Abstract
OBJECTIVE
To describe temporal trends and correlates of glycemic control in youth and young adults (YYA) with youth-onset diabetes.
RESEARCH DESIGN AND METHODS
The study included 6,369 participants with type 1 or type 2 diabetes from the SEARCH for Diabetes in Youth study. Participant visit data were categorized into time periods of 2002–2007, 2008–2013, and 2014–2019, diabetes durations of 1–4, 5–9, and ≥10 years, and age groups of 1–9, 10–14, 15–19, 20–24, and ≥25 years. Participants contributed one randomly selected data point to each duration and age group per time period. Multivariable regression models were used to test differences in hemoglobin A1c (HbA1c) over time by diabetes type. Models were adjusted for site, age, sex, race/ethnicity, household income, health insurance status, insulin regimen, and diabetes duration, overall and stratified for each diabetes duration and age group.
RESULTS
Adjusted mean HbA1c for the 2014–2019 cohort of YYA with type 1 diabetes was 8.8 ± 0.04%. YYA with type 1 diabetes in the 10–14-, 15–19-, and 20–24-year-old age groups from the 2014–2019 cohort had worse glycemic control than the 2002–2007 cohort. Race/ethnicity, household income, and treatment regimen predicted differences in glycemic control in participants with type 1 diabetes from the 2014–2019 cohort. Adjusted mean HbA1c was 8.6 ± 0.12% for 2014–2019 YYA with type 2 diabetes. Participants aged ≥25 years with type 2 diabetes had worse glycemic control relative to the 2008–2013 cohort. Only treatment regimen was associated with differences in glycemic control in participants with type 2 diabetes.
CONCLUSIONS
Despite advances in diabetes technologies, medications, and dissemination of more aggressive glycemic targets, many current YYA are less likely to achieve desired glycemic control relative to earlier cohorts.
Introduction
Optimal glycemic control is the aim of diabetes care. Clinical trials in both type 1 and type 2 diabetes have established the link between glycemic control and the risk for development of diabetes complications (1,2). The SEARCH for Diabetes in Youth (SEARCH) study first reported its cross-sectional analysis evaluating glycemic control in youth with diabetes in 2009, at a time when limited data were available on glycemic control in large populations in the U.S. (3). This initial work highlighted that a substantial percentage of youth with type 1 and type 2 diabetes had poor glycemic control (HbA1c ≥9.5%). SEARCH also helped shed light on disparities in glycemic outcomes in youth, as racial/ethnic minorities are more likely to have higher HbA1c levels compared with non-Hispanic White youth irrespective of the type of diabetes (4).
Since the 2009 SEARCH publication, the landscape of diabetes management has changed dramatically. Most notably, diabetes technology has rapidly evolved, with new technologies being developed and improved every year. The use of continuous subcutaneous insulin infusion and continuous glucose monitoring (CGM) systems in the U.S. has increased, especially among youth with type 1 diabetes (5,6). In addition, to reflect new evidence regarding the risks and benefits of tight glycemic control in children and adolescents with diabetes, recent national and international recommendations endorse lower HbA1c targets, resulting in providers prescribing more intensive diabetes management for all pediatric age groups (7,8).
The T1D Exchange Registry and the Pediatric Diabetes Consortium are two large registry studies in the U.S. that have reported on glycemic control among youth with type 1 diabetes and type 2 diabetes, respectively (9,10). While both have helped to describe the recent state of treatment of youth with diabetes in the U.S., the T1D Exchange Registry and the Pediatric Diabetes Consortium have only been able to comment on glycemic trends over the past decade. Beginning in 2002, SEARCH has recruited a series of incident racially/ethnically and socioeconomically diverse youth cohorts with both type 1 and type 2 diabetes who are well-characterized through a variety of surveys and physical and laboratory assessments soon after diagnosis and have been followed longitudinally. Given the availability of population-based SEARCH longitudinal data related to glycemic control to evaluate the impact of the changing landscape of diabetes management, the study objective was to describe temporal trends in glycemic control by age and diabetes duration in youth-onset diabetes, beginning 1 year after diagnosis. In addition, we sought to identify correlates of glycemic control among youth with type 1 and type 2 diabetes in the 2014–2019 SEARCH cohort of youth and young adults (YYA) to identify groups of patients who may benefit from targeted interventions to improve metabolic control.
Research Design and Methods
Study Population
SEARCH is a population-based registry network that includes five centers located in California, Colorado, Ohio, South Carolina, and Washington. Children and adolescents with diabetes diagnosed before 20 years of age were identified from ongoing surveillance of networks. Comprehensive details pertaining to the recruitment and study visit components of the SEARCH study have previously been published (11). In the first two phases of SEARCH (SEARCH 1 and 2), individuals newly diagnosed with diabetes in 2002–2006 and 2008 were contacted and recruited for a baseline research visit. Incident cases from 2002–2005 were also asked to return for visits at 12, 24, and 60 months after their baseline visit to measure risk factors for diabetes complications. Also, in the first phase of the study (SEARCH 1), prevalent cases of diabetes, diagnosed prior to 2002, were invited for a single visit. In the third phase (SEARCH 3), a subset of SEARCH participants with a duration of diabetes >5 years were recruited for an outcome visit between 2011 and 2015, for whom a single assessment of diabetes-related data collection was completed. In the fourth phase (SEARCH 4), all SEARCH participants aged >10 years were operationally split into a group invited to another study visit between 2015 and 2019 and those who were only invited to complete surveys. Those invited to the in-person research visit included all individuals with type 2 diabetes, all non-Whites, and a random sample of non-Hispanic Whites with type 1 diabetes. Since SEARCH is a population-based study, the study site that recruited the participant was often not the clinical location where participants received their diabetes care.
