details can be found in Supplementary Material.
Statistical Analyses
Baseline characteristics for the full study population and for case and control subjects in the nested case-control setup are displayed as mean values and SD for continuous variables and as proportions for categorical variables.
In the prospective cohort analysis, we estimated hazard ratios (HR) with 95% CI for type 1 diabetes in relation to biomarkers using Cox proportional hazards regression models. Age was the underlying time scale to obtain flexible, nonparametric adjustment for age (19), and person-years were accumulated from age at baseline until age at diabetes diagnosis, death, or end of follow-up, 31 December 2011. These models were adjusted for sex, age (timescale), calendar time, fasting status (overnight fasting vs. nonfasting), socioeconomic status, and country of birth. We investigated the assumption of proportional hazards by applying the χ2 test based on Schoenfeld residuals. If the assumption was violated regarding a confounder, we applied Cox models with interaction between the confounder and age in the model. To estimate and evaluate nonproportional effect and dependence on age, we additionally performed analysis stratified by median and quartiles of age at biomarker measurement.
In these analyses, we used the standardized, continuous variable values to allow that a 1-unit change in HR corresponds to the change of 1 SD in the standardized variable. We additionally dichotomized variables to estimate the HR associated with high compared with low levels of the biomarkers; cutoffs for the high levels were set according to published guidelines or previous publications on the AMORIS cohort (6,15,20). We performed a logarithmic transformation of the continuous variables triglycerides and BMI to approximate normally distributed values.
In addition, we conducted a nested case-control analysis within the cohort for all biomarkers. In this analysis, all incident type 1 diabetes cases were included, as well as 25 randomly selected control subjects per cases based on incidence density sampling through calendar year (21) and individual matching by sex and age-group (5-year interval). We defined the index date based on the date of type 1 diabetes diagnosis for case subjects and the date of selection for the control subjects. We performed conditional logistic regression and estimated associations between the biomarkers and type 1 diabetes with odds ratios (ORs) and 95% CI. All models were adjusted as described above.
To compare biomarker values between case and control subjects at given time points, we created population trajectories using mean biomarker values for each year, up to 25 years prior to diagnosis. These trajectories were based on a single measurement per individual and, hence, represent not alterations of biomarker levels for an individual over time but, rather, the mean value of case and control subjects, respectively; the methodology has previously been described (22). For each biomarker, we estimated weighted means and 95% confidence bands based on matching criteria as described above, for both case and control subjects and for each year prior to type 1 diabetes diagnosis.
All analyses were performed in R Studio with R, version 3.6.2; for details on packages used, see Supplementary Material.
Sensitivity Analysis
To exclude any potentially misclassification of subjects with type 2 diabetes, we repeated the main analyses including only individuals age <30 years and <40 years, respectively, at end of follow-up; 8.9% and 22.6% of type 1 diabetes case subjects had an age of onset ≤30 and ≤40 years, respectively. Furthermore, we performed additional analyses excluding individuals prescribed any oral glucose-lowering therapy, as well as requiring insulin prescription for all case subjects within 1 year of diagnosis.
We additionally performed stratified analyses by running separate models for women and men, and younger and older individuals (with median age or age quartiles of age at blood sampling as cutoffs), to assess the potential variation by sex and age. For details on analysis both limited to fasting samples and adjusted for BMI, see Supplementary Material.
Results
Cohort Analysis
Overall, average age of cohort patients was 44 years at blood sampling. The cohort consisted of slightly more males than females, and the majority of individuals were nonmanual workers. During the follow-up period, 1,122 individuals were diagnosed with type 1 diabetes, with an incidence rate of 10.2 per 100,000 person-years.
Average age of type 1 diabetes case subjects was 53 years at disease onset (age ranging from 8 to 94 years), and 8.4% of all case subjects had a recorded disease onset of <30 years of age. Furthermore, there was a higher proportion of men and a higher proportion of nonmanual workers among type 1 diabetes cases compared with individuals without type 1 diabetes (Table 1). The average time from blood sampling to diagnosis was 11 years.
Table 1.
