Abstract
Palmar and plantar fibromatosis are benign proliferative processes which present as a diffuse thickening or nodules of the hands and/or feet and may lead to flexion contractures, pain, and functional impairment known as Dupuytren and Ledderhose diseases, respectively. Current treatments are noncurative and associated with significant morbidity. Here, we report on the outcomes of 5 patients with advanced disease, no longer surgical candidates, treated with sorafenib. Sorafenib exhibited an expected safety profile. All 5 patients demonstrated objective responses as evaluated by a decrease in tumor size and/or tumor cellularity from baseline and all 5 patients reported subjective pain relief and/or functional improvement. Mechanistically, immunohistochemistry revealed patchy positivity for PDGFRβ, a known target of sorafenib. The outcomes of these 5 patients suggest the safety and efficacy of a relatively well-tolerated oral agent in the treatment of Dupuytren and Ledderhose diseases and suggest the need for future controlled studies.
Keywords: dupuytren disease, ledderhose disease, fibromatosis, tyrosine kinase inhibitor, sarcoma
Palmar and plantar fibromatosis are benign proliferative processes that present as a diffuse thickening or nodules of the hands and/or feet and may lead to Dupuytren or Ledderhose disease. This article describes outcomes of five patients with advanced disease who were treated with sorafenib.
Background
Palmar and plantar fibromatosis, subtypes of superficial fibromatoses, are relatively common benign proliferative processes which present as a diffuse thickening or nodules along the aponeuroses of the hands and/or feet and may lead to flexion contractures, pain, and functional impairment known as Dupuytren and Ledderhose diseases, respectively. Current treatments are noncurative and involve repeated intralesional injections of steroids, collagenase, and/or surgical resection, and are frequently associated with significant morbidity.1
Unlike deep fibromatosis (desmoid tumor), there is evidence that this is a reactive, rather than clonal, process.2 Alterations in Wnt signaling have been implicated in both deep and superficial fibromatoses, however, unlike desmoid tumors (which harbor mutations in CTNNB1 or APC, resulting in β-catenin activation), the underlying biology driving the superficial fibromatoses remains poorly understood.3,4 We recently reported on the activity of the oral multitargeted receptor tyrosine kinase inhibitor sorafenib in desmoid tumor.5 Here we present a case series of 5 patients with Dupuytren and/or Ledderhose disease treated with sorafenib. The results demonstrated an expected side-effect profile, suggest potential efficacy in this population, and provide insight into the underlying biology of the superficial fibromatoses.
Methods and Results
Following approval from our Institutional Review Board (MSK# 16-1101), we retrospectively reviewed the efficacy and safety of off-label sorafenib (initial dose: 200-400 mg orally daily) in 5 patients with advanced Dupuytren (n = 4) and/or Ledderhose (n = 2) disease (2 patients have both palmar and plantar disease). Four patients have recurrent disease after surgical resection (range: 1-4 surgeries) and 3 have the multifocal disease (range: 1-6 lesions). Patients were considered for off-label sorafenib if they had advanced disease which was no longer amenable to or refractory to standard of care therapies and had symptomatic disease resulting in significant pain or functional impairment. As surgery remains a standard of care in this disease, all 5 patients had been previously evaluated by surgeons and were deemed nonoperable due to potential morbidity due to either extensive prior resection(s) and/or the extent of disease infiltration. Importantly, sorafenib exhibited an expected safety profile.5 Three patients temporarily held sorafenib for grade 2 hypertension, all of whom re-started sorafenib with good tolerance after initiation of antihypertensive monotherapy. One patient permanently discontinued sorafenib due to grade 2 hidradenitis suppurativa which failed to improve despite aggressive dermatological management. There were no grade 3 or 4 toxicities seen in this population. At the time of publication, the 3 patients who remain in active follow-up have been dose reduced (range: 200 mg 3 times a week—200 mg daily) with good tolerance.
