Abstract
OBJECTIVE
Despite that periodical monitoring of cobalamin (vitamin B12) in metformin-treated patients with diabetes is recommended, cobalamin-associated mortality benefits or risks remain unclear. We investigated the association between cobalamin intake and related biomarkers and mortality risk in adults with diabetes using metformin or not.
RESEARCH DESIGN AND METHODS
This study included 3,277 adults with type 2 diabetes from the National Health and Nutrition Examination Survey (NHANES) and followed up until 31 December 2015. Weighted Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs for mortality risk.
RESULTS
Among 3,277 participants, 865 all-cause deaths occurred during a median follow-up of 7.02 years. There was no robust relationship between all-cause mortality and serum cobalamin or intake of foods or cobalamin supplements, regardless of metformin treatment (each P ≥ 0.120). The doubling of methylmalonic acid (MMA), a cobalamin-deficiency marker, was significantly associated with higher all-cause (HR 1.31 [95% CI 1.18–1.45], P < 0.001) and cardiac (HR 1.38 [95% CI 1.14–1.67], P = 0.001) mortality. Cobalamin sensitivity was assessed by the combination of binary B12low/high and MMAlow/high (cutoff values: cobalamin 400 pg/mL, MMA 250 nmol/L). Patients with decreased cobalamin sensitivity (MMAhighB12high) had the highest mortality risk. The multivariable-adjusted HRs (95% CIs) of all-cause mortality in MMAlowB12low, MMAlowB12high, MMAhighB12low, and MMAhighB12high groups were 1.00 (reference), 0.98 (0.75–1.28), 1.49 (1.16–1.92), and 1.96 (1.38–2.78), respectively. That association was especially significant in metformin nonusers.
CONCLUSIONS
Serum and dietary cobalamin were not associated with reduced mortality. Decreased cobalamin sensitivity was significantly associated with all-cause and cardiac mortality, particularly among metformin nonusers.
Introduction
Over the past several decades, the prevalence of diabetes has quadrupled worldwide, of which type 2 diabetes accounts for 90% (1). Although cardiovascular diseases (CVDs) remain the leading cause of death in people with type 2 diabetes, a substantial proportion of disease burden cannot be explained by traditional cardiovascular risk factors (2). The identification of novel risk factors in patients with diabetes is still important. Vitamin B12 (cobalamin) is an essential micronutrient that acts as a coenzyme in homocysteine and methylmalonic acid (MMA) metabolism (3,4). Accumulated evidence has supported a biological link between cobalamin deficiency and diabetes that is partly explained by insulin resistance and oxidative stress (5). Clinical studies noted an increased prevalence of cobalamin deficiency among patients with diabetes, especially metformin users, because metformin inhibits cobalamin absorption (6,7). The American Diabetes Association, Chinese Diabetes Society, and International Society for Pediatric and Adolescent Diabetes, therefore, emphasize the risk of cobalamin deficiency in metformin-treated patients and recommend periodic monitoring of serum cobalamin (6,8,9). Nevertheless, the mortality benefits of cobalamin management among patients with diabetes remain unclear.
Previous randomized controlled trials showed nonsignificant cardiovascular benefits of supplementing cobalamin in subjects with and without diabetes (10–13). Meanwhile, several cohort studies found that serum cobalamin is positively associated with elevated mortality in general and inpatient populations (14–17). Recently, Chen et al. (18) reported a linear relationship between serum cobalamin and gestational diabetes mellitus risk in pregnant women. These inconsistent findings present a great challenge to cobalamin management among patients with diabetes. Moreover, an increase in both MMA (marker of cobalamin deficiency) and cobalamin, termed functional cobalamin deficiency, has been identified in patients with diabetes (3,19). This condition cannot be consistently corrected by large-dose cobalamin supplementation, suggesting a decreased sensitivity to cobalamin therapy (20). The heterogenicity of cobalamin sensitivity may explain the inconsistent conclusions for cobalamin levels, yet the knowledge for cobalamin sensitivity is scarce. We hypothesized that diabetes with decreased sensitivity to cobalamin, as a special phenotype associated with a worse prognosis, may be more clinically meaningful than cobalamin intake. To fill these knowledge gaps, we evaluated the association of serum and dietary cobalamin and sensitivity to cobalamin with mortality risk in adults with diabetes and whether the associations were consistent among metformin users and nonusers.
Research Design and Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a continuous, stratified, multistage sampling study conducted by the U.S. Centers for Disease Control and Prevention. Through linkages to follow-up mortality data, NHANES is extensively used as a large prospective cohort with a nationally representative sample (21). NHANES is approved by the research ethics review board of the Centers for Disease Control and Prevention; all participants provide informed consent.
Serum cobalamin and MMA were measured in 1999–2004 and 2011–2014. Diabetes was defined as a self-reported diagnosis by a physician, plasma HbA1c ≥6.5%, or fasting plasma glucose ≥7.0 mmol/L (22). Overall, among 23,469 adults aged ≥20 years, 3,751 were diagnosed with diabetes. We excluded pregnant women (n = 12), possible individuals with type 1 diabetes (defined as those aged <20 years who used only insulin) (n = 49) (23), individuals with unmeasured or ineligible serum cobalamin (n = 385) or MMA (n = 25), and individuals lost to follow-up (n = 3). Thus, 3,277 participants with type 2 diabetes were included in the analysis.
