Abstract
OBJECTIVE
Type 2 diabetes (T2D) is a leading cause of end-stage kidney disease worldwide. Recent studies suggest a more aggressive clinical course of diabetic kidney disease in youth-onset compared with adult-onset T2D. We compared kidney structural lesions in youth- and adult-onset T2D to determine if youth onset was associated with greater early tissue injury.
RESEARCH DESIGN AND METHODS
Quantitative microscopy was performed on kidney tissue obtained from research kidney biopsies in 161 Pima Indians (117 women, 44 men) with T2D. Onset of T2D was established by serial oral glucose tolerance testing, and participants were stratified as youth onset (age <25 years) or adult onset (age ≥25 years). Associations between clinical and morphometric parameters and age at onset were tested using linear models.
RESULTS
At biopsy, the 52 participants with youth-onset T2D were younger than the 109 with adult-onset T2D (39.1 ± 9.9 vs. 51.4 ± 10.2 years; P < 0.0001), but their diabetes duration was similar (19.3 ± 8.1 vs. 17.0 ± 7.8 years; P = 0.09). Median urine albumin-to-creatinine ratio was higher in the youth-onset group (58 [25th–75th percentile 17–470] vs. 27 [13–73] mg/g; P = 0.02). Youth-onset participants had greater glomerular basement membrane (GBM) width (552 ± 128 vs. 490 ± 114 nm; P = 0.002) and mesangial fractional volume (0.31 ± 0.10 vs. 0.27 ± 0.08; P = 0.001) than adult-onset participants. Glomerular sclerosis percentage, glomerular volume, mesangial fractional volume, and GBM width were also inversely associated with age at diabetes onset as a continuous variable.
CONCLUSIONS
Younger age at T2D onset strongly associates with more severe kidney structural lesions. Studies are underway to elucidate the pathways underlying these associations.
Introduction
In the U.S., 13.7 million youth are obese and at increased risk for youth-onset type 2 diabetes (T2D) (1). Between 2002 and 2012, the incidence of youth-onset T2D increased by 4.8% annually (2). Compounding this increase, youth-onset T2D exhibits a more pernicious metabolic phenotype compared with adult-onset T2D, including greater insulin resistance and more rapid deterioration of β-cell function (3,4). These factors result in worse glycemic control and increased risk of complications (1,4–7). We and others have established that youth-onset T2D, which typically manifests during or shortly after puberty, carries significantly greater risk of diabetic kidney disease (DKD) than T1D or adult-onset T2D of similar disease duration (8–12). In the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY2) follow-up study, we demonstrated that the 15-year cumulative incidence of DKD was >50%, documenting the serious health risks encountered by these young adults as they enter what otherwise could be the most productive period of their lives (9–13). Additionally, we found that Pima Indians with youth-onset T2D experienced substantially higher incidence of end-stage kidney disease (ESKD) and mortality than those with adult-onset T2D (14).
Few studies have used kidney biopsies to examine the features and determinants of early DKD in youth-onset T2D, and no studies, to our knowledge, have compared morphometric features of early DKD in youth-onset versus adult-onset T2D. Accordingly, we examined differences in structural lesions on research kidney biopsies performed in adults with youth-onset and adult-onset T2D in an American Indian cohort. We hypothesized that individuals with younger age at T2D onset would have more severe structural lesions associated with progressive DKD. Our findings may provide a morphometric framework to better understand the high rates of DKD progression and ESKD in youth-onset T2D.
Research Design and Methods
Study Participants and Design
Pima Indian persons from the Gila River Indian Community in Arizona have a high prevalence of T2D and a high incidence of ESKD resulting from T2D. Between 1965 and 2007, each member of the community who was at least 5 years old was invited to participate in a longitudinal study of the natural history of diabetes and its complications, with visits every 2 years that included an oral glucose tolerance test after a 75-g oral glucose load. The onset of T2D was determined from these tests and from review of clinical records between these research examinations. In 1988, informative subsets of individuals from this population were selected to undergo more detailed longitudinal studies of kidney pathophysiology in DKD, and many of these individuals continued to be followed for decades (15,16). The current study was conducted in a subset of 161 adult participants from the more detailed longitudinal kidney studies who underwent research kidney biopsies (17). For 132 participants, we also had data on their mothers’ diabetes status over time and, therefore, could identify participants who were and were not exposed to diabetes in utero. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant provided written informed consent.
Youth-Onset Versus Adult-Onset T2D Ascertainment
Participants were stratified as having either youth-onset (age <25 years) or adult-onset T2D (age ≥25 years). Several age-at-onset cutoffs have been used to define youth-onset T2D, including ≤18, <21, and <25 years. We selected <25 years because this age at onset is commonly used by other groups (1,18,19) and is consistent with the United Nations definition for youth (https://www.un.org/en/global-issues/youth). We also examined how the parameters of structural injury were related to age at onset as a continuous variable.