Research visits included questionnaire administration along with collection of anthropometric measurements and a blood sample. HbA1c levels were measured from blood samples obtained at a research study visit. Measurement of HbA1c was performed at the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, which was the central laboratory for the study. Measurements were performed using an automated nonporous ion exchange high-performance liquid chromatography system (Tosoh Bioscience, Montgomeryville, PA). This method has demonstrated to be linear from a total area of 500 to >4,500, indicating that the results are accurate within a large range of number of red cells. If the total area is <500, results are not reported; if the total area is >4,500, the analysis is repeated after sample dilution. A set of quality control samples with low and high levels of HbA1c was analyzed several times per day to monitor the assay performance, and the between-assay coefficients of variation on the two controls were consistently <1.0% and <0.7%, respectively. Aliquots of six whole blood pools prepared in the laboratory with values of 5.0%, 6.0%, 7.0%, 7.5%, 8.0%, and 9.0% stored under liquid nitrogen and analyzed each month for several days to monitor the longitudinal stability of the assay.
SEARCH participants with a diabetes duration >1 year at a study visit were included in the study sample. Diabetes type was based on provider diagnosis within 6 months of diagnosis. Participants with a type 1 diabetes provider diagnosis who were not on insulin were excluded. For all participants, the parent, adolescent or young adult, or both provided consent or assent. The institutional review boards for all sites approved the study protocol.
Statistical Analyses
SEARCH study visits were conducted from 2002 through 2019. All participants with an eligible visit are included in the analytic data set at least once. Participants could have had up to six visits over that time period. A sample of visits for this analysis was randomly selected from the SEARCH data in such a way as to prioritize inclusion in diabetes duration groups (1–4 years, 5–9 years, and ≥10 years) and time periods (2002–2007, 2008–2013, and 2014–2019; the 2002–2007 cohort represents the cohort reported in the initial 2009 SEARCH publication) while ensuring that no participant would have multiple visits within a duration group or time period (Supplementary Fig. 1). By doing this, independence assumptions of statistical methods and analysis stratified by diabetes duration or time period would not be violated due to multiple records per participant. A sensitivity analysis was conducted to verify that the analytic data set was consistent with other randomly drawn samples using the same criteria.
All analyses were stratified by diabetes type. Participant characteristics were summarized using frequencies and percentages for categorical variables or means and SDs for continuous measures. Unadjusted linear regression models stratified by duration group were used to evaluate differences in HbA1c across time periods. Multivariable linear regression models, stratified by duration group, were then used to test differences in HbA1c over time after adjustment for clinical site, age, sex, race/ethnicity, household income, health insurance status, insulin regimen, and disease duration. Repeated-measures linear models were not stratified by diabetes duration (“overall” in tables) to account for the multiple visits (over the three time periods) per participant. Plots were created to investigate the association of age group and time period with HbA1c using results of the fully adjusted stratified multivariable linear models. Multivariable linear models, stratified by diabetes type and adjusted for all covariates, were used to investigate the associations of HbA1c with participant characteristics during the last time period (2014–2019). All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Longitudinal analysis included 6,369 (n = 5,482 type 1 and n = 887 type 2) SEARCH participants. Descriptive characteristics of the study sample are shown in Table 1. While the sex of participants across the three time periods was fairly similar irrespective of diabetes type, the 2014–2019 sample of YYA with type 1 diabetes is comprised of a higher percentage of non-Hispanic Black and Hispanic participants as compared with earlier cohorts due to the SEARCH 4 sampling strategy. Age and average duration of diabetes increased among participants with both type 1 and type 2 diabetes across successive SEARCH cohorts. There was also an increase in insulin pump use and CGM use in the more recent cohorts of participants with type 1 diabetes.