Participant characteristics at the baseline investigation
| Variable | Full study population | Type 1 diabetes case subjects | Control subjects | Difference case vs. control subjects (P) | |||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) or % | N | Mean (SD) or % | N | Mean (SD) or % | ||
| Age (years) | 524,102 | 43.89 (14.39) | 987a | 53.28 (15.67)b | 24,675 | 53.22 (15.64)b | ns |
| Follow-up (years) | 524,102 | 18.87 (5.87) | 987 | 11.26 (6.11) | 24,675 | 11.03 (6.24) | ns |
| Sex (females) | 247,828 | 48.25 | 369 | 37.39 | 9,225 | 37.39 | ns |
| Country of birth (Sweden) | 505,890 | 86.02 | 851 | 86.22 | 21,440 | 86.89 | ns |
| Occupation | |||||||
| Manual workers | 124,871 | 48.85 | 278 | 28.17 | 6,178 | 25.08 | 0.0290 |
| Nonmanual workers | 279,572 | 35.77 | 512 | 51.87 | 13,569 | 54.99 | ns |
| Unclassified/missing | 117,804 | 14.25c | 194 | 19.66 | 4,848 | 19.65 | ns |
| BMI (kg/m2) | 132,207 | 23.72 (3.72) | 228 | 24.96 (4.09) | 5,700 | 24.17 (3.56) | 0.0068 |
| Glucose (mmol/L) | 524,102 | 4.83 (0.68) | 987 | 5.24 (0.98) | 24,675 | 4.80 (0.64) | <0.0001 |
| Fructosamine (mmol/L) | 431,578 | 2.06 (0.19) | 812 | 2.13 (0.20) | 20,300 | 2.07 (0.19) | <0.0001 |
| Triglycerides (mmol/L) | 542,834 | 1.08 (1.74) | 1,040 | 1.66 (1.46) | 26,000 | 1.28 (0.89) | <0.0001 |
| Total cholesterol (mmol/L) | 544,827 | 5.53 (1.16) | 1,040 | 5.55 (1.19) | 26,000 | 5.51 (1.15) | ns |
| ApoB–to–apoA-I ratio | 160,444 | 0.91 (0.31) | 305 | 1.01 (0.37) | 7,625 | 0.92 (0.31) | <0.0001 |
| ApoA-I (g/L) | 184,163 | 1.43 (0.24) | 348 | 1.34 (0.23) | 8,700 | 1.42 (0.23) | <0.0001 |
| ApoB (g/L) | 171,471 | 1.25 (0.36) | 332 | 1.30 (0.37) | 8,300 | 1.26 (0.35) | ns |
| Haptoglobin (g/L) | 411,156 | 1.07 (0.34) | 822 | 1.10 (0.35) | 20,500 | 1.05 (0.32) | <0.0001 |
| Uric acid (μmol/L) | 503,933 | 288.11 (73.45) | 963 | 310.48 (79.25) | 24,075 | 291.37 (69.88) | <0.0001 |
| Albumin (g/L) | 487,892 | 43.25 (2.89) | 930 | 43.36 (2.87) | 23,250 | 43.47 (2.81) | ns |
| Urea (mmol/L) | 383,174 | 5.13 (1.44) | 744 | 5.21 (1.31) | 18,600 | 5.16 (1.28) | ns |
| γ-glutamyl transferase (μkat/L) | 527,829 | 0.44 (0.69) | 1,001 | 0.64 (1.16) | 25,025 | 0.42 (0.57) | <0.0001 |
| Alkaline phosphatase (μkat/L) | 494,381 | 2.73 (1.22) | 951 | 3.05 (1.97) | 23,775 | 2.73 (1.26) | <0.0001 |
| C-reactive protein (mg/L) | 343,978 | 5.96 (16.91) | 582 | 7.96 (30.56) | 14,550 | 5.69 (15.03) | ns |
| Leukocytes (109 cells/L) | 188,057 | 6.58 (2.38) | 323 | 7.03 (2.47) | 8,075 | 6.49 (1.96) | 0.0001 |
ns, not significant at α = 0.05.
Age at recorded diagnosis of type 1 diabetes or selection as control subjects.
Due to the available measurements, the number of case subjects studied varied between biomarkers, e.g., 987 type 1 diabetes case subjects had glucose levels assessed at baseline.
Category “unclassified” also includes individuals age ≤16 years and pensioners.
In using biomarkers as continuous variables, the glycemia-related markers glucose and fructosamine, as well as the markers of lipid metabolism triglycerides, apoB, and apoB–to–apoA-I ratio, were positively associated with risk of developing type 1 diabetes (Table 2). In addition, we found a positive association with type 1 diabetes risk for BMI, uric acid, haptoglobin, C-reactive protein, and leukocyte count, as well as the biomarkers γ-glutamyl transferase and alkaline phosphatase. For apoA-I, on the other hand, we found a negative association with type 1 diabetes risk (Table 2). Overall, we found somewhat stronger associations in older individuals (Supplementary Table 1). Among these biomarkers, the associations for glucose, fructosamine, triglycerides, apoA-I, uric acid, and alkaline phosphatase were independent of age and sex (Table 3 and Supplementary Table 1). Sensitivity analysis using only biomarker values obtained from blood samples drawn after overnight fasting did not change the results (Supplementary Table 1).