Clinical and Kidney Function Measures
Clinical and kidney function measurements were obtained from the research examination performed closest to the kidney biopsy (median time from biopsy 23 days [interquartile range (IQR) 1–76 days]). Data on smoking history were not collected at these examinations because of the very low prevalence of heavy smoking previously reported in this population, which has prevented us from linking smoking convincingly with important health outcomes (20). Blood pressure was measured twice while the participant was resting in the seated position and was averaged. Glomerular filtration rate (GFR) and renal plasma flow (RPF) were measured by the urinary clearance of iothalamate and p-aminohippurate (PAH), respectively. A PAH extraction ratio of 0.85 was used to compute RPF for the 143 participants with GFR ≥80 mL/min and of 0.70 for the 18 participants with GFR <80 mL/min (21). The PAH clearance was divided by the appropriate extraction ratio to obtain the RPF. Filtration fraction was then computed by dividing the GFR by the RPF. Because of the unavailability of PAH, we do not have measures of RPF or filtration fraction for examinations after December 2014 (n = 46). High-performance liquid chromatography was used to measure the concentrations of iothalamate and PAH. Urine albumin concentration was measured by nephelometric immunoassay and urine creatinine by a modified Jaffé reaction until 1 August 2011, when this was replaced by the enzymatic method (17). Because there was a systematic linear difference between assays, results from the Jaffé method were adjusted to be comparable with the enzymatic method based on a formula derived from samples measured by both methods (enzymatic urine creatinine = e[0.1703 + 1.0328 × log(Jaffé urine creatinine]) (17). Albumin excretion was assessed by the urine albumin-to-creatinine ratio (ACR). Urine albumin concentrations below the detection limit of the assay (≤6.8 mg/L) were set to 6.8 mg/L in the analyses. HbA1c was measured by high-performance liquid chromatography. In addition to examining the clinical and laboratory data at the research examination closest to the kidney biopsy, we retrieved HbA1c levels and drug treatment data for participants from all prior research examinations to estimate long-term glycemic control and long-term exposure to renin-angiotensin-aldosterone system (RAAS) blockers. There were no significant differences between age-at-onset groups in either median number of examinations included (n = 9 [IQR 6–17] for youth-onset T2D vs. n = 8 [IQR 1–17] for adult-onset T2D) or median time from first examination to kidney biopsy (8.8 [IQR 5.1–13.9] years for youth-onset T2D vs. 6.0 [IQR 0–14.4] years for adult-onset T2D).
Morphometric Methods
Tissue was available from kidney biopsies performed between 2002 and 2017. Morphometric measurements were undertaken in 2016–17 using standard methods, which are detailed elsewhere (17). Briefly, unbiased systematic uniform random sampling of kidney biopsy tissue sections provided digital images for quantitative morphometric estimates of DKD structural parameters by observers masked to the clinical data. Predefined structural parameters quantified included glomerular basement membrane (GBM) width, cortical interstitial fractional volume, glomerular mesangial fractional volume, glomerular filtration surface density, podocyte number density per glomerulus, and total filtration surface area per glomerulus (22). Over the course of the study, there was an alteration in the protocols for fixation and embedding of biopsy material for light microscopy. Thirty-seven biopsies were fixed with glutaraldehyde and embedded in epon, and the remaining 124 biopsies were fixed with formalin and embedded in paraffin. Mean glomerular volume was estimated using the Weibel-Gomez method for samples embedded in epon and the Cavalieri method for samples embedded in paraffin (17). We applied a correction factor to glomerular volumes estimated by the Weibel-Gomez method to make them comparable to those obtained by the Cavalieri method (mean glomerular volume by Cavalieri = 0.7 mean glomerular volume by Weibel) (17). Total glomerular filtration surface area was the product of surface density and mean glomerular volume. Not all samples provided sufficient tissue for all light microscopy measurements.
Statistical Analysis
Patient characteristics are expressed as means ± SD or medians (25th–75th percentile) for skewed distributions; qualitative variables are presented as frequencies and percentages. Glomerular sclerosis percentage was cube root transformed because of positive skew.
Continuous variables were compared using t tests or Wilcoxon tests; qualitative variables were compared using χ2 tests. Morphometric parameters were compared in participants with youth-onset and adult-onset T2D using linear models adjusted for GFR, HbA1c, sex, age at biopsy, and BMI. Models for glomerular sclerosis percentage and cortical interstitial fractional volume were also adjusted for embedding media. Similar linear models were used to test the association between age at onset as a continuous variable and morphometric parameters. For visualizing results, we plotted the residuals for age at onset and the structural measures of interest from the adjusted models and calculated Pearson correlation coefficients.