Table 1.
Characteristics of YYA who participated in SEARCH study visits by study period and diabetes type
| Type 1 | Type 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| 2002–2007 (N = 3,398) | 2008–2013 (N = 2,184) | 2014–2019 (N = 1,742) | P value | 2002–2007 (N = 379) | 2008–2013 (N = 327) | 2014–2019 (N = 519) | P value | |
| HbA1c (%) | 8.5 (1.5) | 8.9 (1.8) | 9.1 (2.0) | <0.0001 | 8.4 (2.8) | 8.3 (2.8) | 8.9 (2.9) | 0.0034 |
| Diabetes duration, years (SD) | 4.6 (3.6) | 5.8 (2.8) | 8.3 (4.4) | <0.0001 | 2.9 (1.6) | 5.3 (3.1) | 7.0 (4.5) | <0.0001 |
| Insulin regimen (%) | <0.0001 | 0.0002 | ||||||
| Not on insulin | 0 | 0 | 0 | 222 (58.6) | 176 (53.8) | 275 (53.0) | ||
| Insulin pump | 845 (24.9) | 976 (44.7) | 856 (49.1) | 12 (3.2) | 25 (7.6) | 31 (6.0) | ||
| Basal-bolus injections | 1,000 (29.4) | 880 (40.3) | 781 (44.8) | 44 (11.6) | 66 (20.2) | 104 (20.0) | ||
| Other insulin regimens | 1,544 (45.4) | 261 (12.0) | 77 (4.4) | 95 (25.1) | 51 (15.6) | 92 (17.7) | ||
| Unknown | 9 (0.3) | 67 (3.1) | 28 (1.6) | 6 (1.6) | 8 (2.4) | 17 (3.3) | ||
| Medication regimen (%) | 0.3678 | <0.0001 | ||||||
| Insulin only | 3,310 (97.4) | 2,120 (97.1) | 1,685 (96.7) | 63 (16.6) | 61 (18.7) | 119 (22.9) | ||
| Insulin plus oral agent | 88 (2.6) | 64 (2.9) | 57 (3.3) | 94 (24.8) | 90 (27.5) | 122 (23.5) | ||
| Metformin only | 0 | 0 | 0 | 120 (31.7) | 98 (30.0) | 125 (24.1) | ||
| Other oral agent | 0 | 0 | 0 | 58 (15.3) | 27 (8.3) | 26 (5.0) | ||
| None | 0 | 0 | 0 | 44 (11.6) | 51 (15.6) | 117 (22.5) | ||
| Unknown | 0 | 0 | 0 | 0 | 0 | 10 (1.9) | ||
| Blood glucose monitoring frequency (%) | <0.0001 | <0.0001 | ||||||
| Less than once a day | 106 (3.1) | 496 (22.7) | 276 (15.8) | 122 (32.2) | 52 (15.9) | 132 (25.4) | ||
| 1–3 times/day | 646 (19.0) | 474 (21.7) | 304 (17.5) | 169 (44.6) | 95 (29.1) | 95 (18.3) | ||
| ≥4 times/day | 2,506 (73.7) | 674 (30.9) | 341 (19.6) | 64 (16.9) | 63 (19.3) | 55 (10.6) | ||
| CGM* | 0 | 165 (7.6) | 410 (23.5) | 0 | 28 (8.6) | 44 (8.5) | ||
| Unknown | 140 (4.1) | 375 (17.2) | 411 (23.6) | 24 (6.3) | 89 (27.2) | 193 (37.2) | ||
| Sex (%) | 0.5429 | 0.5918 | ||||||
| Female | 1,680 (49.4) | 1,073 (49.1) | 885 (50.8) | 235 (62.0) | 205 (62.7) | 338 (65.1) | ||
| Male | 1,718 (50.6) | 1,111 (50.9) | 857 (49.2) | 144 (38.0) | 122 (37.3) | 181 (34.9) | ||
| Race/ethnicity (%) | <0.0001 | 0.0058 | ||||||
| Non-Hispanic White | 2,622 (77.2) | 1,649 (75.5) | 1,028 (59.0) | 70 (18.5) | 60 (18.3) | 95 (18.3) | ||
| Non-Hispanic Black | 240 (7.1) | 180 (8.2) | 224 (12.9) | 145 (38.3) | 128 (39.1) | 216 (41.6) | ||
| Hispanic | 369 (10.9) | 245 (11.2) | 363 (20.8) | 88 (23.2) | 86 (26.3) | 145 (27.9) | ||
| Native American | 19 (0.6) | 10 (0.5) | 10 (0.6) | 57 (15.0) | 29 (8.9) | 33 (6.4) | ||
| Other/unknown/multiple | 148 (4.4) | 100 (4.6) | 117 (6.7) | 19 (5.0) | 24 (7.3) | 30 (5.8) | ||
| Age, years (SD) | 12.9 (4.4) | 15.1 (5.0) | 18.0 (5.7) | <0.0001 | 17.1 (2.8) | 19.5 (4.3) | 21.4 (5.0) | <0.0001 |
| BMI z-score (SD) | 0.6 (0.9) | 0.6 (0.9) | 0.6 (1.0) | 0.0758 | 1.9 (0.8) | 1.9 (0.7) | 1.9 (0.8) | 0.5024 |
| Household income (%) | <0.0001 | 0.0002 | ||||||
| <$25,000 | 423 (12.4) | 312 (14.3) | 278 (16.0) | 145 (38.3) | 132 (40.4) | 175 (33.7) | ||
| $25,000–49,999 | 695 (20.5) | 357 (16.3) | 306 (17.6) | 83 (21.9) | 54 (16.5) | 92 (17.7) | ||
| $50,000–74,999 | 695 (20.5) | 362 (16.6) | 231 (13.3) | 42 (11.1) | 28 (8.6) | 33 (6.4) | ||
| >$75,000 | 1,335 (39.3) | 863 (39.5) | 555 (31.9) | 31 (8.2) | 30 (9.2) | 38 (7.3) | ||
| Do not know/refused | 250 (7.4) | 290 (13.3) | 372 (21.4) | 78 (20.6) | 83 (25.4) | 181 (34.9) | ||
| Health insurance (%) | <0.0001 | 0.0002 | ||||||
| None | 43 (1.3) | 60 (2.7) | 49 (2.8) | 18 (4.7) | 47 (14.4) | 53 (10.2) | ||
| Other | 54 (1.6) | 70 (3.2) | 88 (5.1) | 32 (8.4) | 21 (6.4) | 37 (7.1) | ||
| Medicaid/Medicare | 568 (16.7) | 431 (19.7) | 425 (24.4) | 144 (38.0) | 131 (40.1) | 229 (44.1) | ||
| Private | 2,733 (80.4) | 1,623 (74.3) | 1,180 (67.7) | 185 (48.8) | 128 (39.1) | 200 (38.5) | ||
Not collected prior to 2011 and at baseline visits in 2012 and 2016.