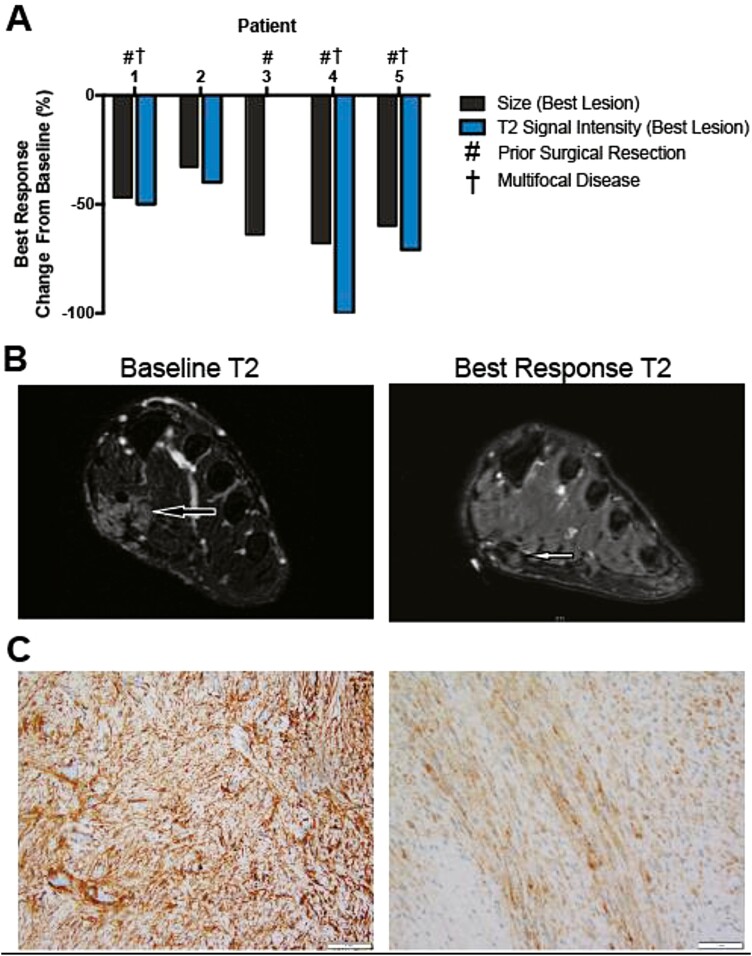
All 5 patients demonstrated objective responses as evaluated by a decrease in tumor size (WHO criteria) and/or tumor cellularity (T2 fat-saturated signal intensity) from baseline, including 1 pathological complete response not captured in interval imaging (Fig. 1A and B).6 While any subanalysis is limited by small sample size, there was no significant difference in tumor response in plantar versus palmar lesions. Importantly, all 5 patients reported subjective pain relief and/or functional improvement. Disease responses were durable while on sorafenib (mean follow-up = 17.0 months; range 11-27 months), including during periods of dose reduction. However, 4 of 5 patients experienced disease progression during treatment breaks and 1 patient was lost to follow up. In the 4 evaluable samples, immunohistochemistry was negative for nuclear accumulation of beta-catenin, VEGFR2, PDGFRα, and TGFβR3; 3 of 4 samples were patchy positive for PDGFRβ, a known target of sorafenib (Fig. 1C).
Figure 1.
Sorafenib results in decrease in tumor size and cellularity in superficial fibromatosis. (A) Response of 5 patients with plantar (n = 4) and/or palmar (n = 2) fibromatosis to sorafenib (starting dose 200-400 mg orally daily); 4 of which have recurrent disease after surgical resection and 3 of which have multifocal disease. Response is measured as percent change from baseline tumor size (WHO criteria) and tumor cellularity (MRI T2 fat-saturated signal intensity) in the single tumor with the best response. Tumor cellularity is a measure of tumor collagenization, and therefore, a surrogate marker of tumor growth potential.6 Change in tumor size of the lesion with the best response from baseline (black bars; best lesion: 33%-68%; mean of all lesions: 26%-64%). Change in tumor cellularity of the lesion with the best response from baseline (blue bars; best lesion: 0%-100%; mean of all lesions: 0%-62%). (B) Representative MRI T2 fat-saturated images of baseline and best response lesion from patient 4. (C) Representative images of PDGFRβ immunohistochemistry in superficial fibromatoses demonstrates patchy positivity.
