Abstract
OBJECTIVE
There is mounting evidence regarding the cardiovascular benefits of sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RA) among patients with atherosclerotic cardiovascular disease (ASCVD) and type 2 diabetes mellitus (T2DM). There is paucity of data assessing real-world practice patterns for these drug classes. We aimed to assess utilization rates of these drug classes and facility-level variation in their use.
RESEARCH DESIGN AND METHODS
We used the nationwide Veterans Affairs (VA) health care system data set from 1 January 2020 to 31 December 2020 and included patients with established ASCVD and T2DM. Among these patients, we assessed the use of SGLT2i and GLP-1 RA and the facility-level variation in their use. Facility-level variation was computed using median rate ratios (MRR), a measure of likelihood that two random facilities differ in use of SGLT2i and GLP-1 RA in patients with ASCVD and T2DM.
RESULTS
Among 537,980 patients with ASCVD and T2DM across 130 VA facilities, 11.2% of patients received an SGLT2i while 8.0% of patients received a GLP-1 RA. Patients receiving these cardioprotective glucose-lowering drug classes were on average younger and had a higher proportion of non-Hispanic Whites. Overall, median (10th–90th percentile) facility-level rates were 14.92% (9.31–22.50) for SGLT2i and 10.88% (4.44–17.07) for GLP-1 RA. There was significant facility-level variation among SGLT2i use—MRRunadjusted: 1.41 (95% CI 1.35–1.47) and MRRadjusted: 1.55 (95% CI 1.46 –1.63). Similar facility-level variation was observed for use of GLP-1 RA—MRRunadjusted: 1.34 (95% CI 1.29–1.38) and MRRadjusted: 1.78 (95% CI 1.65–1.90).
CONCLUSIONS
Overall utilization rates of SGLT2i and GLP-1 RA among eligible patients are low, with significantly higher residual facility-level variation in the use of these drug classes. Our results suggest opportunities to optimize their use to prevent future adverse cardiovascular events among these patients.
Introduction
Over the last decade, there has been accumulating evidence demonstrating the cardiovascular benefits of various classes of glucose-lowering agents (1). These drug classes not only differ in their glycemic profile but also have a varied impact on cardiovascular disease end points. Moreover, data have suggested differential cardiovascular benefit of such cardioprotective glucose-lowering drug classes among patients with a history of ischemic heart disease (IHD), heart failure (HF), and chronic kidney disease (CKD) (2,3). Across the different classes of glucose-lowering agents, the two predominant classes that have emerged to have shown benefit toward secondary prevention of atherosclerotic cardiovascular disease (ASCVD) are the sodium–glucose cotransporter 2 inhibitors (SGLT2i) and the glucagon-like peptide 1 receptor agonists (GLP-1 RA) (4). Although the exact mechanisms behind the observed cardiovascular benefits associated with these agents remain a topic of ongoing investigation, the hypothesized mechanisms are linked to pathways of blood pressure lowering, effects on vascular tone, anti-inflammatory pathways, cardiometabolic effects, and improvement in hemodynamics as well as overall fluid balance (5–7). Hence, the American Diabetes Association has recommended the use of these drug classes for patients with established ASCVD as part of the glucose-lowering regimen, independent of baseline glycemic control or use of concurrent glucose-lowering medications such as metformin (8).
With the increasing global burden of ASCVD and its associated cardiovascular morbidity and mortality (9), introduction of SGLT2i and GLP-1 RA has provided another tool within the armamentarium of secondary prevention therapies for such patients. Despite encouraging evidence regarding the use of these cardioprotective glucose-lowering drug classes funneling into the cardiovascular community, a degree of therapeutic inertia in adopting these agents into routine clinical practice may be observed. Even though investigators have evaluated practice patterns and use of these drug classes among patients with HF and diabetes (10), assessment of real-world use patterns among patients with ASCVD and diabetes remains scarce. Furthermore, there is a paucity of contemporary data regarding facility-level variation in the use of these cardioprotective glucose-lowering drug classes among patients with ASCVD and diabetes. Therefore, our primary aim was to conduct a cross-sectional nationwide analysis from the Veterans Affairs (VA) health care system to evaluate facility-level variation in use of SGLT2i and GLP-1 RA among patients with established ASCVD and type 2 diabetes mellitus (T2DM).
Research Design and Methods
Cohort Development and Patient Population
We used data from the nationwide VA administrative and clinical data set. Our database comprised veterans aged ≥18 and a diagnosis of ASCVD. Patients with at least one primary care visit between 1 January 2020 and 31 December 2020 at 130 VA facilities and their associated community-based outpatient clinics were included in our cohort. In cases where a patient had multiple primary care visits during the study duration, the last primary care visit in the year 2020 was defined as the index visit and used to anchor our analyses. Patients with ASCVD included patients with a prior history of IHD, peripheral arterial disease (PAD), or ischemic cerebrovascular disease (ICVD). ICD-10-CM diagnosis and procedure codes and Current Procedural Terminology (CPT) codes were used to identify patients with ASCVD (Supplementary Table 1).
The Institutional Review Boards at the Baylor College of Medicine and the Michael E. DeBakey VA Medical Center approved the study protocol. We also obtained a waiver for informed consent.