Serum and Dietary Cobalamin, Supplement Use, MMA, and Cobalamin Sensitivity
All specimens were tested in the central laboratory of NHANES. Serum cobalamin was measured using the commercial Quantaphase II Folate/B12 Radioassay Kit (Bio-Rad Laboratories) from 1999 to 2004 and an automated electrochemiluminescence immunoassay (Elecsys E170; Roche) from 2011 to 2014. Both assays had similar coefficients of variation (<5%) and limits of detection (20–30 pg/mL). An in-house comparison was conducted between two assays on 284 specimens to assess the differences. Because Deming regression considered errors in both methods, cobalamin values obtained by Roche assay were converted to Bio-Rad cobalamin levels according to NHANES recommendations.
Dietary intake was assessed by 24-h food recall for 1 (1999–2002) or 2 (since 2003) inconsecutive days. Primary dietary recall was conducted by trained interviewers at NHANES mobile examination centers. A standard set of protocols and tools were used to assist in assessing the volume and dimensions of the food consumed. The second recall was collected by telephone 3–10 days later. Nutrition ingredients from foods were estimated using the Food and Nutrient Database for Dietary Studies (21).
Data on the use of dietary supplements containing cobalamin were acquired by standardized questionnaires (21). All participants were asked what dietary supplements they used in the past 30 days. The ingredient information was validated from the bottles and labels to minimize the misclassification error.
MMA was measured in venous plasma and/or serum by gas chromatography-mass spectrometry in the 1999–2004 cycles or by liquid chromatography-tandem mass spectrometry in the 2011–2014 cycles. According to the in-house comparison, liquid chromatography-tandem mass spectrometry showed excellent correlation (r = 0.99) and agreement with the measurements by gas chromatography-mass spectrometry (24). MMA concentrations in pairs of serum and plasma were validated to be comparable (25). The overall coefficient of variation was 4–10%, and the average recovery rate was 96.0% (25). Detailed laboratory procedures are provided in the Supplementary Materials.
According to previous studies, functional cobalamin deficiency (impaired sensitivity to cobalamin therapy) was defined as MMA >250 nmol/L and B12 > 400 pg/mL (19,20). Patients with diabetes were categorized by the combination of binary serum cobalamin and MMA to assess cobalamin sensitivity, which included four groups: MMAlowB12low (MMA ≤250 nmol/L, cobalamin ≤400 pg/mL), MMAlowB12high (MMA ≤250 nmol/L, cobalamin >400 pg/mL), MMAhighB12low (MMA >250 nmol/L, cobalamin ≤400 pg/mL), and MMAhighB12high (MMA >250 nmol/L, cobalamin >400 pg/mL).
Covariates
Baseline data on demographics, smoking, comorbidities, and medications were acquired using standardized protocols (25). The duration of diabetes, antidiabetic medications, and diabetic peripheral complications (e.g., foot ulcer/sore, paresthesia of the lower extremity, retinopathy) were extracted from diabetes questionnaires. Blood pressure was calculated as the mean of three eligible values. Hypertension was defined as using antihypertensive therapy or average blood pressure ≥140/90 mmHg. Total cholesterol (TC), HDL cholesterol (HDL-C), HbA1c, plasma glucose, and serum creatinine were measured and calibrated to correct for changes across years according to laboratory methods (23). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Urine albumin and creatinine were tested using a fluorescence immunoassay and Jaffe reaction, respectively. Urine albumin excretion was presented as the urinary albumin-to-creatinine ratio (UACR) (in mg/g).
Outcomes
Participants were linked to the National Death Index through 31 December 2015 to identify mortality status (25). The follow-up period was defined from baseline until death or the end of follow-up, whichever came first. The leading cause of death was identified in line with ICD-10 codes, including death as a result of heart disease (I00–I09, I11, I13, and I20–I51), malignant neoplasms (C00–C97), and others.
Statistical Analyses
For the power estimate, the ratio of the samples (low exposure/high exposure) was assumed to be 1:1 and the relative risk between the low and high levels of cobalamin or MMA to be 0.85 or 1.15, according to previous relevant studies (14,21,25). The cumulative mortality risk rate in this study was ∼50%. For cobalamin or MMA as a categorical variable, a power of 0.95 would be achieved for relative risk ≤0.85 or ≥1.15 among 3,277 participants at the 0.05 level of significance.
All analyses were conducted in compliance with the analytical guidelines of the NHANES data set, as we previously reported (25). Masked variance in the primary sampling unit, pseudostrata, and sampling weights were used to account for the multistage-sampling design and to ensure nationally representative estimates. Numerical and categorical variables are expressed as weighted means and percentages. Because of skewed distribution and wide ranges, log2-transformed serum cobalamin and MMA levels were used for correlation analyses.
Weighted Kaplan-Meier curves for survival status were plotted across cobalamin or MMA strata. Hazard ratios (HRs) and 95% CIs were assessed by survey-weighted Cox proportional hazards regression models for the associations between serum cobalamin (both continuous and by tertiles), cobalamin supplements (use vs. nonuse), cobalamin intake from foods (both continuous and by tertiles), MMA level (both continuous and by tertiles), and cobalamin sensitivity (four groups by the binary cobalamin and MMA levels) and all-cause, cardiac-specific, and cancer-related mortality. Two models adjusted for conventional risk factors were included. Model 1 was adjusted for demographics (age, sex, and race/ethnicity). Model 2 was additionally adjusted for lifestyle and clinical covariates (smoking, BMI, TC/HDL-C ratio, hypertension, cancer, and CVD), diabetes-related variables (HbA1c, eGFR, UACR, duration of diabetes, diabetic complications, and metformin use), and serum cobalamin (continuous, only for MMA in model 2). Models for cobalamin supplements were further adjusted for cobalamin intake from foods (continuous) and vice versa.