Table 2.
HRs and 95% CIs of type 1 diabetes for continuous biomarkers
| Biomarker | Person-years | Case subjects, n (%) | HR (95% CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Biomarkers related to glycemia | ||||
| Glucose (mmol/L) | 9,887,018 | 987 (0.19) | 1.66 (1.59–1.73) | 1.72 (1.65–1.80) |
| Fructosamine (mmol/L) | 7,956,801 | 813 (0.19) | 1.50 (1.40–1.61) | 1.45 (1.35–1.56) |
| Biomarkers of lipid metabolism | ||||
| Triglycerides (mmol/L) | 10,196,681 | 1,040 (0.19) | 1.49 (1.41–1.58) | 1.49 (1.40–1.58) |
| Total cholesterol (mmol/L) | 10,227,990 | 1,040 (0.19) | 1.05 (0.98–1.12) | 1.06 (0.99–1.13) |
| ApoB–to–apoA-I ratio | 2,810,460 | 305 (0.19) | 1.36 (1.25–1.49) | 1.35 (1.23–1.48) |
| ApoA-I (g/L) | 3,306,276 | 348 (0.19) | 0.65 (0.58–0.73) | 0.67 (0.59–0.76) |
| ApoB (g/L) | 3,035,459 | 332 (0.19) | 1.20 (1.08–1.34) | 1.17 (1.05–1.31) |
| Biomarkers related to inflammation | ||||
| Haptoglobin (g/L) | 8,145,669 | 823 (0.20) | 1.16 (1.09–1.24) | 1.16 (1.09–1.24) |
| Uric acid (μmol/L) | 9,537,617 | 964 (0.19) | 1.43 (1.35–1.52) | 1.46 (1.36–1.57) |
| Albumin (g/L) | 9,202,623 | 930 (0.19) | 1.02 (0.95–1.09) | 0.97 (0.90–1.04) |
| C-reactive protein (mg/L) | 5,964,550 | 582 (0.17) | 1.05 (1.01–1.09) | 1.04 (1.01–1.08) |
| Leukocytes (109 cells/L) | 3,138,493 | 323 (0.17) | 1.08 (1.05–1.12) | 1.08 (1.05–1.12) |
| Other biomarkers | ||||
| Urea (mmol/L) | 7,276,004 | 745 (0.19) | 1.10 (1.03–1.18) | 1.05 (0.97–1.14) |
| γ-glutamyl transferase (μkat/L) | 9,844,951 | 1,001 (0.19) | 1.09 (1.07–1.11) | 1.09 (1.07–1.11) |
| Alkaline phosphatase (μkat/L) | 9,238,047 | 952 (0.19) | 1.16 (1.14–1.19) | 1.16 (1.13–1.19) |
| BMI (kg/m2) | 2,387,455 | 230 (0.17) | 1.31 (1.15–1.48) | 1.28 (1.12–1.46) |
HRs were estimated using adjusted Cox models with attained age as the underlying timescale and date of birth as the time origin. We used standardized, continuous variable values to allow that a 1-unit change in HR corresponds with the change of 1 SD in the standardized variable. Model 1 was not adjusted for any additional confounders; model 2 was adjusted for sex, age at first blood sampling, fasting status (overnight fasting vs. nonfasting), occupation, and country of birth.
Table 3.