Among this selected cohort of patients with ASCVD, we further used ICD-10-CM diagnosis codes to identify patients with concomitant T2DM (Supplementary Table 2). At least one inpatient ICD-10-CM diagnosis code or two outpatient diagnosis codes pertaining to T2DM were required to classify patients as having T2DM. Additionally, we used the following clinical parameters to identify patients with a diagnosis of T2DM: hemoglobin (Hb) A1c of ≥6.5%, fasting plasma glucose of ≥126 mg/dL, or random plasma glucose of ≥200 mg/dL or higher, or use of diabetes medications within 2 years prior to the index visit. Patients with limited life expectancy were excluded from our cohort. Limited life expectancy was defined as patients receiving hospice care within the preceding 12 months or those with a history of metastatic cancer diagnosed in the last 5 years (n = 32,106) (11).
Study Outcomes and Measures
Using the VA pharmacy data sets, we ascertained the use of cardioprotective glucose-lowering drug classes within our cohort. Given the primary aim of our analysis, we focused our efforts on assessing the use of SGLT2i and GLP-1 RA. The use of these medications was assessed via an existing prescription for the corresponding medication within 180 days before or up to 100 days after the index primary care provider (PCP) visit. The prescribed SGLT2i included canagliflozin, dapagliflozin, and empagliflozin, and the prescribed GLP-1 RA included exenatide, liraglutide, lixisenatide, semaglutide, and dulaglutide.
Covariates
We evaluated patient-level characteristics, including patient age, sex, race, history of hypertension, and BMI. The estimated glomerular filtration rate (eGFR) (using the Chronic Kidney Disease Epidemiology Collaboration equation) (12) and HbA1c were also assessed for all patients. We assessed the use of aspirin, any statin therapy, high-intensity statin therapy, angiotensin receptor blocker (ARB) or ACE inhibitor therapy. We assessed the number of primary care, cardiology, and endocrinology visits in the 12 months preceding the index PCP visit. Facility- and provider-level variables were also evaluated, including receipt of care via a physician PCP (vs. an advanced practice provider [nurse practitioner or a physician assistant]) and receipt of care at a teaching facility (vs. a nonteaching facility).
Statistical Analysis
We conducted descriptive analyses to assess the distribution of various patient-level, facility-level, and provider-level variables across all patients with ASCVD and concomitant T2DM. We used the χ2 test to analyze categorical variables, and continuous variables were analyzed using an unpaired, two-tailed t test. We used a two-sided P < 0.05 to define statistical significance. We assessed the use of SGLT2i across patients with an eGFR ≥30 mL/min/1.73 m2, whereas the use of GLP-1 RA was assessed across all patients (irrespective of eGFR) with T2DM and ASCVD. Subsequently, we assessed facility-level rates of SGLT2i use and GLP-1 RA use across all eligible VA facilities. Facilities with <25 patients with ASCVD and concomitant T2DM during the study period were excluded from assessment of facility-level utilization rates. Similarly, we assessed Veterans Integrated Services Networks (VISNs)-level rates of SGLT2i use and GLP-1 RA use across all VISNs.
We first assessed median (interquartile range [IQR]) utilization rates of SGLT2i and GLP-1 RA across all eligible facilities. Thereafter, we constructed regression models to quantify facility-level variation among the use of these cardioprotective glucose-lowering drug classes. We first calculated median rate ratios (MRRs), which are well-established measures of facility-level variation (11,13–17). The variation in utilization rates of SGLT2i and GLP-1 RA were computed as unadjusted MRRs. Adjusted MRRs were calculated after adjusting for patient age, sex, race, BMI, HbA1c, eGFR, presence of hypertension, IHD (vs. presence of PAD or ICVD), receipt of care via physician PCP (vs. advanced practice provider), receipt of care at a teaching facility (vs. nonteaching facility), number of PCP visits, and number of cardiology visits in 12 months preceding the index PCP visit (18). Using similar methodology, we assessed variation across the 18 VISNs and computed MRRs to depict VISN-level variation in use of SGLT2i and GLP-1 RA. The resultant adjusted MRRs represented the likelihood of two random facilities or two random VISNs differing in use of SGLT2i and GLP-1 RA among similar eligible patients with ASCVD and concomitant T2DM. Interpretation of the MRR value was such that a value of 1 corresponded to no facility-level or VISN-level variation in use of these cardioprotective glucose-lowering drug classes, whereas a MRR value of 1.6 signified 60% probability of differing in use of these drugs among two hypothetically similar patients with ASCVD and T2DM at two random facilities or across two random VISNs. Based on prior published literature (16), we determined an MRR of ≥1.2 is considered a threshold for significant facility-level variation given that some degree of variation in care is to be expected. All of our analyses were conducted using SAS 9.1.3 software (SAS Institute, Cary, NC) and Stata 14 software (StataCorp, College Station, TX).
Results
Baseline Characteristics
After excluding patients with limited life expectancy (n = 32,106), we identified 1,203,461 patients with ASCVD. Among these patients, 537,980 patients had ASCVD and concomitant T2DM and constituted of our primary cohort. The overall use of glucose-lowering drug classes within our cohort was as follows: insulin, 35.9%; biguanides, 47.2%; sulfonylureas, 21.6%; thiazolidinediones, 2.6%; dipeptidyl peptidase 4 inhibitors, 9.6%; GLP-1 RA, 8.0%; and SGLT2i, 11.2%. A higher use of SGLT2i was noted among patients aged <65 years compared with those aged ≥65 (17.9% vs. 10.7%, P < 0.01). Similarly, patients aged <65 years had a higher use of GLP-1 RA compared with patients ≥65 years of age (11.3% vs. 7.4%, P < 0.01).