Additionally, restricted cubic splines based on multivariable-adjusted Cox regression were conducted to visualize the linear or nonlinear relationship between cobalamin or MMA and all-cause mortality. We investigated whether the relationship between MMA and mortality risk varied in the low versus high cobalamin group (cutoff 400 pg/mL), and the survey-weighted Wald test was used to assess the significance of interaction effects. Because of the well-established relationship between metformin use and cobalamin deficiency, we separately investigated the association between mortality risk and cobalamin in metformin users and nonusers.
For sensitivity analyses, we first reanalyzed the association of serum cobalamin with mortality according to the previously reported cutoff values: normal range of cobalamin (≥339 pg/mL [≥250 pmol/L]), insufficient cobalamin (203–339 pg/mL), and cobalamin deficiency (<203 pg/mL [<150 pmol/L]) (3,4). Second, because of the significant difference in the baseline characteristics across MMA tertiles, we repeated the association between MMA and mortality after selecting individuals with identical characteristics in the tertile 3 versus tertile 1 subgroups using a propensity score matching approach with a 1:1 ratio (26). Paired participants were selected using the nearest neighbor matching algorithm and nonreplacement method with a caliper size of 0.2 SDs of the logit of the propensity score. Third, we excluded patients with first-diagnosed diabetes or without any antidiabetic agent use and repeated the Cox proportional hazards regression analysis in metformin users and nonusers. Two-sided P < 0.05 was considered significant. Stata 15 statistical software was used for the analyses.
Results
Baseline Characteristics
The study included 3,277 adults aged ≥20 years with type 2 diabetes, representing ∼20.1 million U.S. adults with diabetes after weighting. The baseline characteristics of all patients with diabetes are shown in Table 1. The mean age was 59.3 years, and 51.7% were men. Approximately 25.0%, 39.5%, and 27.4% of patients had a history of CVD, metformin use, and diabetic peripheral complications, respectively. Dietary supplements containing cobalamin were used by 35.8% of patients with diabetes, and the mean value of cobalamin intake from foods was 4.8 μg/day. The mean concentrations of serum cobalamin and MMA were 599.5 pg/mL and 201.5 nmol/L, respectively. The baseline characteristics across serum cobalamin (by tertiles), cobalamin supplements (use vs. nonuse), cobalamin intake from foods (by tertiles), and MMA (by tertiles) are shown in Supplementary Tables 1–4. Participants in a higher MMA tertile were more likely to have diabetic complications and longer disease duration. During a median follow-up of 7.02 years, 865 of the 3,277 patients with type 2 diabetes died, including 200 (23.1%) cardiac-specific deaths and 130 (15.0%) cancer-related deaths.
Table 1.
Baseline characteristics of patients with type 2 diabetes in the NHANES study
| Characteristic | Mean ± SE or n (%) | |
|---|---|---|
| Patients, n | 3,277 | |
| Age (years) | 59.3 ± 0.3 | |
| Male | 1,708 (51.7) | |
| Race/ethnicity | ||
| Non-Hispanic White | 1,174 (61.0) | |
| Non-Hispanic Black | 848 (15.0) | |
| Hispanic Mexican | 548 (8.8) | |
| Other | 707 (15.2) | |
| Smoking status | ||
| Never | 1,586 (47.6) | |
| Former | 1,140 (34.7) | |
| Current | 545 (17.7) | |
| BMI (kg/m2) | 32.8 ± 0.2 | |
| TC/HDL-C ratio | 2.4 ± 0.1 | |
| Hypertension | 2,346 (69.1) | |
| CVD | 855 (25.0) | |
| Cancer | 422 (14.8) | |
| eGFR (mL/min/1.73 m2) | 84.9 ± 0.6 | |
| UACR (mg/g) | 133.2 ± 11.5 | |
| HbA1c | ||
| % | 7.4 ± 0.04 | |
| mmol/mol | 57.6 ± 0.5 | |
| Duration of diabetes (years) | 8.8 ± 0.3 | |
| Diabetic complications | 983 (27.4) | |
| Retinopathy | 573 (15.2) | |
| Foot ulcer | 123 (3.5) | |
| Peripheral neuropathy | 535 (15.1) | |
| Metformin use | 1,242 (39.5) | |
| B12 supplements | 1,047 (35.8) | |
| B12 intake from foods (μg/day) | 4.8 ± 0.2 | |
| Serum B12 (pg/mL) | 599.5 ± 7.4 | |
| Circulating MMA (nmol/L) | 201.5 ± 7.2 | |
Data were adjusted for survey weights of NHANES. The observed numbers for categorical variables were unweighted.
