Abstract
Background
Injury to articular cartilage cause certain degree of disability due to poor self-repair ability of cartilage tissue. To promote cartilage regeneration, it is essential to develop a scaffold that properly mimics the native cartilage extracellular matrix (ECM) in the aspect of compositions and functions.
Methods
A mussel-inspired strategy was developed to construct an ECM-mimicking hydrogel scaffold by incorporating polydopamine-modified hyaluronic acid (PDA/HA) complex into a dual-crosslinked collagen (Col) matrix for growth factor-free cartilage regeneration. The adhesion, proliferation, and chondrogenic differentiation of cells on the scaffold were examined. A well-established full-thickness cartilage defect model of the knee in rabbits was used to evaluated the efficacy and functionality of the engineered Col/PDA/HA hydrogel scaffold.
Results
The PDA/HA complex incorporated-hydrogel scaffold with catechol moieties exhibited better cell affinity than bare negatively-charged HA incorporated hydrogel scaffold. In addition, the PDA/HA complex endowed the scaffold with immunomodulation ability, which suppressed the expression of inflammatory cytokines and effectively activated the polarization of macrophages toward M2 phenotypes. The in vivo results revealed that the mussel-inspired Col/PDA/HA hydrogel scaffold showed strong cartilage inducing ability to promote cartilage regeneration.
Conclusions
The PDA/HA complex-incorporated hydrogel scaffold overcame the cell repellency of negatively-charged polysaccharide-based scaffolds, which facilitated the adhesion and clustering of cells on the scaffold, and therefore enhanced cell-HA interactions for efficient chondrogenic differentiation. Moreover, the hydrogel scaffold modulated immune microenvironment, and created a regenerative microenvironment to enhance cartilage regeneration.
The translational potential of this article
This study gives insight into the mussel-inspired approach to construct the tissue-inducing hydrogel scaffold in a growth-factor-free manner, which show great advantage in the clinical treatment. The hydrogel scaffold composed of collagen and hyaluronic acid as the major component, providing cartilage ECM-mimicking environment, is promising for cartilage defect repair.
Keywords: Hydrogel, Mussel-inspired strategy, Extracellular matrix mimicking, Cell affinity, Cartilage regeneration
1. Introduction
Articular cartilage is an avascular tissue with a complicated structure and shows limited self-repair capacity following trauma or disease because of lack of access to abundant nutrients or circulating progenitor cells [1]. Therefore, the regeneration of defected cartilage remains one of the main challenges in the orthopedic field. Although several strategies, including debridement and lavage, microfracture, and autografts, have been clinically employed to repair cartilage defects, these strategies still have certain limitations such as low repair efficiency, poor integration with healthy cartilage, and fibrous tissue formation [[2], [3], [4]]. Accordingly, cartilage tissue engineering has been developed, which aims to combine extracellular matrix (ECM) mimicking scaffolds with physicochemical stimuli for cartilage reconstruction [5,6]. Studies have shown that cartilage ECM not only provides mechanical stability to the cartilage tissue but also contains various bioactive components to maintain the phenotype of chondrocytes and induce chondrogenic differentiation of mesenchymal stem cells [[7], [8], [9]]. On the other hand, the cartilage defects can lead to active inflammatory conditions in the cartilage tissue, which interferes with repairing process and retards the tissue regeneration effect of the implanted scaffolds [10,11]. During the inflammatory response, macrophages are one of the most crucial immune cells, which can be activated into different polarization status (pro-inflammatory M1 or anti-inflammatory M2 phenotype) in response to localized environmental cues [12]. Previous studies have reported that implanting the certain scaffolds can stimulate activation of M2 macrophages, which act as regenerative homeostasis promoters by releasing prochondrogenic cytokines to enhance tissue healing [13,14]. Thus, it is important to fabricate a scaffold mimicking the natural cartilage ECM, while showing the ability to regulate inflammatory microenvironment at cartilage defects and guide successful cartilage regeneration.
Hydrogel scaffolds, possessing characteristics, such as high-water absorption, adjustable porosity, bionic mechanical behavior, biodegradation, and biocompatibility, similar to those of cartilage ECM, are one of promising candidate materials for cartilage tissue engineering. Both natural and synthetic polymers have been employed for the development of hydrogels. Owing to their notable biocompatibility and biodegradability, naturally derived biopolymers are considered smart materials for the design of cartilage ECM-mimicking scaffolds to support tissue regeneration [[15], [16], [17]]. For example, collagen (Col), as the major component of cartilage ECM, has been widely adopted as an ideal biomaterial for cartilage tissue engineering due to its good biocompatibility, low antigenicity, and excellent biodegradability [18,19]. Significantly, Col with adhesive motifs can provide a biomimetic microenvironment for cell adhesion, spreading, and proliferation [20]. However, the clinical applications of Col-based hydrogels or scaffolds are limited due to their poor cartilage regeneration ability [21,22]. Therefore, current strategies utilized blending with functional natural/synthetic polymers to enhance the bioactivity of scaffolds [23]. For example, Liu et al. [24] developed a collagen type I to II blend (Col I/II) hydrogel, which provided favorable conditions for the chondrogenic differentiation of bone marrow-derived mesenchymal stem cells (MSCs) and cartilage repair. Liu et al. [25] prepared injectable thiolated icariin functionalized hyaluronic acid (HA)/collagen hydrogel, which maintained chondrocyte phenotype and promoted the production of the cartilage extracellular matrix. HA, a nonsulfated glycosaminoglycan (GAG) containing repeating disaccharide units, is another crucial component of cartilage ECM. In the ECM, HA can provide biological cues, such as CD44 interactions, based on the role of HA in cellular signaling and thus can promote the metabolism of chondrocytes [26,27]. Moreover, it acts as a lubricant in diarthrodial joints. Thus, an HA-containing scaffold is considered as one of the most promising biomaterials for cartilage tissue engineering. Lin et al. [28] developed a photo-crosslinked gelatin/HA hybrid scaffold and proved that the scaffold can stimulate chondrogenesis by activating cellular signaling pathways. Pfeifer et al. [29] investigated different compositions of HA–gelatin in a composite scaffold and demonstrated that the scaffold with a higher amount of HA supported the chondrogenic differentiation of human MSCs. Zhu et al. [30] also reported that a high concentration of HA in an elastin-like protein–HA hydrogel led to increased marker gene expression and sulfated GAG deposition. However, these HA-based scaffolds showed poor affinity to MSCs or chondrocytes because their negative charges prevented cell adhesion [31], thus limiting HA–cell interactions.
The development of an ECM-mimicking scaffold with cell/tissue affinity that can recruit native cells and facilitate cartilage repair is still imperative. Recently, a facile and versatile method inspired by mussel adhesion chemistry has provided a new way to increase the cell affinity of ECM scaffolds. Polydopamine (PDA) with catechol groups exhibits high binding affinity to amine and thiol functional groups on cell membranes and shows versatile adhesion to various substrates [32,33]. Thus, PDA has been employed in the synthesis of biologically functional scaffolds that promote the differentiation of stem cells into nerve cells [34,35] or osteoblasts [36,37]. In our previous studies, we have also demonstrated that mussel-inspired adhesive hydrogels are favorable for cell adhesion and tissue regeneration [[38], [39], [40]]. Additionally, our previous studies have demonstrated that the catechol groups can effectively inhibit inflammatory response through regulating the activity of macrophages [41], which is essential for tissue regeneration. Thus, PDA provides a possibility of improving the cell affinity and immunomodulatory effect of scaffolds.
