Introduction and Background
The Precision Medicine in Diabetes Initiative (PMDI) was launched in 2018 by the American Diabetes Association (ADA) in collaboration with the European Association for the Study of Diabetes (EASD). The PMDI has subsequently partnered with other organizations including the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), JDRF, and the Diabetes Technology Society. The mandate of the PMDI is to establish consensus on the viability and ultimate clinical implementation of precision medicine for the diagnosis, prevention, treatment, prognosis, and monitoring of diabetes. This process is designed to take place through systematic evaluation of available scientific evidence, expert consultation, and broad stakeholder engagement (Fig. 1). The mandate of the PMDI is pursued with the overarching goal of facilitating longer, healthier lives for people living with diabetes, identifying those at risk, and preventing diabetes through multiple interventional approaches.
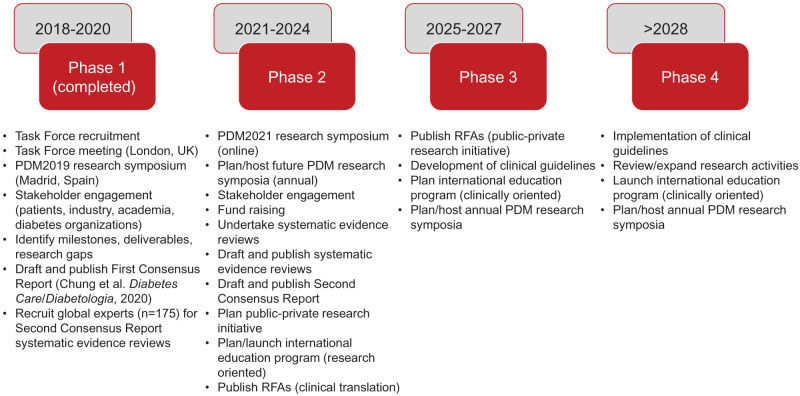
Figure 1.
Key milestones and timelines of the ADA/EASD PMDI. Adapted from Chung et al. (1). RFA, request for applications.
The PMDI held its first international scientific conference in Madrid, Spain, in October 2019. Approximately 100 delegates participated, with attendees from North America, Europe, the Middle East, Asia, and Africa, with diverse representation (including academia, industry, funders, and people with diabetes). The conference provided the foundation and framework from which a first Consensus Report on precision medicine in diabetes was developed and published in 2020 (1). The Consensus Report named five key pillars, or domains, of precision diabetes medicine: precision diagnostics, precision prevention, precision treatment, precision prognostics, and precision monitoring. Across each domain, the article also identified critical gaps in knowledge and evidence required for the scientific advancement, implementation, and ongoing evaluation of precision medicine in diabetes. Newly formed PMDI working groups (composed of ∼175 internationally leading clinicians and researchers in precision diabetes medicine [Supplementary Appendix]) have since initiated multiple, ongoing evidence-based evaluations in diabetes related to the five key domains.
The second international scientific conference organized by the PMDI was held in April 2021 (www.pdm2021.org). This meeting, hosted virtually owing to the coronavirus disease 2019 (COVID-19) pandemic, included more than 3,000 participants from 67 countries. Building on the foundation and framework established at the first conference, diverse stakeholders in the development and implementation of precision diabetes medicine exchanged new data, ideas, and opinions. Over 3 days, the meeting delivered interactive state-of-the-science overviews of all key domains of precision diabetes medicine in research and practice. The PMDI working groups undertaking systematic reviews provided progress updates and summaries of existing evidence and identified critical gaps in current scientific knowledge. Plenary presentations discussed the interface of precision medicine in diabetes with the social determinants of health and disparities in care as well as the impact of racial discrimination and other forms of health inequity. Early-career investigators showcased novel findings and contributions to the five key domains. Daily keynote lectures, delivered by people living with diabetes from around the globe, highlighted patient perspectives and offered insights into the opportunities for precision diabetes medicine to improve the day-to-day experience of living with diabetes. A moderated poster session, with abstracts selected by reviewers, was a focus of trainees and early-career investigators, while other abstracts were available for viewing online.