When compared with patients with ASCVD and T2DM not receiving SGLT2i (n = 477,488), those receiving SGLT2i (n = 60,492) (Table 1) were on average younger (mean age 69.2 vs. 73.0 years) and had a higher proportion of non-Hispanic Whites (72.8% vs. 70.6%) and a lower proportion of non-Hispanic Blacks (13.0% vs. 15.6%). Overall, patients receiving SGLT2i had a higher prevalence of hypertension (92.9% vs. 90.5%), worse glycemic control (mean HbA1c 8.0% vs. 7.2%), and better renal function (mean eGFR 68.4 vs. 63.0 mL/min/1.73 m2). Overall, prevalence of IHD was higher among patients receiving SGLT2i (85.6% vs. 78.8%), while prevalence of PAD (20.6% vs. 24.6%) and ICVD (23.9% vs. 27.4%) were lower among patients receiving SGLT2i compared with those not receiving SGLT2i. A higher proportion of patients receiving SGLT2i also received other cardioprotective agents, including aspirin (92.3% vs. 91.5%), any statin (93.1% vs. 86.1%), high-intensity statin (67.3% vs. 51.4%), and ACE inhibitor or ARB therapy (73.5% vs. 56.5%). A higher proportion of patients receiving SGLT2i received care at teaching facilities (35.2% vs. 33.9%) and had a higher mean number of PCP visits (9.6 vs. 7.4), cardiology visits (1.07 vs. 0.67), and endocrinology visits (0.42 vs. 0.17) in the 12 months preceding the index PCP visit. All comparisons were observed to be statistically significant with P < 0.01.
Table 1.
Baseline characteristics of patients with ASCVD and concomitant T2DM receiving SGLT2i versus those not receiving SGLT2i
| SGLT2i users | SGLT2i nonusers | ||
|---|---|---|---|
| n = 60,492 (11.2%) | n = 477,488 (88.8%) | P value | |
| Age, mean (SD), years | 69.2 (8.0) | 73.0 (9.0) | <0.01 |
| Male sex, n (%) | 59,278 (98.0) | 468,945 (97.6) | <0.01 |
| Race, n (%) | <0.01 | ||
| Non-Hispanic White | 44,081 (72.8) | 336,925 (70.6) | |
| Non-Hispanic Black | 7,836 (13.0) | 74,688 (15.6) | |
| Others | 8,575 (14.2) | 65,875 (13.8) | |
| Hypertension, n (%) | 56,201 (92.9) | 431,971 (90.5) | <0.01 |
| BMI, mean (SD), kg/m2 | 32.5 (6.1) | 31.1 (6.3) | <0.01 |
| HbA1c, mean (SD), % | 8.0 (1.4) | 7.2 (1.4) | <0.01 |
| IHD, n (%) | 51,751 (85.6) | 376,058 (78.8) | <0.01 |
| PAD, n (%) | 12,485 (20.6) | 117,547 (24.6) | <0.01 |
| ICVD, n (%) | 14,455 (23.9) | 130,924 (27.4) | <0.01 |
| Aspirin, n (%) | 47,234 (92.3) | 344,310 (91.5) | <0.01 |
| Any statin, n (%) | 56,333 (93.1) | 411,301 (86.1) | <0.01 |
| High-intensity statin, n (%) | 40,687 (67.3) | 245,451 (51.4) | <0.01 |
| ACE inhibitor or ARB therapy, n (%) | 44,441 (73.5) | 269,618 (56.5) | <0.01 |
| Physician PCP, n (%) | 46,463 (76.8) | 359,817 (75.4) | <0.01 |
| eGFR, mean (SD), mL/min/1.73 m2 | 68.4 (19.4) | 63.0 (22.8) | <0.01 |
| Receipt of care at a teaching facility, n (%) | 21,290 (35.2) | 161,969 (33.9) | <0.01 |
| Visits in the 12 months prior to the index PCP visit | |||
| PCP visits (primary care), mean (SD), n | 9.6 (6.8) | 7.4 (6.2) | <0.01 |
| Endocrinology visits, mean (SD), n | 0.42 (1.28) | 0.17 (0.85) | <0.01 |
| Cardiology visits, mean (SD), n | 1.07 (2.33) | 0.67 (1.75) | <0.01 |
Patients with ASCVD and T2DM receiving GLP-1 RA (n = 43,118) (Table 2) were on average younger (mean age 69.6 vs. 72.8 years) and had a greater proportion of non-Hispanic Whites (74.0% vs. 70.6%) and a lower proportion of non-Hispanic Blacks (12.5% vs. 15.6%) compared with those not receiving GLP-1 RA (n = 494,862). Patients receiving GLP-1 RA had a higher mean BMI (33.9 vs. 31.0 kg/m2) and worse glycemic control (mean HbA1c 8.0% vs. 7.2%). The prevalence of hypertension (94.0% vs. 90.5%) and IHD (83.4% vs. 79.2%) were higher, while prevalence of PAD (23.7% vs. 24.0%) and ICVD (25.6% vs. 27.2%) were lower among patients receiving GLP-1 RA. A higher proportion of patients receiving GLP-1 RA also received other cardioprotective agents, including any statin (92.7% vs. 86.4%), high-intensity statin (65.5% vs. 52.1%), and ACE inhibitor or ARB therapy (69.9% vs. 57.4%). Compared with patients not receiving GLP-1 RA, those receiving this medication had a higher mean number of PCP visits (11.0 vs. 7.4), cardiology visits (1.0 vs. 0.69), and endocrinology visits (0.63 vs. 0.16) in the 12 months leading up to the index PCP visit. P < 0.01 for all comparisons.