Associations Between Dietary and Serum Cobalamin, Supplement Use, MMA, and Mortality Risk
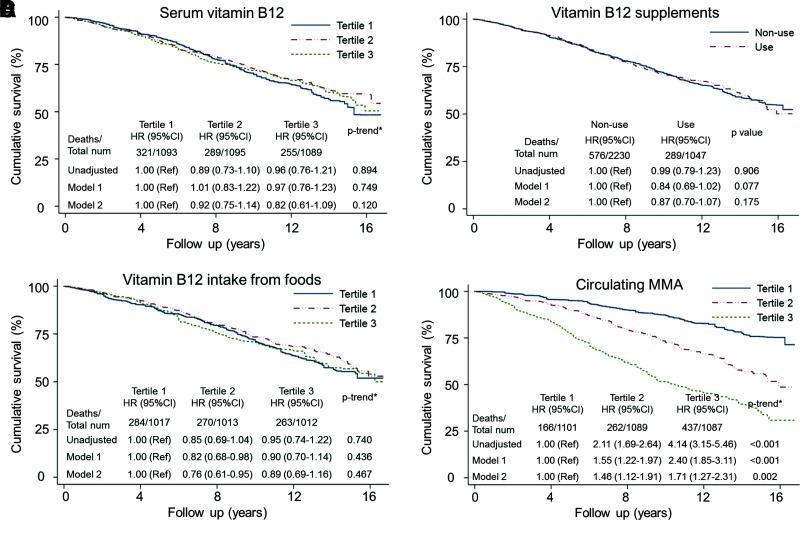
Serum cobalamin was not significantly associated with all-cause, cardiac-specific, or cancer-specific mortality in patients with diabetes according to weighted Kaplan-Meier plots and a series of Cox proportional hazards regression analyses (Fig. 1 and Supplementary Tables 5–7). Compared with the lowest tertile of serum cobalamin, the multivariable-adjusted HRs and 95% CIs in the highest tertile were 0.82 (0.61–1.09) for all-cause mortality (P for trend = 0.120), 1.04 (0.66–1.63) for cardiac-specific mortality (P for trend = 0.104), and 0.69 (0.36–1.32) for cancer-related mortality (P for trend = 0.215). Furthermore, the potential impact of sources of cobalamin intake was considered. Those who took cobalamin supplements did not have a significantly reduced risk of all-cause (HR 0.87 [95% CI 0.70–1.07], P = 0.175) or cause-specific mortality (each P ≥ 0.339), compared with nonusers. Although patients with higher cobalamin intake from foods had reduced cardiac-specific mortality risk in age-, sex-, and race/ethnicity-adjusted models (tertile 3 vs. tertile 1: HR 0.55 [95% CI 0.34–0.91], P for trend = 0.022), all associations with all-cause and cause-specific mortality became nonsignificant after multivariable adjustment (each P ≥ 0.133).
Figure 1.
Weighted Kaplan-Meier plots and HRs illustrating the relationship between all-cause mortality and serum cobalamin (vitamin B12), supplement use, dietary intake from foods, and MMA levels. A: Serum vitamin B12. B: Vitamin B12 supplements. C: Vitamin B12 intake from foods. D: Circulating MMA. HR (95% CI) was estimated by weighted Cox proportional hazards regression analyses. Model 1 was adjusted for age, sex, and race/ethnicity (n = 3,277). Model 2 was additionally adjusted for smoking, BMI, hypertension, cancer, CVD, TC/HDL-C ratio, serum cobalamin (continuous, only for MMA in model 2), eGFR, HbA1c, metformin, duration of diabetes, UACR, and diabetic complications (n = 2,935). Models for cobalamin supplements were further adjusted for cobalamin intake from foods (μg/day, continuous) and vice versa. *P value for trend across the tertiles of vitamin B12 or MMA. num, number; Ref, reference.
In contrast, higher MMA, a biomarker of cobalamin deficiency, was positively associated with increased all-cause and cardiac-specific mortality, not cancer-specific mortality, after multivariable adjustment (Fig. 1 and Supplementary Tables 5–7). After full adjustment for age, sex, race, race/ethnicity, smoking status, BMI, hypertension, TC/HDL-C ratio, CVD, cancer, eGFR, HbA1c, metformin use, duration of diabetes, UACR, diabetic peripheral complications, and serum cobalamin, the dose-dependent relationship between MMA and mortality risk remained significant. All-cause and cardiac-specific mortality rates increased by 31% and 38%, respectively, with a doubling of MMA levels (both P ≤ 0.001). Consistently, the multivariable-adjusted HRs (95% CIs) across the MMA tertiles were 1.00 (reference), 1.46 (1.12–1.91), and 1.71 (1.27–2.31) for all-cause mortality (P for trend = 0.002) and 1.00 (reference), 1.49 (0.80–2.81), and 1.85 (1.02–3.36) for cardiac-specific mortality (P for trend = 0.040).
According to restricted cubic spline, the association between all-cause mortality and MMA still followed a dose-response pattern, while there was no significant linear or nonlinear association for serum cobalamin levels or cobalamin intake from foods (Supplementary Fig. 1). For sensitivity analysis, serum cobalamin levels were categorized according to predefined cutoffs of cobalamin deficiency, and the association remained statistically nonsignificant (Supplementary Table 8). Compared with patients with diabetes and cobalamin levels ≥339 pg/mL, the adjusted HRs (95% CIs) for all-cause mortality among those with cobalamin levels from 203–339 pg/mL and <203 pg/mL were 1.21 (0.92–1.61) and 1.24 (0.82–1.87), respectively (P for trend = 0.094). After propensity score matching, 393 pairs of adults with diabetes in tertile 3 versus tertile 1 of MMA were well balanced (Supplementary Table 9). Compared with participants in tertile 1, the increased mortality risk in tertile 3 remained significant (HR 2.09 [95% CI 1.55–2.80], P < 0.001) (Supplementary Table 10).
