Abstract
OBJECTIVE
The role of the gut in diabetes remission after Roux-en-Y gastric bypass (RYGB) is incompletely understood. We assessed the temporal change in insulin secretory capacity after RYGB, using oral and intravenous (IV) glucose, in individuals with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Longitudinal, prospective measures of β-cell function were assessed after oral glucose intake and graded glucose infusion in individuals with severe obesity and diabetes studied at 0, 3 (n = 29), 12 (n = 24), and 24 (n = 20) months after RYGB. Data were collected between 2015 and 2019 in an academic clinical research center.
RESULTS
The decreases in body weight, fat mass, waist circumference, and insulin resistance after surgery (all P < 0.001 at 12 and 24 months) did not differ according to diabetes remission status. In contrast, both the magnitude and temporal changes in β-cell glucose sensitivity after oral glucose intake differed by remission status (P = 0.04): greater (6.5-fold; P < 0.01) and sustained in those in full remission, moderate and not sustained past 12 months in those with partial remission (3.3-fold; P < 0.001), and minimal in those not experiencing remission (2.7-fold; P = not significant). The improvement in β-cell function after IV glucose administration was not apparent until 12 months, significant only in those in full remission, and only ∼33% of that observed after oral glucose intake. Preintervention β-cell function and its change after surgery predicted remission; weight loss and insulin sensitivity did not.
CONCLUSIONS
Our data show the time course of changes in β-cell function after RYGB. The improvement in β-cell function after RYGB, but not changes in weight loss or insulin sensitivity, drives diabetes remission.
Introduction
The role of the gut in diabetes remission after Roux-en-Y gastric bypass (RYGB) (1) is incompletely understood. The rapid postoperative calorie restriction, the decreased glucose toxicity, and the enhancement of the incretin effect all improve islet function, independent of weight loss (2–6); whereas insulin sensitivity improves as a function of weight loss (7). The recovery of β-cell function measured during an oral stimulus, observed after RYGB (5), is not seen after a purely restrictive surgery such as gastric banding, even after matched weight loss (8,9), highlighting the importance of gastrointestinal factors. Here, we sought to characterize temporal changes in β-cell function after both and oral and an intravenous (IV) glucose challenges in individuals with variable states of type 2 diabetes (T2D) remission after RYGB. We hypothesized that after RYGB 1) the improvement in β-cell function after oral glucose intake will be greater in magnitude and occur sooner than after IV glucose administration; and 2) the improvement in body composition and in insulin sensitivity would not differ by diabetes remission status.
Research Design and Methods
Participants
Individuals with obesity and T2D, scheduled to have RYGB at St Luke's Roosevelt Hospital, New York City, provided written informed consent before participating. Of the 36 consented individuals, 29 returned at 3 months (3M; 1 never had surgery, 2 had unplanned vertical sleeve gastrectomy, and 4 were lost to follow-up), 24 returned at 12 months (12M), and 20 at 24 months (24M) (see the CONSORT diagram in Supplementary Material).
Study Design
This study was a longitudinal prospective study of participants studied before and at 3M, 12M, and 24M after RYGB. All participants had T2D before surgery (10). After surgery, the same criteria (10) were used to define full remission (HbA1c <5.7% [39 mmol/mol], fasting glucose <100 mg/dL, 120-min glucose test level <140 mg/dL, not taking any diabetes medication); persistent diabetes (HbA1c ≥6.5% [48 mmol/mol], fasting glucose ≥126 mg/dL, and/or 120-min glucose-test level ≥200 mg/dL, with or without diabetes medication); and partial remission (HbA1c, 5.7%–6.4% [39 to 46 mmol/mol], fasting glucose 100–125 mg/dL and/or 120-min glucose-test level 140–199 mg/dL, not taking diabetes medications). Diabetes status was assessed at each study time point. For prediction models of long-term diabetes remission, data from the latest available study time point postsurgery was used to divide participants into those in full remission (F-REM; n = 5), partial remission (P-REM; n = 16), and those not in remission (N-REM; n = 8). For all participants but five, who only came back at 3M, the last assessment was at 12M (n = 24) or 24M (n = 20). Before surgery, GLP-1 analogs, DPP-4 inhibitors, and thiazolidinediones were discontinued at least 2 months before the first study visit and replaced as needed by sulfonylureas or insulin. All oral antidiabetes medications were withheld for 3 days prior to each visit; the last shot of insulin was given 24 h before each study visit.
Surgery
All RYGB surgeries were done by the same bariatric team. The jejunum was divided 30 cm from the ligament of Treitz, anastomosed to a 30-mL proximal pouch, and re-anastomosed 150 cm distal to the gastrojejunostomy (2). Diet before or after surgery was neither monitored nor controlled for, and participants followed dietary recommendations from the bariatric team; none followed a liquid diet at the time of visits.
Experimental Procedures
Oral Glucose Tolerance Test
Participants underwent a 3-h oral glucose tolerance test (OGTT; 75 g of glucose in 222 mL noncarbonated drink) after a 12-h overnight fast at each visit. The glucose solution was consumed over 15 min; blood samples, collected in chilled EDTA tubes at 0, 15, 30, 45, 60, 90, 120, and 180 min using an antecubital IV catheter, were centrifuged at 4°C and stored at −80°C until assayed.
