There is a growing body of evidence for sodium–glucose cotransporter 2 (SGLT2) inhibitors, supporting their use in a wide spectrum of patient populations. Their indications have now expanded beyond patients with type 2 diabetes (T2D) to patients with heart failure and chronic kidney disease, regardless of T2D status (1–3). Several mechanisms are postulated to explain their favorable effects, such as their natriuretic, diuretic, and glucosuric properties, among many others. Consistent evidence suggests a relationship between SGLT2 inhibitors and increased hematocrit (Hct)/hemoglobin/erythropoietin levels (4). Whether this reflects hemoconcentration due to diuretic effects, expansion of red blood cell (RBC) mass due to increased erythropoietin, or both remains unclear. In BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), empagliflozin was associated with increased Hct (5). In Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF), anemia was more frequently corrected with dapagliflozin than with placebo (3). To that effect, we analyzed data from the longer and larger Dapagliflozin Effect on Cardiovascular Events trial (DECLARE-TIMI 58), which included a broader population of patients with T2D, to assess effects of dapagliflozin (DAPA) versus placebo on Hct (1).
DECLARE-TIMI 58 was a randomized, double-blinded, placebo-controlled trial of DAPA in 17,160 patients with T2D with, or at high risk for, atherosclerotic cardiovascular (CV) disease (1). After the demonstration of the efficacy of empagliflozin in the EMPA-REG OUTCOME trial (5), including reduced risk for the composite of CV death/myocardial infarction/stroke and for hospitalization for heart failure and attenuated progression of chronic kidney disease, similar findings were corroborated with DAPA in the DECLARE-TIMI 58 trial on heart failure and kidney outcomes but without superior efficacy for CV death/myocardial infarction/stroke.
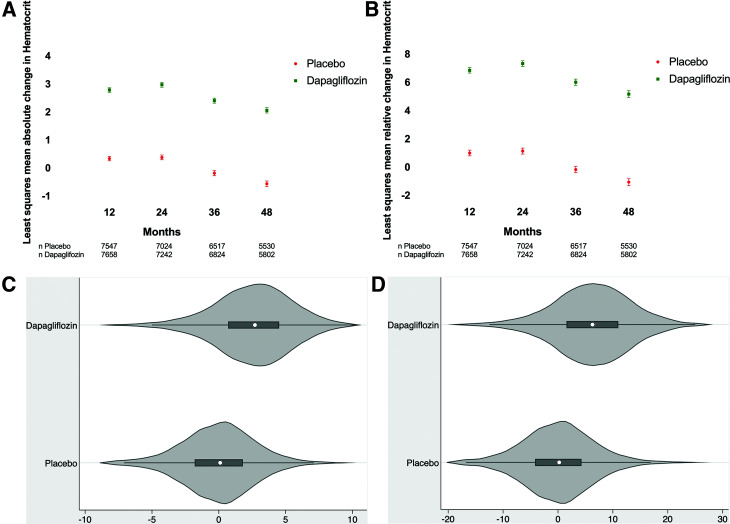
In DECLARE-TIMI 58, DAPA versus placebo increased Hct levels at 12, 24, 36, and 48 months (Fig. 1A and B). For instance, at 4 years, the median (interquartile range) Hct in the DAPA group had increased from a baseline of 42.1% (39.4, 44.8) to 44.6% (41.5, 47.5), reflecting a 5.7% (0.9, 10.5) relative change. Over the same time period, the placebo group baseline median Hct of 42.1% (39.4, 44.8) remained stable at 41.8% (38.9, 44.5). When comparing DAPA with placebo in the first, second, and third Hct tertiles, the relative percent change from baseline in median Hct was 3.1% (1.1, 5.2) vs. 0.7% (−1.4, 2.5), 3.1% (1.1, 5.2) vs. 0.7% (−1.4, 2.5), and 1.8% (−0.3, 3.5) vs. −1.2% (−3.2, 0.6), respectively. Stratified by baseline estimated glomerular filtration rate, relative percent change from baseline in median Hct was 5.6% (0.5, 10.9) vs. −1.0% (−5.8, 3.7), 2.4% (0.5, 4.3) vs. −0.4% (−2.4, 1.5), and 2.4% (0.5, 4.3) vs. −0.1% (−2.0, 1.8) in the first, second, and third estimated glomerular filtration rate tertiles, respectively. At 1 and 4 years, the placebo-subtracted least squares mean estimates of absolute Hct percent were 2.5 (SE 0.05) and 2.6 (SE 0.06), corresponding to relative percent changes of 5.9% (SE 0.12) and 6.2% (SE 0.15), respectively (P < 0.001 in all cases). Absolute and relative percent changes in Hct by randomized group at 1 year are presented in Fig. 1C and D. Diuretic use at the end of the study was 41.6% (3,571/8,582) and 44.3% (3,798/8,578) in the DAPA and placebo groups, respectively (P < 0.001).