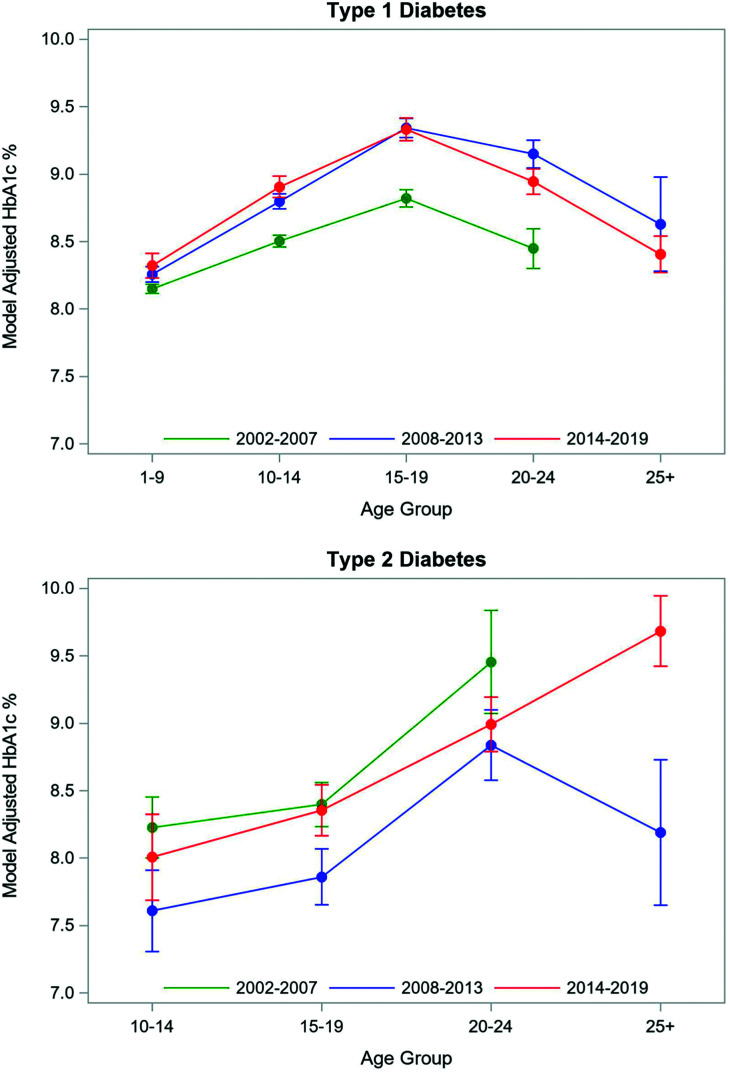
The estimated average adjusted HbA1c for the 2014–2019 cohort of YYA with type 1 diabetes was 8.8 ± 0.04% (72 mmol/mol) (Table 2). There was a statistically significant difference in HbA1c among the three cohorts, with the average adjusted HbA1c for the 2014–2019 cohort with type 1 diabetes being 0.3% higher than the mean HbA1c for the 2002–2007 cohort (8.5 ± 0.03% [70 mmol/mol]). When examined by diabetes duration, YYA with type 1 diabetes and a diabetes duration of 5–9 years exhibited a temporal trend of worse glycemic control in recent years (2002–2007: 8.6% [70 mmol/mol] vs. 2008–2013: 9.1% [76 mmol/mol] vs. 2014–2019: 9.2% [77 mmol/mol]). There was also a statistically significant increase in HbA1c among the 10–14-, 15–19-, and 20–24-year-old age groups of YYA with type 1 diabetes when comparing mean HbA1c in 2014–2019 to 2002–2007 (Fig. 1A and Supplementary Table 1). In 2014–2019, SEARCH participants had relatively comparable glycemic control compared with similarly aged YYA with type 1 diabetes in 2008–2013, except for the 20–24-year-old age group, which had a statistically significant lower adjusted HbA1c (8.7% [72 mmol/mol]) in 2014–2019 compared with 2008–2013 (8.9% [74 mmol/mol]).
Table 2.
Model adjusted* mean HbA1c stratified by diabetes duration
| HbA1c (%) | P value | |||
|---|---|---|---|---|
| 2002–2007 | 2008–2013 | 2014–2019 | ||
| Type 1 | ||||
| Overall | 3,398: 8.5 (0.03) | 2,184: 8.9 (0.03) | 1,742: 8.8 (0.04) | <0.0001 |
| 1–4 years | 2,288: 8.5 (0.04) | 684: 8.6 (0.06) | 380: 8.7 (0.09) | 0.0444 |
| 5–9 years | 765: 8.6 (0.07) | 1,320: 9.1 (0.05) | 670: 9.2 (0.07) | <0.0001 |
| ≥10 years | 345: 8.6 (0.13) | 180: 9.3 (0.13) | 692: 9.0 (0.08) | 0.0005 |
| Type 2 | ||||
| Overall | 379: 8.7 (0.14) | 327: 8.3 (0.13) | 519: 8.6 (0.12) | 0.0330 |
| 1–4 years | 336: 8.4 (0.14) | 148: 7.9 (0.19) | 190: 8.2 (0.19) | 0.1349 |
| 5–9 years | 43: 9.6 (0.44) | 154: 8.8 (0.21) | 167: 9.2 (0.20) | 0.2600 |
| ≥10 years | — | 25: 8.4 (0.60) | 162: 10.1 (0.35) | 0.0140 |
Data are n: least squares means (SE).
Adjusted for age, clinical site, disease duration, health insurance status, household income, insulin regimen, race/ethnicity, and sex.
Figure 1.
Model adjusted for age, clinical site, disease duration, health insurance status, household income, insulin regimen, race/ethnicity, and sex. Mean HbA1c by age group across study periods.
In the multivariate analysis of participants with type 1 diabetes from the 2014–2019 cohort, glycemic control (i.e., HbA1c) was significantly associated with race/ethnicity, age, BMI, insulin regimen, blood glucose monitoring frequency, and household income (Table 3). Non-Hispanic Black and Native American YYA with type 1 diabetes had higher HbA1c levels than non-Hispanic White participants (Supplementary Fig. 2). Other statistically significant correlates of poorer glycemic control in the multivariate model for T1D included younger age, lower BMI z-score, not using an insulin pump, infrequent self-monitoring of blood glucose, and lower household income.
Table 3.