Graded Glucose Infusion
Patients were admitted after a 12-h overnight fast; antecubital IV catheters were placed in each arm. If fasting glucose level was >150 mg/dL (8.33 mmol/L), a 0.6 unit/kg bolus of IV regular insulin followed by a continuous infusion of 0.01 unit/kg/h was initiated until the blood glucose level reached ≤150 mg/dL, at which point the infusion was stopped. The graded glucose infusion (GGI) started 60 min later (time 0) with an infusion of 20% dextrose at 2 mg/kg/min. Every 30 min, the infusion rate was increased to 4, 6, 8, and 12 mg/kg/min, with blood samples collected every 10 min. The order of OGTT and GGI was randomized, with at least 1 week separating the two tests.
Body Composition by Three-Dimensional Photonic Scanner
Fat mass and waist circumference were measured by the validated three-dimensional photonic scanner (Hamamatsu Photonics KK, Hamamatsu City, Shizuoka, Japan) (11,12). Seven participants had missing data at 12M or 24M and were omitted from the analysis.
Biomarkers
Plasma glucose level was determined by the glucose oxidase method with an Analox glucose analyzer (Lunenburg, MA). Plasma insulin and C-peptide levels were measured by radioimmunoassay (EMD Millipore, St Charles, MO, USA) in the Diabetes Research Center Biomarkers Core. Intra- and interassay coefficients of variance ranged from 3.4% to 7.4%.
Calculations
Total area under the curve (tAUC) and incremental area under the curve (iAUC) during the OGTT and GGI were calculated using the trapezoidal method (13). HOMA-IR was calculated as follows: (fasting insulin µU/mL × fasting glucose mmol/L)/22.5 (14). β-Cell function was assessed using the following: the prehepatic insulin secretion rates (ISRs), determined by C-peptide deconvolution using a two-compartment model (15); β-cell glucose sensitivity (BCGS) was calculated as the slope of the relationship between ISR and corresponding plasma glucose, using fasting glucose to peak glucose values (usually at 30 min) during the OGTT, and fasting glucose to highest glucose value (at 180 min) for the GGI; the early OGTT insulinogenic index (eIGI), calculated as (insulin30 min − insulin0 min)/(glucose30 min − glucose0 min) and the total insulinogenic index (tIGI), calculated as insulintAUC/glucosetAUC. The disposition index (DI), calculated as the product of either BCGS after oral glucose challenge (O-BCGS) or BCGS after GGI (GGI-BCGS) and 1/HOMA-IR. Fasting insulin clearance rate was calculated as following: fasting ISR/fasting insulin; and ICR during OGTT as (ISRtAUC/insulintAUC) − (V*[(insulinend time − insulinstart time)/insulintAUC]), where V is the volume of distribution of insulin and estimated as 0.14 L/kg (16).
Nomenclature
Condition refers to the months when participants were studied: presurgery, 3M, 12M, and 24M after surgery; time, in minutes, refers to the time point at which blood samples were collected during OGTT or GGI. Groups were defined on the basis of diabetes remission status at the latest time point after surgery: F-REM, P-REM, and N-REM. All variables derived from OGTT are labeled with an O- (e.g., O-ISR for ISR calculated during the OGTT). Variables calculated during the GGI are labeled with the prefix GGI- (e.g., GGI-ISR).
Statistical Analysis
Continuous variables were tested for normality and log transformed if found to have a nonparametric distribution. ANOVA tests were used to discern differences of mean values between before and after surgery within and between remission groups.
The general linear model with repeated measure was used to study the effect of time since surgery (condition effect) and of remission status (condition × group effect) on changes in outcome variables and to study changes in metabolic and hormonal variables occurring during the OGTT and GGI glucose challenges (time effect).
Pearson and Spearman correlations and linear regression were used to determine the predictive value of baseline clinical characteristics, baseline β-cell function or its early change at 3M or total change at 12M and 24M, and percent weight loss on glucose control post-RYGB. Multinomial logistic regression was used to determine these variables’ predictive potential on remission status at the latest time point. Data are expressed as mean ± SD except in figures where mean ± SEM are reported.
Power analysis was based on the change of β-cell function after RYGB in individuals in diabetes remission (4). We determined that eight participants would be needed to see a difference of BCGS between pre- and post-RYGB of 1.74 with a SD of 1.22 (effect size d = 1.43 [i.e., 1.74/1.22], and α = 0.05).
Results
Baseline Participant Characteristics
Participants were either Hispanic (n = 22) or Black (n = 7), predominantly women (79%), aged 42.9 ± 8.3 years (range 21–61 years), with known T2D duration of 7.7 ± 7.3 years (range 1 month to 26 years) (Supplementary Table 1). There were no baseline differences in participants’ characteristics and no difference at 3M in weight, weight loss, and values for HbA1c, HOMA-IR, fasting and 120-min glucose, O-glucose AUC, and O-BCGS between those who completed the study and participants who dropped out after the 3M study time point (data not shown).
There were no baseline differences in body composition and insulin resistance between remission groups (Table 1); however, as expected, preintervention indicators of β-cell function (i.e., O-BCGS, O-ISR, and GGI-ISR) were greatest in F-REM and P-REM, compared with N-REM (P < 0.05) (Supplementary Table 2).
Table 1.