To address the clinical need for an effective biomaterial for cartilage regeneration, this study employed a mussel-inspired strategy to construct a cartilage ECM-mimicking hydrogel scaffold with excellent cell affinity and immunomodulatory ability to promote cartilage regeneration (Fig. 1). To mimic the structure and functions of native cartilage ECM, a PDA-functionalized HA (PDA/HA) complex was firstly incorporated into a Col matrix, which was then chemical cross-linked and freeze-dried to obtain a Col/PDA/HA hydrogel scaffold. In this scaffold, the Col matrix were dual cross-linked to form matrices with stable mechanical properties; these matrices mimic the loose or dense connective fibers of the ECM, providing a 3D structural support for cell attachment. The negatively charged HA segments of the scaffold mimic the GAG component of the ECM, which upregulates the expression of cartilage-specific genes in cells. The functionalization of HA by PDA grafts the adhesive catechol groups on the HA chain; this is inspired by the structures of glycoproteins comprising polysaccharide chains linked to a protein core. In our case, the PDA/HA complex with adhesive catechol moieties exhibit higher cell affinity than bare HA to enhance the adhesion and clustering of cells, which can efficiently direct the bone marrow MSCs (BMSCs) toward osteogenic lineages. In addition, the PDA/HA complex endows the scaffold immunomodulatory ability that regulates macrophage transition to M2, which further creates a regenerative microenvironment for promoting cartilage regeneration. A well-established full-thickness cartilage defect model of the knee in rabbits was used to analyze the efficacy and functionality of the engineered Col/PDA/HA hydrogel scaffold, confirming that this scaffold created an appropriate ECM microenvironment to promote cell adhesion, induce chondrogenic differentiation ability of BMSCs, and repair cartilage defects in a growth factor-free manner.
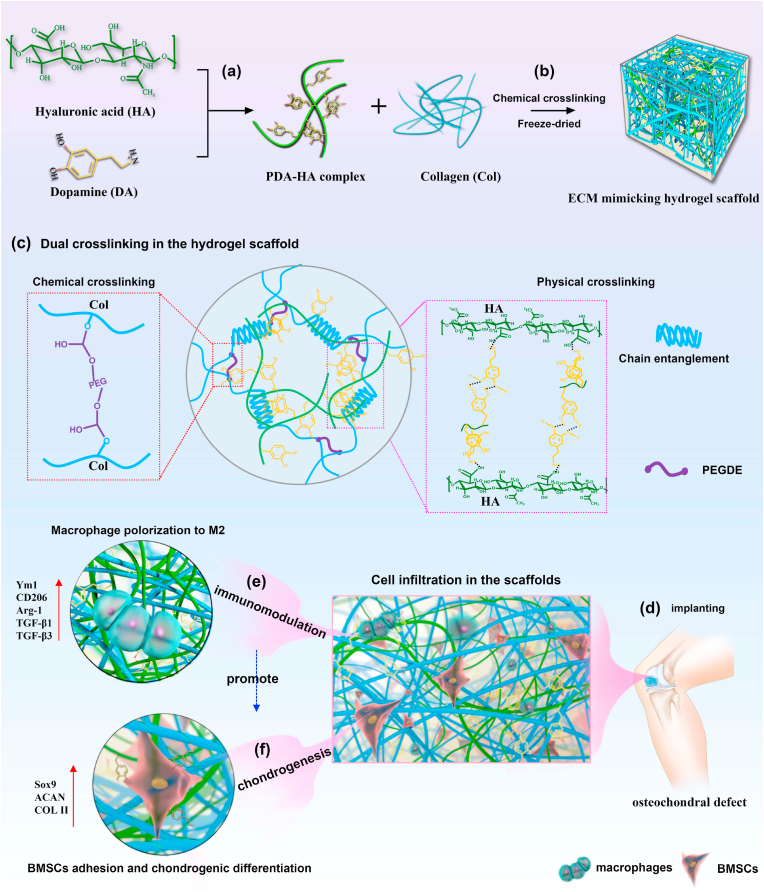
Fig. 1.
Schematics of fabrication process of mussel-inspired ECM mimicking Col/PDA/HA hydrogel scaffold for growth-factor-free cartilage regeneration. (a) HA was functionalized by catechol groups to form the PDA/HA complex. (b) The Col/PDA/HA hydrogel scaffold was formed by chemical-crosslinking the PDA/HA complex and collagen and subsequent freeze-drying. (c) Illustrations of dual crosslinking in the Col/PDA/HA hydrogel scaffolds. (d) The hydrogel scaffold was implanted into the cartilage defect, which favored cell infiltration and tissue ingrowth due to its cell affinity. (e) The scaffold drove the macrophages towards the M2 phenotype and facilitated chondrogenesis. (f) The scaffold established a regenerative microenvironment to enhance cartilage regeneration.
2. Results
2.1. Fabrication of the Col/PDA/HA hydrogel scaffold
The formation of the ECM-mimicking Col/PDA/HA hydrogel scaffold was achieved by incorporating the PDA/HA complex into a chemically cross-linked Col network. The preparation process of the Col/PDA/HA hydrogel scaffold is shown in Fig. 1. Initially, the HA chains were modified by PDA under alkaline conditions (pH = 8.0), where PDA was grafted on the HA chains via noncovalent interactions (hydrogen bonding and electrostatic interactions) to form the PDA/HA complex. The PDA/HA complex was subsequently added into the Col solution and then cross-linked by poly (ethylene glycol) diglycidyl ether (PEGDE) to form the Col/PDA/HA hydrogels. Finally, the cartilage ECM-like Col/PDA/HA hydrogel scaffold was obtained after freeze-drying the Col/PDA/HA hydrogels. Additionally, to demonstrate the advantages of the Col/PDA/HA hydrogel scaffold, we also prepared Col only, Col/HA, and Col/PDA scaffolds.
2.2. Characterizations of the Col/PDA/HA hydrogel scaffold
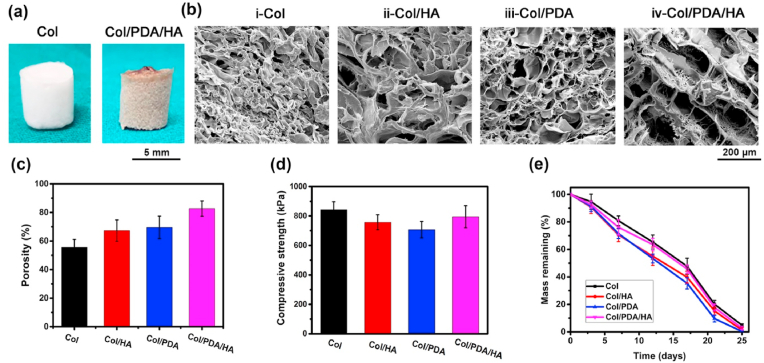
Using the abovementioned protocol, we fabricated a 3D Col/PDA/HA hydrogel scaffold with physicochemical cross-linking. As shown in Fig. 2a, the Col/PDA/HA hydrogel scaffold was brown in color due to the presence of PDA, compared with the white Col scaffold. Scanning electron microscopy (SEM) images indicated that the incorporation of PDA and HA affected the microstructures of the scaffolds. The Col only scaffold has a dense and disordered porous structure (Fig. 2b–i). When HA or PDA were separately incorporated into Col, the resulting Col/HA and Col/PDA scaffolds exhibited a loose structure (Fig. 2b–ii, b-iii). When the PDA/HA complex was incorporated into Col matrix, the resulting Col/PDA/HA hydrogel scaffold showed highly porous and interconnected pore structures with pore size of approximately 200 μm (Fig. 2b–iv). The Col/PDA/HA scaffold exhibited a higher level of porosity (up to 82%) compared to the Col/PDA (65%), Col/HA (63%), and Col only (57%) scaffolds (Fig. 2c). This is because the incorporation of the PDA/HA complex hampered the entanglement of Col chains during the freezing–thawing process, thus decreasing the physical cross-linking density of the Col/PDA/HA scaffold. The porous structure and pore size of the Col/PDA/HA hydrogel scaffold mimic the architecture of the natural ECM, which is favorable for cell attachment and new cartilage tissue ingrowth [42,43]. Compression tests were used to characterize the mechanical strength of the scaffolds, and the results revealed that the Col/PDA/HA scaffolds had a compressive strength of 800 kPa, which was similar to the Col scaffolds (Fig. 2d).
Fig. 2.
Characterization of the hydrogel scaffolds. (a) Digital images of Col (white) and Col/PDA/HA (brown) scaffolds. (b) SEM images of the scaffolds with different compositions. (c) Porosity of various scaffolds. (d) Compressive strength of different scaffolds. (e) In vitro degradation profiles of different scaffolds in PBS containing collagenase (2 μg/ml).