Associations of HbA1c with participant characteristics: SEARCH 2014–2019 cohort*
| Type 1 (n = 1,805 observations used) | Type 2 (n = 478 observations used) | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P value | Estimate | 95% CI | P value | |
| Sex | 0.07 | 0.44 | ||||
| Female | Reference | Reference | ||||
| Male | −0.16 | −0.33, 0.01 | −0.18 | −0.62, 0.27 | ||
| Race/ethnicity | <0.0001 | 0.34 | ||||
| Non-Hispanic White | Reference | Reference | ||||
| Non-Hispanic Black | 1.22 | 0.94, 1.51 | 0.4 | −0.23, 1.03 | ||
| Hispanic | 0.19 | −0.06, 0.44 | 0.45 | −0.32, 1.22 | ||
| Native American | 1.21 | 0.09, 2.33 | 0.66 | −0.50, 1.83 | ||
| Other/unknown/multiple | 0.08 | −0.27, 0.43 | 1.06 | −0.01, 2.14 | ||
| Age (years) | −0.06 | −0.08, −0.04 | <0.0001 | 0.02 | −0.06, 0.10 | 0.58 |
| Diabetes duration (years) | 0.01 | −0.03, 0.04 | 0.7545 | 0 | −0.11, 0.11 | 0.94 |
| BMI z-score | −0.23 | −0.32, −0.14 | <0.0001 | −0.43 | −0.72, −0.14 | 0.004 |
| Insulin regimen | <0.0001 | |||||
| Insulin pump | Reference | |||||
| Basal-bolus injections | 0.42 | 0.23, 0.60 | ||||
| Other insulin regimens | 0.83 | 0.39, 1.26 | ||||
| Unknown | 0.5 | −0.19, 1.19 | ||||
| Medication regimen | <0.0001 | |||||
| Insulin only | Reference | |||||
| Insulin plus oral agent | −0.26 | −0.89, 0.37 | ||||
| Metformin only | −3.09 | −3.77, −2.42 | ||||
| Other oral agent | −1.91 | −2.98, −0.83 | ||||
| None | −2.9 | −3.57, −2.24 | ||||
| Unknown | −1.77 | −3.35, −0.19 | ||||
| Blood glucose monitoring frequency | <0.0001 | 0.49 | ||||
| Less than once a day | Reference | Reference | ||||
| 1–3 times/day | 0.43 | 0.13, 0.73 | −0.16 | −0.83, 0.52 | ||
| 4 or more times/day | −0.31 | −0.60, −0.02 | −0.47 | −1.26, 0.31 | ||
| CGM | −0.48 | −0.76, −0.20 | −0.21 | −1.07, 0.65 | ||
| Unknown | −1.13 | −1.47, −0.80 | −0.72 | −1.57, 0.12 | ||
| Household income | <0.0001 | 0.33 | ||||
| <$25,000 | Reference | Reference | ||||
| $25,000–49,999 | −0.12 | −0.41, 0.18 | −0.19 | −0.83, 0.46 | ||
| $50,000–74,999 | −0.55 | −0.88, −0.22 | −0.56 | −1.52, 0.39 | ||
| >$75,000 | −0.72 | −1.02, −0.42 | −0.81 | −1.76, 0.14 | ||
| Do not know/refused | −0.24 | −0.53, 0.05 | 0.08 | −0.44, 0.60 | ||
| Health insurance | 0.27 | 0.33 | ||||
| Private | Reference | Reference | ||||
| Medicaid/Medicare | 0.22 | −0.01, 0.45 | −0.43 | −0.96, 0.10 | ||
| Other | 0.12 | −0.27, 0.52 | 0.16 | −0.73, 1.06 | ||
| None | 0.28 | −0.26, 0.82 | −0.19 | −0.96, 0.10 | ||
Models adjusted for variables in the table as well as SEARCH site.
The 2014–2019 cohort with type 2 diabetes had an adjusted HbA1c of 8.6 ± 0.12% (70 mmol/mol). There was a statistically significant difference in HbA1c level across the three time periods for participants with type 2 diabetes. The adjusted HbA1c level for the 2014–2019 cohort (8.6% [70 mmol/mol]) was relatively comparable to the 2002–2007 cohort (8.7% [72 mmol/mol] but was higher than the 2008–2013 cohort (8.3% [67 mmol/mol]). When examined by diabetes duration, YYA with type 2 diabetes with a diabetes duration of ≥10 years exhibited a temporal trend of worse glycemic control (2008–2013: 8.4% [68 mmol/mol] vs. 2014–2019: 10.1% [87 mmol/mol]). There was no statistically significant difference in the 2014–2019 cohort when compared with similarly aged YYA groups in the other two cohorts (Fig. 1B and Supplementary Table 1). Among participants with type 2 diabetes in the 2014–2019 cohort, the multivariate results revealed that HbA1c was associated with BMI and medication regimen, with those on metformin having a lower HbA1c as compared with those on insulin.
Conclusions
Many YYA with diabetes in the U.S. are not meeting desired glycemic targets despite increased availability of advanced diabetes technologies, newer therapies, and more aggressive glycemic targets over time. Overall, we found that adjusted mean HbA1c has increased for YYA with type 1 diabetes since the start of the SEARCH study, while the adjusted mean HbA1c in YYA with type 2 diabetes is relatively unchanged when comparing the SEARCH 2002–2007 to the most recent 2014–2019 SEARCH cohort. This contrasts with other countries where improved glycemic control and outcomes have been observed in YYA with diabetes. The SWEET project, which includes 22 centers from Europe, Australia, Canada, and India, demonstrated an improvement in glycemic control; individuals <25 years of age with type 1 diabetes had a mean adjusted HbA1c that declined from 8.4% [68 mmol/mol] to 7.9% [63 mmol/mol] between 2008–2010 and 2016–2018 (12). Similarly, the National Paediatric Diabetes Audit in England and Wales also reported a decline in median HbA1c of 8.6% [71 mmol/mol] in 2011/2012 to 7.8% [61.5 mmol/mol] in 2019/2020 in the pediatric population with diabetes (13).