Decreased Sensitivity to Cobalamin and Increased Mortality Risk in Adults With Diabetes
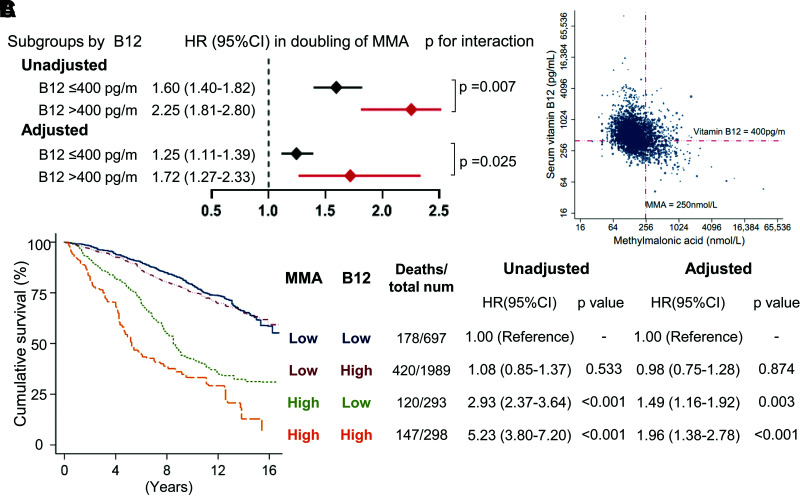
We first investigated whether the increased mortality associated with MMA varied among patients with high or low serum cobalamin (Fig. 2A). A significant interaction was noted between serum cobalamin and MMA for all-cause mortality (P for interaction ≤ 0.025). The adjusted HRs (95% CIs) per doubling of MMA for all-cause mortality were 1.25 (1.11–1.39) among patients with cobalamin ≤400 pg/mL vs. 1.72 (1.27–2.33) among those with cobalamin >400 pg/mL.
Figure 2.
The association of all-cause mortality with decreased sensitivity to cobalamin (vitamin B12) in patients with diabetes. A: HRs per doubling of MMA for all-cause mortality in B12 subgroups. P value for the interaction between vitamin B12 and MMA was estimated by the survey-weighted Wald test, with 0.007 and 0.025 for unadjusted and adjusted models, respectively. B: Scatter diagram of serum vitamin B12 and MMA among patients with diabetes. The correlation coefficient between serum vitamin B12 and MMA is r = −0.323 (P < 0.001). Dashed lines represent serum vitamin B12 400 pg/mL and MMA 250 nmol/L. Among adults with diabetes and MMA >250 nmol/L, 47.0% had serum vitamin B12 >400 pg/mL. C: All-cause mortality associated with the increase in both serum vitamin B12 and MMA. Both indicators were categorized into high vs. low levels and combined into four groups according to the prespecified cutoff values (MMA >250 nmol/L and cobalamin >400 pg/mL). All HRs (95% CI) were estimated by weighted Cox proportional hazards regression analyses. A multivariable model was adjusted for age, sex, race/ethnicity, smoking, BMI, hypertension, cancer, CVD, TC/HDL-C ratio, eGFR, HbA1c, metformin, duration of diabetes, UACR, and diabetic periphery complications. num, number.
According to the scatter plot shown in Fig. 2B, 47.0% of adults with diabetes had serum cobalamin >400 pg/mL among those with MMA >250 nmol/L. Weighed Kaplan-Meier curves showed that patients with both MMA >250 nmol/L and cobalamin >400 pg/mL had the highest cumulative mortality rate (Fig. 2C). After adjustment for the mentioned covariates, an increase of both MMA and cobalamin still predicted a higher risk of all-cause mortality (Fig. 2C). Compared with patients with MMAlowB12low, the multivariate-adjusted HRs (95% CIs) of total mortality in the MMAlowB12high, MMAhighB12low, and MMAhighB12high groups were 0.98 (0.75–1.28), 1.49 (1.16–1.92), and 1.96 (1.38–2.78), respectively.
Metformin Use and Mortality Risk Associated With Decreased Cobalamin Sensitivity
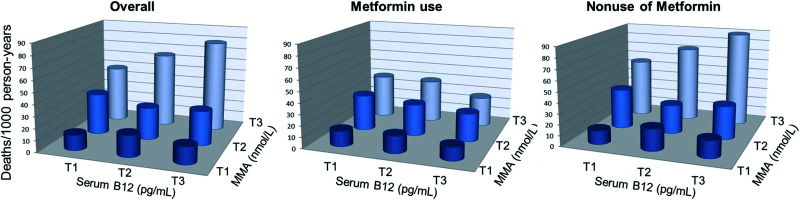
We conducted a cross-tabulation analysis of MMA tertiles and B12 tertiles for mortality rates (deaths/1,000 person-years) stratified by metformin treatment (Fig. 3). As tertiles of MMA and cobalamin increased, so did the risk of all-cause mortality among metformin nonusers but not metformin users. Compared with metformin nonusers in the first tertile of MMA and the first tertile of cobalamin, those in the third tertile of MMA and third tertile of cobalamin had the highest rate of all-cause mortality (89.5 vs. 12.8/1,000 person-years, P < 0.001).