Progress and Current Focus
Based on emerging milestones, as well as the long-term road map outlined in the first Consensus Report (1), the PMDI will continue to focus on advancing the vision of precision diabetes medicine in practice, with a view to enhancing clinical diabetes guidelines through evidence-based precision medicine recommendations. Major next steps include 1) highlighting the research and clinical domains of interest in precision diabetes medicine (diagnosis, prevention, treatment, prognosis, and monitoring); 2) engaging with key stakeholders in areas germane to precision diabetes medicine, including clinicians, researchers in academia and industry, and those working with health care data science and informatics, health policy and reform, and social sciences; 3) identifying the gaps and future steps toward the clinical application of precision medicine; and 4) establishing a dialogue with people with diabetes to keep lived experiences and unmet needs at the forefront of the vision for precision diabetes medicine.
Research Themes and Directions
The PMDI is working toward a second Consensus Report on precision diabetes medicine. This follow-up to the first Consensus Report will summarize and integrate findings across the multiple systematic evidence reviews spanning the five key domains of precision diabetes medicine outlined in the first Consensus Report (1). Common themes are already evident from the research in the different PMDI working groups. The crosscutting nature of the PMDI framework (precision diagnosis, prevention, treatment, prognosis, and monitoring) has revealed opportunities for synergy, including connecting research efforts that might otherwise be siloed by traditional clinical categories of diabetes (e.g., type 1 diabetes, type 2 diabetes, gestational diabetes, and monogenic diabetes). The leading research themes and directions emerging from the work so far in the different PMDI working groups can be summarized as follows.
First, precision diabetes medicine involves an evolution of existing approaches to diabetes care and the use of decision support tools, moving from a one-size-fits-all paradigm to one in which diverse and high-resolution data are used to enhance clinical decisions at the subgroup, if not individual, level. Although the term “personalized medicine” is sometimes used in this context, doing so may oversell the potential of this approach to tailor the medical model to the individual; thus, here we consider personalized medicine to be synonymous with “individualized medicine,” as defined in the ADA Standards of Medical Care in Diabetes (2). Despite many differences between the clinical settings of type 1 diabetes, type 2 diabetes, gestational diabetes, and monogenic diabetes, the systematic evidence evaluation focuses on the key topics below.
Identification of Key Patient-Level Markers for Enhanced Precision in Diabetes Medicine
Markers may include traditional biomarkers, such as information about a person’s genome, epigenome, transcriptome, proteome, metabolome, and clinical phenotype, as well as proxies for their social circumstances, behavior, psychological state, and/or physical environment. These biomarkers may be used to cluster individuals in subgroups or assign probabilities for improved risk stratification or prediction of disease progression, to predict treatment responses more precisely, or to direct other targeted health interventions, including preventative efforts. A key consideration is the varying availability and utility of different biomarkers across clinical or research settings as well as careful interpretation of markers to support inclusivity and equity and to minimize unintentional bias propagated through language (3).
Prioritization of Outcomes and Clarity in the Definition(s) of Success in Diabetes Therapy or Other Health Interventions for Individuals With Diabetes
Outcomes to guide the targeting or tailoring of an intervention may include traditional clinical measures (e.g., glycemic control) as well as patient-oriented outcomes (e.g., treatment satisfaction and quality of life) and indicators of cost-effectiveness.
Opportunities for Genetic Data to Improve Clinical Care for Diabetes
The use of genetic/genomic scoring has revealed multiple, novel approaches for precision diabetes medicine, particularly as it relates to the domains of precision diagnosis, treatment, and prognosis. Integration of genetic data into study design could inform best practices in precision diabetes medicine, by including key biomarkers and clinical characteristics in the selection of interventions, outcomes and subgroup assignment in the target population.
Collection and Use of New Multisource Data
Numerous opportunities germane to precision medicine exist by analyzing just-in-time information from wearables and other technologies, helping people with diabetes as well as clinicians to adapt behavior and treatment, and data from dynamic and/or time-series testing. Individuals living with diabetes will be key partners in research efforts to collect, manage, and utilize these data for large-scale studies.
Emphasis on Clinical Utility
Looking ahead to real-world implementation, a key aspect for evaluation is how the association of (bio)markers, genotypes, and phenotypes with clinical outcomes and treatment responses can be applied in clinical settings to enhance clinical care and decision-making as well as health policy. Decision support yielded by advancements in precision diabetes medicine must be integrated into clinical and community workflows such that it is easily understandable and pragmatic and confers value for end users.