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC), R statistical software version 3.6.3, and GraphPad Prism software version 8.0 (La Jolla, CA). P values <0.05 were considered statistically significant. Analyses were considered exploratory and hypothesis generating, and we did not adjust for multiple comparisons.
Data and Resource Availability
The data sets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Results
Cohort Description
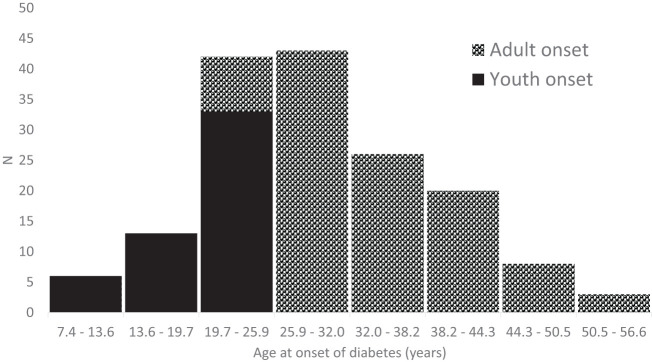
Of the 161 participants (117 women, 44 men), 52 had youth-onset T2D and 109 had adult-onset T2D. Participant characteristics stratified by youth-onset/adult-onset T2D are summarized in Table 1. Average age at T2D diagnosis among those defined as having youth-onset T2D was 19.8 ± 4.2 (range 7.4–24.7) years; among those with adult-onset T2D, it was 34.4 ± 7.5 (range 25.0–56.6) years (Fig. 1). Participants with youth-onset T2D were younger, on average, at the time of biopsy than those with adult-onset T2D (39.1 ± 9.9 vs. 51.4 ± 10.2 years; P < 0.0001), but their diabetes duration at biopsy was similar (19.3 ± 8.1 vs. 17.0 ± 7.8 years; P = 0.09). There were slightly more women diagnosed with youth-onset T2D than adult-onset T2D (81 vs. 69%; P = 0.11), and HbA1c was higher (10.2 ± 2.3 vs. 9.2 ± 2.1%; P = 0.006) and BMI lower (34.0 ± 8.9 vs. 36.9 ± 7.3 kg/m2; P = 0.03) in the youth-onset group at the research examination closest to the kidney biopsy. Long-term glycemic control prior to the kidney biopsy was also poorer in the youth-onset T2D group, with the HbA1c from studies conducted prior to the kidney biopsy averaging 10.0 ± 1.8% compared with 8.9 ± 1.8% in the adult-onset T2D group (P = 0.001). Median ACR (58 [IQR 17–470] vs. 27 [13–73] mg/g; P = 0.02) and mean GFR (155 ± 56 [range 49–305] vs. 138 ± 52 [40–320] mL/min; P = 0.06) were also higher in the youth-onset group. Use of RAAS blockers did not differ significantly between the youth- and adult-onset T2D groups regardless of whether their use was determined at the examination closest to the kidney biopsy or at all research examinations conducted prior to the kidney biopsy.
Table 1.
Description of the population
| Youth-onset T2D (n = 52)* | Adult-onset T2D (n = 109)† | P | |
|---|---|---|---|
| Age at diabetes onset, years | 19.8 ± 4.2 | 34.4 ± 7.5 | — |
| Female sex, % | 81 | 69 | 0.11‡ |
| Insulin therapy, % | 52 | 44 | 0.35‡ |
| RAAS therapy, % | 54 | 62 | 0.30‡ |
| Ever had RAAS therapy, %§ | 75 | 80 | 0.49‡ |
| Age at biopsy, years | 39.1 ± 9.9 | 51.4 ± 10.2 | <0.0001 |
| Diabetes duration at biopsy, years | 19.3 ± 8.1 | 17.0 ± 7.8 | 0.09 |
| HbA1c, % | 10.2 ± 2.3 | 9.2 ± 2.1 | 0.006 |
| HbA1c, mmol/mol | 88 ± 25 | 77 ± 23 | 0.006 |
| Mean HbA1c over course of study, %§ | 10.0 ± 1.8 | 8.9 ± 1.8 | 0.0004 |
| Mean HbA1c over course of study, mmol/mol§ | 86 ± 20 | 74 ± 19 | 0.0004 |
| BMI, kg/m2 | 34.0 ± 8.9 | 36.9 ± 7.3 | 0.03 |
| SBP, mmHg | 123 ± 14 | 122 ± 16 | 0.59 |
| DBP, mmHg | 77 ± 11 | 74 ± 10 | 0.13 |
| Cholesterol, mg/dL | 155 ± 35 | 158 ± 38 | 0.73 |
| Triglycerides, mg/dL | 153 (97–233) | 128 (107–198) | 0.54ǁ |
| ACR, mg/g | 58 (17–470) | 27 (13–73) | 0.02ǁ |
| GFR, mL/min | 155 ± 56 | 138 ± 52 | 0.06 |
| RPF, mL/min | 734 ± 265 | 685 ± 246 | 0.34 |
| Filtration fraction | 0.21 ± 0.07 | 0.20 ± 0.05 | 0.45 |
| Exposed to diabetes in utero, % | 13 | 9 | 0.51‡ |
Data are presented as percentage, mean ± SD, or median (IQR). P values were determined using t tests unless otherwise indicated.