HRs and 95% CIs of type 1 diabetes for continuous biomarkers (analyses stratified by sex and age of onset)
| Biomarker | Men | Women | Age of onset ≤30 years | Age of onset ≤40 years | ||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI) | N | HR (95% CI) | N case subjects | HR (95% CI) | N case subjects | HR (95% CI) | |
| Biomarkers related to glycemia | ||||||||
| Glucose (mmol/L) | 276,274 | 1.65 (1.56–1.75) | 247,828 | 1.85 (1.73–1.98) | 83 | 1.67 (1.40–1.99) | 222 | 1.54 (1.37–1.74) |
| Fructosamine (mmol/L) | 226,584 | 1.46 (1.33–1.60) | 204,967 | 1.44 (1.28–1.62) | 71 | 1.56 (1.21–2.00) | 30 | 1.49 (1.27–1.74) |
| Biomarkers of lipid metabolism | ||||||||
| Triglycerides (mmol/L) | 286,762 | 1.46 (1.35–1.57) | 256,072 | 1.53 (1.37–1.71) | 86 | 1.04 (0.82–1.31) | 228 | 1.12 (0.98–1.29) |
| Total cholesterol (mmol/L) | 287,889 | 1.04 (0.95–1.13) | 256,938 | 1.06 (0.94–1.19) | 86 | 1.05 (0.79–1.41) | 228 | 1.02 (0.86–1.21) |
| ApoB–to–apoA-I ratio | 89,616 | 1.34 (1.19–1.51) | 70,828 | 1.34 (1.14–1.58) | 13 | 1.49 (1.05–2.12) | 47 | 1.15 (0.85–1.56) |
| ApoA-I (g/L) | 103,016 | 0.62 (0.53–0.73) | 81,147 | 0.74 (0.61–0.90) | 16 | 0.59 (0.33–1.05) | 54 | 0.72 (0.53–0.99) |
| ApoB (g/L) | 95,262 | 1.13 (0.98–1.31) | 76,209 | 1.19 (0.99–1.43) | 18 | 1.45 (0.84–2.51) | 54 | 0.98 (0.69–1.39) |
| Biomarkers related to inflammation | ||||||||
| Haptoglobin (g/L) | 217,344 | 1.11 (1.03–1.21) | 193,812 | 1.25 (1.13–1.39) | 65 | 1.01 (0.79–1.30) | 173 | 1.10 (0.95–1.28) |
| Uric acid (μmol/L) | 264,102 | 1.43 (1.31–1.56) | 239,831 | 1.50 (1.33–1.69) | 82 | 1.06 (0.79–1.42) | 213 | 1.12 (0.94–1.33) |
| Albumin (g/L) | 256,437 | 0.91 (0.83–1.00) | 241,455 | 1.03 (0.92–1.16) | 81 | 0.82 (0.66–1.02) | 203 | 0.91 (0.79–1.05) |
| C-reactive protein (mg/L) | 176,509 | 1.05 (1.01–1.09) | 167,469 | 1.02 (0.94–1.12) | 51 | 0.96 (0.64–1.43) | 131 | 0.92 (0.64–1.31) |
| Leukocytes (109 cells/L) | 80,434 | 1.08 (1.03–1.13) | 107,623 | 1.08 (1.03–1.14) | 22 | 1.02 (0.63–1.65) | 55 | 1.11 (0.83–1.49) |
| Other | ||||||||
| Urea (mmol/L) | 198,705 | 1.01 (0.91–1.12) | 184,443 | 1.11 (0.98–1.27) | 53 | 1.07 (0.85–1.34) | 144 | 0.99 (0.80–1.23) |
| γ-glutamyl transferase (μkat/L) | 276,793 | 1.09 (1.07–1.11) | 251,036 | 1.10 (1.07–1.13) | 86 | 0.97 (0.55–1.69) | 226 | 1.06 (0.94–1.19) |
| Alkaline phosphatase (μkat/L) | 257,418 | 1.15 (1.11–1.19) | 236,962 | 1.18 (1.13–1.23) | 78 | 1.13 (1.00–1.27) | 208 | 1.11 (1.01–1.21) |
| BMI (kg/m2) | 58,584 | 1.41 (1.17–1.70) | 73,623 | 1.12 (0.92–1.37) | 15 | 0.99 (0.56–1.74) | 48 | 1.19 (0.89–1.60) |
Restricted analysis by sex and restricted analysis by age of onset; values determined in overnight fasting samples. HRs were estimated using adjusted Cox models with attained age as the underlying timescale and date of birth as the time origin. Models were adjusted for sex, age at first blood sampling, fasting status (overnight fasting vs. nonfasting), occupation, and country of birth.
To exclude potential misclassification of type 2 diabetes, we repeated the analyses including only individuals with age of onset <30 years. For this subgroup, the associations with type 1 diabetes risk for the biomarkers glucose, fructosamine, apoB–to–apoA-I ratio, and alkaline phosphatase remained (Table 3). Restricting the analysis to case subjects without any oral glucose-lowering therapy yielded similar associations for the majority of biomarkers (Supplementary Table 1). Finally, in repeating the analysis including only case subjects with insulin prescribed within 1 year of diagnosis (only possible for 28.3% of cases, diagnosed from 2005 onward when the PDR was started), the associations with glucose, triglycerides, and uric acid remained significant (Supplementary Table 1).
We obtained similar results when comparing high with low levels of biomarkers (Supplementary Table 2). Similar to biomarkers used as continuous variables, the associations for high levels of glucose, fructosamine, triglycerides, apoA-I, uric acid, γ-glutamyl transferase, and alkaline phosphatase were independent of age and sex of the individuals, and overall, we found more pronounced associations in individuals with older age (Supplementary Table 3 and Supplementary Table 4).
