Abstract
Background
Amid continued uncertainty about the management of cancer patients during the pandemic, this study sought to obtain real-world data on the use of immune checkpoint inhibitors (ICIs) before COVID-19 diagnosis and its association with severity and survival outcomes in cancer patients who contracted COVID-19.
Methods
Cancer patients diagnosed with COVID-19 were identified from a large electronic health record database; those treated with ICIs before COVID-19+ diagnosis were matched in a 1:2 ratio to those not treated with ICIs, using a 2-step matching procedure. A descriptive analysis examined the difference in COVID-19 mortality (30-day and overall) and severity outcomes between the 2 cohorts, and overall survival was compared.
Results
Among 17 545 adults ≥18 years with cancer who tested positive for COVID-19 between February 20, 2020, and January 28, 2021, in the US, 228 ICI-treated patients were matched to 456 non-ICI-treated patients, comprising the 2 study cohorts. Clinical characteristics differed significantly between the 2 cohorts before matching, with metastatic disease, lung cancer, a history of smoking, and the presence of pulmonary comorbidities being more common in the ICI-treated cohort; after matching, the 2 cohorts were similar. There were no significant differences between the ICI-treated and non-ICI-treated cohorts for 30-day mortality (12.7% vs. 14.9%, P = .235), overall mortality (22.4% vs. 22.4%, P = 1.000), hospitalization (38.6% vs. 39.0%, P = .912), or emergency department visits (16.7% vs. 14.7%, P = .500). Overall survival was similar between the 2 cohorts.
Conclusion
This analysis adds to the clinical evidence base that use of ICIs before SARS-CoV-2 infection does not affect COVID-19 severity or survival outcomes, supporting the continued use of ICIs in cancer patients during the pandemic.
Keywords: checkpoint inhibitors, COVID-19, cancer
At the intersection of COVID-19 and cancer lies a population vulnerable to infection because of underlying disease and possible immunosuppression. This article examines whether cancer patients receiving immune checkpoint inhibitors (ICIs) were at greater risk of severe COVID-19 outcomes than cancer patients who were not receiving ICIs.
Implications for Practice.
A key question in oncology practice during the global COVID-19 pandemic is whether immune checkpoint inhibitor (ICI) treatment leads to different COVID-19 outcomes. To date, further data is needed to clarify the potential impact—negative, positive, or neither—of ICI use in the context of COVID-19. This study adds to the evidence base that use of ICIs before COVID-19 infection does not affect the disease severity or survival outcomes. The study not only filled an important evidence gap but also supported clinicians to make evidence-based decisions that enabled cancer patients to continue to benefit from ICI without added risk.
Introduction
This has been a tumultuous year for health care delivery. Upended by patients wary of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and by physicians and hospitals stretched by the urgent needs of COVID-19-positive (+) patients, telehealth prevailed while many elective procedures, treatments, and examinations were disrupted, reprioritized, or delayed.1-3
At the intersection of COVID-19 and cancer lies a population vulnerable to infection because of underlying disease and possible immunosuppression.1,2 Studies early in the pandemic found that in addition to increased vulnerability to infection, cancer patients are at higher risk for severe COVID-19 outcomes, including increased mortality.2,4,5 Data from international registry studies by the COVID-19 and Cancer Consortium (CCC19) and the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) indicate an increased risk of mortality in older patients, males, smokers, and among patients with comorbidities and show that patients diagnosed earlier in the pandemic had worse outcomes than those diagnosed later.4-6
Given that cancer patients with COVID-19 have elevated risks of disease severity and mortality, there is a compelling need to characterize the impact of various cancer treatments on COVID-19 outcomes to guide clinical decision-making. While immune checkpoint inhibitors (ICIs) or chemotherapy were found to be risk factors for severe COVID-19 outcomes by some researchers, others found no such association and support the safety of ICI treatment during the pandemic.4,7-16 For example, analysis of data in multiple cancer types collected from Memorial Sloan Kettering Cancer Center (MSKCC) between March 2020 and April 2020 found an association between ICI treatment and higher frequencies of hospitalizations and severe respiratory illness.13 In contrast, several studies reported that ICIs do not negatively impact COVID-19 outcomes; immunotherapy administered to cancer patients within 4 weeks of contracting COVID-19 was found to have no significant impact on mortality.7,9 Studies on lung cancer patients at MSKCC found that disease severity was not affected by prior administration of programmed death-ligand 1 (PD-[L]1) blockade (with or without chemotherapy).10,11 The TERAVOLT registry demonstrated that immunotherapy did not worsen outcomes for patients with thoracic neoplasms and COVID-19.4 It should be noted that contemporary evidence of the impact of ICIs on COVID-19 outcomes is emerging and remains unknown. Preliminary evidence generated from real-world studies have yielded conflicting results because of small cohorts and limited data collected early in the pandemic.