In addition to differences in adjusted HbA1c over time (Table 2), examination by age group demonstrated significant differences in glycemic control (Fig. 1). We found that adjusted mean HbA1c levels for YYA with type 1 diabetes have increased over time across many age groups. Similar to the 2002–2007 SEARCH cohort, adjusted mean HbA1c was highest among 15–19-year-old participants with type 1 diabetes at 9.3% (78 mmol/mol) for the 2014–2019 cohort. However, it is notable that the adjusted HbA1c for this age group in the most recent SEARCH cohort was 0.5% (5 mmol/mol) higher when compared with 2002–2007 (8.8% [73 mmol/mol]). A comparable increase in adjusted mean HbA1c of 0.4% (4 mmol/mol) was observed in emerging adults, as the adjusted HbA1c for 20–24-year-old participants increased from 8.3% (67 mmol/mol) in the 2002–2007 cohort to 8.7% (72 mmol/mol) in the most recent cohort. The mean glycemic control for these age groups is comparable to what has recently been reported by the U.S. T1D Exchange Registry (15–18 years old: 9.3% [78 mmol/mol]; 18–25 years old: 8.9% [74 mmol/mol]), which reported a worsening in glycemic control in U.S. YYA with diabetes between 2010–2012 and 2016–2018 (9). While there have been significant advances in type 1 diabetes management (14), the burden of diabetes self-care remains demanding. Our findings highlight that the need to monitor blood glucose levels frequently or continuously and administer insulin reliably multiple times per day while balancing diet, physical activity, and other life activities continues to be challenging for adolescents and emerging adults with type 1 diabetes.
SEARCH has provided important information about YYA with type 2 diabetes in the U.S over the past two decades (3,15,16). Results from this study demonstrate that despite a growing recognition of the more rapidly progressive decline in β-cell function (17) and accelerated development of diabetes complications in youth-onset type 2 diabetes compared with adult-onset type 2 diabetes (18), glycemic control in U.S. YYA with type 2 diabetes has not improved over time. The adjusted mean HbA1c of YYA with type 2 diabetes with an average diabetes duration of 7.0 years in our sample was 8.6% (70 mmol/mol), which is comparable to the 8.4% (68 mmol/mol) reported for the Pediatric Diabetes Consortium Type 2 Diabetes Registry for youth with type 2 diabetes with a diabetes duration of ≥4 years (10).
The observed pattern of worsening glycemic control with increasing duration of type 2 diabetes, independent of many other potential correlates, was likely driven in part by the progressive loss of β-cell function in these participants. The adjusted HbA1c of 10.1% (87 mmol/mol) for type 2 diabetes participants with a disease duration of ≥10 years in the most recent SEARCH cohort is very concerning and provides further confirmation of the high degree of treatment failure in YYA with type 2 diabetes, as well as the need for aggressive intervention (19). Given the evidence that lifestyle intervention alone to achieve or maintain normal blood glucose levels in type 2 diabetes is often unsuccessful in YYA, efforts are needed to support medical providers providing care for YYA with type 2 diabetes to become more comfortable with recommended care and the use of pharmacologic agents beyond metformin and insulin, such as liraglutide, in this population (7).
Previous research, including data from meta-analyses and large international registries, has demonstrated that the use of insulin pumps in youth with type 1 diabetes is associated with lower HbA1c as compared with multiple daily injections (20–22). Separately, CGM alone has been associated with improved glycemic control, with the benefits increasing as CGM use increases (23). Similarly, we found that 2014–2019 SEARCH participants using an insulin pump had an HbA1c level that was 0.4% (4 mmol/mol) lower than those managing their type 1 diabetes with basal-bolus injections. CGM users had an ∼0.2% lower HbA1c level even when compared with those who were monitoring their glucose four or more times per day with fingerstick blood glucose checks. It is worth noting, however, that despite the literature supporting the benefits of pump therapy and CGM systems in the YYA population, universal adoption has not been achieved (24,25). For example, while insulin pump use nearly doubled from 2002–2007 (24.9%) to 2014–2019 (49.1%), there was a minimal increase in the 2014–2019 cohort from the 44.7% found to be using insulin pumps in 2008–2013. Previous studies have shown that household income and parental education are predictive of insulin pump use (24,26). Given the challenges that YYA with type 1 diabetes face in achieving the goals of therapy, addressing differences in access and use of diabetes technologies offers a potential avenue to help the YYA population meet glycemic targets.
Robust diabetes education, diabetes device training, and follow-up of YYA and families are essential to help achieve target glycemic outcomes. Historically, structured, person-centered, and empowerment-based education programs for diabetes self-management and diabetes technology use have been delivered mostly in-person by a certified diabetes specialist. With the expansion of telehealth services during the coronavirus disease 2019 pandemic, virtual training sessions to provide diabetes education and start diabetes technology have been shown to be feasible (27–30). The benefits of virtual training can include scheduling flexibility, access to individuals who live in more remote locations, and reaching individuals who experience challenges traveling to appointments and thus help to alleviate health equity issues (29).
We also found that lower BMI was associated with worse glycemic control in participants with type 1 diabetes. This is consistent with observations from the SEARCH for Diabetes in Youth study published over a decade ago (3), as well as more recent studies from Europe (31). While both nonautomated and automated insulin pump delivery systems are associated with improved glycemic control relative to multiple daily insulin injections, some studies have reported that their use may be associated with higher BMI (31,32). The insulin resistance accompanying potential overweight and obesity warrants further examination with implementation of insulin pump therapy.