Nested Case-Control Study Analysis
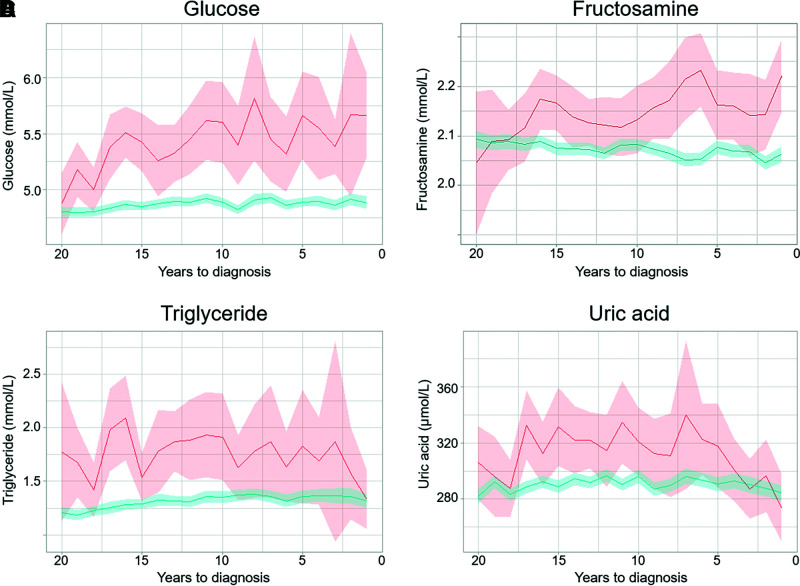
The case-control analyses yielded biomarker–type 1 diabetes associations similar to those obtained with the full cohort (Supplementary Table 5). By plotting mean biomarker levels by time to index year, we found that type 1 diabetes case subjects had consistently higher levels of glucose, fructosamine, triglycerides, and uric acid up to 15 years before diagnosis compared with control subjects (Fig. 1). The mean levels of other biomarkers did not differ significantly between case and control subjects prior to diagnosis (Supplementary Fig. 2); spikes occurring in some of the plotted biomarker levels are likely due to small numbers of case subjects at certain time points.
Figure 1.
Trajectories of biomarker values during the 20 years before the diagnosis of type 1 diabetes. Weighted mean biomarkers values and 95% confidence bands are plotted by time to index date for type 1 diabetes case subjects (red) and control subjects (blue). Graphs for additional biomarkers can be found in Supplementary Fig. 1.
Glucose levels were higher in type 1 diabetes case subjects, with an average of 5.42 mmol/L (95% CI 5.16–5.68) at 15 years before diagnosis and an average difference of 0.57 mmol/L in comparison with control subjects. Two years prior to diagnosis, the average glucose levels reached 5.67 mmol/L (95% CI 4.94–6.39), indicating prediabetes. Fructosamine levels were elevated in type 1 diabetes cases 15 years prior to diagnosis (average 2.17 mmol/L [95% CI 2.11–2.22]), and the difference between case and control subjects increased inversely to time of diagnosis (average difference 5 years prior to diagnosis 0.08 mmol/L [95% CI 0.03–0.14]).
Triglyceride levels were elevated in type 1 diabetes case subjects up to two decades prior to diagnosis, with average levels of 2.09 mmol/L (95% CI 1.70–2.49) and an average difference of 0.81 mmol/L (95% CI 0.46–1.15) compared with control subjects 16 years prior to diagnosis. Similarly, levels of uric acid were elevated in type 1 diabetes cases 15 years prior to diagnosis compared with control subjects (average difference 42.34 µmol/L [95% CI 18.83–65.84]), but these differences between case and control subjects decreased again and leveled off in the years preceding type 1 diabetes diagnosis.
To account for the influence of BMI on biomarkers of lipid metabolism, we repeated the analysis for the biomarkers triglyceride, apoA-I, and the apoB–to–apoA-I ratio with additional adjustment for BMI; the HRs were comparable, but the CIs were wider (Supplementary Table 5B).
Conclusions
In our population-based, longitudinal study, we found that biomarkers related to glycemia, lipid metabolism, and inflammation were associated with future type 1 diabetes risk. Furthermore, the levels of glucose, fructosamine, triglycerides, and uric acid were elevated in case subjects up to 15–20 years prior to diagnosis compared with randomly selected population control subjects. These results indicate that metabolic conditions influencing the development of type 1 diabetes in adults may operate decades before disease manifestation.