Table 2.
Baseline characteristics of patients with ASCVD and concomitant T2DM receiving GLP-1 RA versus those not receiving GLP-1 RA
| GLP-1 RA users | GLP-1 RA nonusers | ||
|---|---|---|---|
| n = 43,118 (8.0%) | n = 494,862 (92.0%) | P value | |
| Age, mean (SD), years | 69.6 (8.0) | 72.8 (9.0) | <0.01 |
| Male sex, n (%) | 41,681 (96.7) | 483,542 (97.7) | <0.01 |
| Race, n (%) | <0.01 | ||
| Non-Hispanic White | 31,902 (74.0) | 349,104 (70.6) | |
| Non-Hispanic Black | 5,374 (12.5) | 77,450 (15.6) | |
| Others | 5,842 (13.5) | 68,608 (13.8) | |
| Hypertension, n (%) | 40,526 (94.0) | 447,646 (90.5) | <0.01 |
| BMI, mean (SD), kg/m2 | 33.9 (6.5) | 31.0 (6.2) | <0.01 |
| HbA1c, mean (SD), % | 8.0 (1.5) | 7.2 (1.4) | <0.01 |
| IHD, n (%) | 35,960 (83.4) | 391,849 (79.2) | <0.01 |
| PAD, n (%) | 10,219 (23.7) | 119,813 (24.2) | 0.11 |
| ICVD, n (%) | 11,018 (25.6) | 134,361 (27.2) | <0.01 |
| Aspirin, n (%) | 32,878 (91.9) | 358,666 (91.6) | 0.06 |
| Any statin, n (%) | 39,950 (92.7) | 427,684 (86.4) | <0.01 |
| High-intensity statin, n (%) | 28,247 (65.5) | 257,891 (52.1) | <0.01 |
| ACE inhibitor or ARB therapy, n (%) | 30,142 (69.9) | 283,917 (57.4) | <0.01 |
| Physician PCP, n (%) | 32,461 (75.3) | 373,819 (75.5) | 0.24 |
| eGFR, mean (SD), mL/min/1.73 m2 | 62.0 (22.5) | 63.8 (22.5) | <0.01 |
| Receipt of care at a teaching facility, n (%) | 14,950 (34.7) | 168,309 (34.0) | <0.01 |
| Visits in the 12 months prior to index PCP visit | |||
| PCP visits, mean (SD), n | 11.0 (7.6) | 7.4 (6.1) | <0.01 |
| Endocrinology visits, mean (SD), n | 0.63 (1.60) | 0.16 (0.82) | <0.01 |
| Cardiology visits, mean (SD), n | 1.00 (2.26) | 0.69 (1.79) | <0.01 |
Facility-Level Variation
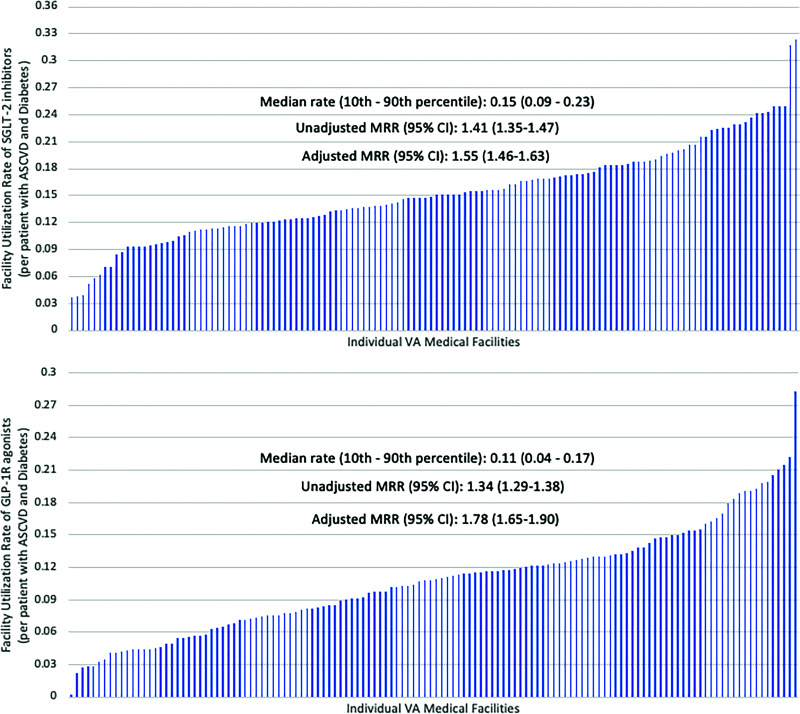
Across all 130 VA medical facilities, the median (10th–90th percentile) utilization rate (Fig. 1) for SGLT2i was 14.92% (9.31–22.50%) among patients with ASCVD and T2DM with an eGFR ≥30 mL/min/1.73 m2. Significant facility-level variation in the rate of SGLT2i use was noted with MRRunadjusted: 1.41 (95% CI 1.35–1.47). After adjusting for various patient-, provider-, and facility-level characteristics, the facility-level variation in use of SGLT2i increased to MRRadjusted: 1.55 (95% CI 1.46–1.63).