Participants’ characteristics by remission status
| Presurgery | 3M | 12M | 24M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-REM | P-REM | N-REM | F-REM | P-REM | N-REM | F-REM | P-REM | N-REM | F-REM | P-REM | N-REM | |
| Sex (men/women) | 1/4 | 3/13 | 2/6 | 1/4 | 3/13 | 2/6 | 1/4 | 1/12 | 2/4 | 0/3 | 0/11 | 1/5 |
| Age (years) | 46.4 ± 6.23 | 40.6 ± 9.61 | 45.3 ± 5.42 | 46.4 ± 6.23 | 40.6 ± 9.61 | 45.3 ± 5.42 | 46.4 ± 6.23 | 42.0 ± 10.1 | 44.8 ± 5.31 | 48.0 ± 4.36 | 41.1 ± 10.6 | 44.8 ± 5.31 |
| Ethnicity (Hispanic/Black) | 4/1 | 13/3 | 5/3 | 4/1 | 13/3 | 5/3 | 4/1 | 10/3 | 4/2 | 2/1 | 8/3 | 5/1 |
| Oral medication (n) | 3/5 | 12/16 | 7/8 | 0/5 | 6/16 | 8/8 | 0/5 | 0/13 | 4/6 | 0/3 | 0/11 | 3/6 |
| Using insulin (n) | 0/5 | 3/16 | 6/8 | 0/5 | 0/16 | 2/8 | 0/5 | 0/13 | 3/6 | 0/3 | 0/11 | 2/6 |
| T2D duration (years)¥ | 1.48 ± 0.99a | 7.38 ± 7.95b | 12.2 ± 5.17b | 1.48 ± 0.99a | 7.38 ± 7.95b | 12.2 ± 5.17b | 1.46 ± 1.00a | 8.38 ± 8.47b | 13.9 ± 3.44b | 1.96 ± 0.81 | 8.16 ± 8.46 | 11.2 ± 5.47 |
| HbA1c (%)¥ (mmol/mol) | 7.34 ± 0.85a 57 | 7.68 ± 1.17a 60 | 8.41 ± 0.88a 68 | 5.56 ± 0.52a** 37 | 5.96 ± 0.38a*** 42 | 7.39 ± 1.09b 57 | 5.51 ± 0.19a*** 37 | 5.87 ± 0.29a*** 41 | 7.34 ± 1.25b 57 | 5.42 ± 0.13a** 36 | 5.97 ± 0.23a*** 42 | 7.86 ± 1.71b 62 |
| BMI (kg/m2) | 42.2 ± 4.29a | 43.3 ± 4.97a | 40.6 ± 4.10a | 34.1 ± 4.14a | 36.2 ± 4.68a** | 33.9 ± 3.94a* | 29.2 ± 5.22a** | 32.7 ± 5.12a*** | 29.5 ± 5.16a** | 29.8 ± 6.82a* | 27.6 ± 5.47a*** | 33.5 ± 6.02a*** |
| BMI (% loss) | 19.16 ± 3.44 | 16.44 ± 3.76 | 16.61 ± 3.25 | 31.00 ± 7.67 | 24.84 ± 4.91 | 27.54 ± 9.66 | 33.1 ± 11.1 | 22.5 ± 9.76 | 29.9 ± 11.3 | |||
| Weight (kg) | 113 ± 12.2a | 118 ± 17.3a | 107 ± 25.9a | 91.2 ± 11.5a | 98.8 ± 14.8a** | 89.5 ± 22.4a | 78.1 ± 14.3a** | 88.6 ± 14.8a*** | 79.0 ± 25.8a | 78.7 ± 22.2a* | 89.2 ± 17.1a*** | 68.2 ± 16.9a* |
| Weight loss (%) | 19.2 ± 3.25a | 16.4 ± 3.8a | 16.61 ± 3.3a | 31.0 ± 7.6a | 24.8 ± 4.9a | 27.5 ± 9.6a | 33.1 ± 11.1a | 22.5 ± 9.8a | 29.9 ± 11.3a | |||
| Fat mass (kg) | 60.0 ± 10.8a | 58.2 ± 12.9a | 52.2 ± 8.8a | 43.8 ± 7.9a* | 46.7 ± 13.7a | 42.9 ± 8.2a | 40.3 ± 5.7a* | 39.5 ± 8.1a** | 33.2 ± 1.0a** | 36.8 ± 6.0a** | 43.2 ± 12.4a* | 32.4 ± 1.0a* |
| Fat mass loss (%) | 25.5 ± 13.6a | 20.2 ± 16.1a | 20.6 ± 11.6a | 33.8 ± 20.8a | 30.9 ± 15.3a | 31.5 ± 16.8a | 23.4 ± 13.5a | 26.5 ± 13.8a | 39.7 ± 2.5a | |||
| Waist circumference (cm) | 121 ± 5.7a | 125 ± 12.5a | 122 ± 12.3a | 103 ± 5.8a* | 111 ± 18.7a | 109 ± 13.4a | 96.7 ± 13.2a* | 105 ± 13.1a* | 86.1 ± 16.7a | 97.4 ± 11.2a* | 106 ± 15.7a* | 79.4 ± 12.5a** |
| Hip circumference (cm)¥ | 133 ± 12.9a | 130 ± 9.6a | 120 ± 6.7a | 119 ± 11.4a | 118 ± 16.2a | 111 ± 5.6a* | 113 ± 9.6a | 114 ± 10.3a* | 98.2 ± 15.9a** | 115 ± 12.0a | 115 ± 11.2a | 91.5 ± 10.4a** |
| Waist-to-hip ratio¥ | 0.91 ± 0.07a | 0.96 ± 0.06a | 1.00 ± 0.10a | 0.87 ± 0.06a | 0.95 ± 0.15a | 0.99 ± 0.11a | 0.85 ± 0.07a | 0.92 ± 0.06a | 0.87 ± 0.03a | 0.88 ± 0.03a | 0.93 ± 0.06a | 0.87 ± 0.04a |
| SBP (mmHg)¥ | 131 ± 17.1a | 126 ± 20.8a | 124 ± 12.4a | 119 ± 15.9a | 126 ± 20.4a | 133 ± 15.9a | 116 ± 21.2a | 124 ± 24.2a | 121 ± 12.2a | 119 ± 22.3a | 125 ± 21.2a | 114 ± 15.0a |
| DBP (mmHg) | 77.0 ± 11.9a | 76.1 ± 15.4a | 78.8 ± 11.2a | 68.8 ± 12.2a | 76.2 ± 10.5a | 82.6 ± 5.7a | 67.2 ± 15.0a | 75.9 ± 11.3a | 76.5 ± 7.5a | 76.7 ± 10.0a | 76.2 ± 8.8a | 72.0 ± 9.7a |
| HOMA-IR¥ | 9.34 ± 2.91a | 13.5 ± 6.13a | 12.2 ± 8.49a | 2.29 ± 1.12a*** | 3.61 ± 2.10a*** | 5.07 ± 3.09a* | 1.98 ± 0.84a*** | 2.33 ± 0.53a*** | 4.11 ± 2.00b** | 1.58 ± 0.86a** | 2.41 ± 2.04a*** | 2.99 ± 0.90a** |
Data are given as mean ± SD. Different letter superscripts indicate statistically significant differences between subgroups by one-way ANOVA post hoc multiple comparison tests at P < 0.05. For within-group differences from presurgery,
P < 0.05;
P < 0.01;
P < 0.001.