Racial and ethnic disparities in glycemic control among YYA with type 1 diabetes are well documented (3,4,33,34). Our finding that non-Hispanic Black and Native American YYA with type 1 diabetes had an HbA1c level that was 1.2% (13 mmol/mol) higher than non-Hispanic White YYA, confirms the need to address inequities in diabetes care (35). As we work to address disparities in diabetes care, socioeconomic challenges must also be addressed. We found YYA participants in higher household income categories had improved HbA1c levels. In an era of rapidly rising insulin prices (36) and estimated mean out-of-pocket costs for medical care of ∼$2,500 annually even for those on private health insurance (37), solutions to insulin and diabetes technology affordability are needed.
Recognizing that treating patients with diabetes earlier and more intensively has the potential to confer long-term improvements in public health (38–41), our finding that the majority of YYA with diabetes continue to not meet established glycemic targets represents a missed opportunity for improving lifetime outcomes for patients with diabetes. The transition from pediatric to adult diabetes care is a high-risk period during which there is an increased rate of disengagement from care (42). For YYA with type 1 and type 2 diabetes, unique adherence challenges can range from incomplete knowledge and understanding of treatment regimens and risk for future health to premature shift in responsibility for management from parents to YYA (43). Thus, efforts should be made to successfully transfer YYA with diabetes to an adult-oriented diabetes specialist, who is aware of how best to meet the unique needs of YYA with diabetes and the more aggressive clinical course in this patient population.
While SEARCH is the largest multiethnic population-based study of pediatric diabetes in the U.S., there are limitations to the interpretation of the results. First, data are available only for participants who attended study visits, which might limit the generalizability of our results. Second, we were unable to meaningfully estimate the impact of increased CGM use on glycemic control since we did not have complete information about blood glucose monitoring frequency. However, despite a relatively small confirmed sample of CGM users in the 2014–2019 cohort, we found that CGM users had lower HbA1c levels than those who did not use CGM. Third, we had a relatively small sample size of YYA with type 2 diabetes, particularly in the earlier years of the SEARCH study, highlighting the need for continued longitudinal studies to better characterize trends in glycemic control in YYA with type 2 diabetes. Fourth, since SEARCH is an observational study, we are unable to account for unmeasured residual confounding. Fifth, while we adjusted for race/ethnicity in the models to help account for the sampling variability, it is possible that this adjustment may not completely account for the race-based sampling differences across cohorts.
Data from this large population-based multicenter study confirm that YYA with type 1 and type 2 diabetes in the U.S. are not demonstrating improved glycemic control over time and highlight the need for systematic approaches in the U.S. to support YYA with diabetes, such as those that many European countries have implemented. The establishment and growth of the T1D Exchange Quality Improvement Collaborative (44), which now includes >40 U.S. pediatric and adult diabetes clinics, offers a promising framework to improve health care delivery and dissemination of best practices to accelerate improvement in diabetes outcomes in the U.S. (45). Recognizing that lower HbA1c levels in childhood and young adulthood is associated with lower risk and rate of microvascular and macrovascular complications, this study further underscores the urgent need for implementation of effective treatment strategies to improve metabolic status in YYA with diabetes.
Article Information
Funding. SEARCH 4: The SEARCH for Diabetes in Youth Cohort Study is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK127208-01 and 1UC4DK108173) and supported by the Centers for Disease Control and Prevention. The Population Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, and U18DP006139) is funded by the Centers for Disease Control and Prevention (DP-15-002) and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. SEARCH 1–3: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati Children’s Hospital Medical Center (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171) also provided support. The time contributed by F.S.M. was supported by a K23 Career Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK119465).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.S.M. contributed to the study design, interpretation of the data, and drafted the manuscript. K.A.S. and C.P. contributed to the study design and interpretation of the data. S.I. contributed to the study design and conducted the analyses. D.D., J.M.L., A.R., E.J.M.-D., and L.D. contributed to interpretation of the data. All authors critically reviewed and edited revisions before approving the final version. C.P. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.17052590.
A complete list of the members of the SEARCH for Diabetes in Youth Study can be found in the supplementary material online.