DBP, diastolic blood pressure; SBP, systolic blood pressure.
n = 44 for cholesterol and triglycerides; n = 46 for exposed to diabetes in utero; n = 37 for RPF and filtration fraction.
n = 108 for ACR and n = 102 for cholesterol and triglycerides; n = 86 for exposed to diabetes in utero; n = 78 for RPF and filtration fraction.
χ2 test.
Median observation time prior to biopsy examination was 8.8 (IQR 5.1–13.9) years for youth-onset and 6.0 (IQR 0–14.4) years for adult-onset T2D (P = 0.63 for difference between groups).
Wilcoxon rank sum test.
Figure 1.
Distribution of age at onset of diabetes.
Structural Differences in Youth-Onset T2D Versus Adult-Onset T2D
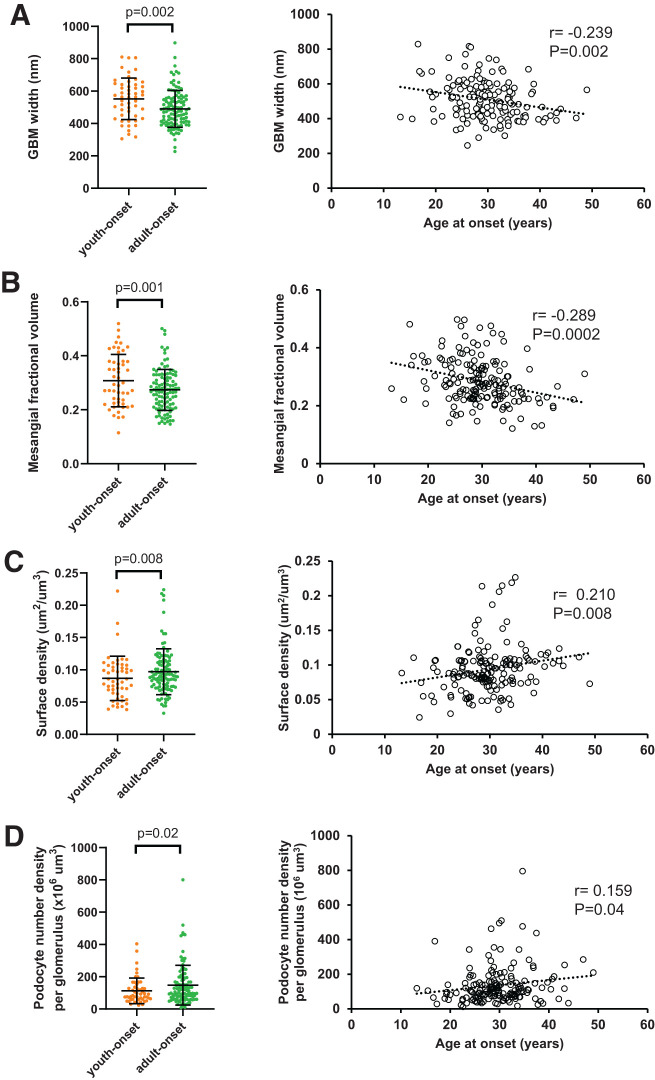
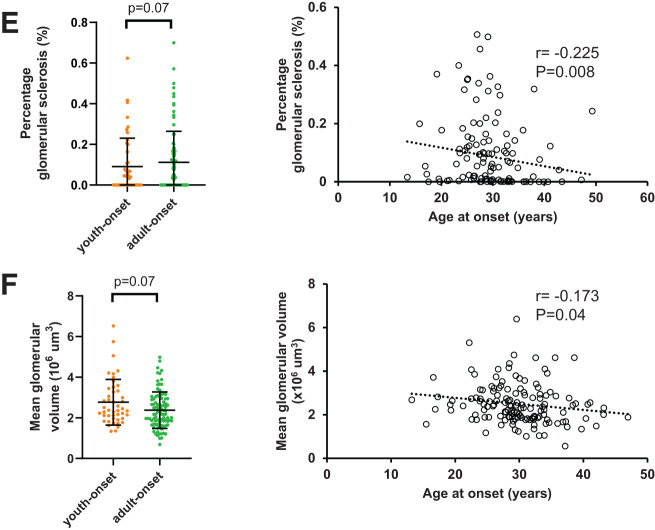
Compared with participants with adult-onset T2D, those with youth-onset T2D exhibited greater GBM width (mean ± SD 552 ± 128 vs. 490 ± 114 nm; P = 0.002) and mesangial fractional volume (0.31 ± 0.10 vs. 0.27 ± 0.08; P = 0.001) after adjusting for sex, age at biopsy, HbA1c, GFR, and BMI (Fig. 2). Differences in cortical interstitial fractional volume, mean glomerular volume, and glomerular sclerosis percentage were not statistically significant after multivariable adjustments. These findings are similar to the univariable analyses, with the exception that glomerular sclerosis percentage was not statistically significantly different between the two groups in univariable analyses (Table 2 and Supplementary Table 1). When age at T2D onset was examined as a continuous variable, younger age at T2D onset was associated significantly with greater glomerular sclerosis percentage, podocyte numeric density per glomerulus, mean glomerular volume, mesangial fractional volume, and GBM width and lower glomerular filtration surface density after multivariable adjustments (Fig. 2 and Supplementary Table 2). Younger age at T2D onset was not associated significantly with cortical interstitial fractional volume or total glomerular filtration surface area in multivariable models.