Not normally distributed; statistical analysis was done with log-transformed data. DPB, diastolic blood pressure; SBP, systolic blood pressure.
Effect of Surgery on Diabetes Remission
As predicted, the use of all diabetes medications decreased after surgery. HbA1c decreased by 1.65% at 3M and remained at this improved level up to 24M (P < 0.0001) (Supplementary Table 1). Although the decrease in HbA1c was clinically relevant in all groups (−0.5% to −1.9%), it was statistically significant only in F-REM and P-REM (Table 1).
Effect of Surgery on Body Composition and Insulin Sensitivity
Body weight, BMI, waist and hip circumferences, and fat mass decreased after surgery (condition effect, P < 0.001) without differences by remission status (condition × group, P = not significant). HOMA-IR decreased after surgery (P < 0.0001), without group effect (condition × group, P = 0.49) (Table 1). HOMA-IR was strongly associated with fat mass (r = 0.45; P < 0.001), waist circumference (r = 0.49; P < 0.001), and body weight (r = −0.43; P = 0.001) across conditions.
Post-RYGB Changes in Glycemia and β-Cell Function During OGTT
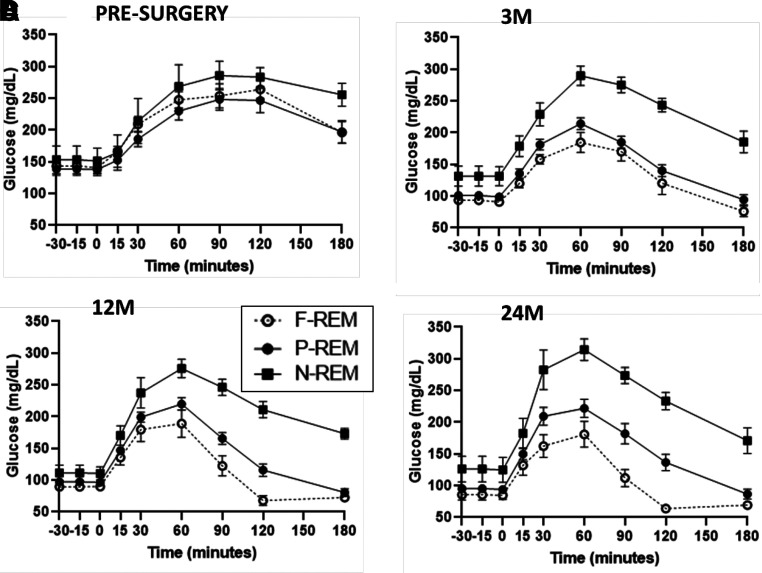
The postoperative improvement in glycemia during the OGTT (condition effect, P < 0.001) varied by remission status (condition × group × time, P = 0.004) (Fig. 1A). Improvement was greatest in F-REM, moderate in P-REM, and minimal in N-REM (Fig. 1B–D). For the entire cohort, compared with presurgery, the temporal pattern of change in β-cell function was an immediate and significant improvement at 3M, further increased at 12M, and returned to 3M levels at 24M (Table 2). Hypoglycemia (48–65 mg/dL) was documented during the OGTT in three of five participants with F-REM at 12M and 24M but was symptomatic in only one participant. The O-ISR (i.e., tAUC-ISR and iAUC-ISR) increased at all conditions after surgery, compared with presurgery (condition effect P = 0.012 and P = 0.002, respectively) (Table 2), without group differences (condition × group P = 0.23 and P = 0.34, respectively) (Supplementary Table 2), with peak increase observed at 12M (Table 2).
Figure 1.
Changes in glycemia during the OGTT at (A) presurgery, (B), 3M, (C) 12M, and (D) 24M in F-REM (open circles), P-REM (black circles), and N-REM (black squares). Overall condition × group × time effect by general linear model test of within-subjects contrasts, P = 0.004.
Table 2.