Figure 3.
All-cause mortality rates and sensitivity to cobalamin among metformin users or nonusers. Cross-tabulation analysis of serum cobalamin tertiles and MMA tertiles for all-cause mortality rates (deaths/1,000 person-years) in patients with diabetes who were metformin users or nonusers. The top tertile of serum cobalamin combined with MMA had the highest rate of all-cause mortality in metformin nonusers (P < 0.001) but not in metformin users (P = 0.133).
Whether we added metformin use into the multivariable-adjusted model, the association of cobalamin intake or relevant biomarkers with all-cause, cardiac-specific, and cancer-specific mortality was unchanged (Supplementary Tables 4–6). We further conducted a stratification analysis grouped by metformin use. Serum cobalamin and both sources of cobalamin intake were not significantly associated with mortality risk in both metformin users and nonusers. Interestingly, elevated mortality associated with decreased cobalamin sensitivity was confined to patients with diabetes who did not use metformin but not for those who did (Supplementary Fig. 2). With the MMAlowB12low group treated as the reference, the adjusted HRs (95% CIs) for all-cause mortality in the MMAlowB12high, MMAhighB12low, and MMAhighB12high groups among metformin nonusers were 1.03 (0.72–1.46), 1.74 (1.29–2.35), and 2.29 (1.55–3.39), respectively, whereas the HRs (95% CIs) among metformin users were 0.90 (0.58–1.40), 1.31 (0.74–2.31), and 1.28 (0.36–4.52), respectively. Excluding individuals with first-diagnosed diabetes or not administered antidiabetic agents, a robust relationship between decreased cobalamin sensitivity and mortality risk remained in metformin nonusers (Supplementary Table 11).
Conclusions
In this prospective cohort study of U.S. adults with type 2 diabetes, serum and dietary cobalamin were not significantly associated with mortality risk in metformin users and nonusers. In contrast, circulating MMA, a typical biomarker of cobalamin deficiency, was positively associated with all-cause and cardiac-specific mortality. Of note, approximately one-half of adults with diabetes and an increase in MMA had sufficient cobalamin levels. We found that decreased cobalamin sensitivity, not cobalamin deficiency, was significantly associated with increased mortality risk. Furthermore, that association was especially significant in metformin nonusers.
To our knowledge, this study is the first to evaluate the associations of cobalamin with mortality in patients with diabetes. Although two clinical trials revealed that vitamin B12 intake improves glycemic control, insulin resistance, and peripheral neuropathy in patients with type 2 diabetes, a small sample size (<20 patients/group) may have been a limitation (27,28). Actually, most of the trials did not reveal significant neurological and cardiovascular benefits of cobalamin supplementation for patients with and without diabetes (10–13,29). Our study also showed that serum and dietary cobalamin levels were not significantly associated with decreased all-cause or cause-specific mortality in patients with type 2 diabetes. Although Chen et al. (21) noted that the intake of multiple nutrients from foods, not supplements, was associated with lower mortality in the general population, in our study, neither cobalamin supplement use nor intake from foods was associated with mortality benefits among patients with diabetes. Ruminant meat and milk are the primary sources of cobalamin intake, which is commonly adequate in developed countries (30). The U.S., Europe, and Austria recommend 2.4–3.0 μg/day of cobalamin intake for adults (30). Thus, the average cobalamin intake from foods (4–5 μg/day) in U.S. patients with diabetes seems to be sufficient to meet health needs. Taken together, our results do not support the mortality benefits of intensive cobalamin management in patients with diabetes.
The association between MMA accumulation and diabetes has been noted in some pilot studies (19,31). A seemingly contradictory result in our study was that MMA accumulation but not cobalamin deficiency (<203 pg/mL) was positively associated with increased mortality risk. Impaired sensitivity to cobalamin may explain the inconsistency. Our recent cohort study also found that increased cardiovascular mortality associated with MMA accumulation was especially significant in the general population with higher serum cobalamin (25).
Compared with functional cobalamin deficiency emphasizing the insufficient active form of cobalamin, impaired sensitivity may be more suitable to describe the obtuse response to cobalamin therapy (3,32). Adenosylcobalamin, the active coenzyme required for MMA metabolism, was not recommended for correcting B12 deficiency (3). Besides activated cobalamin, MMA metabolism highly relies on healthy mitochondria and mitochondrial enzymes (3). Although the underlying mechanism of decreased cobalamin sensitivity is not fully understood, oxidative stress and mitochondrial dysfunction seem to be striking features (19,20). Additional biological research targeting mitochondria may provide some clues about the understanding of impaired cobalamin sensitivity.
Solomon (19) previously found functional cobalamin deficiency, defined as MMA >250 nmol/L and cobalamin >400 pg/mL, in 47 patients with diabetes. We also demonstrated that cobalamin sensitivity was robustly associated with all-cause and cardiac-specific mortality in this prospective cohort of type 2 diabetes. According to prior cohort studies, the increased mortality associated with serum cobalamin in general and in-patient populations may be partly explained by the weakened response to cobalamin (14–17). In our findings, among patients with diabetes with MMA >250 nmol/L, approximately one-half had serum cobalamin >400 pg/mL, indicating that substantial MMA accumulation cannot be attributed to insufficient cobalamin (3). Given that cobalamin deficiency was defined as a serum level <203 pg/mL and that >299 pg/mL (some reported 339 pg/mL) was considered cobalamin adequacy, the actual prevalence of decreased cobalamin sensitivity in adults with diabetes seemed to be worse (3,4). Altogether, compared with cobalamin levels, impaired cobalamin sensitivity was a dramatically distinctive phenotype among patients with diabetes and should be preferentially investigated.