Figure 2.
Association of kidney structure with age at onset of diabetes. A: GBM width. B: Mesangial fractional volume. C: Glomerular filtration surface density. D: Podocyte number density per glomerulus. E: Glomerular sclerosis percentage. F: Mean glomerular volume. Left column shows association of structural measure in youth-onset and adult-onset T2D with P values from generalized linear models adjusted for age, sex, HbA1c, BMI, and GFR. Right column shows correlation of residuals for each structural measure and age at onset of diabetes adjusted for age, sex, HbA1c, BMI, and GFR. In panel E, models are based on cube root values because of positive skew of measure and are adjusted for embedding media in addition to other covariates.
Table 2.
Structural differences of diabetic kidney injury in youth-onset versus adult-onset T2D after multivariable adjustments
| Youth-onset T2D (n = 52)* | Adult-onset T2D (n = 109)† | P | P ‡ | P § | |
|---|---|---|---|---|---|
| GBM width, mm | 552 ± 128 | 490 ± 114 | 0.002 | 0.0001 | 0.002 |
| Mesangial fractional volume | 0.31 ± 0.10 | 0.27 ± 0.08 | 0.03 | 0.0002 | 0.001 |
| Cortical interstitial fractional volumeǁ | 0.17 ± 0.04 | 0.18 ± 0.05 | 0.53 | 0.39 | 0.40 |
| Mean glomerular volume, × 106 μm3 | 2.77 ± 1.13 | 2.38 ± 0.90 | 0.03 | 0.08 | 0.07 |
| Glomerular sclerosis, %ǁ¶ | 0.04 (0.00–0.11) | 0.05 (0.00–0.17) | 0.52 | 0.09 | 0.07 |
| Glomerular surface density, μm2/μm3 | 0.09 ± 0.03 | 0.10 ± 0.04 | 0.08 | 0.003 | 0.008 |
| Podocyte number density per glomerulus, 106 μm3 | 113 ± 79 | 148 ± 123 | 0.03 | 0.02 | 0.02 |
| Total glomerular filtration surface area, 105 μm2 | 0.23 ± 0.10 | 0.23 ± 0.12 | 0.78 | 0.11 | 0.13 |
Data are presented as mean ± SD or median (IQR). P values are for univariate analyses and were determined using t tests.
Except n = 47 for glomerular sclerosis percentage; n = 46 for mean glomerular volume and total filtration surface area; n = 45 for cortical interstitial fractional volume.
Except n = 89 for glomerular sclerosis percentage; n = 92 for mean glomerular volume and total filtration surface area; n = 86 for cortical interstitial fractional volume.
Adjusted for sex and age at biopsy.
Adjusted for sex, age at biopsy, HbA1c, GFR, and BMI.
Models also adjusted for embedding media.
Cube root transformation because of positive skew.
Exposure to Diabetes In Utero
While there was no significant difference in the proportion of participants exposed to diabetes in utero in the two age-at-onset groups (Table 1), those exposed to diabetes in utero had a younger mean age at onset of diabetes (21.9 ± 9.3 years) compared with the nonexposed (mean 29.5 ± 8.7 years) in the subset of 132 participants with data on maternal diabetes status during pregnancy (P = 0.003).
Sensitivity Analyses
When ACR was added to the multivariable models, the results for GBM width, mesangial fractional volume, surface density, and number density of podocytes per glomerulus remained statistically significantly different between the youth-onset and adult-onset groups. In addition, GBM width, mesangial fractional volume, glomerular filtration surface density, glomerular sclerosis percentage, and podocyte number density per glomerulus remained statistically significantly associated with age at onset of T2D as a continuous variable (data not shown).