Contributor Information
Collaborators: SEARCH for Diabetes in Youth Study:, Jean M. Lawrence, Peggy Hung, Corinna Koebnick, Xia Li, Eva Lustigova, Kristi Reynolds, David J. Pettitt, Elizabeth J. Mayer-Davis, Amy Mottl, Joan Thomas, Malaka Jackson, Lisa Knight, Angela D. Liese, Christine Turley, Deborah Bowlby, James Amrhein, Elaine Apperson, Bryce Nelson, Dana Dabelea, Anna Bellatorre, Tessa Crume, Richard F. Hamman, Katherine A. Sauder, Allison Shapiro, Lisa Testaverde, Georgeanna J. Klingensmith, David Maahs, Marian J. Rewers, Paul Wadwa, Stephen Daniels, Michael G. Kahn, Greta Wilkening, Clifford A. Bloch, Jeffrey Powell, Kathy Love-Osborne, Diana C. Hu, Lawrence M. Dolan, Amy S. Shah, Debra A. Standiford, Elaine M. Urbina, Catherine Pihoker, Irl Hirsch, Grace Kim, Faisal A. Malik, Lina Merjaneh, Alissa Roberts, Craig Taplin, Joyce Yi-Frazier, Natalie Beauregard, Cordelia Franklin, Carlo Gangan, Sue Kearns, Mary Klingsheim, Beth Loots, Michael Pascual, Carla Greenbaum, Giuseppina Imperatore, Sharon H. Saydah, Barbara Linder, Santica M. Marcovina, Alan Chait, Noemie Clouet-Foraison, Jessica Harting, Greg Strylewicz, Ralph D’Agostino, Jr., Elizabeth T. Jensen, Lynne E. Wagenknecht, Ronny A. Bell, Ramon Casanova, Jasmin Divers, Maureen T. Goldstein, Leora Henkin, Scott Isom, Kristin Lenoir, June Pierce, Beth Reboussin, Joseph Rigdon, Andrew Michael South, Jeanette Stafford, Cynthia Suerken, Brian Wells, and Carrie Williams
References
- 1. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petitti DB, Klingensmith GJ, Bell RA, et al.; SEARCH for Diabetes in Youth Study Group . Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr 2009;155:668–72.e1, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open 2018;1:e181851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeSalvo DJ, Miller KM, Hermann JM, et al.; T1D Exchange and DPV Registries . Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller KM, Hermann J, Foster N, et al.; T1D Exchange and DPV Registries . Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: international comparison in the German and Austrian DPV and U.S. T1D Exchange Registries. Diabetes Care 2020;43:e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S180–S199 [DOI] [PubMed] [Google Scholar]
- 8. DiMeglio LAAC, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):105–114 [DOI] [PubMed] [Google Scholar]
- 9. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nambam B, Silverstein J, Cheng P, et al.; Pediatric Diabetes Consortium . A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: enrollment data from the Pediatric Diabetes Consortium Type 2 Diabetes Registry. Pediatr Diabetes 2017;18:222–229 [DOI] [PubMed] [Google Scholar]
- 11. Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerhardsson P, Schwandt A, Witsch M, et al. The SWEET Project 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol Ther 2021;23:491–499 [DOI] [PubMed] [Google Scholar]
- 13. Royal College of Paediatrics and Child Health . National Paediatric Diabetes Audit Annual Report 2019-20: Care Processes and Outcomes, 2021. Accessed 19 October 2021. Available from https://www.rcpch.ac.uk/resources/npda-annual- reports
- 14. Sherr JL, Tauschmann M, Battelino T, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetes technologies. Pediatr Diabetes 2018;19(Suppl. 27):302–325 [DOI] [PubMed] [Google Scholar]
- 15. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea D, Bell RA, D’Agostino R Jr., et al. Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 17. TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016;39:1635–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941–951 [DOI] [PubMed] [Google Scholar]
- 21. Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 2009;10:52–58 [DOI] [PubMed] [Google Scholar]
- 22. Szypowska A, Schwandt A, Svensson J, et al.; SWEET Study Group . Insulin pump therapy in children with type 1 diabetes: analysis of data from the SWEET registry. Pediatr Diabetes 2016;17(Suppl. 23):38–45 [DOI] [PubMed] [Google Scholar]
- 23. Laffel LM, Kanapka LG, Beck RW, et al.; CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group; CDE10 . Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Commissariat PV, Boyle CT, Miller KM, et al. Insulin pump use in young children with type 1 diabetes: sociodemographic factors and parent-reported barriers. Diabetes Technol Ther 2017;19:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin MH, Connor CG, Ruedy KJ, et al.; Pediatric Diabetes Consortium . Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther 2013;15:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care 2021;44:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinsker JE, Singh H, McElwee Malloy M, et al. A virtual training program for the Tandem t:slim X2 Insulin Pump: implementation and outcomes. Diabetes Technol Ther 2021;23:467–470 [DOI] [PubMed] [Google Scholar]
- 28. Vigersky RA, Velado K, Zhong A, Agrawal P, Cordero TL. The effectiveness of virtual training on the MiniMed™ 670G System in people with type 1 diabetes during the COVID-19 pandemic. Diabetes Technol Ther 2021;23:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messer LH, Berget C, Ernst A, Towers L, Slover RH, Forlenza GP. Initiating hybrid closed loop: a program evaluation of an educator-led Control-IQ follow-up at a large pediatric clinic. Pediatr Diabetes 2021;22:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gómez AM, Henao D, Parra D, et al. Virtual training on the hybrid close loop system in people with type 1 diabetes (T1D) during the COVID-19 pandemic. Diabetes Metab Syndr 2021;15:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Birkebaek NH, Kahlert J, Bjarnason R, et al.; Nordic Childhood Diabetes Registry Study Group, NordicDiabKids . Body mass index standard deviation score and obesity in children with type 1 diabetes in the Nordic countries. HbA1c and other predictors of increasing BMISDS. Pediatr Diabetes 2018;19:1198–1205 [DOI] [PubMed] [Google Scholar]
- 32. Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM; Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON) . Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 2018;39:629–663 [DOI] [PubMed] [Google Scholar]
- 33. Redondo MJ, Libman I, Cheng P, et al.; Pediatric Diabetes Consortium . Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care 2018;41:1017–1024 [DOI] [PubMed] [Google Scholar]
- 34. Willi SM, Miller KM, DiMeglio LA, et al.; T1D Exchange Clinic Network . Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017;389:1453–1463 [DOI] [PubMed] [Google Scholar]
- 36. Cefalu WT, Dawes DE, Gavlak G, et al.; Insulin Access and Affordability Working Group . Conclusions and recommendations. Diabetes Care 2018;41:1299–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chua KP, Lee JM, Conti RM. Out-of-pocket spending for insulin, diabetes-related supplies, and other health care services among privately insured US patients with type 1 diabetes. JAMA Intern Med 2020;180:1012–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med 2008;359:1618–1620 [DOI] [PubMed] [Google Scholar]
- 39. de Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC Study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 41. Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters A; American Diabetes Association Transitions Working Group . Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care 2011;34:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr 2010;22:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes 2020;38:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ginnard OZB, Alonso GT, Corathers SD, et al. Quality improvement in diabetes care: a review of initiatives and outcomes in the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]