Figure 1.
Effect of DAPA vs. placebo on Hct in the DECLARE-TIMI 58 trial. A: Least squares mean absolute change in Hct from baseline over time. B: Least squares mean relative change in Hct from baseline over time. C: Absolute Hct change from baseline to 1 year. D: Relative percent change of Hct from baseline to 1 year.
The present observations with regard to DAPA effects on Hct are in line with previously reported data from the EMPA-REG OUTCOME trial of empagliflozin but extend them to a broader population with longer follow-up duration. While the EMPA-REG OUTCOME investigators speculated that the increase in Hct was due to plasma volume contraction and hemoconcentration (5), others have postulated that the increase in Hct with SGLT2 inhibitors is more likely due to hematopoiesis/RBC mass expansion associated with increased erythropoietin (4). The lower diuretic use in the DAPA group argues against this being a pure hemoconcentration effect. A prominent theory as to how SGLT2 inhibition induces erythropoietin, leading to increased RBC mass, includes the reversal of relative tissue hypoxia surrounding the proximal convoluted tubules as a result of the diminished action of the ATP/oxygen-consuming Na+/K+ ATPase pump, a secondary effect of SGLT2 inhibition. This, in turn, may result in the restoration of the erythropoietin production capacity of fibroblasts surrounding the proximal convoluted tubules, stimulating hematopoiesis (4). In addition, inhibiting the highly metabolically efficient glucose and sodium reuptake by SGLT2 relegates their reclamation to more distal mechanisms with less energy efficiency (e.g., sodium–hydrogen exchanger, SGLT1, and epithelial sodium channel) and potentially inducing regional tissue hypoxia in zone 3 of the renal medulla, further inducing erythropoietin from interstitial fibroblasts. The present findings support but cannot address these hypotheses, limited by lack of measurement of erythropoietin levels, RBC mass, reticulocyte count, and plasma volume. Further explorations regarding the consistent finding of Hct increase across the SGLT2 inhibitor class, its clinical relevance, and its mechanistic underpinnings are necessary.
Article Information
Funding. A.A.K. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL12547.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Duality of Interest. S.D.W. discloses grants from Amgen, Arena, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Merck, and Sanofi and reports consulting fees from Arena, AstraZeneca, Aegerion, Allergan, Angelmed, Boehringer Ingelheim, Boston Clinical Research Institute, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Icon Clinical, Janssen, Lexicon, Merck, Servier, St. Jude Medical, and Xoma. S.D.W.’s spouse, Dr. Caroline Fox, is an employee of Merck. S.D.W. is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. I.R. discloses the following relationships: advisory board for AstraZeneca, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Inc., and Sanofi; consultant for AstraZeneca, Insuline Medical, Medial EarlySign, Ltd., CamerEyes, Ltd., Exscopia, Orgenesis, Ltd., BOL Pharma, Glucome, Ltd., DarioHealth, Diabot, and Concenter BioPharma; speaker’s bureau for AstraZeneca, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Inc., and Sanofi; and stock/shareholder of Glucome, Ltd., Orgenesis, Ltd., DarioHealth, and CamerEyes, Ltd. O.M. discloses the following relationships: advisory board for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca, and BOL Pharma; research grant support, through Hadassah Hebrew University Hospital, from Novo Nordisk and AstraZeneca; speaker’s bureau for AstraZeneca, Novo Nordisk, Eli Lilly and Company, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, and Janssen. S.A.M. discloses the following: research grant support, through Brigham and Women’s Hospital, from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, and Zora Biosciences. D.L.B. discloses the following relationships: advisory board for Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; board of directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; inaugural chair, American Heart Association Quality Oversight Committee; data monitoring committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the Portico Resheathable Transcatheter Aortic Valve System US IDE Trial [PORTICO-IDE], funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis [ExCEED] trial, funded by Edwards), Contego Medical (Chair, Protection Against Emboli During Carotid Artery Stenting Using the Neuroguard IEP System [PERFORMANCE 2]), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation [ENVISAGE] trial, funded by Daiichi Sankyo), Novartis, and Population Health Research Institute; honoraria from Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting [RE-DUAL PCI] clinical trial steering committee funded by Boehringer Ingelheim; Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome [AEGIS-II] executive committee funded by CSL Behring), Belvoir Publications (editor in chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease [PRONOUNCE] trial, funded by Ferring Pharmaceuticals), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (Continuing Medical Education steering committees), MJH Life Sciences, Population Health Research Institute (for the Cardiovascular OutcoMes for People Using Anticoagulation StrategieS [COMPASS] operations committee, publications committee, steering committee, and U.S. national co-leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (Continuing Medical Education steering committees); and other for Clinical Cardiology (deputy editor), National Cardiovascular Data Registry-Acute Coronary Treatment and Intervention Outcomes Network [NCDR-ACTION] Registry Steering Committee (chair), Veterans Health Administration Clinical Assessment, Reporting and Tracking System for Cath Labs [VA CART] Research and Publications Committee (chair); research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Eli Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, and 89Bio; royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, and Svelte; trustee for American College of Cardiology; and unfunded research for FlowCo, Merck, and Takeda. L.A.L. has received research funding from, has provided continuing medical education on behalf of, and/or has acted as an advisor to AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Lexicon, Merck, Novo Nordisk, Pfizer, Sanofi, and Servier. J.P.H.W. has received honoraria/lecture fees from AstraZeneca, Boehringer Ingelheim, Lilly, Napp, Mundipharma, Sanofi, and Takeda. He has undertaken consultancy contracted via the University of Liverpool for AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Napp, Novo Nordisk, Mundipharma, Rhythm Pharmaceuticals, Sanofi, and Saniona and is a named grant holder (at University of Liverpool) for research grants for clinical trials from AstraZeneca and Novo Nordisk. I.G.-N. discloses being an employee at BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden. M.S.S. declares research grant support, through Brigham and Women’s Hospital, from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, Intarcia, IONIS, The Medicines Company, MedImmune, Merck, Novartis, Pfizer, and Quark Pharmaceuticals and consulting for Althera, Amgen, Anthos Therapeutics, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, DalCor, Dr. Reddy’s Laboratories, Fibrogen, IFM Therapeutics, Intarcia, MedImmune, Merck, and Novo Nordisk. Additionally, he is a member of the TIMI Study Group, which has also received institutional research grant support, through Brigham and Women’s Hospital, from Abbott, Regeneron, Roche, and Zora Biosciences. D.K.M. discloses personal fees from Boehringer Ingelheim, Janssen Research and Development LLC, Sanofi US, Merck & Co., Merck Sharp & Dohme, Eli Lilly USA, NovoNordisk, GlaxoSmithKline, AstraZeneca, Lexicon Pharmaceuticals, Eisai, Pfizer, Metavant, Applied Therapeutics, Afimmune, CSL Behring, Bayer, and Esperion. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.A.K. wrote the manuscript and researched data. S.D.W., I.R., S.A.M., O.M., D.L.B., L.A.L., J.P.H.W., I.G.-N., and M.S.S. reviewed/edited the manuscript. D.K.M. wrote the manuscript and researched data. D.K.M. is the guarantor of this manuscript and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 2. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 3. Docherty KF, Curtain JP, Anand IS, et al.; DAPA-HF Investigators and Committees . Effect of dapagliflozin on anaemia in DAPA-HF. Eur J Heart Fail 2021;23:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation 2019;139:1985–1987 [DOI] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]