Figure 1.
Utilization rates and facility-level variation of SGLT2i and GLP-1 RA.
Similarly, throughout all 130 nationwide VA facilities, the median (10th–90th percentile) prescription rate for GLP-1 RA among patients with ASCVD and concomitant T2DM was 10.88% (4.44–17.07%). We observed a significant facility-level variation in the rate of GLP-1 RA use with MRRunadjusted: 1.34 (95% CI 1.29–1.38), which persisted despite covariate adjustment—MRRadjusted: 1.78 (95% CI 1.65–1.90).
VISN-Level Variation
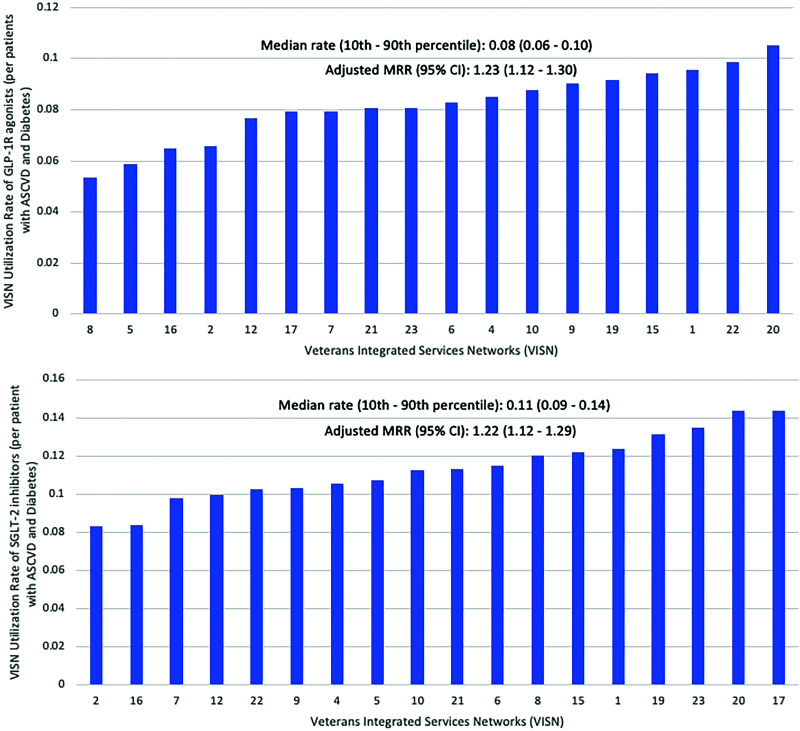
Across all 18 VISNs, the median (10th–90th percentile) utilization rate (Fig. 2) for SGLT2i was 11.3% (9.4–13.8%) among patients with ASCVD and T2DM. Significant VISN-level variation was observed despite adjusting for aforementioned covariates with MRRadjusted: 1.22 (95% CI 1.12–1.29). Similarly, the median (10th–90th percentile) utilization rate for GLP-1 RA across all VISNs was 8.2% (6.3–9.6%), while VISN-level variation was observed to be MRRadjusted: 1.23 (95% CI 1.12–1.30) (Fig. 2).
Figure 2.
Utilization rates and VISN-level variation of GLP-1 RA and SGLT2i.
Conclusions
In our nationwide cohort of patients with ASCVD and concomitant T2DM, we demonstrated an overall low use of SGLT2i and GLP-1 RA along with a significantly high facility-level and VISN-level variation in the use of these drug classes. The utilization rates were 14.9% for SGLT2i and 10.9% for GLP-1 RA. A greater use of these drug classes was observed among younger and White patients with worse glycemic control and more endocrinology, cardiology, and PCP visits. Across nationwide VA facilities, there exists a 55% facility-level and 22% VISN-level variation in SGLT2i use despite adjustment for patient-, provider-, and facility-level covariates. Similarly, we demonstrated a 78% adjusted facility-level variation and 23% adjusted VISN-level variation in use of GLP-1 RA among similar patients with ASCVD and concomitant T2DM receiving care at two random facilities or across two random VISNs in the VA health care system.
Therapeutic inertia within the realm of CVD prevention is a well-established barrier to widespread and consistent implementation of novel pharmacotherapeutic advances (19). Similar has been the case with cardioprotective glucose-lowering drug classes such as SGLT2i and GLP-1 RA. Although data supporting the use of these drugs in reducing cardiovascular death and major adverse cardiovascular events (MACE) have been established (20–23), these drug classes remain underused and infrequently prescribed by primary care clinicians and specialists (24). Therapeutic inertia can sometimes be attributed to the recent introduction of novel drug classes and lack of real-world experience, but our results provide concerning evidence regarding persistently low use of these drug classes even several years since their approval by the U.S. Food and Drug Administration.