The in vitro degradation performance of the hydrogels was also evaluated using pH 7.4 PBS with collagenase (2 μg/ml). As shown in Fig. 2e, all the scaffolds completely degraded after 25 days of incubation with collagenase, and the Col/PDA/HA hydrogel scaffold had a degradation profile comparable to that of the pure Col scaffold. This phenomenon indicated that the incorporation of the PDA/HA complex did not change the biodegradation behavior of the Col matrix for further in vivo application.
2.3. Anti-inflammation and immunomodulation
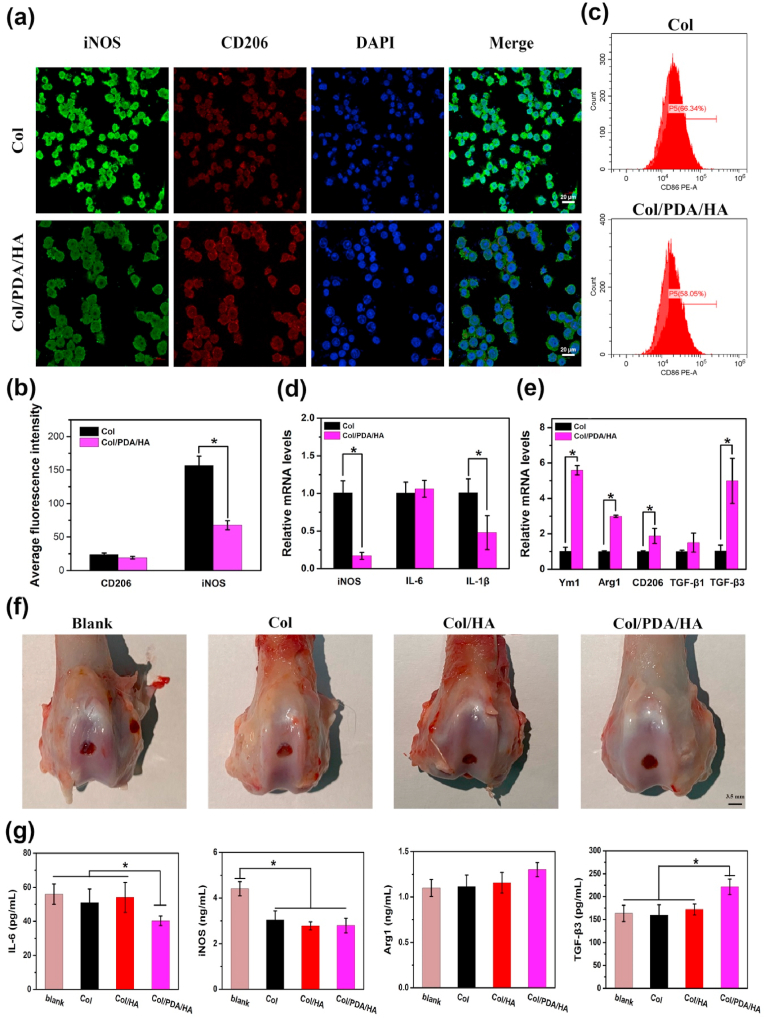
The Col/PDA/HA hydrogel scaffold displayed anti-inflammation and immunomodulation ability. RAW 264.7 macrophages stimulated with lipopolysaccharide (LPS) were seeded on the hydrogel scaffold to determine the anti-inflammation and immunomodulatory effects. After 48 h of culturing, immunofluorescence staining of iNOS (a marker of M1 macrophages) and CD206 (a biomarker of M2 macrophages) was performed for the cells treated with Col and Col/PDA/HA hydrogel scaffolds and observed under a confocal laser scanning microscope (CLSM). As shown in Fig. 3a, the intensity of iNOS-positive cells on Col/PDA/HA hydrogel scaffold was lower than that on Col hydrogel scaffold, while the intensity of CD206-positive cells on Col/PDA/HA hydrogel was higher than that on Col hydrogel scaffolds, which was consistent with the statistical results of fluorescent intensity (Fig. 3b). The results of flow cytometry also revealed that the less CD86 (M1 marker) positive macrophages were detected in the Col/PDA/HA group (Fig. 3c), proving the anti-inflammation activity of PDA/HA-incorporated hydrogel scaffold. To further evaluate the macrophages polarization status, the real-time polymerase chain reaction (RT-PCR) analysis was used to evaluated the expression level of M1 and M2 macrophages marker genes. The results showed that the expression of M2 macrophages marker genes, including Ym1, CD206, Arg1, TGF-β1, and TGF-β3, were significantly elevated by Col/PDA/HA group in contrast to Col group (Fig. 3e). Meanwhile, the expression of M1 macrophages marker genes, including the iNOS and IL-1β remarkably decreased in Col/PDA/HA group (Fig. 3d). The local immunomodulatory effect of the Col/PDA/HA hydrogel scaffold was further evaluated via a in vivo full-thickness cartilage defect model in rabbits. The Col and Col/HA hydrogel scaffolds were also implanted as controls. The defects without any treatment were set as blank control. After 7 days of treatments, the sample-implanted-defects were harvested for gross observation and the synovial fluid were collected for assessing the expression levels of immune-related cytokines. As shown in Fig. 3f, the surface of the Col/PDA/HA-treated defect was the smoothest among all groups. Enzyme linked immunosorbent assay (ELISA) indicated that decreased levels of pro-inflammatory cytokines (IL-6, iNOS) and increased levels of anti-inflammatory cytokine (Arg1) were found from the synovial fluid in the Col/PDA/HA-treated groups (Fig. 3g). Especially, the Col/PDA/HA hydrogel scaffold activated the M2 macrophages to secrete more pro-chondrogenic cytokines (TGF-β1, TGF-β3) (Fig. 3e, g), which were important cytokines to induce the chondrogenesis of BMSCs and regulate cartilage regeneration. All these results demonstrated that Col/PDA/HA hydrogel scaffold modulated the inflammatory environment by effectively inhibiting inflammatory response and activating macrophages polarization to M2 phenotype, which provided a locally pro-chondrogenic microenvironment for cartilage regeneration.
Fig. 3.
Immunomodulatory effect of the Col/PDA/HA hydrogel scaffold. (a) Representative immunofluorescence images of iNOS, and CD206, and nucleic staining of RAW 264.7 macrophages after 48 h of culturing on Col and Col/PDA/HA hydrogel scaffolds. (b) Quantitative analysis of fluorescence intensity of iNOS and CD206. (c) Flow cytometry analysis of RAW 264.7 macrophages treated with Col and Col/PDA/HA hydrogel scaffolds. The relative mRNA levels of (d) pro-inflammatory cytokines (iNOS, IL-6, IL-1β) and (e) anti-inflammatory cytokines (Ym1, Arg1, TGF-β1, TGF-β3) in macrophages. (f) Gross observation of the cartilage defects after 7 days of implantation in different treated groups. (g) ELISA assay of proinflammation cytokines (IL-6, iNOS) and anti-inflammation cytokines (TGF-β3, Arg1) in different treated groups. Error bars were means ± SD, ∗p < 0.05.