Conclusions
In Pima Indians with T2D, youth-onset T2D was associated with more severe structural lesions of diabetic kidney injury than adult-onset T2D of similar duration. The structural lesions most prominently associated with youth-onset T2D, mesangial expansion and thickened GBM, were among the key structural predictors of declining kidney function identified previously in this population (23). The youth-onset T2D group also had a more severe clinical phenotype, reflected by their higher mean HbA1c and higher median ACR. These findings illustrate the extreme phenotype of DKD in youth-onset T2D and provide a structural framework for the higher rates of DKD and ESKD observed in youth-onset T2D compared with adult-onset T2D noted in past studies (5,6,11,14). Collectively, these data may help explain the serious personal and public health consequences of this diagnosis.
The structural lesions observed on research kidney biopsies in the Pima Indians are exclusively attributable to diabetes (24) and are more homogenous than those reported in other populations with T2D (25,26). They are present very early, typically before the onset of clinically detectable kidney disease (17,23,24). We have also shown that these early mild lesions strongly predict the subsequent decline in kidney function characteristic of DKD (23). Moreover, study participants who have undergone serial kidney biopsy have shown worsening of the structural parameters, and these structural changes correlate with a decline in GFR and increase in albuminuria (17). Although safety concerns prevented us from acquiring kidney tissue in nondiabetic Pima Indians, comparison with structural parameters measured in kidney tissue from healthy living donors demonstrated significantly higher mesangial fractional volume and GBM thickness in Pima Indians with diabetes with normoalbuminuria and normal GFR than in healthy donors (24).
Diabetes in Pima Indian children and adolescents was first identified in the mid-1960s with the initiation of the longitudinal population study. Studies confirmed that this youth-onset diabetes was entirely T2D, because it was characterized by ongoing insulin secretion and lack of insulin dependence, absent or low levels of islet cell and glutamic acid decarboxylase antibodies, and absence of strong linkage or association with maturity-onset diabetes of youth loci (16). The prevalence of youth-onset T2D in Pima Indians has increased in recent years, in part because of a growing incidence of exposure to intrauterine diabetes as well as an increasing prevalence and severity of obesity in childhood and adolescence (27). These trends have also led to an increase in the frequency of DKD in midlife and to a fivefold greater risk of diabetic ESKD between the ages of 25 and 54 years among Pima Indians with youth-onset T2D compared with those with older-onset diabetes (14). The increasing frequency of DKD in midlife among Pima Indians exposed to intrauterine diabetes is explained largely by their earlier age at onset of diabetes, but this exposure may also adversely affect nephron development (28). Low birth weight may also adversely affect nephron endowment in Pima Indians, enhancing the risk of DKD in those who subsequently develop T2D (29). A rise in youth-onset T2D is now reported in populations worldwide, and its impact on DKD is becoming increasingly apparent (2).
The prospective longitudinal data in the TODAY study showed that DKD develops early in the course of youth-onset T2D, with >50% developing microalbuminuria by 15 years of diabetes (11). These rates are higher than those reported in adults with T1D and adult-onset T2D (30). Findings from the SEARCH for Diabetes in Youth also indicated that youth-onset T2D associated with over twofold greater odds of DKD than youth-onset T1D (5). In the landmark International Diabetic Nephropathy Study (IDNS), investigators performed research kidney biopsies on 243 youth with T1D (mean age 16.8 years) (31). The morphometric data generated from IDNS were instrumental in the understanding of early DKD pathogenesis in T1D. To our knowledge, no prior structural data exist on those with youth-onset T2D, who carry a substantially higher risk of DKD than those with T1D (32).
The reasons for the more severe structural lesions of diabetic kidney injury in youth-onset T2D are unknown but may be affected by the extreme metabolic phenotype observed in these patients, including severe insulin resistance and rapid worsening of β-cell function, as well as the challenging socioeconomic circumstances of many of these patients (33). Exposure to diabetes in utero is associated with younger age at onset of diabetes (34). In this study, we did not see any difference in the prevalence of exposure in utero in the youth-onset and adult-onset groups, possibly because of the low number of participants exposed to diabetes in utero. Therefore, we do not view in utero exposure as an important mechanism explaining the observed difference in kidney structure described here. Ongoing studies by our group that leverage tissue-level RNA sequencing and metabolomics will expand our understanding of the molecular and metabolic determinants contributing to the more severe structural lesions in youth-onset versus adult-onset T2D. Such investigations hold promise to uncover novel therapeutic targets that can mitigate the high risk of DKD in youth-onset T2D.