Another plausible reason for low utilization rates of SGLT2i and GLP-1 RA may be secondary to the phenomenon of attribution given the scope of drug prescribing ranging across various medical specialties, including nephrology, primary care, cardiology, or endocrinology (25). Thereby, the initiation of these drugs may be delayed due to deferment to other medical specialties such as endocrinology subspecialists. This was demonstrated in an observational analysis by Vaduganathan et al. (24), whereby most SGLT2i were prescribed by endocrinologists and PCPs while only ∼5% were prescribed by cardiologists. Furthermore, patients themselves may not be familiar with the concept or data behind the use of these glucose-lowering drug classes to prevent future adverse cardiovascular events. Therefore, patients with optimally controlled diabetes or those receiving insulin therapy may express hesitancy in starting these cardioprotective glucose-lowering drug classes, thereby contributing to their lower use in patients likely to benefit from them.
Our results highlight high levels of nationwide facility-level and VISN-level variation in the use of SGLT2i and GLP-1 RA among similar patients with ASCVD and T2DM. We demonstrated facility-level variation to be in the magnitude of 55%–70% while VISN-level variation was ∼20% for the use of these drug classes. Moreover, this level of variability persisted despite adjusting for patient-, facility-, and provider-level variables. There are several plausible reasons behind this observation. Providers’ familiarity and clinical comfort with indications, net clinical benefit, and associated adverse effects of these pharmacotherapies may create a level of variability in prescribing patterns at an individual clinician level. Given the relative recent introduction of these cardioprotective glucose-lowering drug classes, prescribing these agents may require having a thorough understanding of the Criteria for Use (CFU) and accordingly submitting a prior-authorization drug request (PADR), if needed. CFU are developed by the U.S. Department of Veterans Affairs and outline how and for which patient population certain drugs may be used. Similar to private health insurance entities, PADR refers to the process that allows the VA pharmacy staff to review the necessity and indication for the requested nonformulary medication prior to its approval. These additional steps in prescribing these drug classes may also deter initiation as well as introduce a greater degree of variation in prescribing patterns. The variability in prescribing patterns may be further amplified by institutional culture and practice cultures of the individual providers.
Our results demonstrate that patients receiving care at teaching hospitals had significantly higher rates of SGLT2i and GLP-1 RA use. Although our analysis were adjusted for this variable, residual confounding may be plausible given the observational nature of our study and thus impact the overall variation because teaching hospitals may be more inclined to widespread dissemination and earlier adoption of guideline-concordant glucose-lowering therapy. As with other primary and secondary cardiovascular prevention therapies (26,27), the responsibility entails cultivating a patient-provider relationship, environment of mutual trust, more inclusive formulary choices available to clinicians, and clinician motivation to inculcate these therapies and practices within the therapeutic regimen to prevent future MACE. Variability in such patient-provider relationships may have downstream effects on initiation or continuation of these cardioprotective glucose-lowering drug classes, thereby introducing the level of facility-level variation as observed in our analysis. Additionally, the residual level of facility-level variation, despite adjustment of various covariates, suggests a substantial proportion of variation residing at the level of individual providers, which may be difficult to mitigate. The baseline low rates of nationwide use may also statistically lend themselves to a greater degree of overall variation.
Finally, the significantly lower degree of variation across VISNs compared with across individual facilities may again suggest individual facility-level factors that contribute to the observed variation. Although facilities within the VA health care system may have similar management structure, at the individual facility level, factors such as processes for reviewing nonformulary requests, formulary versus nonformulary medications, level of pharmacy support, nuances within set CFU, and processing of PADR may all introduce variability, as observed in our analysis.
Our analysis provides contemporary insights from the nationwide VA health care system with regards to utilization rates and facility-level variation of SGLT2i and GLP-1 RA among patients with established ASCVD and T2DM. The VA health care system represents one of the largest health care systems in the U.S., providing health care services to >9 million veterans each year (28). Among the patient population within the VA health care system, the prevalence of T2DM has been estimated to be ∼13.3% (1.2 million veterans) (29), which is similar to the nationwide prevalence noted in the non-VA health care sectors (∼10.5%) (30). On the basis of prior analyses evaluating patients with T2DM, the prevalence of ASCVD has been observed to be 21–30% both within the VA and the non-VA health care systems (13,31,32), thereby suggesting a similar level of ASCVD burden within patients with T2DM, irrespective of the VA or non-VA health care system. Our findings of overall low utilization rates (∼10–15%) of SGLT2i and GLP-1 RA are similar to what has been reported by prior investigator groups. Using the Anthem pharmacy and medical claims database, Nelson et al. (33) reported the use of SGLT2i or GLP-1 RA to be 9.9%, while Arnold et al. (34) reported the use of SGLT2i and GLP-1 RA to be 9.0% and 7.9%, respectively, based on their U.S.-based registry. The subtle differences in utilization rates (SGLT2i: 14.9% in our analysis compared with ∼9% in prior analyses; GLP-1 RA: 10.9% in our analysis compared with ∼8% in the analysis by Arnold et al. [34]) may be secondary to the studied cohort years (the 2020 cohort year in our analysis compared with the 2018 cohort year in prior analyses). Although different patient samples, the slight increase between 2018 (prior analyses) and 2020 (our analysis) cohorts may represent an overall slow but steady dissemination as well as uptake of these therapies within patients with T2DM and ASCVD. Finally, it is noteworthy that other secondary prevention measures among this patient population have also experienced, albeit to a lesser extent, suboptimal utilization rates and facility-level variation. For example, among patients with T2DM and ASCVD, an overall utilization rate and facility-level variation for high-intensity statin therapy has been observed to be ∼ 60% and ∼30%, respectively (13), while optimal blood pressure control has been observed in ∼50% of patients with a facility-level variation of ∼25% (35).