Second, each domain of precision diabetes medicine is complex. The task of the multiple systematic reviews to summarize and grade the current evidence base for precision diabetes medicine, and the most important gaps, remains an enormous effort. One example of the scale and range of questions to be explored in the existing literature was presented by Dr. Ewan Pearson on behalf of the type 2 diabetes precision treatment working group (2). For each of seven classes of antihyperglycemic medications (e.g., metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, thiazolidinediones, sodium–glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and basal insulin), the working group plans to evaluate effects across different classes of markers, including ethnicity, other phenotypic markers (e.g., sex), metabolic status (e.g., BMI), and genomic/epigenomic features. Outcomes spanning six categories must be assessed for each medication class and marker interaction. These outcomes include glycemic progression monitored using HbA1c, treatment failure, adverse drug reaction, cardiovascular outcomes, renal outcomes, and mortality.
Third, the approach to precision diabetes internationally must take account of regional, cultural, genetic, and other differences in populations around the world. This includes taking account of differences in diabetes phenotypes across global populations (4–6) or across subpopulations (7). For precision prognosis and treatment, there are known differences in the relative prevalence of certain risk or response genotypes across racial and ethnic subgroups (8), e.g., thiazolidinediones are metabolized by CYP450 2C8 enzyme (encoded by the CYP2C8 gene), with the CYP2C8*3 allele altering thiazolidinedione pharmacokinetics. The CYP2C8*3 variant (rs10509681) is associated with reduced glycemic response to rosigliltazone (9), with the frequency of CYP2C8*3 in people of European ancestry (0.12) differing from the frequency in people of African ancestry (0.01) (https://bravo.sph.umich.edu/freeze8/hg38/). An understanding of these differences will be required for precision interventions and optimal clinical management, particularly in the context of additional social or cultural differences that may exist.
In addition to establishing the objectives and tasks for the PMDI working groups, the first Consensus Report also outlined several overarching considerations for the long-term vision of precision diabetes medicine as a newly emerging field. The PDM2021 conference continued and expanded discussions of three key considerations, reinforcing the importance of these themes in ongoing and future work to realize the potential of precision diabetes medicine in practice. Below, we provide a brief update on each of the key themes: equity, implementation, and cross-sector partnerships.
Equity in Precision Diabetes Medicine
A key aspect of equity in precision diabetes medicine is to integrate an understanding of social determinants of health (10) and racial discrimination in diabetes (11), highlighted in part by Arleen Tuchman, Professor of History at Vanderbilt University (12).
It is essential that the clinical benefits of precision diabetes medicine are made available to all individuals in need, with special attention focused on subgroups of populations that have been historically marginalized or underrepresented in scientific and clinical research. The attention to equity should begin with considerations of fair data collection and spans the scientific and translational process, ending with equitable implementation of new interventions.
The Road to Implementation
A crosscutting theme for implementing precision diabetes medicine in practice is that of precision monitoring. Precision monitoring may extend beyond glucose monitoring, and it may be integrated to better understand safety and enhance the efficacy of precision diagnostics, prevention, prognosis, and treatment.
New approaches to collecting and storing data, in addition to careful maintenance of data privacy, will be needed to support the scaling of precision diabetes medicine research as well as its implementation, dissemination, and ongoing evaluation both at the individual and population levels. Broad uptake and sustained innovation will be contingent on the infrastructures in place to collect and securely store large and growing amounts of complex data relevant to diabetes care in real time. People living with diabetes will also have to be engaged in the use of their data and provide informed consent for the use of their data for ongoing research in addition to their clinical care. Together, these hurdles underscore the importance of adopting a systems-based approach for the delivery of precision diabetes medicine that considers a full view of information flow, knowledge generation, and practice change.
Following completion of the systematic review of existing scientific evidence, multiple working groups have discussed the need for future trial designs to rigorously test precision diabetes medicine interventions. Trial data will be critical to evaluate and test superiority to usual care as well as cost-effectiveness. However, the complexities of undertaking randomized clinical trials to assess precision medicine approaches, particularly the multidimensional nature of precision approaches, will necessitate innovative trial designs. These complexities will also place greater emphasis on data obtained from secondary analysis of existing randomized clinical trials and registry data. Thus, there is a challenge and opportunity to address precision diabetes medicine questions using novel trial designs and real-world data.