Youth-onset T2D is also characterized by a suboptimal response to currently approved medical therapies (35), compounded by major challenges in adherence and management because of age and socioeconomic factors (36). The current mainstays of therapy to mitigate DKD risk in adult-onset T2D include RAAS blockade and sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1–RA) (37–39). Neither sodium–glucose cotransporter 2 inhibitors nor GLP-1–RA were available to participants at the time of our study. Moreover, despite these therapeutic advances, the only U.S. Food and Drug Administration–approved medications for youth-onset T2D are metformin and insulin, with the recent addition of GLP-1–RA. Importantly, the TODAY and Restoring Insulin Secretion (RISE) trials have shown us that kidney physiology may be different in youth-onset T2D versus adult-onset T2D (3,4). Understanding the unique pathophysiology of DKD in youth-onset T2D is a necessary first step to design efficient trials that focus on this high-risk group (3,4).
This study has important strengths and limitations. Strengths include the large number of participants with youth- and adult-onset T2D and comprehensive morphometric examinations of tissue from research kidney biopsies. In addition, serial oral glucose tolerance testing permitted accurate ascertainment of T2D onset and duration. Small differences in the mean diabetes duration between the youth- and adult-onset T2D groups may affect the extent of structural injury, but its impact is likely to be small relative to the effects of the more aggressive metabolic phenotype in the youth-onset group. Moreover, the evidence of more severe kidney lesions in the participants with youth-onset T2D is consistent with epidemiologic data showing higher rates of DKD progression and ESKD in this high-risk group (8,14). In the present analysis, more women than men participated, which may confound our findings because of potential sexual dimorphism of structural lesions in DKD. The time interval between the clinical measurements (GFR, RPF, and ACR) and the kidney biopsies was between 0.03 and 12.6 months. Because structural changes of diabetic kidney injury typically take several years to manifest, potential confounding by the temporal variability of hemodynamic and morphometric parameters was likely modest. The issue of confounding is complicated by the possibility that some potential confounders may also be on the causal pathway between age at T2D onset and kidney structural injury. Accordingly, we reported adjusted and unadjusted relationships between the T2D-onset groups and structural injury. By any of these approaches, the structural differences observed between the youth-onset and adult-onset T2D groups were preserved, suggesting a robust relationship between youth-onset T2D and greater structural injury. We did not have glucose measures during pregnancy, so we were not able to identify cases of gestational diabetes. Furthermore, we did not have data on birth weight for a majority of participants, which prevented us from evaluating the impact of being large or small for gestational age on structural parameters of diabetic kidney injury. Historic glycemic control may be an important confounder of the differences observed in structural lesions between youth-onset T2D and adult-onset T2D, but this cannot be meaningfully examined in our cross-sectional analyses and would be better addressed in larger population cohorts. Although we were unable to assess glycemic control throughout the entire diabetes course in these individuals, HbA1c was higher in youth-onset T2D than in adult-onset T2D at the research examination closest to the kidney biopsy and, on average, at all research examinations conducted prior to the kidney biopsy, suggesting that differences in glycemic control may explain at least some of the differences in kidney structure that we observed. Similarly, although we cannot determine the full duration of treatment with RAAS blockers in study participants, we did not observe a statistically significant difference with this treatment in the two groups by two different approaches, suggesting that RAAS therapy did not contribute appreciably to the structural differences we observed. Finally, youth-onset T2D affects many racial and ethnic groups. Although Pima Indians are just one such group, findings in this population have consistently been generalizable to other racial and ethnic groups (16).
In conclusion, younger age at T2D onset was associated strongly with the glomerular structural lesions that best predict progression to ESKD. Uncovering the metabolic and molecular mechanisms contributing to these structural differences may provide new therapeutic targets for DKD in youth-onset T2D.
Article Information
Acknowledgments. The authors acknowledge the work of Lois I. Jones, Enrique Diaz, Bernadine Waseta, Camille Waseta, Julie Paul, and Joey de Keizer.
Funding. Financial support for this work was provided by the American Diabetes Association (Clinical Science Award 1-08-CR-42) and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). P.B. receives salary and research support from NIDDK (R01 DK129211, R21 DK129720, K23 DK116720, UC DK114886, and P30 DK116073), JDRF (2-SRA-2019-845-S-B and 3-SRA-2017-424-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Center for Women’s Health Research at University of Colorado, and Department of Pediatrics, Section of Endocrinology, and Barbara Davis Center for Diabetes at University of Colorado School of Medicine.
Duality of Interest. P.B. has acted as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, and Horizon Pharma and serves on the advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX Therapeutics. P.J.S. has acted as a consultant for AstraZeneca. M.M. reports having consultancy agreements with Amicus Therapeutics, Avrobio, Bayer, Boeheringer Ingelheim, Freeline Therapeutics, Sangamo Therapeutics, and Sanofi/Genzyme; receiving honoraria from Amicus Therapeutics, Freeline Therapeutics, and Sanofi/Genzyme; receiving research funding from Amicus Therapeutics and Sanofi/Genzyme; and serving on the North American Fabry Registry Board. B.N. reports having consultancy agreements with Amicus Therapeutics, Avrobio, 4D Molecular Therapeutics, Freeline Therapeutics, Sangamo Therapeutics, and Sanofi; serving as a scientific advisor for or member of Amicus Therapeutics, Freeline Therapeutics, and Sanofi; and receiving research funding and honoraria from Amicus Therapeutics and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. H.C.L., R.G.N., and P.B. wrote the manuscript and researched data. H.C.L., L.P., T.V., C.S., and P.J.S. assisted in analyses, contributed to discussion, and reviewed/edited the manuscript. B.N. and M.M. were responsible for morphometric analyses, contributed to discussion, and reviewed/edited the manuscript. R.G.N. designed the study. H.C.L., R.G.N., and P.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.17069885.