Change of β-cell function during oral and GGI challenges
| Presurgery | 3M | 12M | 24M | |
|---|---|---|---|---|
| O-ISR AUC (pmol/kg*180 min)¥ | 763 ± 433 | 995 ± 539 | 1,175 ± 703* | 1,165 ± 758 |
| O-ISR iAUC (pmol/kg*180 min)¥ | 406 ± 302 | 718 ± 473 | 868 ± 557* | 822 ± 670* |
| O-BCGS (pmol/kg/min/mmol)¥ | 0.48 ± 0.44 | 1.19 ± 0.97** | 1.68 ± 1.19*** | 1.25 ± 1.41 |
| O-DI¥ | 0.05 ± 0.05 | 0.47 ± 0.52*** | 0.80 ± 0.74*** | 0.65 ± 0.80*** |
| O-early IGI¥ | 79.51 ± 128.3 | 140.8 ± 161.6* | 136.3 ± 146.7 | 128.9 ± 136.8 |
| O-total IGI¥ | 47.44 ± 66.18 | 64.27 ± 81.09 | 55.99 ± 53.78 | 50.48 ± 59.49 |
| GGI-ISR AUC (pmol/kg*180 min)¥ | 542 ± 353 | 518 ± 291 | 565 ± 331 | 643 ± 392 |
| GGI-ISR iAUC (pmol/kg*180 min)¥ | 255 ± 274 | 294 ± 241 | 565 ± 331 | 375 ± 334 |
| GGI-BCGS (pmol/kg/min/mmol)¥ | 0.30 ± 0.31 | 0.33 ± 0.32 | 0.54 ± 0.57 | 0.52 ± 0.57 |
| GGI-DI¥ | 0.04 ± 0.05 | 0.14 ± 0.15** | 0.25 ± 0.28*** | 0.33 ± 0.40*** |
Data given as mean ± SD. Differences from presurgery by one-way ANOVA post hoc multiple comparison tests,
P < 0.05;
P < 0.01;
P < 0.001.
Not normally distributed; statistical analysis was done with log-transformed data. Data were collected during the oral glucose challenge or during the GGI. iAUC, incremental area under the curve.
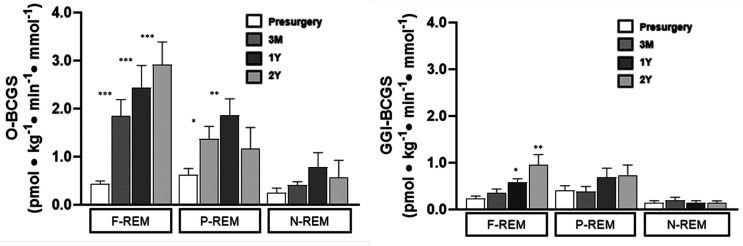
O-BCGS increased after surgery (condition effect P < 0.001) by 2.7-fold at 3M (P = 0.008) and by 3.8-fold at 12M (P < 0.001), and reverted to 3M values at 24M (2.8-fold, P = 0.42 vs. 3M) (Table 2). O-BCGS strongly correlated with 2-h postprandial glucose level (Supplementary Fig. 2). Both the magnitude and temporal changes in O-BCGS varied by remission status (condition × group, P = 0.04). They were greater (5.2- to 6.5-fold; P < 0.01) with a sustained increase in F-REM, moderate increase and not sustained past 12M in P-REM (2.3- to 3.3-fold, P < 0.001), and minimal and very transient in N-REM (0.8- to 2.7-fold; P < 0.05) (Fig. 2A). The overall trend and statistical significance (Supplementary Fig. 1), and the variation according to remission status, were similar for the O-DI (Table 2 and Supplementary Table 2).
Figure 2.
Change in O-BCGS and after GGI-BCGS according to diabetes remission. Mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 vs. presurgery by one-way ANOVA with post hoc multiple comparison tests.
Interestingly, the pattern of change was different for the IGI (both eIGI and tIGI). The improvement in eIGI after surgery (condition effect, P < 0.01) (Table 2), did not differ by group (condition × group P = 0.85) (Supplementary Table 2).
The changes in O-BCGS and O-DI were associated with fat mass (r = 0.37, P = 0.001; r = −0.49, P < 0.001, respectively) and waist circumference (r = −0.41, P < 0.001; r = −0.55, P < 0.001, respectively) across conditions, and, but only for O-DI, with absolute and percent weight loss (r = 0.34, P = 0.004; r = 0.33, P = 0.007, respectively).
Change in β-Cell Function During GGI After Surgery
Seven participants at the presurgery condition, and only one at 3M, required a small insulin dose (bolus with or without insulin infusion) because of elevated glucose prior to initiating the GGI. Glucose changes during GGI are presented in Supplementary Figure 4. Compared with O-BCGS, the increase in GGI-BCGS after surgery was of much smaller magnitude and statistically nonsignificant: 1.1-fold at 3M, 1.8-fold at 12M, and 1.7-fold at 24M (condition effect P = 0.188; condition × group effect, P = 0.440) (Fig. 2B and Table 2). GGI-BCGS increased significantly only in F-REM at 12M (P = 0.020) and 24M (P = 0.002) compared with presurgery (Fig. 2B and Supplementary Table 2).