2.4. In vitro cell affinity
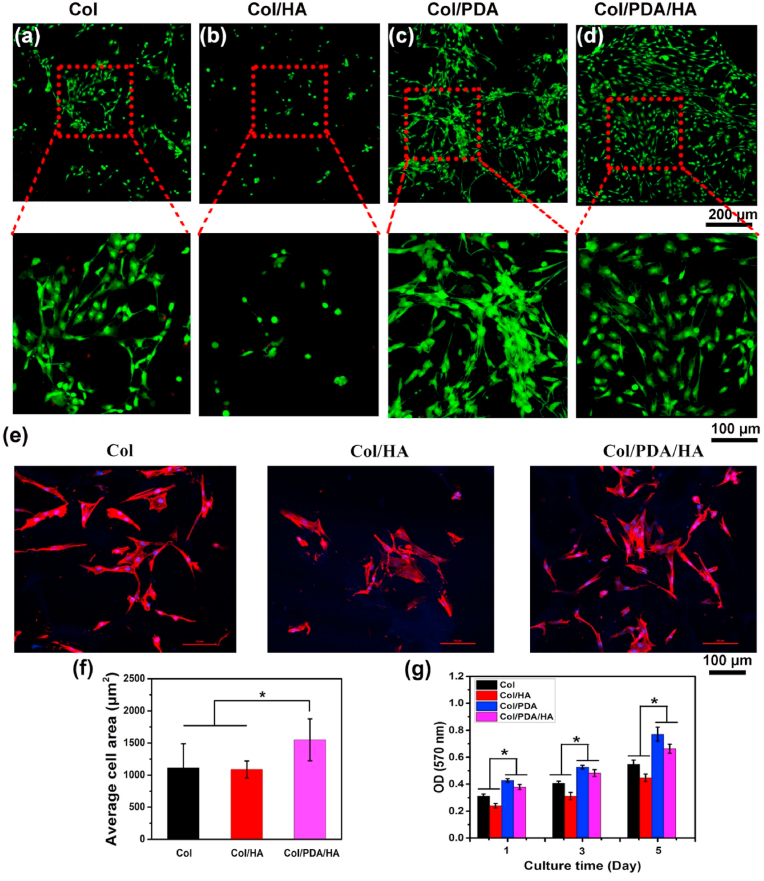
The Col/PDA/HA hydrogel scaffolds exhibited excellent cytocompatibility and cell affinity favoring cell adhesion, spreading, and proliferation. To demonstrate this, BMSCs were seeded on this scaffold and cultured in a basic stem cell maintenance medium. BMSCs were also separately cultured on the Col only, Col/HA, and Col/PDA hydrogel scaffolds for comparison. The morphologies of the BMSCs adhered on various scaffolds were observed using the CLSM on day 3 after stained by LIVE/DEAD Viability/Cytotoxicity Kit assay. As shown in Fig. 4a and b, only few round-shaped cells were observed on the Col/HA scaffold. In contrast, many cells were found on the surface of the Col/PDA and Col/PDA/HA hydrogel scaffolds. Magnified image showed that the majority of cells on the Col/PDA and Col/PDA/HA hydrogel scaffolds displayed typical spindle-like morphology (Fig. 4c and d), proving that the PDA component in the scaffolds facilitated cell adhesion and spreading. The cellular morphology on various hydrogel scaffolds was further evaluated by F-actin cytoskeleton staining. As shown in Fig. 4e, BMSCs cultured on the Col/PDA/HA and Col hydrogel scaffolds displayed more elongated cell morphology with extensive actin filaments that linked adjacent cells. In contrast, the cells on the Col/HA hydrogel scaffold were dispersive, and some of cells displayed spherical morphology with smaller spreading areas (Fig. 4f). Then, the cell proliferation was investigated via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on days 1, 3, and 5. The proliferation of the BMSCs increased on all scaffolds from day 1–5 (Fig. 4g), indicating good biocompatibility of these scaffolds. However, the cells adhered to the Col/HA scaffold showed a lower proliferation rate when compared with the Col only scaffold; this confirmed that the incorporation of HA into the Col only scaffold could hamper cell adhesion. Contrarily, the cells adhered to the Col/PDA/HA hydrogel scaffold showed a higher proliferation rate than those adhered to the Col only and Col/HA scaffolds. The above results proved that the PDA functionalization could improve the cell affinity of HA, and therefore the Col/PDA/HA hydrogel scaffold had better cell affinity to promote the proliferation of BMSCs.
Fig.4.
In vitro cytocompatibility evaluation of the hydrogel scaffolds (a–d) CLSM images of live/dead stained BMSCs on the four kinds of hydrogel scaffolds (Col, Col/HA Col/PDA, and Col/PDA/HA) after 3 days of culturing in primary medium. Live and dead cells were stained green and red, respectively. (e) Phalloidin (red) and DAPI (blue) staining of BMSCs on Col, Col/HA and Col/PDA/HA hydrogel scaffolds on day 3. (f) Quantitative analysis of spreading area of BMSCs on various hydrogel scaffolds. (g) MTT assay of the proliferation of BMSCs on hydrogel scaffolds after 1, 3, and 5 days of culturing in primary medium. Error bars were means ± SD, ∗p < 0.05.
2.5. In vitro chondrogenic differentiation of BMSCs on the Col/PDA/HA hydrogel scaffold
The Col/PDA/HA hydrogel scaffold with high cell affinity also provided cell-supportive physicochemical and structural cues for stimulating chondrogenic differentiation of BMSCs. To evaluate the chondrogenic inducibility the Col/PDA/HA hydrogel scaffold, BMSCs were seeded on this scaffold and cultured in a chondrogenic induction medium. First, cellular morphologies in different culture stages were observed using SEM (Fig. 5). On day 3, the BMSCs adhered to the Col/PDA/HA and Col/PDA scaffolds spread better than those adhered to the Col/HA scaffold and showed a typical spindle and elongated shape (Fig. 5a–i). The observations from SEM images were in agreement with those from CLSM images (Fig. 4a–d), indicating that the presence of PDA enhanced the cell affinity of the scaffolds, and Col/PDA/HA hydrogel scaffold favored the initial adhesion and spreading of BMSCs [44]. After 14 days of culture in the induction medium, the morphological changes of the BMSCs adhered to various scaffolds were observed. The BMSCs cultured on the Col/PDA/HA hydrogel scaffold displayed a round shape (Fig. 5a–iv), suggesting that the BMSCs acquired chondrocyte phenotypes. However, the BMSCs cultured on the Col only scaffold still retained the spindle shapes, and The BMSCs adhered to the Col/HA scaffold also showed a spreading shape, indicating that no differentiation occurred. The morphological transition of BMSCs indicated that the HA component itself in the scaffold could not exert its function to induce any effect on cell morphology.
Fig. 5.
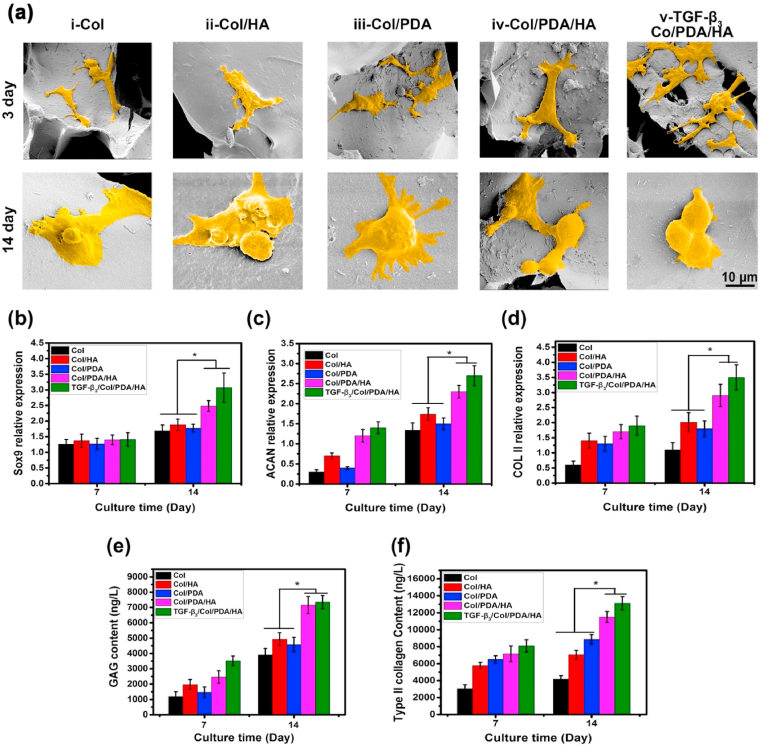
Chondrogenic differentiation of BMSCs on scaffolds. (a) False color SEM images of BMSCs seeded on the four kinds of scaffolds after 3 and 14 days of culturing in chondrogenic medium without addition of TGF-β3. The TGF-β3-loaded Col/PDA/HA hydrogel scaffolds were tested as controls. The relative mRNA levels of chondrogenic markers, including (b) SOX 9, (c) ACAN, and (d) Col II in BMSCs on hydrogel scaffolds at day 7 and 14. Relative production of (e) GAG and (f) type II collagen for BMSCs cultured on the hydrogel scaffolds at 7 and 14 days. Error bars were means ± SD, ∗p < 0.05.