We found increased incidence of type 1 diabetes associated with higher levels of triglycerides, apoB, and especially elevated apoB–to–apoA-I ratio. Studies in childhood type 1 diabetes also found dysregulation of lipid metabolism prior to diagnosis (11,12,23); consistent with our findings, data from the German BABYDIAB study showed increased levels of triglycerides and other lipids in autoantibody-positive children (11), whereas downregulated lipid levels, including triglycerides, were found in children progressing to type 1 diabetes in the Finnish Type 1 Diabetes Prevention and Prediction Study (DIPP) (12,23). Notably, these differences were primarily detected in early infancy prior to the appearance of autoantibodies (11,12). High triglycerides and apoB–to–apoA-I ratio and low apoA-I levels have been described to be associated with insulin resistance and adiposity (24,25), and our findings could thus reflect that elevated lipid levels contribute to development of type 1 diabetes in adults via adverse effects on insulin sensitivity. In addition, alkaline phosphatase and uric acid levels were elevated in individuals who later developed type 1 diabetes, and these biomarkers are also associated with reduced insulin sensitivity, adiposity, and the metabolic syndrome (26,27). Finally, we observed a positive association between BMI and the risk of type 1 diabetes, which is in line with previous findings regarding type 1 diabetes in children (28,29), including a recent Mendelian randomization study (30). Taken together, these results provide support for the contribution of reduced insulin sensitivity, adiposity, and other elements of the metabolic syndrome to the development of type 1 diabetes in adults. Hypothetically, this implies that the onset of type 1 diabetes may be delayed by efforts to contain insulin sensitivity, e.g., weight loss or increased physical activity. Our findings differ somewhat from observations in children, which could reflect that insulin resistance plays a less crucial role in the development of childhood type 1 diabetes compared with type 1 diabetes with adult onset. To our knowledge, this is the first prospective study investigating glycemic, lipid, and other metabolic markers in relation to type 1 diabetes with adult onset, and validation studies are clearly warranted.
In theory, the moderate elevations in biomarkers observed in individuals who subsequently develop diabetes may reflect genetic susceptibility. It is indeed possible that individuals with HLA risk genotypes or family history of diabetes have a phenotype that is inherently more diabetogenic than that of others (31,32), including higher glucose or lipid levels. On the contrary, most previous studies indicate that sporadic type 1 diabetes is associated with more severe metabolic disturbances compared with familial type 1 diabetes (33,34), but these characteristics have primarily been assessed at time of diagnosis; prospective data, on the other hand, are scarce. Notably, the downregulation of lipids observed before type 1 diabetes diagnosis in children was found to be independent of HLA genotype (23). Furthermore, the influence of environmental factors may also be exerted through alterations of lipid metabolism or inflammation, and such factors could potentially include dietary factors or virus infections in addition to overweight and obesity. Since we did not have information on either genetic or lifestyle factors, these hypotheses will need to be explored in future studies.
We previously reported on positive associations between these biomarkers (e.g., glucose, fructosamine, triglycerides, apoB–to–apoA-I ratio, and uric acid) and incidence of type 2 diabetes in the AMORIS cohort (6). Our findings regarding type 2 diabetes were strikingly similar to those seen with type 1 diabetes, but biomarker associations with type 2 diabetes were generally stronger and with indications of an even longer preclinical phase. This provides support for the hypothesis of a partly shared pathogenesis of diabetes with adult onset (35), with and without an autoimmune component. In terms of genetic background, it should, however, be noted that the overlap between type 1 and type 2 diabetes appears to be minor (36).
The strengths of our study include the population-based design and the size of the study, including >500,000 individuals and up to 1,000 type 1 diabetes cases and the long follow-up period of up to 25 years. Furthermore, the biomarker measurements were performed on fresh blood samples in the same laboratory with a well-documented and consistently implemented methodology, assuring high quality of the biomarkers. The use of the PR in combination with the NDR and the PDR allowed a high coverage of all patients with type 1 diabetes (37) and, together with the Swedish Cause of Death Register and population registers, ensured minimal loss to follow-up. Our approach to defining type 1 diabetes cases was rigorous and included several steps to ensure exclusion of type 2 diabetes. Notably, we could verify a type 1 diabetes diagnosis using multiple sources and exclude all individuals who at any time, in any register, received a type 2 diabetes diagnosis. In addition to being exclusively registered with type 1 diabetes, the additional criteria of insulin treatment or age ≤30 years at diagnosis was used. It should be noted that a diagnosis of type 1 diabetes based on information in NDR has been shown to be accurate in 97% of cases with age of onset <30 years (16). We additionally performed sensitivity analyses restricted to 1) samples obtained after overnight fasting, 2) case subjects both age <30 years and age <40 years at end of follow-up, and 3) case subjects without any record of oral glucose-lowering drugs; most associations were comparable between the main and the restricted analysis, although there was less precision due to smaller numbers in the sensitivity analyses. Furthermore, in separate analyses including only case subjects with a record of insulin dispensed during the year of diagnosis, the associations with glucose, triglycerides, and uric acid remained. In support of our method, incidence of type 1 diabetes with onset at ages between 15 and 29 years ranged between 5 and 13 per 100,00 person-years (2), which aligns well with our cohort (10 per 100,000 person-years). It is possible that diagnosis was timed incorrectly; this would most likely lead to an overestimation of the time between biomarker assessment and diagnosis. To circumvent this, we used three different national registers for identification of cases, using the first recording as time of diagnosis.