For the entire cohort, the GGI-DI increased significantly (P = 0.002 at 3M, P < 0.001 at 12 and 24M) with a maximum >8-fold increase at 24M (Table 2 and Supplementary Fig. 1). This pattern of improvement differed from the O-DI, which peaked at 12M. GGI-DI improved significantly in F-REM and P-REM at 3M (P < 0.05 for both) and 12M (P < 0.01 and P < 0.001, respectively), and peaked at 24M (P < 0.01 and P < 0.001, respectively), but did not improve in N-REM (Supplementary Table 2 and Fig. 2B).
The changes in GGI-BCGS and GGI-DI across conditions were associated with fat mass (r = −0.29, P = 0.012; r = −0.50, P < 0.00, respectively), waist circumference (−0.34, P = 0.003; −0.55, P < 0.001, respectively), and weight loss (r = 0.25, P = 0.039; r = 0.42, P < 0.001, respectively).
Comparison of Oral vs IV-Derived β-Cell Function Biomarkers
GGI-BCGS was lower than O-BCGS at presurgery (P < 0.001) and remained lower at 3M (P = 0.001), 12M (P < 0.001), and 24M (P = 0.20) (Table 2 and Fig. 2). The absolute change in O-BCGS from pre- to postsurgery was more than 20 times greater than the change in GGI-BCGS at 3M (0.70 ± 0.77 vs. 0.03 ± 0.31; P < 0.001) and 40 times greater at 12M (1.21 ± 0.93 vs. 0.03 ± 0.44; P < 0.001). This large difference in the increase in BCGS after the oral versus the IV glucose challenge persisted but to a significantly lesser degree at 24M, with the change in O-BCGS at 24M only approximately four times greater (0.82 ± 1.38 vs. 0.22 ± 0.61; P = 0.057).
Interestingly, the magnitude of the difference between O-BCGS and GGI-BCGS differed by remission status, being greater in F-REM compared with P-REM and N-REM. In F-REM, O-BCGS was larger than GGI-BCGS at all time points (P < 0.05 at presurgery, 12M, and 24M; P < 0.01 at 3M). In P-REM, O-BCGS was greater than GGI-BCGS at 3M and 12M (P ≤ 0.001) but not at 24M. In N-REM, however, the difference between O-BCGS and GGI-BCGS was minimal and significant only at 12M (P < 0.001) (Supplementary Table 2). Although the O-DI and the GGI-DI did not differ significantly at presurgery, the magnitude of the increase after surgery was twice as great for O-DI (16-fold) than for GGI-DI (>8-fold) (Table 2 and Supplementary Fig. 1).
As noted, GLP-1 concentrations during the OGTT (peak and AUC) increased by two to three times at all time points after RYGB (data not shown). The magnitude (peak and AUC) of the GLP-1 postprandial release did not differ by diabetes remission and did not change overtime (data not shown).
Insulin Clearance
As we have reported previously (16), insulin clearance under fasting conditions increased significantly and continuously at 3M, 12M, and 24M, when it more than tripled the presurgical value (P < 0.001), with no difference by group (condition × group effect, P = 0.42). The O-ICR also increased after surgery, maximally at 24M (P < 0.001) again but with no difference by group (condition × group effect, P = 0.10) (Table 1 and Supplementary Table 1).
Predictors of Diabetes Remission and Glucose Control
Bivariate predictive models were constructed using a stepwise regression or multinomial logistic regression based on clinical (diabetes duration, insulin use) and β-cell function (O-BCGS, GGI-BCGS) predictors with P < 0.05 during univariate linear regression or likelihood ratio (LR) tests, respectively (Supplementary Tables 3 and 4). A single clinical and β-cell function variable was added to each model, for a maximum of two baseline, early change, and total change predictors at once.
In a multinomial regression model, presurgery diabetes duration with either baseline O-BCGS or GGI-BCGS were the best predictors of remission status (LR test, P = 0.001 and P = 0.003, respectively). When BCGS was added to a model with presurgery insulin use, insulin use fell out of the model (insulin use was not significant by LR tests), leaving O-BCGS and GGI-BCGS as strong predictors of remission status (LR test, P = 0.003 and P = 0.013, respectively) (Supplementary Table 5B). Baseline O-BCGS was not a significant predictor of long-term HbA1c when paired with either insulin use or diabetes duration (O-BCGS not significant and fell out of the model; P = 0.45 and P = 0.24, respectively). The best predictors of postsurgery HbA1c were presurgery insulin use, with the early change in GGI-BCGS (P < 0.001; R2=0.51), and with both the early and long-term change in O-BCGS (P < 0.001, R2=0.58; P < 0.001, R2=0.55, respectively). Diabetes duration paired with either early or long-term change in O-BCGS also significantly predicted HbA1c (P = 0.002, R2=0.43; P = 0.003, R2=0.43) (Supplementary Table 5A).
Conclusions
The following are the main findings of the present study in individuals with severe obesity and T2D: 1) presurgery β-cell function is a strong predictor of T2D remission; 2) the temporality and magnitude of the improvement in β-cell function after RYGB are highly associated with remission status; 3) the greater magnitude of the improvement in β-cell function after oral glucose compared with IV glucose is more pronounced in F-REM; and 4) weight loss, fat mass loss, and decreases in waist circumference and insulin resistance after surgery are not key determinants of diabetes remission.
Our data confirm that the high degree of insulin resistance, a hallmark of T2D, improved significantly postsurgery, despite participants remaining in the obesity range at 12M with a mean BMI of 31 kg/m2. However, neither presurgery nor the large decreases in body weight (∼30% at 1 and 2 years), fat mass, and waist circumference, and the resulting improvement in insulin sensitivity differ according to diabetes remission status. So although the weight loss–dependent improvement in insulin sensitivity after surgery (7) surely plays a role in overall metabolism, it does not differentiate patients in remission from N-REM and does not appear to be a key determinant of the diabetes remission phenotype.