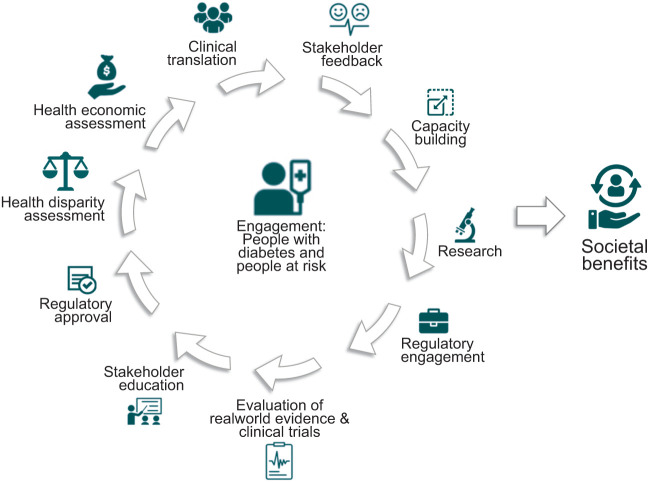
The process of implementing precision diabetes medicine in practice has yet to be fully determined, but with clinical implementation already a reality for precision medicine in monogenic forms of diabetes and precision medicine for complex forms of the disease on the horizon, there is a growing need for consensus on best practices for implementation. Recognizing that this is unlikely to be entirely linear and instead a process of ongoing refinement dependent on continuing feedback, new data generation, updated scientific evidence or care guidelines, and available therapies, each step is likely to form part of a cycle (Fig. 2), which, with the passage of time, should lead primarily to benefits for people living with diabetes and their families as well as more broadly for society as a whole. Implementation efforts can be strengthened with the incorporation of existing theories and frameworks (13). Moreover, learning health systems may provide ideal environments to integrate various data and patient preferences to test approaches to care, drive new research questions, and enhance knowledge translation relevant to precision diabetes medicine in real-world settings (14).
Figure 2.
Key steps in the iterative process of implementing the PDMI into clinical practice.
Building Partnerships
The translation of precision diabetes medicine into practice requires engagement of many different stakeholders. Figure 3 depicts a map of important stakeholder groups, many of which are already engaged with the PMDI. These include care providers and researchers, the leading international scientific societies in the field, people living with diabetes, industry, and governments (national, regional, and local). In the future, it is likely that additional stakeholders will be identified.
Figure 3.
Overview of key stakeholders germane to the success of the ADA/EASD PMDI. IT, information technology; pharma, pharmaceutical industry; med tech, medical technology; data sci, data science.
Collaborative relationships between senior industry scientists and academia, including the leading scientific societies, will help develop new approaches to early prevention of diabetes as well as pipelines for new drug development. Indeed, public-private partnerships in precision diabetes medicine will be critical, as research and development of precision medicine approaches will be resource-intensive and require wide-ranging expertise. Moreover, industry engagement will be essential to ensure precision medicines and technologies meet regulatory standards and can be produced and distributed at scale to those most in need.
The voices of people living with diabetes need to be put at the center of any research and policy development in this field. Discussions during the PDM2021 conference underscored once again the importance of obtaining input from people living with all types of diabetes, beginning with gestational diabetes through to diabetes as it presents from childhood to old age; this input can be used to guide research priorities, identify new problems and develop solutions, and to ultimately ensure that precision diabetes medicine will enhance health and well-being of individuals at risk for or living with diabetes.
Future Perspective
Interest in the potential for precision medicine in the prevention, treatment, and ongoing management of diabetes continues to grow across many of the most important stakeholder groups, including people with diabetes. At the same time, there is a growing recognition of the complexity of precision diabetes medicine, including the unique challenges that distinguish it from other clinical domains in which precision medicine has been applied. At the conclusion of the second PMDI conference, it was clear that there is great potential for diabetes care to move to a more precise approach, yet precision medicine for diabetes will not develop in a single sweeping set of changes to clinical practice. Instead, the field of precision diabetes medicine will comprise many different veins of research and implementation work, each at different stages of development and progressing at their own paces. So far, few of these precision approaches are close to “prime-time” readiness. As this nascent field continues to evolve, it is also likely that new opportunities for precision diabetes will continue to be identified.