The chondrogenic markers (Sox9, ACAN, and COL II) of the BMSCs cultured on various scaffolds were examined via RT-PCR analysis after 7 and 14 days of culture in chondrogenic media (Fig. 5b–d). On day 7, the expression of the Sox9 gene was similar in all groups. However, the expression of the ACAN and COL II genes in the Col/HA and Col/PDA/HA groups was significantly higher than that in the Col and Col/PDA groups, and Col/PDA/HA groups achieved the highest gene expression level among all samples. After 14 days of culture, the expression of Sox9, ACAN, and COL II genes in all groups were increased. The same trend was found in the expression level of these three genes: Col/PDA/HA > Col/HA > Col/PDA ≈ Col. Note that the expression levels in Col/PDA/HA group were same as those in the TGF-β3-loaded scaffold-treated group. The GAG and type-II collagen contents also confirmed the trends (Fig. 5e and f). All these results showed that Col/PDA/HA hydrogel scaffold improved the adhesion, spreading, and proliferation of BMSCs at early proliferation stage, and effectively induced the chondrogenic differentiation of BMSCs.
2.6. In vivo cartilage repair by the Col/PDA/HA hydrogel scaffolds
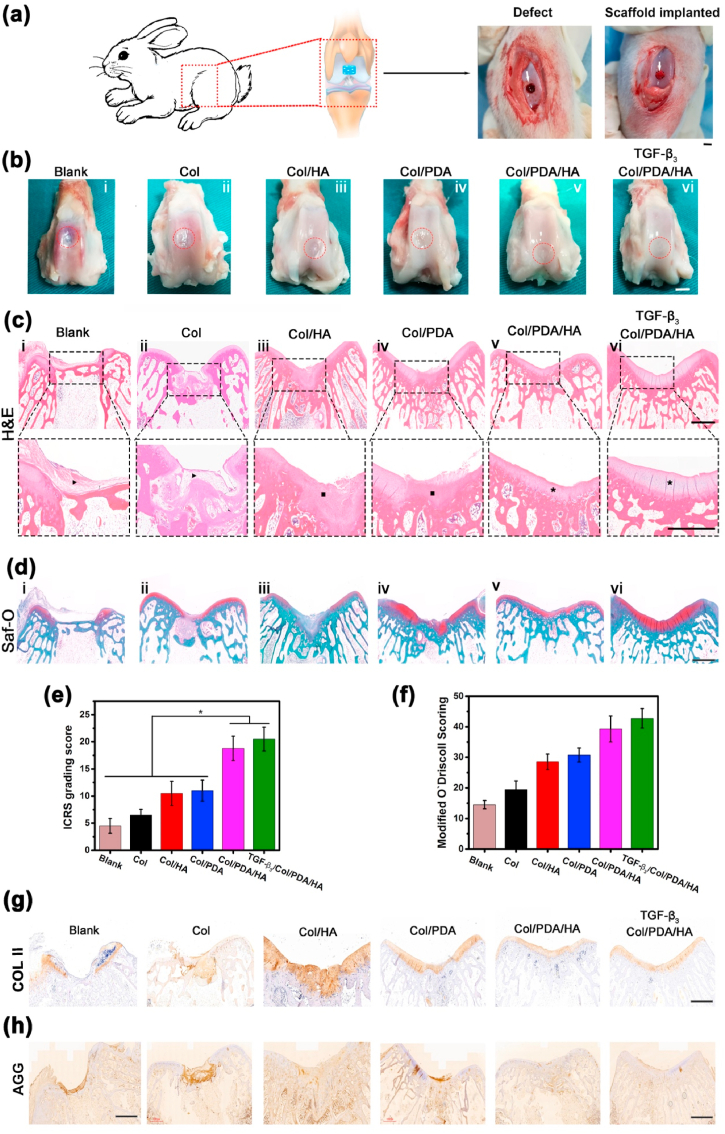
The Col/PDA/HA hydrogel scaffold was implanted into a critical-sized full-thickness cartilage defect to evaluate its ability to support cartilage regeneration in vivo (Fig. 6a). The TGF-β3-loaded Col/PDA/HA hydrogel scaffolds were also implanted as positive groups. After 12 weeks of implantation, macroscopic gross morphology examination showed that the defects in all groups were mostly filled with regenerated tissues (Fig. 6b). In the blank groups, the defects were filled with loose fibrous tissue, and a boundary between the defect and surrounding cartilage was observed (Fig. 6b–i). In the Col scaffold-treated groups, the defects were filled with denser tissue; however, the surface of the defect was rough (Fig. 6b–ii). In the Col/HA scaffold- and Col/PDA scaffold-treated groups, although the repair tissue underneath integrated with the surrounding tissue, the surface seemed fibrillated with a concavity in the center (Fig. 6b–iii, iv). In the Col/PDA/HA hydrogel scaffold-treated group, the defects were filled with cartilage-like tissue having a smooth and congruent surface that integrated well with the surrounding host cartilage (Fig. 6b–v), which was comparable to TGF-β3-loaded Col/PDA/HA hydrogel scaffold-treated group (Fig. 6b–vi). The International Cartilage Repair Society (ICRS) score were calculated for macroscopic evaluation. As shown in Fig. 6e, the ICRS scores of the Col/HA scaffold- (10.5 ± 2.2) and Col/PDA scaffold (11.0 ± 1.9)-treated groups were higher than those of blank (4.5 ± 1.4) and Col scaffold (6.5 ± 1.1)-treated groups. The Col/PDA/HA hydrogel scaffold-treated group had a considerably higher score (18.8 ± 2.3), and the TGF-β3-loaded Col/PDA/HA hydrogel scaffold-treated group presented the highest score (20.5 ± 2.2).
Fig. 6.
In vivo cartilage repair by the Col/PDA/HA hydrogel scaffolds. (a) Schematic illustration of implanting hydrogel scaffold into a full-thickness cylindrical defect in the patellar groove of rabbit. Digital photos of the defect with 3.5 mm in diameter and 5 mm in depth (left), and hydrogel scaffold-implanted defect (right). (b) Macroscopic observation of repaired cartilages after 12 weeks of post-healing in different groups. Histological of repaired cartilages in different scaffolds-treated groups at 12 weeks after surgery. (c) H&E and (d) Safranin-O staining of post-sacrifice defects. Dashed lines indicate approximate margins of defect sites. Black arrows indicate fibrous tissue, Blacks squares indicate newly-regenerated cartilage tissue. Black asterisks indicate with a well-organized cartilage tissue. (e) ICRS macroscopic and (f) Modified O'Driscoll histological scores of repaired cartilages at 12 weeks. Immunohistochemical expression of (g) Type II collagen (COL II) and (h) aggrecan (AGG) in defect sites at 12 weeks. Scar bar: 3.5 mm (b), 1 μm (c, d, g, h). Data are presented as mean ± SD, ∗p < 0.05.
The quality of regenerated cartilage in each group was further evaluated by histological analysis after H&E and Safranin-O staining (Fig. 6c and d). In the blank group, only thin fibrous tissue was present on the surface of the defect, and the surrounding host cartilage was moderately degenerated (Fig. 6c–i). In the Col scaffold-treated group, loose fibrous tissue filled the defect (Fig. 6c–ii). In the Col/PDA scaffold and Col/HA scaffold-treated groups, the defects were partially repaired, and some newly-regenerated cartilage tissue was observed at the defect sites (Fig. 6c–iii, iv). In the Col/PDA/HA hydrogel scaffold-treated group, the defect was completely covered with a well-organized and thick cartilage layer, which was in line and well-integrated with the neighboring host cartilage (Fig. 6c–v). The magnified image showed typical hierarchical zonal cell alignments, indicating that articular cartilage was formed in the defect treated with the Col/PDA/HA hydrogel scaffold. Additionally, the Safranin-O staining images showed almost no red staining in the blank, Col only scaffold-, and Col/HA scaffold-treated defects, indicating no GAG deposition at the defect sites (Fig. 6d). Light red staining was observed in the Col/PDA scaffold-treated groups, implying partial cartilage formation. In sharp contrast, the Safranin-O staining of the defects treated by the Col/PDA/HA hydrogel scaffold was similar to that of the normal surrounding cartilage. In addition, tidemark, an important indicator of the formation of mature cartilage, appeared in the defects treated with the Col/PDA/HA and TGF-β3-loaded Col/PDA/HA ECM scaffolds, suggesting the maturity of the new regenerated cartilage tissues. Nevertheless, the modified O'Driscoll modified score for histological evaluation of cartilage regeneration was significantly better in the Col/PDA/HA hydrogel scaffold-treated groups than in the Col only, Col/PDA, and Col/HA scaffold-treated groups (Fig. 6f).