Accumulated studies have indicated that the aging process and vitamin B12 metabolism and function interact (3,19,33). Pannérec et al. (34) provided convincingly physiopathological evidence, based on elderly individuals and aged mice, that aging reduces intestinal uptake and renal reabsorption of cobalamin. Besides absorption, the process of cobalamin involved in MMA metabolism includes endocytosis transport, intracellular release through lysosomes, and activation in mitochondria (3). Lysosomal and mitochondrial dysfunction usually occurs in chronic pathology, including aging and diabetes, which disturbed the intracellular bioavailability of cobalamin (3,35), which may be a partial interpretation of the limited benefit of cobalamin supplementation (including intramuscular and intravenous injection) found in previous clinical trials (3,19,33). Conversely, cobalamin-deficiency marker MMA triggered mitochondrial oxidative stress, which also aggravated the aging process (25). We also noted that adults with diabetes were more likely to be older in the higher MMA tertile. Additional research on the physiopathological influence of cobalamin metabolic/functional process is needed.
Metformin is one of a few traditional first-line antidiabetic drugs associated with cardiovascular benefits (7). The link between metformin use and cobalamin deficiency has been well established (6,31). However, the side effects of metformin occurring via cobalamin deficiency are controversial. For example, some studies found that long-term metformin use was related to a 70–93% increase in the risk of anemia or dementia, whereas opposite findings were reported among 25,393 patients with type 2 diabetes in whom metformin was associated with a 24% reduced risk of dementia (7,36,37). In our analysis, the null association between mortality risk and cobalamin intake was consistent among metformin users and nonusers, indicating that current recommendations of monitoring and correcting B12 deficiency in metformin-treated patients may have limited mortality benefits (6). Interestingly, the increased mortality associated with decreased sensitivity to cobalamin was especially significant in metformin nonusers. Thus, metformin may reduce the mortality risk associated with decreased cobalamin sensitivity, which was consistent with the cardiovascular benefits of metformin (6,7). Other and our previous studies supported that mitochondrial dysfunction is a critical mediator of diabetic cardiopathy (38). The alleviation of mitochondrial impairment and redox disorder by metformin may partly explain our findings (39). Nonetheless, whether metformin improves cobalamin sensitivity needs validation by clinical trials.
The strengths of this study include the collection of data from a nationally representative sample via well-designed and validated protocols, which facilitate the generalization and repeatability of our findings. Several limitations should also be considered. First, cobalamin intake from foods was assessed using one or two 24-h dietary recalls. Although the repeatability of protocols was validated, long-term dietary habits or changes should be considered in further studies (21). Second, MMA levels were determined using plasma and/or serum specimens, which may be of limited concern because previous studies demonstrated comparability in pairs of plasma and serum samples (25). Third, although the multivariable analysis was adjusted for traditional and diabetes-related factors, we could not rule out residual confounding from unmeasured variables. Finally, serum homocysteine, another marker of cobalamin deficiency, was not included in our analysis because it had not been determined since 2005. However, compared with MMA, homocysteine metabolism also needs folate and vitamin B6. MMA had favorable specificity and sensitivity to reflect cobalamin status (3,4). Thus, the combination of MMA and cobalamin was used to assess cobalamin function (19,20).
In conclusion, cobalamin intake and serum concentration were not associated with mortality risk independent of metformin use. However, impaired cobalamin sensitivity accounted for one-half of MMA accumulation among adults with diabetes and was significantly associated with elevated all-cause and cardiac-specific mortality, especially in metformin nonusers. Our findings may provide novel insights into cobalamin management in patients with diabetes. Current guidelines recommend that the periodic assessment of serum cobalamin in metformin-treated patients may have limited mortality benefits, while the mortality risk associated with decreased cobalamin sensitivity may be largely underrecognized.
Article Information
Acknowledgments. The authors thank all the NHANES participants and researchers for their substantial contributions.
Funding. B.Y. was supported by the National Key R&D Program (grant 2016YFC1301100) and the National Natural Science Foundation of China (grant 81827806). S.F. was supported by the National Natural Science Foundation of China (grants 82170262 and 81870353).
This manuscript was completed independently.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. S.W., X.W., and J.G. organized all data. S.W., Y.Z., and M.T. analyzed and visualized the results. S.W. and B.Y. conceived and designed the study. Y.W., X.W., and S.F. developed the protocols. M.T., S.F., and B.Y. reviewed and edited the manuscript. S.F. and B.Y. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
S.W. and Y.W. contributed equally to this article.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16965025.