The limitations of this study include the lack of repeated measurements for the biomarkers. We did not have information on family history of diabetes, genetic susceptibility toward HLA, measurements of autoantibodies, and insulin and C-peptide levels with which to estimate insulin resistance and β-cell function. We did not have complete data on BMI; thus, our additional analyses with adjustment for BMI are based on a smaller number of individuals. Due to the nature of the available data, it is possible that our findings could at least partly be explained by the presence of individuals with misclassified type 2 diabetes within our population with type 1 diabetes. These could, for instance, include the insulin-deficient subgroup of adult-onset diabetes as defined by Ahlqvist et al. (38), who were autoantibody negative but whose clinical features were similar to those of patients with autoimmune-deficient diabetes. Furthermore, we were unable to separate type 1 diabetes case subjects with adult onset from case subjects with latent autoimmune diabetes in adults. We acknowledge that the moderate effect sizes and the similarity with previous findings in a cohort with type 2 diabetes suggest that potential misclassification of subjects may have contributed to the observed associations. Future studies with an even stricter case definition, e.g., including autoantibody testing, are clearly needed to confirm our findings.
In conclusion, elevated levels of various biomarkers related to glycemia, lipid metabolism, and inflammation are associated with an increased risk of developing type 1 diabetes in adulthood, and these biomarkers may be elevated already 15–20 years preceding diagnosis.
Article Information
Funding. This study was supported by the Gunnar and Ingmar Jungner Foundation for Laboratory Medicine, Stockholm, Sweden (Dnr.1118/12-1). S.C. is supported by grants from the Swedish Research Council (2018-03035) and FORTE (Swedish Research Council for Health, Working Life and Welfare) (2018-00337).
The funding sources were not involved in the study design; the collection, analysis, or interpretation of data; or in the writing of the report or the decision to submit the manuscript for publication.
Duality of Interest. K.H. is supported by a Novo Nordisk postdoctoral fellowship run in partnership with Karolinska Institutet. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. The conception and the design of the study were a collaborative effort by N.H., H.M., G.W., and S.C. S.C. conceptualized the research objectives and thoroughly revised the manuscript. K.H. analyzed data, developed the study objectives, and drafted the manuscript. All authors contributed to the interpretation of the results and critically revised and approved the final version of the manuscript. K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the virtual 57th Annual Meeting of the European Association for the Study of Diabetes, 28 September–1 October 2021 and at the virtual Scandinavian Society for the Study of Diabetes Meeting 2021, 11 March 2021–12 March 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16967647.