Our study has several limitations. The inherent nature of the clinical data sets meant we were unable to reliably assess and adjust for additional variables such as doses of respective cardioprotective glucose-lowering drug classes. Optimal control of other cardiometabolic risk factors, such as hypertension, could not be ascertained from our data set, which may influence clinicians to focus their efforts in optimizing these risk factors prior to introducing SGLT2i or GLP-1 RA for cardiovascular prevention. Similarly, we were unable to assess for overall pill burden for patients included in our cohort, which may also influence the decision to initiate therapy. Intolerances and adverse effects to these therapies were not assessed in our cohort, which may have also contributed to the observed utilization rates and facility-level variation. Given the evolving nature of this field and changes in the American Diabetes Association guidance between 2020 and 2021 with regards to eligibility of patients with optimally controlled HbA1c on metformin therapy, it is plausible that patients within our 2020 cohort may not have met the standard of care use criteria, thereby decreasing utilization rates. Given the nature of our data set, we were unable to account for patient refusal resulting in lower use of these therapies. Finally, owing to our cohort of predominately elderly White men, the generalizability of our findings to other racial minorities and the nonveteran population may be limited.
Our findings serve as a benchmark for future research, guideline work, and implementation efforts in the area of cardioprotective glucose-lowering drug classes for secondary prevention of ASCVD. It is noteworthy that compared with recent outcomes trials whereby patients with high-risk features for ASCVD were included, our analysis consisted of all patients with T2DM and established ASCVD, thereby signifying a higher-risk population that may derive higher benefit compared with patients with diabetes and high-risk features. Research initiatives, especially qualitative investigations, are needed to clearly ascertain reasons behind such variation in practice patterns. As clinical data demonstrating cardiovascular benefit with the use of glucose-lowering drug classes (36) continue to accumulate, a paradigm shift is needed to redirect clinical focus on approaching these drugs not only as glucose-lowering agents but also as therapies for cardiovascular disease prevention. A paradigm shift in the hierarchy of glucose-lowering drug classes is needed. In addition to focusing on glucose-lowering properties, first-line therapies for patients with T2DM and established ASCVD should include drug classes such as GLP-1 RA and SGLT2i which supplement cardiovascular prevention in addition to their antiglycemic effects. Lastly, policy driven initiatives on a national level as well as focused efforts by guideline writing committees are necessary for further promoting these drug classes as therapies for cardiovascular prevention thereby increasing its utilization and narrowing the current level of facility-level variation.
Conclusion
In recent years, SGLT2i and GLP-1 RA have emerged as a cornerstone preventative therapy for patients with ASCVD and concomitant T2DM. In our contemporary analysis from a large nationwide VA cohort, we observed an alarmingly low use of these secondary cardiovascular prevention therapies. Moreover, there was a significant residual facility-level variation in the use of these therapies despite adjusting for patient-, provider-, and facility-level covariates. Our findings serve as foundational evidence for future research initiatives in this arena as well as implementation efforts to increase utilization rates of these drug classes while simultaneously lowering the observed variation in care in this high-risk patient population.
Article Information
Funding. C.M.B. has received grant/research support from National Institutes of Health, American Heart Association, and American Diabetes Association. This work was supported by a Department of Veterans Affairs (VA) Health Services Research & Development Service Investigator Initiated Grants (IIR 16-072, IIR 19-069), the Houston VA Health Services Research & Development Center for Innovations grant (CIN13-413), and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK110341). Support for VA/Centers for Medicare & Medicaid Services data was provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Duality of Interest. S.S.V. discloses honorarium from the American College of Cardiology (associate editor for Innovations, acc.org). C.J.L. has served as a consultant and promotional speaker for AstraZeneca of their SGLT2i. C.M.B. discloses grant/research support (all significant, paid to institution, not individual) from Akcea, Amgen, Esperion, Novartis, Regeneron, and Sanofi-Synthelabo, and as a consultant for Abbott Diagnostics, Akcea, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Intercept, Matinas BioPharma, Merck, Novartis, Regeneron, and Sanofi-Synthelabo. Y.B. has received a research grant from AstraZeneca. S.N. has received honorarium from Bayer, Boehringer Ingelheim, Tricida, and Reata Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M. and S.S.V. contributed to conception, design, and interpretation of data, drafting and revising of the manuscript, and final approval of the manuscript submitted. D.J.R. and L.C. contributed to analysis and interpretation of data and final approval of the manuscript submitted. M.T.L., M.A.R., J.M.A., E.M.V., M.E.M., K.R.d.E.S., S.D.N., C.J.L., Y.B., C.M.B., and L.A.P. contributed to interpretation of data, revising of manuscript, and final approval of the manuscript. S.S.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs or the U.S. Government.