Precision diabetes medicine remains to be fully probed; however, it remains clear that the field is not just concerned with genetics, nor is it solely concerned with glucose. Just as clinical diabetes care concerns itself with the whole person, so too must the field of precision diabetes medicine consider a complete phenotype via the measurement of markers relevant to the underlying pathophysiology, clinical care, and larger physical and social environments. The challenge will be to conceptualize and study the most clinically actionable phenotypes of diabetes, including those that are informed by population-level heterogeneity but can be operationalized as part of clinical care.
At the same time, the development of precision diabetes medicine should not detract from efforts to promote the most fundamental best practices in diabetes care. For example, it is clear that many people with diabetes do not have access to modern drugs, technologies, and care systems (10). These disparities in diabetes care and outcomes, in addition to the obstacles posed by fragmented health care systems, are a huge challenge and have been stressed by PMDI contributors from all areas of the world. Further, there has actually been worsening in key parameters of care over the most recent decade, where a recent report from the National Health and Nutrition Examination Survey (NHANES) database shows that glycemic control and blood pressure control declined in adults with diabetes since 2010, in contrast to good progress in the preceding decade (15). Leading contributors to the PMDI have stressed the importance of keeping focus on the gaps between the current state of diabetes care and current evidence-based approaches to prevent morbidity and mortality associated with the disease.
The PMDI has also drawn attention to the larger systems that provide care and support for individuals living with diabetes. For example, the organization of electronic medical records may need to be revised to display relevant phenotyping information while protecting other sensitive genetic or biomarker data that may be collected. Standardization of key genetic, phenotypic, and outcome measurements is also a critically important issue that will require attention and commitment from many contributors in the field of diagnostics and technology. The increasing use of continuous glucose monitoring and other wearable technologies provides a wealth of new individual clinical information that can inform the selection and dose of treatment, yet these data must be rendered through precision monitoring to decision support for other domains of precision diabetes medicine.
Cross-disciplinary or multifaceted interventions may require collaboration across different care providers or stakeholders in diabetes care, including those within the health care system and those within the community. Creative approaches may be needed to adapt new knowledge to different settings, as care and support systems vary greatly between countries and regions. Above all, systems-level innovation to usher in a new era of precision diabetes medicine will require clear channels of communication to bridge language, ideas, and values (e.g., patient preferences, clinical outcomes, health equity, and cost-effectiveness) across all participants in the development of systems of care, with implications for the undergraduate and postgraduate education of the diabetes clinicians of the future.
Conclusions
In conclusion, the application of precision medicine across the five key pillars, or domains, of diabetes care (diagnosis, prevention, treatment, prognosis, and monitoring) is an ambitious, complex, yet critical undertaking for improving the health of people living with diabetes around the globe. While there remain significant challenges ahead, the second international scientific conference organized by PMDI highlighted areas of progress and emerging synergies across the PMDI working groups as well as evolution across the themes of equity, monitoring, implementation, and cross-sector partnerships. As the PMDI continues to work toward its mandate, regional and international collaborations among leading clinicians and researchers in precision diabetes medicine will continue to strengthen the initiative. Continued dialogue with individuals spanning diverse personal and professional backgrounds, as well as new cross-sector partners, will further support the efforts to improve the lives of people living with diabetes through more precise approaches to prevention and care.
Article Information
Acknowledgments. The authors are grateful to the ADA and EASD for their support of the PMDI. The authors acknowledge the contribution of Dr. William T. Cefalu (formerly of the ADA and now at the NIDDK) in establishing the PMDI from its outset. The ADA staff supported the preparation and running of both the first PDM conference in Madrid in 2019 and the second virtual PDM2021 conference this year. The authors also acknowledge the ongoing scientific contributions of the volunteer participants in the PMDI working groups (Supplementary Appendix).