References
- 1. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruno G, Gruden G, Songini M. Incidence of type 1 diabetes in age groups above 15 years: facts, hypothesis and prospects for future epidemiologic research. Acta Diabetol 2016;53:339–347 [DOI] [PubMed] [Google Scholar]
- 3. Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 4. Hjort R, Ahlqvist E, Carlsson P-O, et al. Overweight, obesity and the risk of LADA: results from a Swedish case-control study and the Norwegian HUNT Study. Diabetologia 2018;61:1333–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juhl CB, Bradley U, Holst JJ, Leslie RD, Yderstraede KB; Action LADA Consortium . Similar weight-adjusted insulin secretion and insulin sensitivity in short-duration late autoimmune diabetes of adulthood (LADA) and type 2 diabetes: Action LADA 9 [corrected]. Diabet Med 2014;31:941–945 [DOI] [PubMed] [Google Scholar]
- 6. Malmström H, Walldius G, Carlsson S, et al. Elevations of metabolic risk factors 20 years or more before diagnosis of type 2 diabetes: experience from the AMORIS study. Diabetes Obes Metab 2018;20:1419–1426 [DOI] [PubMed] [Google Scholar]
- 7. Achenbach P, Bonifacio E, Ziegler A-G. Predicting type 1 diabetes. Curr Diab Rep 2005;5:98–103 [DOI] [PubMed] [Google Scholar]
- 8. Sørgjerd EP, Skorpen F, Kvaløy K, Midthjell K, Grill V. Time dynamics of autoantibodies are coupled to phenotypes and add to the heterogeneity of autoimmune diabetes in adults: the HUNT study, Norway. Diabetologia 2012;55:1310–1318 [DOI] [PubMed] [Google Scholar]
- 9. Lundgren VM, Isomaa B, Lyssenko V, et al. GAD antibody positivity predicts type 2 diabetes in an adult population. Diabetes 2010;59:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolandsson O, Hampe CS, Sharp SJ, et al. Autoimmunity plays a role in the onset of diabetes after 40 years of age. Diabetologia 2020;63:266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pflueger M, Seppänen-Laakso T, Suortti T, et al. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes 2011;60:2740–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamichhane S, Ahonen L, Dyrlund TS, et al. Dynamics of plasma lipidome in progression to islet autoimmunity and type 1 diabetes - Type 1 Diabetes Prediction and Prevention Study (DIPP). Sci Rep 2018;8:10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walldius G, Malmström H, Jungner I, et al. Cohort profile: the AMORIS cohort. Int J Epidemiol 2017;46:1103–1103i [DOI] [PubMed] [Google Scholar]
- 14. Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem 1998;44:1641–1649 [PubMed] [Google Scholar]
- 15. Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies--cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One 2014;9:e111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–735 [DOI] [PubMed] [Google Scholar]
- 19. Cologne J, Hsu W-L, Abbott RD, et al. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 2012;23:565–573 [DOI] [PubMed] [Google Scholar]
- 20. Mariosa D, Hammar N, Malmström H, et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 2017;81:718–728 [DOI] [PubMed] [Google Scholar]
- 21. Vandenbroucke JP, Pearce N. Case-control studies: basic concepts. Int J Epidemiol 2012;41:1480–1489 [DOI] [PubMed] [Google Scholar]
- 22. Heianza Y, Arase Y, Fujihara K, et al. Longitudinal trajectories of HbA1c and fasting plasma glucose levels during the development of type 2 diabetes: the Toranomon Hospital Health Management Center Study 7 (TOPICS 7). Diabetes Care 2012;35:1050–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oresic M, Simell S, Sysi-Aho M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008;205:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sierra-Johnson J, Romero-Corral A, Somers VK, et al. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J 2007;28:2637–2643 [DOI] [PubMed] [Google Scholar]
- 25. Maahs DM, Nadeau K, Snell-Bergeon JK, et al. Association of insulin sensitivity to lipids across the lifespan in people with Type 1 diabetes. Diabet Med 2011;28:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bjornstad P, Snell-Bergeon JK, McFann K, et al. Serum uric acid and insulin sensitivity in adolescents and adults with and without type 1 diabetes. J Diabetes Complications 2014;28:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011;60:1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verbeeten KC, Elks CE, Daneman D, Ong KK. Association between childhood obesity and subsequent type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2011;28:10–18 [DOI] [PubMed] [Google Scholar]
- 29. Meah FA, DiMeglio LA, Greenbaum CJ, et al. The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 2016;59:1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Censin JC, Nowak C, Cooper N, Bergsten P, Todd JA, Fall T. Childhood adiposity and risk of type 1 diabetes: a Mendelian randomization study. PLoS Med 2017;14:e1002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobi T, Massier L, Klöting N, et al. HLA class II allele analyses implicate common genetic components in type 1 and non-insulin-treated type 2 diabetes. J Clin Endocrinol Metab 2020;105:e245–e254 [DOI] [PubMed] [Google Scholar]
- 32. McKeigue PM, Spiliopoulou A, McGurnaghan S, et al. Persistent C-peptide secretion in type 1 diabetes and its relationship to the genetic architecture of diabetes. BMC Med 2019;17:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veijola R, Reijonen H, Vähäsalo P, et al. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. Childhood Diabetes in Finland (DiMe) Study Group. J Clin Invest 1996;98:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turtinen M, Härkönen T, Parkkola A, Ilonen J; Finnish Pediatric Diabetes Register . Characteristics of familial type 1 diabetes: effects of the relationship to the affected family member on phenotype and genotype at diagnosis. Diabetologia 2019;62:2025–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferrara-Cook C, Geyer SM, Evans-Molina C, et al. Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care 2020;43:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inshaw JRJ, Sidore C, Cucca F, et al. Analysis of overlapping genetic association in type 1 and type 2 diabetes. Diabetologia 2021;64:1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rawshani A, Landin-Olsson M, Svensson A-M, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia 2014;5:1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]