This article contains supplementary material online at https://doi.org/10.2337/figshare.17052728.
References
- 1. Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 2020;173:278–286 [DOI] [PubMed] [Google Scholar]
- 2. Zhu J, Yu X, Zheng Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol 2020;8:192–205 [DOI] [PubMed] [Google Scholar]
- 3. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 2021;398:262–276 [DOI] [PubMed] [Google Scholar]
- 5. Lee MMY, Petrie MC, McMurray JJV, Sattar N. How do SGLT2 (sodium-glucose cotransporter 2) inhibitors and GLP-1 (glucagon-like peptide-1) receptor agonists reduce cardiovascular outcomes? Completed and ongoing mechanistic trials. Arterioscler Thromb Vasc Biol 2020;40:506–522 [DOI] [PubMed] [Google Scholar]
- 6. Heuvelman VD, Van Raalte DH, Smits MM. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovasc Res 2020;116:916–930 [DOI] [PubMed] [Google Scholar]
- 7. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020;17:761–772 [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S111–S124 [DOI] [PubMed] [Google Scholar]
- 9. Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015;201(Suppl. 1):S1–S7 [DOI] [PubMed] [Google Scholar]
- 10. Dhillon P, Grover H, Haq S, et al. Current utilization trends of SGLT-2 inhibitors in type 2 diabetics with heart failure. J Endocr Soc 2021;5(Suppl. 1):A408–A409 [Google Scholar]
- 11. Woodard LD, Landrum CR, Urech TH, Profit J, Virani SS, Petersen LA. Treating chronically ill people with diabetes mellitus with limited life expectancy: implications for performance measurement. J Am Geriatr Soc 2012;60:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsushita K, Mahmoodi BK, Woodward M, et al. Chronic Kidney Disease Prognosis Consortium . Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pokharel Y, Akeroyd JM, Ramsey DJ, et al. Statin use and its facility-level variation in patients with diabetes: insight from the Veterans Affairs National Database. Clin Cardiol 2016;39:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pokharel Y, Gosch K, Nambi V, et al. Practice-level variation in statin use among patients with diabetes: insights from the PINNACLE registry. J Am Coll Cardiol 2016;68:1368–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol 2015;65:111–121 [DOI] [PubMed] [Google Scholar]
- 16. Hira RS, Kennedy K, Jneid H, et al. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol 2014;63:2876–2877 [DOI] [PubMed] [Google Scholar]
- 17. Shah NR, Ahmed ST, Winchester DE, et al. Facility-level variation in stress test utilization in veterans with ischemic heart disease. JACC Cardiovasc Imaging 2019;12:1292–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein H. Multilevel Statistical Models. Hoboken, John Wiley & Sons, 2011 [Google Scholar]
- 19. Dixon DL, Sharma G, Sandesara PB, et al. Therapeutic inertia in cardiovascular disease prevention: time to move the bar. J Am Coll Cardiol 2019;74:1728–1731 [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 21. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 22. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: systematic review and meta-analysis of cardiovascular outcomes trials. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 23. Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–3372 [DOI] [PubMed] [Google Scholar]
- 25. Gulsin GS, Graham-Brown MPM, Davies MJ, McCann GP. Emerging glucose-lowering therapies: a guide for cardiologists. Heart 2020;106:18–23 [DOI] [PubMed] [Google Scholar]
- 26. Fürthauer J, Flamm M, Sönnichsen A. Patient and physician related factors of adherence to evidence based guidelines in diabetes mellitus type 2, cardiovascular disease and prevention: a cross sectional study. BMC Fam Pract 2013;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrovic K, Blank TO. The Andersen–Newman Behavioral Model of Health Service Use as a conceptual basis for understanding patient behavior within the patient–physician dyad: the influence of trust on adherence to statins in older people living with HIV and cardiovascular disease. Cogent Psychol 2015;2:1038894 [Google Scholar]
- 28. U.S. Government Accountability Office . 2015a. High-risk series: An update report to Congressional committees. Managing risks and improving VA health care. Accessed 22 October 2021. Available from https://www.gao.gov/assets/gao-15-290.pdf
- 29. Mahtta D, Ahmed ST, Shah NR, et al. Facility-level variation in cardiac stress test use among patients with diabetes: findings from the Veterans Affairs National Database. Diabetes Care 2020;43:e58–e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 31. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rungby J, Schou M, Warrer P, Ytte L, Andersen GS. Prevalence of cardiovascular disease and evaluation of standard of care in type 2 diabetes: a nationwide study in primary care. Cardiovasc Endocrinol 2017;6:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson AJ, Ardissino M, Haynes K, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc 2021;10:e016835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnold SV, de Lemos JA, Rosenson RS, et al.; GOULD Investigators . Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease: insights from Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD). Circulation 2019;140:618–620 [DOI] [PubMed] [Google Scholar]
- 35. Rehman H, Akeroyd JM, Ramsey D, et al. Facility-level variation in diabetes and blood pressure control in patients with diabetes: findings from the Veterans Affairs national database. Clin Cardiol 2017;40:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z. Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern Med 2021;181:1043–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]