References
- 1. Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 2016;39:823–829 [DOI] [PubMed] [Google Scholar]
- 2. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consortium R; RISE Consortium; RISE Consortium Investigators . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. RISE Consortium . Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2019;42:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The association between age of onset of type 2 diabetes and the long-term risk of end-stage kidney disease: a national registry study. Diabetes Care 2020;43:1788–1795 [DOI] [PubMed] [Google Scholar]
- 9. Bjornstad P, Nehus E, El Ghormli L, et al.; TODAY Study Group . Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis 2018;71:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjornstad P, Laffel L, Lynch J, et al.; TODAY Study Group . Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for Type 2 Diabetes in Adolescents and Youth (TODAY) study. Diabetes Care 2019;42:1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjornstad P, Caprio S, Goland R, et al. Incidence of complications and comorbidities in young people with type 2 diabetes. N Engl J Med 2021;385:416–42634320286 [Google Scholar]
- 12. Seegmiller JC, Wolfe BJ, Albtoush N, et al. Tubular secretion markers, glomerular filtration rate, effective renal plasma flow, and filtration fraction in healthy adolescents. Kidney Med 2020;2:670–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjornstad P, Maahs DM, Cherney DZ, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 2014;37:3033–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426 [DOI] [PubMed] [Google Scholar]
- 15. Nelson RG, Bennett PH, Beck GJ, et al.; Diabetic Renal Disease Study Group . Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 1996;335:1636–1642 [DOI] [PubMed] [Google Scholar]
- 16. Nelson RG, Knowler WC, Kretzler M, et al. Pima Indian contributions to our understanding of diabetic kidney disease. Diabetes 2021;70:1603–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Looker HC, Mauer M, Saulnier PJ, et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. J Am Soc Nephrol 2019;30:1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett T, Jalaludin MY, Turan S, Hafez M; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel . Rapid progression of type 2 diabetes and related complications in children and young people—a literature review. Pediatr Diabetes 2020;21:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weghuber D, Barrientos-Pérez M, Kovarenko M. Youth-onset type 2 diabetes manifestations in other specialties: its many disguises. Ann Nutr Metab 2019;74:339–347 [DOI] [PubMed] [Google Scholar]
- 20. Nelson RG, Sievers ML, Knowler WC, et al. Low incidence of fatal coronary heart disease in Pima Indians despite high prevalence of non-insulin-dependent diabetes. Circulation 1990;81:987–995 [DOI] [PubMed] [Google Scholar]
- 21. Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int 2000;58:1228–1237 [DOI] [PubMed] [Google Scholar]
- 22. Squarer A, Lemley KV, Ambalavanan S, et al. Mechanisms of progressive glomerular injury in membranous nephropathy. J Am Soc Nephrol 1998;9:1389–1398 [DOI] [PubMed] [Google Scholar]
- 23. Fufaa GD, Weil EJ, Lemley KV, et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 2016;11:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997;99:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 1996;39:1569–1576 [DOI] [PubMed] [Google Scholar]
- 26. Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:1064–1070 [DOI] [PubMed] [Google Scholar]
- 27. Tanamas SK, Reddy SP, Chambers MA, et al. Effect of severe obesity in childhood and adolescence on risk of type 2 diabetes in youth and early adulthood in an American Indian population. Pediatr Diabetes 2018;19:622–629 [DOI] [PubMed] [Google Scholar]
- 28. Pavkov ME, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Nelson RG. Effect of intrauterine diabetes exposure on the incidence of end-stage renal disease in young adults with type 2 diabetes. Diabetes Care 2010;33:2396–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol 1998;148:650–656 [DOI] [PubMed] [Google Scholar]
- 30. Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 2008;336:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drummond KN, Kramer MS, Suissa S, et al.; International Diabetic Nephropathy Study Group . Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes 2003;52:1818–1824 [DOI] [PubMed] [Google Scholar]
- 32. Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2007;30:2593–2598 [DOI] [PubMed] [Google Scholar]
- 33. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2648–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 38. Mann JFE, Ørsted DD, Brown-Frandsen K, et al.; LEADER Steering Committee and Investigators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–848 [DOI] [PubMed] [Google Scholar]
- 39. Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]