Discussion
This case series explores the use of sorafenib in patients with advanced Dupuytren and/or Ledderhose disease not amenable to further local interventions due to procedural morbidity. Sorafenib exhibited an expected side-effect profile.5 Importantly, all 5 patients demonstrated an objective response which was associated with substantial symptomatic improvement. These responses were durable even with dose reductions of sorafenib, suggesting that lower doses, which are associated with significantly lower rates of adverse events, may be efficacious. This is important as ongoing treatment was required to maintain disease response in this case series.
The outcomes of these 5 patients suggest the efficacy of a relatively well-tolerated oral agent in the treatment of Dupuytren and/or Ledderhose disease, offering the potential of sparing patients the morbidity of repeated invasive procedures, and suggest the need for future controlled studies. This study highlights the power of re-purposing oncological drugs in a benign condition and aims to aid our understanding into the underlying biology; given the pleiotropic effects of sorafenib, further investigation is required to better define the mechanism driving disease response, possibly through inhibition of the PDGF pathway.
Contributor Information
Joshua D Schoenfeld, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
Narasimhan P Agaram, Department of Pathology, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
Robert A Lefkowitz, Department of Radiology, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
Ciara M Kelly, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
John H Healey, Department of Surgery, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
Mrinal M Gounder, Department of Medicine, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA.
Funding
This research was supported by the Draper Family Fund, Gounder Philanthropic Research Fund, and the National Cancer Institute, National Insititue of Health (P30CA008748) - core grant (CCSG shared resources and core facility).
Conflict of Interest
Mrinal Gounder: Bayer (RF), Bayer, Karyopharm, Epizyme, Springworks, Daiichi, Amgen, Tracon, Flatiron, Medscape, Physicians Education Resource, Guidepoint, GLG, UpToDate (C/A), Athenex, Ayala, Bayer, Boehringer Ingelheim, Daiichi, Epizyme, Flatiron Health, GLG, Guidepoint, Karyopharm, Rain, Springworks, Third Bridge, Tracon, TYME (C/A); Athenex, Ayala, Bayer, Boehringer Ingelheim, Daiichi, Epizyme, Flatiron Health, GLG, Guidepoint, Medscape, More Health, Karyopharm, Physicians Education Resource, Rain, Springworks, Third Bridge, touchIME, Tracon, TYME (H); Ciara Kelly: Merck, Amgen, Servier, Exicure, Xencor, Kartos (RF), Exicure, Kartos (C/A), Immunicum (SAB), Daicchi Sankyo (E--spouse); John Healey: Daicchi Sankyo (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/Design: M.M.G. Provision of study material or patients: M.M.G., C.M.K., and J.H.H. Collection and/or assembly of data: J.D.S., N.P.A., and R.A.L. Data analysis and interpretation: J.D.S., N.P.A., R.A.L., and M.M.G. Manuscript writing: J.D.S. and M.M.G. Final approval of manuscript: all authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Denkler KA, Vaughn CJ, Dolan EL, Hansen SL. Evidence-based medicine: options for Dupuytren’s contracture: incise, excise, and dissolve. Plast Reconstr Surg. 2017;139(1):240e-255e. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Zhu H. Clonal analysis of palmar fibromatosis: a study whether palmar fibromatosis is a real tumor. J Transl Med. 2006;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dolmans GH, Werker PM, Hennies HC, et al. ; Dutch Dupuytren Study Group; German Dupuytren Study Group; LifeLines Cohort Study; BSSH-GODD Consortium. Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011;365(4):307-317. [DOI] [PubMed] [Google Scholar]
- 4. Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod Pathol. 2001;14(7):695-701. [DOI] [PubMed] [Google Scholar]
- 5. Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379(25):2417-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cassidy MR, Lefkowitz RA, Long N, et al. Association of MRI T2 signal intensity with desmoid tumor progression during active observation: a retrospective cohort study. Ann Surg. 2020;271(4):748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.