Funding. PMDI has received funding support from the ADA (for ongoing work, including both conferences) as well as existing research funding from P.W.F. at Lund University (for conferences and for administrative and librarian support for the working groups as well as for the cost of software licenses required to perform the systematic reviews). No formal funding relationships with governmental structures (e.g., NIDDK), foundations, or pharmaceutical/device companies exist currently. These organizations, however, as well as EASD, have provided intellectual support thus far. For expansion of the program (including support for the working groups and the proposed research and outreach programs), additional sources of support are being sought by the ADA and EASD. A.R.K. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant KL2TR002490. Z.S.-A. was supported during this work by the Canadian Institutes of Health Research Canada Graduate Doctoral Scholarship. M.-F.H. was supported by an American Diabetes Association Pathways to Stop Diabetes Accelerator Award (1-15-ACE-26). L.J. is supported by the National Key Research and Development Program of China (2016YFC1304901) and Beijing Municipal Science and Technology Commission Funding (Z201100005520013). V.M. has received funds from NIH, National Institute for Health Research, and Indian Government funding agencies, including the Indian Council of Medical Research, Department of Biotechnology, and Department of Science and Technology. L.H.P. is funded by grants from the University of Chicago Diabetes Research Center: P30 DK020595, R01DK104942, and U54DK118612. S.S.R. is funded by the following grants from NIH: U01-DK062418, DP3-DK111906, and R01-DK122586. P.W.F. has been supported by an Irish Research Council award from the Swedish Foundation for Strategic Research and European Research Council award ERC-2015-CoG-681742_NASCENT.
Duality of Interest. V.M. has acted as a consultant and speaker for and has received research or educational grants from Novo Nordisk, MSD, Eli Lilly, AstraZeneca, Novartis, Boehringer Ingelheim, LifeScan J&J, Sanofi, Roche Diagnostics, Abbott, and several Indian pharmaceutical companies, including USV, Dr. Reddy’s Laboratories, and Sun Pharma. P.W.F. has served on advisory panels for Eli Lilly, Sanofi Aventis, Novo Nordisk A/S, and Zoe Ltd., has received honoraria from Lilly, iMedica, Novo Nordisk A/S, and UBS, and has received research funding from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk A/S, Sanofi Aventis, and Servier. As of May 2021, P.W.F. is an employee of the Novo Nordisk Foundation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.J.N., S.S.R., and P.W.F. (together with Dr. William T. Cefalu) conceptualized the overall PMDI. These three, along with L.H.P. and R.H.E., have led the development of the PMDI as its Executive Committee. C.G. represents the ADA on the Executive Committee. All authors were involved in the planning and execution of the PDM2021 conference. J.J.N. wrote the manuscript. All authors contributed to manuscript review and editing. J.J.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16965562.
References
- 1. Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:1617–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearson E. Precision treatment in type 2 diabetes: can we predict response and outcomes to diabetes therapies? PDM2021: a virtual conference. Day 3, workshop 1. Accessed 4 November 2021. Available from https://pdm2021.org/video/day3/workshop1/5.%20Ewan%20Pearson.mp4?ws1&tp52
- 3. Flanagin A, Frey T; AMA Manual of Style Committee . Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 2021;326:621–627 [DOI] [PubMed] [Google Scholar]
- 4. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 5. Zou X, Zhou X, Zhu Z, Ji L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol 2019;7:9–11 [DOI] [PubMed] [Google Scholar]
- 6. Anjana RM, Baskar V, Nair ATN, et al. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: a data-driven cluster analysis: the INSPIRED study. BMJ Open Diabetes Res Care 2020;8:e001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet 2017;389:2252–2260 [DOI] [PubMed] [Google Scholar]
- 8. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2013;13:814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dawed AY, Donnelly L, Tavendale R, et al. CYP2C8 and SLCO1B1 variants and therapeutic response to thiazolidinediones in patients with type 2 diabetes. Diabetes Care 2016;39:1902–1908 [DOI] [PubMed] [Google Scholar]
- 10. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuchman AM. Diabetes: A History of Race and Disease. New York, Yale University Press, 2020 [Google Scholar]
- 12. Tuchman A. Precision diabetes care—a historical perspective on race and disease. PDM2021: a virtual conference. Day 3, plenary session. Accessed 4 November 2021. Available from https://pdm2021.org/video/day3/plenary_session/2.%20Arleen%20Tuch man.mp4?ps3&tp39
- 13. Nilsen P. Making sense of implementation theories, models, and frameworks. Implement Sci 2020;10:53–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA 2016;315:1941–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med 2021;384:2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]