The quality of regenerated cartilage was further evaluated through the immunohistochemical (IHC) staining for type II collagen (COL II, Fig. 6g) and aggrecan (AGG, Fig. 6h). COL II and AGG did not stain the neotissue in the blank group. Light staining was observed in the Col group. More positive staining of COL II and AGG was shown in the Col/HA group. Intensive COL II but light AGG staining was observed in the Col/PDA group, which indicated that calcification might happen in the cartilage layer. In sharp contrast, intensive staining of both COL II and AGG were detected in the Col/PDA/HA hydrogel scaffold-treated groups, same as that in the TGF-β3-loaded scaffold-treated groups, indicating good production of ECM and integrative cartilage repair in the Col/PDA/HA hydrogel scaffold-treated groups. These results also confirmed the phenotypic stability of the newly formed cartilage in the Col/PDA/HA hydrogel scaffold treated-groups. In vivo cartilage defect regeneration experiments suggested that the superior cartilage repair ability of the Col/PDA/HA hydrogel scaffold resulted in high quality of the repaired cartilage tissue as compared to that in the case of the Col/HA scaffold.
3. Discussions
Owing to the limited self-repair capacity of cartilage, cartilage regeneration is still a clinical challenge. Bioactive scaffolds have been explored as a promising platform for repairing the damaged cartilage. Generally, the Col only scaffold is an ideal scaffold for cartilage tissue engineering. Although the Col only scaffold has cell affinity, it lacks chondrogenic inducibility. Moreover, HA is an active component of GAG in the ECM of cartilage [[45], [46], [47]]. However, the negative charge of HA inhibits cell attachment, and therefore, it is difficult for HA to induce chondrogenic differentiation of the BMSCs. In vitro cell culture results confirmed that the adhesion and spreading of the BMSCs on the Col/HA scaffold were difficult because of the presence of HA that repelled the cells and inhibited cell mobility [30,48,49]. After PDA functionalization, the resulting Col/PDA/HA hydrogel scaffold exhibited improved cell affinity, which intrinsically promoted cell adhesion and spreading. This was because the PDA functionalization introduced abundant reactive catechol groups having high binding affinity to the diverse nucleophiles (e.g., amines, thiols, and imidazoles) of peptides and proteins on the cell surfaces. In the previous studies, PDA-modified HA was generally prepared based on EDC/NHS method [50,51] or thiol-prefunctionalized HA [52,53]. The efficiency of these chemical modifications is limited in terms of the low substitution of catechol groups and tedious preparation process, resulting in insufficient grafting ratio of catechol groups and a fast degradation rate of HA due to chain broken during grafting process. Compared with these previous studies, the advantages of PDA/HA complex in this study are as following. (1) The procedure of preparation of PDA/HA complex in the current study are simply and biocompatible without using additional chemical agents. (2) The main interactions between PDA and HA in the complex was non-covalent interactions, which would not break the HA chains. (3) The catechol groups were efficiently incorporated into the complex. Thus, the Col/PDA/HA hydrogel scaffold proposed herein possesses several technical advantages, including strong cell adhesion due to the presence of PDA in addition to HA, immunomodulatory ability to drive macrophage polarization, promotion of the chondrogenic differentiation of BMSCs, and enhancement of cartilage regeneration in vivo, over conventional scaffolds. The good cartilage regeneration ability of the Col/PDA/HA hydrogel scaffold is attributed to the following reasons.
First, the Col hydrogel scaffold created a cell affinitive 3D microenvironment to support cell adhesion, spreading, and proliferation. The incorporation of PDA-modified HA complex did not alter the cell affinity of the scaffold. SEM observation illustrated that the BMSCs were firmly adhered to the surface of the Col and Col/PDA/HA hydrogel scaffolds (Fig. 5a), suggesting that these cells could be effectively stimulated without further migration. The MTT assay indicated that the Col/PDA/HA hydrogel scaffold could further enhance cell proliferation and increase the cell density in a shorter time (5 days) as compared to the case of the Col/HA and Col hydrogel scaffolds. Thus, the PDA in the hydrogel scaffold plays an important role in directing cell–matrix interactions during BMSCs condensation, a process mediated by surface contacts that results in the aggregation of progenitor cells [54,55].
Second, the Col/PDA/HA hydrogel scaffold exhibited the anti-inflammation and immune modulation ability. Generally, the damage on cartilage initiates inflammatory process, which interfere with the cartilage regeneration process. In this study, the Col/PDA/HA hydrogel scaffold was demonstrated to be able to inhibit inflammation and drive the macrophages of transition from an inflammatory state to a regenerative state, by enhancing the secretion of specific chemokines and cytokines, including Ym1, CD206, Arg-1, TGF-β1, and TGF-β3 (Fig. 3), which therefore exerted multiple functions in tissue regeneration process [56]. Especially, the secretion of TGF-β3 and TGF-β1 was enhanced in the macrophages treated by Col/PDA/HA hydrogel scaffold, which could then mediate chondrogenic differentiation of BMSCs [57,58]. The in vivo examination further confirmed that the immunomodulatory ability of the Col/PDA/HA hydrogel was ascribed to the introduction of PDA, which was in accordance with our previous studies. The reactive catechol groups of PDA actively alleviate the inflammatory response by regulating the activity of macrophages [41]. Therefore, when the Col/PDA/HA hydrogel scaffold was implanted into the defect area, the inflammatory process was inhibited and part of the macrophage population was induced to polarize into M2 macrophages. Thus, the Col/PDA/HA hydrogel scaffold created a regenerative microenvironment for recruiting repairing cells and favoring cartilage tissue regeneration.
Third, the PDA/HA complex endowed biological functions to the Col matrix, which induced the differentiation of the BMSCs into chondrocytes and maintained the chondrocyte phenotype. Most importantly, the cell-affinitive PDA/HA complex overcame the cell–repellent properties of HA, and enhanced the adhesion and clustering of cells. As well-documented, the cellular microenvironment played an important role in promoting BMSCs proliferation and differentiation, and better cell–matrix interactions has profound impact on cell differentiation. In Col/PDA/HA hydrogel scaffold, the Col and PDA could form strong interactions with functional groups of the cell membrane to effectively promote the cell adhesion and spreading [32,59,60]. Thus, the HA segments in the Col/PDA/HA hydrogel scaffold effectively upregulated the chondrogenic gene expression. The proliferation, morphological changes, and cartilage-related gene expression of the BMSCs on the Col/PDA/HA hydrogel scaffold exhibited an increasing trend, suggesting that the PDA/HA complex is more effective in promoting the chondrogenic differentiation of BMSCs in vitro than bare HA. Consequently, the Col/PDA/HA hydrogel scaffold created a suitable biomimetic ECM microenvironment to efficiently induce the chondrocyte differentiation of BMSCs. After implantation, this scaffold could capture and recruit cells and endogenous growth factors from the interstitial fluid of the surrounding tissues to the defect sites. This is an important process in achieving effective cluster formation at an early stage of cartilage regeneration and promotes the formation of cartilage at the defect sites without any chondrogenic cytokines [61,62]. Based on these above results, it was concluded that the Col/PDA/HA hydrogel scaffold could effectively enhance cartilage regeneration by facilitating the adhesion and clustering of cells on the scaffold as well as modulating immune microenvironment due to the synergy effects of PDA, hyaluronic acid, and collagen.