Contrary to weight loss and insulin sensitivity, presurgery β-cell function and its temporal variation after RYGB emerged as the strongest predictor of the magnitude of the reversal of hyperglycemia, as shown previously (5,6). Not surprisingly, individuals with the worst β-cell function at presurgery had the longest known diabetes duration, were more likely to be using insulin, had higher HbA1c values, and were the least likely to experience remission after surgery. Although insulin resistance did not differ between groups at presurgery, presurgery β-cell function, calculated both after an oral and IV glucose challenge, was significantly more impaired in the N-REM group than in the F-REM or P-REM groups. Moreover, the temporal changes of β-cell function assessed during the OGTT were vastly different according to remission status; in the F-REM, robust, immediate (3M) continuous and sustained (24M) improvement occurred, whereas in the P-REM or N-REM groups, the lesser improvement was only transient (at 3M and/or 12M). These changes were independent of weight and fat-mass losses or change in insulin sensitivity, or even change in insulin clearance, which were all similar in all three groups.
We (2) and others (17) have shown the importance of the incretins in restoring insulin secretion during an oral glucose challenge after RYGB. We also previously showed that the recovery of the β-cell function measured during an isoglycemic IV glucose clamp was only partial when compared with individuals with severe obesity and normal glucose tolerance or with lean individuals, even in individuals with full clinical diabetes remission studied up to 3 years after RYGB (4,6). In the present study, therefore, we assessed β-cell function after both oral glucose and GGI. As expected, we observed a striking difference in the improvement of β-cell function according to the route of the glucose challenge, with a three to four times greater increase after oral than IV glucose administration. In addition, we show that the improvement in BCGS during the GGI was quite limited in magnitude, significant only in F-REM, and delayed, starting only 1 year postsurgery. This is contrary to the rapid recovery of β-cell function after oral glucose shown previously after 1 month (4,6) and here at 3 months postsurgery. We also observed striking differences in the temporal changes in β-cell function according to remission status. Regardless of the route of the glucose stimulus, participants in the F-REM group demonstrated a continuous and sustained improvement of their β-cell function, even after body weight stabilized between years 1 and 2. The substantial improvement in the β-cell function of P-REM, however, was only transient and not sustained beyond year 1, despite absence of weight regain.
Interestingly, the gap between BCGS after oral and IV glucose administration, or the greater O-BCGS compared with GGI-BCGS was associated with remission status; it was primarily observed in F-REM and P-REM, and less so in the N-REM. On the basis of past work (2), these data suggest a larger incretin effect in patients with diabetes remission. In F-REM, O-BCGS tended to increase overtime, which suggest some temporality of the involvement of the gut in the recovery of β-cell function. The accelerated nutrient transit is the main mechanism by which postprandial GLP-1 release is enhanced after RYGB (18). Because nutrient transit is unlikely to change significantly over time after surgery, the observed temporal change in O-BCGS could be related to either some degree of intestinal adaptation or reprogramming (19) or to change in the sensitivity to insulinotropic hormones after RYGB observed in individuals without diabetes (20,21).
Our data hint at the mechanisms by which RYGB improves β-cell function. Calorie restriction (22) rapidly decreases fasting glucose and clearly plays a role after RYGB when food and calorie intake decrease significantly (23). The resulting removal of glucose toxicity can also enhance BCGS (24). Chronic hyperglycemia is toxic to the β-cell (25) and BCGS is a key determinant of postprandial glucose level. This close temporal bidirectional relationship of BCGS and postprandial glucose levels, illustrated in Supplementary Figure 2, was previously highlighted by Nannipieri et al. (5) in a similar cohort.
A third mechanism is the large and sustained decrease of peripheral insulin resistance, which decreases the workload of the β-cell. These three mechanisms—calorie restriction, decreased glucose toxicity, and reduced insulin resistance—should equally play a role in improving BCGS after oral and IV glucose challenges. However, the modest improvement in IV glucose-challenge BCGS after RYGB, which was significant only in individuals in the F-REM group, speaks to the minimal parts played by these mechanisms. It could also indicate alteration of BCGS to IV-administered glucose, observed by others in individuals after RYGB who did not have diabetes (26).
On the contrary, the role of the incretins is likely to be quite significant and explains the extraordinary temporal recovery of O-BCGS after RYGB, which was of significantly greater magnitude in patients in remission. The postprandial incretin release is immediate (2,17) and sustained after RYGB (6), largely due to the rerouting of nutrients to the lower part of the gut (18). In individuals with T2D, the blunted incretin effect on insulin secretion is very rapidly recovered 1 month after RYGB (2). The contribution of endogenous GLP-1 to the recovery of BCGS shortly after RYGB was elegantly illustrated in an experiment using the GLP-1 receptor antagonist exendin9–39 at 1 week and 3 months after RYGB (17) or in cross-sectional cohorts (27,28) in individuals without diabetes or who were in full diabetes remission. By comparing patients in remission and N-REM, the present study sheds new light on the possible role of the incretins in diabetes remission: First, the O-BCGS improves significantly more in patients in remission than in N-REM, whereas weight loss and change in insulin sensitivity, and even circulating GLP-1 concentrations (data not shown), were identical in the three groups; second, the differential improvement of O-BCGS over GGI-BCGS is more marked in patients in remission than N-REM, suggesting a better incretin effect.