References
- 1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88–98 [DOI] [PubMed] [Google Scholar]
- 2. Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 2018;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green R, Allen LH, Bjørke-Monsen AL, et al. Vitamin B12 deficiency. Nat Rev Dis Primers 2017;3:17040. [DOI] [PubMed] [Google Scholar]
- 4. Hannibal L, Lysne V, Bjørke-Monsen AL, et al. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front Mol Biosci 2016;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boachie J, Adaikalakoteswari A, Samavat J, Saravanan P. Low vitamin B12 and lipid metabolism: evidence from pre-clinical and clinical studies. Nutrients 2020;12:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S32–S36 [DOI] [PubMed] [Google Scholar]
- 7. Donnelly LA, Dennis JM, Coleman RL, et al. Risk of anemia with metformin use in type 2 diabetes: a MASTERMIND study. Diabetes Care 2020;43:2493–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia W, Weng J, Zhu D, et al.; Chinese Diabetes Society . Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev 2019;35:e3158. [DOI] [PubMed] [Google Scholar]
- 9. Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes 2018;19(Suppl. 27):28–46 [DOI] [PubMed] [Google Scholar]
- 10. Lonn E, Yusuf S, Arnold MJ, et al.; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators . Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006;354:1567–1577 [DOI] [PubMed] [Google Scholar]
- 11. Kwok T, Lee J, Ma RC, et al. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin Nutr 2017;36:1509–1515 [DOI] [PubMed] [Google Scholar]
- 12. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev 2018;12:CD011906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rafnsson SB, Saravanan P, Bhopal RS, Yajnik CS. Is a low blood level of vitamin B12 a cardiovascular and diabetes risk factor? A systematic review of cohort studies. Eur J Nutr 2011;50:97–106 [DOI] [PubMed] [Google Scholar]
- 14. Flores-Guerrero JL, Minovic I, Groothof D, et al. Association of plasma concentration of vitamin B12 with all-cause mortality in the general population in the Netherlands. JAMA Netw Open 2020;3:e1919274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cappello S, Cereda E, Rondanelli M, et al. Elevated plasma vitamin B12 concentrations are independent predictors of in-hospital mortality in adult patients at nutritional risk. Nutrients 2016;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sviri S, Khalaila R, Daher S, et al. Increased vitamin B12 levels are associated with mortality in critically ill medical patients. Clin Nutr 2012;31:53–59 [DOI] [PubMed] [Google Scholar]
- 17. Soohoo M, Ahmadi SF, Qader H, et al. Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol Dial Transplant 2017;32:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Zhang Y, Chen H, et al. Association of maternal folate and vitamin B12 in early pregnancy with gestational diabetes mellitus: a prospective cohort study. Diabetes Care 2021;44:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon LR. Diabetes as a cause of clinically significant functional cobalamin deficiency. Diabetes Care 2011;34:1077–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomon LR. Functional cobalamin (vitamin B12) deficiency: role of advanced age and disorders associated with increased oxidative stress. Eur J Clin Nutr 2015;69:687–692 [DOI] [PubMed] [Google Scholar]
- 21. Chen F, Du M, Blumberg JB, et al. Association among dietary supplement use, nutrient intake, and mortality among U.S. adults: a cohort study. Ann Intern Med 2019;170:604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kramer H, Boucher RE, Leehey D, et al. Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care 2018;41:775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in U.S. adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care 2020;43:1227–1233 [DOI] [PubMed] [Google Scholar]
- 24. Mineva EM, Zhang M, Rabinowitz DJ, Phinney KW, Pfeiffer CM. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal Bioanal Chem 2015;407:2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Liu Y, Liu J, et al. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol 2020;37:101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vetter TR, Mascha EJ. Bias, confounding, and interaction: lions and tigers, and bears, oh my! Anesth Analg 2017;125:1042–1048 [DOI] [PubMed] [Google Scholar]
- 27. Satapathy S, Bandyopadhyay D, Patro BK, Khan S, Naik S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: a multi-arm randomized controlled clinical trial. Complement Ther Med 2020;53:102526. [DOI] [PubMed] [Google Scholar]
- 28. Farvid MS, Homayouni F, Amiri Z, Adelmanesh F. Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res Clin Pract 2011;93:86–94 [DOI] [PubMed] [Google Scholar]
- 29. Jayabalan B, Low LL. Vitamin B supplementation for diabetic peripheral neuropathy. Singapore Med J 2016;57:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gille D, Schmid A. Vitamin B12 in meat and dairy products. Nutr Rev 2015;73:106–115 [DOI] [PubMed] [Google Scholar]
- 31. Wile DJ, Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care 2010;33:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vollbracht C, McGregor GP, Kraft K. Supraphysiological vitamin B12 serum concentrations without supplementation: the pitfalls of interpretation. QJM 2020;113:619–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomes AP, Ilter D, Low V, et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 2020;585:283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pannérec A, Migliavacca E, De Castro A, et al. Vitamin B12 deficiency and impaired expression of amnionless during aging. J Cachexia Sarcopenia Muscle 2018;9:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao H, Brunk UT, Garner B. Age-related lysosomal dysfunction: an unrecognized roadblock for cobalamin trafficking? Cell Mol Life Sci 2011;68:3963–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011;24:485–493 [DOI] [PubMed] [Google Scholar]
- 37. Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 2012;60:916–921 [DOI] [PubMed] [Google Scholar]
- 38. Wang S, Zhao Z, Fan Y, et al. Mst1 inhibits Sirt3 expression and contributes to diabetic cardiomyopathy through inhibiting Parkin-dependent mitophagy. Biochim Biophys Acta Mol Basis Dis 2019;1865:1905–1914 [DOI] [PubMed] [Google Scholar]
- 39. Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol 2020;34:101517. [DOI] [PMC free article] [PubMed] [Google Scholar]