4. Conclusions
In summary, we have fabricated a mussel-inspired Col/PDA/HA hydrogel scaffold mimicking the native ECM of cartilage. This scaffold possesses a suitable porous structure, mechanical strength, good cell affinity, immunomodulatory ability, and chondrogenic inducibility, which provides a biologically active, growth factor-free microenvironment favorable for cell growth, proliferation, and chondrogenic differentiation. Such a mussel-inspired strategy solves the problem of poor cell affinity of traditional polysaccharide-based ECM scaffolds, and prepares cartilage ECM-mimicking scaffolds as next-generation biomaterials for cartilage regeneration.
Funding
This work was financially supported by the Key-Area Research and Development Program of GuangDong Province (2019B010941002), NSFC (82072071, 31800798), Shenzhen Funds of the Central Government to Guide Local Scientific and Technological Development (2021SZVUP123), Fundamental Research Funds for Central Universities (2682020ZT79), the Scientific and Technological Innovation Project financially supported by the Pilot National Laboratory for Marine Science and Technology (Qingdao), and the Projects of Nanjing Normal University (184080H202B283). The authors wish to acknowledge the assistance on materials characterization received from Analytical & Testing Center of the Southwest Jiaotong University.
Ethical statement
Animal surgical procedures were performed in accordance with the protocols approved by the Institutional Animal Ethics Committee of Southwest Jiaotong University and laboratory animal administration rules of China.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.02.006.
Contributor Information
Lu Han, Email: hanlu@ouc.edu.cn.
Xiong Lu, Email: luxiong_2004@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supporting Information contains compositions of various hydrogel scaffolds and details of experiments and characterizations.
References
- 1.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filardo G., Perdisa F., Roffi A., Marcacci M., Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11(1):42. doi: 10.1186/s13018-016-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhard J.C., Vunjak-Novakovic G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther. 2016;7(1):1–9. doi: 10.1186/s13287-016-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Z., Li Y., Gao X., Lei G., Huard J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthritis Cartilage. 2018;26(9):1153–1161. doi: 10.1016/j.joca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Baei P., Daemi H., Mostafaei F., Sayahpour F.A., Baharvand H., Eslaminejad M.B. A tough polysaccharide-based cell-laden double-network hydrogel promotes articular cartilage tissue regeneration in rabbits. Chem Eng J. 2021;418:129277. doi: 10.1016/j.cej.2021.129277. [DOI] [Google Scholar]
- 6.Yang C., Yu Y., Wang X., Wang Q., Shang L. Cellular fluidic-based vascular networks for tissue engineering. Eng Regener. 2021;2:171–174. doi: 10.1016/j.engreg.2021.09.006. [DOI] [Google Scholar]
- 7.Yin H., Wang Y., Sun Z., Sun X., Xu Y., Li P., et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109. doi: 10.1016/j.actbio.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Rathan S., Dejob L., Schipani R., Haffner B., Möbius M.E., Kelly D.J. Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage tissue engineering. Adv Healthcare Mate. 2019;8(7):1801501. doi: 10.1002/adhm.201801501. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W., Ling C., Liu H., Zhang A., Mao L., Wang J., et al. Tannic acid-mediated dual peptide-functionalized scaffolds to direct stem cell behavior and osteochondral regeneration. Chem Eng J. 2020;396:125232. doi: 10.1016/j.cej.2020.125232. [DOI] [Google Scholar]
- 10.Zhang Y., Pizzute T., Pei M. Anti-inflammatory strategies in cartilage repair. Tissue Eng B Rev. 2014;20(6):655–668. doi: 10.1089/ten.teb.2014.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houard X., Goldring M.B., Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji X., Lei Z., Yuan M., Zhu H., Yuan X., Liu W., et al. Cartilage repair mediated by thermosensitive photocrosslinkable TGFβ1-loaded GM-HPCH via immunomodulating macrophages, recruiting MSCs and promoting chondrogenesis. Theranostics. 2020;10(6):2872. doi: 10.7150/thno.41622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai M., Sui B., Hua Y., Zhang Y., Bao B., Lin Q., et al. A well defect-suitable and high-strength biomimetic squid type II gelatin hydrogel promoted in situ costal cartilage regeneration via dynamic immunomodulation and direct induction manners. Biomaterials. 2020;240:119841. doi: 10.1016/j.biomaterials.2020.119841. [DOI] [PubMed] [Google Scholar]
- 14.Dai M., Sui B., Xue Y., Liu X., Sun J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91–103. doi: 10.1016/j.biomaterials.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Cheng G., Dai J., Dai J., Wang H., Chen S., Liu X., et al. Extracellular matrix imitation utilizing nanofibers-embedded biomimetic scaffolds for facilitating cartilage regeneration. Chem Eng J. 2021;410:128379. doi: 10.1016/j.cej.2020.128379. [DOI] [Google Scholar]
- 16.Cai L., Xu D., Chen H., Wang L., Zhao Y. Designing bioactive micro-/nanomotors for engineered regeneration. Eng Regener. 2021;2:109–115. doi: 10.1016/j.engreg.2021.09.003. [DOI] [Google Scholar]
- 17.Mansilla M.O., Salazar-Hernandez C., Perrin S.L., Scheer K.G., Cildir G., Toubia J., et al. 3D-printed microplate inserts for long term high-resolution imaging of live brain organoids. Biomed Eng. 2021;3(1):1–14. doi: 10.1186/s42490-021-00049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8(2):42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugliano M., Vanbellinghen X., Schwinté P., Benkirane-Jessel N., Keller L. Combined jellyfish collagen type II, human stem cells and Tgf-β3 as a therapeutic implant for cartilage repair. J Stem Cell Res Ther. 2017;7(382):2. doi: 10.4172/2157-7633.1000382. [DOI] [Google Scholar]
- 20.Lin K., Zhang D., Macedo M.H., Cui W., Sarmento B., Shen G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv Funct Mater. 2019;29(3):1804943. doi: 10.1002/adfm.201804943. [DOI] [Google Scholar]
- 21.Levingstone T.J., Thompson E., Matsiko A., Schepens A., Gleeson J.P., O'Brien F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016;32:149–160. doi: 10.1016/j.actbio.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese G., Gulino R., Giuffrida R., Forte S., Figallo E., Fabbi C., et al. In vivo evaluation of biocompatibility and chondrogenic potential of a cell-free collagen-based scaffold. Front Physiol. 2017;8:984. doi: 10.3389/fphys.2017.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irawan V., Sung T.-C., Higuchi A., Ikoma T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regener Med. 2018;15(6):673–697. doi: 10.1007/s13770-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilmer C.E., Battistoni C.M., Cox A., Breur G.J., Panitch A., Liu J.C. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater Sci Eng. 2020;6(6):3464–3476. doi: 10.1021/acsbiomaterials.9b01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Yang J., Luo Z., Li D., Lu J., Wang Q., et al. Development of an injectable thiolated icariin functionalized collagen/hyaluronic hydrogel to promote cartilage formation in vitro and in vivo. J Mater Chem B. 2019;7(17):2845–2854. doi: 10.1039/C9TB00211A. [DOI] [PubMed] [Google Scholar]