The best predictors of remission were presurgery BCGS with or without diabetes duration. The best predictors of long-term HbA1c were presurgery insulin use or diabetes duration, paired with changes in BCGS resulting from the surgery. Neither weight loss, fat-mass loss, nor change in insulin sensitivity predicted remission. These data confirm the importance of β-cell functional reserve, as shown by others (5,29) and confirm previous data in larger cohorts, using various scores, based on presurgery clinical variables (30,31), with or without change in β-cell function postsurgery (32–34). Interestingly, in a different homogenous cohort of individuals with diabetes of short duration (<2 years), with good glycemic control and taking few medications (i.e., with good β-cell reserve), both weight loss and preintervention β-cell function contributed to remission status (6).
Our study has many strengths. Data were from a single bariatric center with standardized gastric bypass technique, longitudinal measures of β-cell function in individuals with and without diabetes remission were obtained, both oral and IV glucose stimuli were used concomitantly in the same participant, and up to 2-year follow-up was conducted. Some limitations need to be acknowledged, including the lack of a sleeve gastrectomy group, given that this is now more frequently performed, and the relative short-term follow-up, because relapse is often observed at 5 years postsurgery (35).
In summary, our data confirm that presurgery β-cell functional reserve is a key determinant of diabetes remission postsurgery. The data also show the temporal variability in the improvement, or lack of, in β-cell function after RYGB. Finally, the greater recovery of β-cell function after oral versus IV glucose stimulus underlines the key role of gut factors (i.e., the incretins) to restore β-cell function in diabetes remission.
Article Information
Acknowledgments. The authors thank the St Luke’s Roosevelt Hospital bariatric team, James McGinty, John Harvey, Ninan Koshy, Koji Park, Scott Belsley, Toni Colarusso, Vicky Drake, for referring their patients to the study. The authors thank Betsy Rojas and Esmeralda Pierrini who assisted with recruitment.
Funding. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grants UL1TR000040; NIH R01DK098056-04, P30DK26687, P30DK063608, and UL1TR000040. A.S. was supported by NIH grant F32DK113747.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.L. designed the study, supervised data collection and analysis, wrote the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. V.M., R.D., A.S., and N.N. assisted with data collection. V.M., M.P., A.S., and C.L. assisted with some data analyses. All coauthors edited and approved the manuscript.
Footnotes
Clinical trial reg. no. NCT02287285, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.16989505.
References
- 1. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 2. Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holter MM, Dutia R, Stano SM, et al. Glucose metabolism after gastric banding and gastric bypass in individuals with type 2 diabetes: weight loss effect. Diabetes Care 2017;40:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nannipieri M, Mari A, Anselmino M, et al. The role of β-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372–E1379 [DOI] [PubMed] [Google Scholar]
- 6. Dutia R, Brakoniecki K, Bunker P, et al. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 2014;63:1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med 2020;383:721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes 2010;34:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012;122:4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Gallagher D, Thornton JC, Yu W, Horlick M, Pi-Sunyer FX. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. Am J Clin Nutr 2006;83:809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah A, Prasad M, Devjani S, et al. Anthropometrics by three-dimensional photonic scanner in patients with obesity before and after bariatric surgery. Obes Surg 2021;31:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care 1995;18:245–250 [DOI] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 15. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991;88:934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah A, Holter MM, Rimawi F, et al. Insulin clearance after oral and intravenous glucose following gastric bypass and gastric banding weight loss. Diabetes Care 2019;42:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013;62:3044–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pournaras DJ, Aasheim ET, Bueter M, et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis 2012;8:371–374 [DOI] [PubMed] [Google Scholar]
- 19. Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013;341:406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to insulinotropic gut hormones is reduced after gastric bypass surgery. Gut 2019;68:1838–1845 [DOI] [PubMed] [Google Scholar]
- 21. Dirksen C, Bojsen-Møller KN, Jørgensen NB, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013;56:2679–2687 [DOI] [PubMed] [Google Scholar]
- 22. Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998;21:2–8 [DOI] [PubMed] [Google Scholar]
- 23. Wardé-Kamar J, Rogers M, Flancbaum L, Laferrère B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg 2004;14:1070–1079 [DOI] [PubMed] [Google Scholar]
- 24. Yki-Järvinen H. Glucose toxicity. Endocr Rev 1992;13:415–431 [DOI] [PubMed] [Google Scholar]
- 25. Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 26. Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab 2018;20:872–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiménez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013;36:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg 2013;257:894–899 [DOI] [PubMed] [Google Scholar]
- 30. Panunzi S, Carlsson L, De Gaetano A, et al. Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 2016;39:166–174 [DOI] [PubMed] [Google Scholar]
- 31. Sjöholm K, Carlsson LMS, Taube M, le Roux CW, Svensson PA, Peltonen M. Comparison of preoperative remission scores and diabetes duration alone as predictors of durable type 2 diabetes remission and risk of diabetes complications after bariatric surgery: a post hoc analysis of participants from the Swedish Obese Subjects Study. Diabetes Care 2020;43:2804–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aron-Wisnewsky J, Sokolovska N, Liu Y, et al. The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia 2017;60:1892–1902 [DOI] [PubMed] [Google Scholar]
- 33. Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ligon C, Shah, A, Prasad M, Laferrère B. Pre-intervention clinical determinants and measured β-cell function as predictors of type 2 diabetes remission after Roux-en-Y gastric bypass surgery. Diabetes Care 2021;44:2427–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]