- 26.Wolf K.J., Kumar S. Hyaluronic acid: incorporating the bio into the material. ACS Biomater Sci Eng. 2019;5(8):3753–3765. doi: 10.1021/acsbiomaterials.8b01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S.-C., Chen C.-H., Wang J.-Y., Lin Y.-S., Chang J.-K., Ho M.-L. Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater. 2018;66:224–237. doi: 10.1016/j.actbio.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Lin H., Beck A.M., Shimomura K., Sohn J., Fritch M.R., Deng Y., et al. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regener. Med. 2019;13(8):1418–1429. doi: 10.1002/term.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeifer C.G., Berner A., Koch M., Krutsch W., Kujat R., Angele P., et al. Higher ratios of hyaluronic acid enhance chondrogenic differentiation of human MSCs in a hyaluronic acid–gelatin composite scaffold. Materials. 2016;9(5):381. doi: 10.3390/ma9050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu D., Wang H., Trinh P., Heilshorn S.C., Yang F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials. 2017;127:132–140. doi: 10.1016/j.biomaterials.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y., Wang P., Li X., Xu Y., Lu G., Jiang Q., et al. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold. Acta Biomater. 2020;111:197–207. doi: 10.1016/j.actbio.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 33.Liu C., Xu X., Cui W., Zhang H. Metal-organic framework (MOF)-based biomaterials in bone tissue engineering. Eng Regener. 2021;2:105–108. doi: 10.1016/j.engreg.2021.09.001. [DOI] [Google Scholar]
- 34.Yang K., Lee J.S., Kim J., Lee Y.B., Shin H., Um S.H., et al. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials. 2012;33(29):6952–6964. doi: 10.1016/j.biomaterials.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Huang Z., Pu X., Chen X., Yin G., Wang Y., et al. Polydopamine/carboxylic graphene oxide-composited polypyrrole films for promoting adhesion and alignment of Schwann cells. Colloids Surf, B. 2020:110972. doi: 10.1016/j.colsurfb.2020.110972. [DOI] [PubMed] [Google Scholar]
- 36.Ko E., Yang K., Shin J., Cho S.-W. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules. 2013;14(9):3202–3213. doi: 10.1021/bm4008343. [DOI] [PubMed] [Google Scholar]
- 37.Xie C., Lu X., Wang K., Yuan H., Fang L., Zheng X., et al. Pulse electrochemical driven rapid layer-by-layer assembly of polydopamine and hydroxyapatite nanofilms via alternative redox in situ synthesis for bone regeneration. ACS Biomater Sci Eng. 2016;2(6):920–928. doi: 10.1021/acsbiomaterials.6b00015. [DOI] [PubMed] [Google Scholar]
- 38.Gan D., Xu T., Xing W., Wang M., Fang J., Wang K., et al. Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J Mater Chem B. 2019;7(10):1716–1725. doi: 10.1039/c8tb01664j. [DOI] [PubMed] [Google Scholar]
- 39.Han L., Wang M., Li P., Gan D., Yan L., Xu J., et al. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl Mater Interfaces. 2018;10(33):28015–28026. doi: 10.1021/acsami.8b05314. [DOI] [PubMed] [Google Scholar]
- 40.Gan D., Wang Z., Xie C., Wang X., Xing W., Ge X., et al. Mussel-inspired tough hydrogel with in situ nanohydroxyapatite mineralization for osteochondral defect repair. Adv Healthcare Mater. 2019;8(22):1901103. doi: 10.1002/adhm.201901103. [DOI] [PubMed] [Google Scholar]
- 41.Jia Z., Gong J., Zeng Y., Ran J., Liu J., Wang K., et al. Bioinspired conductive silk microfiber integrated bioelectronic for diagnosis and wound healing in diabetes. Adv Funct Mater. 2021;31(19):2010461. doi: 10.1002/adfm.202010461. [DOI] [Google Scholar]
- 42.Li Z., Ramay H.R., Hauch K.D., Xiao D., Zhang M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 43.Li S., Tallia F., Mohammed A.A., Stevens M.M., Jones J.R. Scaffold channel size influences stem cell differentiation pathway in 3-D printed silica hybrid scaffolds for cartilage regeneration. Biomater Sci. 2020;8:4458–4466. doi: 10.1039/C9BM01829H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J., Li Y., Liu Y., Li D., Zhang L., Wang Q., et al. Influence of hydrogel network microstructures on mesenchymal stem cell chondrogenesis in vitro and in vivo. Acta Biomater. 2019;91:159–172. doi: 10.1016/j.actbio.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 45.Han L., Grodzinsky A.J., Ortiz C. Nanomechanics of the cartilage extracellular matrix. Annu Rev Mater Res. 2011;41:133–168. doi: 10.1146/annurev-matsci-062910-100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balakrishnan B., Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011;111(8):4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 47.Chang C.-H., Liu H.-C., Lin C.-C., Chou C.-H., Lin F.-H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials. 2003;24(26):4853–4858. doi: 10.1016/S0142-9612(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 48.Bagheri S., Bagher Z., Hassanzadeh S., Simorgh S., Kamrava S.K., Nooshabadi V.T., et al. Control of cellular adhesiveness in hyaluronic acid-based hydrogel through varying degrees of phenol moiety cross-linking. J Biomed Mater Res. 2020 doi: 10.1002/jbm.a.37049. [DOI] [PubMed] [Google Scholar]
- 49.Li L., Yu F., Zheng L., Wang R., Yan W., Wang Z., et al. Natural hydrogels for cartilage regeneration: modification, preparation and application. J Orthopaedic Trans. 2019;17:26–41. doi: 10.1016/j.jot.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv R., Bei Z., Huang Y., Chen Y., Zheng Z., You Q., et al. Mussel-inspired flexible, wearable, and self-adhesive conductive hydrogels for strain sensors. Macromol Rapid Commun. 2020;41(2):1900450. doi: 10.1002/marc.201900450. [DOI] [PubMed] [Google Scholar]
- 51.Guo Z., Xia J., Mi S., Sun W. Mussel-inspired naturally derived double-network hydrogels and their application in 3D printing: from soft, injectable bioadhesives to mechanically strong hydrogels. ACS Biomater Sci Eng. 2020;6(3):1798–1808. doi: 10.1021/acsbiomaterials.9b01864. [DOI] [PubMed] [Google Scholar]
- 52.Yegappan R., Selvaprithiviraj V., Mohandas A., Jayakumar R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf B Biointerfaces. 2019;177:41–49. doi: 10.1016/j.colsurfb.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 53.Yu Q.H., Zhang C.M., Jiang Z.W., Qin S.Y., Zhang A.Q. Mussel-inspired adhesive polydopamine-functionalized hyaluronic acid hydrogel with potential bacterial inhibition. Glob Challen. 2020;4(2):1900068. doi: 10.1002/gch2.201900068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee D.J., Lee Y.-T., Zou R., Daniel R., Ko C.-C. Polydopamine-laced biomimetic material stimulation of bone marrow derived mesenchymal stem cells to promote osteogenic effects. Sci Rep. 2017;7(1):1–14. doi: 10.1038/s41598-017-13326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perikamana S.K.M., Lee J.K., Shin Y.M., Ahmad T., Kim S-j, Park K.M., et al. Oxygen-dependent generation of a graded polydopamine coating on nanofibrous materials for controlling stem cell functions. J Mater Chem B. 2017;5(44):8865–8878. doi: 10.1039/C7TB00995J. [DOI] [PubMed] [Google Scholar]
- 56.Tsukamoto Y., Helsel W., Wahl S. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981;127(2):673–678. [PubMed] [Google Scholar]
- 57.Shen B., Wei A., Tao H., Diwan A.D., Ma D.D. BMP-2 enhances TGF-β3–mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng. 2009;15(6):1311–1320. doi: 10.1089/ten.tea.2008.0132. [DOI] [PubMed] [Google Scholar]
- 58.Luo Z., Jiang L., Xu Y., Li H., Xu W., Wu S., et al. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials. 2015;52:463–475. doi: 10.1016/j.biomaterials.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Lu H., Yan L., Wang K., Fang L., Zhang H., Tang Y., et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017;9(4):e372. doi: 10.1038/am.2017.33. [DOI] [Google Scholar]
- 60.Han L., Lu X., Wang M., Gan D., Deng W., Wang K., et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017;13(2):1601916. doi: 10.1002/smll.201601916. [DOI] [PubMed] [Google Scholar]
- 61.Taraballi F., Bauza G., McCulloch P., Harris J., Tasciotti E. Concise review: biomimetic functionalization of biomaterials to stimulate the endogenous healing process of cartilage and bone tissue. Stem Cells Transl Med. 2017;6(12):2186–2196. doi: 10.1002/sctm.17-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Yuan X., Yu K., Meng H., Zheng Y., Peng J., et al. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 2018;171:118–132. doi: 10.1016/j.biomaterials.2018.04.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.