Concerns about the effects of ICI treatment on COVID-19 severity and mortality arise in part from lingering uncertainty about the physiological similarities between immune-related adverse events induced by ICIs and the COVID-19-induced cytokine-release syndrome (CRS) or cytokine storm.1,2,17,18 While patients infected with SARS-CoV-2 could theoretically benefit from the enhanced T-cell activity induced by treatment with ICIs, allowing greater immunologic control of viral infection, there is concern that ICIs could potentiate immune hyperactivation in some patients, resulting in poorer outcomes by triggering CRS or ICI-related pneumonitis.1,2,17 Some of these concerns arise from preclinical data which show that PD-L1/PD-1 blockade is associated with an exacerbation of inflammation during acute viral infection.19
There is an urgent need for real-world evidence from larger cohorts of cancer patients treated with ICIs to better guide clinicians about the safety of their use during the pandemic. The present study sought to fill the evidence gap by examining whether cancer patients receiving ICIs were at greater risk of severe COVID-19 outcomes, including mortality, than cancer patients who were not receiving ICIs. We used recent data from a large cohort of United States (US) patients infected with SARS-CoV-2 in the Optum deidentified electronic health record (EHR) database.
Methods
Data and Patient Selection
The study population and data were extracted from the Optum deidentified COVID-19 EHR dataset, a COVID-19-enriched subset of an Optum EHR-based database, which is payer agnostic and sourced directly from US providers. It represents patient-level clinical and administrative data from large integrated delivery networks of ambulatory practices, which were deidentified in compliance with the HIPAA (Health Insurance Portability and Accountability Act) Expert Method and managed according to Optum customer data use agreements. The data are updated on a monthly basis.
COVID-19+ cases were confirmed either by a positive laboratory test or by an International Classification of Diseases, 10th Revision (ICD-10) diagnostic code. Following the World Health Organization and Centers for Disease Control and Prevention coding guidelines, patients defined as having a confirmed COVID-19+ diagnosis were those who had at least one of the following: a diagnosis code of U07.1, U07.2; a positive diagnostic test for COVID-19; or a diagnosis code of B97.29 without a negative molecular SARS-CoV-2 test within a 14-day window.20 The index date was defined as the first instance of a laboratory-confirmed or presumptive COVID-19+ diagnosis according to the above criteria. The baseline period was defined as the 180 days leading up to the index date. For the current analysis, all confirmed COVID-19+ cases between February 20, 2020, and January 28, 2021, were included.
To identify adult patients who contracted COVID-19 and who also had cancer, patients had to meet all 3 of the following criteria: (1) be at least 18 years of age in the year of the confirmed COVID-19+ diagnosis; (2) have any ICD-10 cancer diagnosis up to 180 days before the index date; and (3) have valid and complete follow-up information without missing data on date of death.
Identification of Study Cohorts
To examine the impact of ICI treatment on COVID-19 outcomes, we further defined 2 study cohorts: an ICI-treated cohort and a non-ICI-treated cohort. Patients in the ICI-treated cohort were those who had received any type of ICI cancer treatment (any class, with or without chemotherapy) within 90 days before the index date. These included PD1 (cemiplimab, nivolumab, and pembrolizumab), PD-L1 (atezolizumab and avelumab), and CTLA-4 (ipilimumab) antibodies. ICI-based regimens were identified by drug codes or string search in the data (Supplementary Table S1). Patients in the non-ICI-treated cohort were those who had no evidence of any ICI treatment at any time in the dataset. Thus, patients who only had evidence of ICI treatment after the index date or 90 days or more before the index date were further excluded from the analysis.
Statistical Analysis
Baseline demographic and clinical characteristics were reported for the ICI-treated and non-ICI-treated cohorts. Categorical variables were summarized using counts and percentages, and continuous variables were summarized using means and SDs. The primary study outcomes were mortality (30-day and anytime) and severity of COVID-19, measured by hospitalization and emergency department (ED) visit rates (occurred within 7 days before or within 30 days after COVID-19+ diagnosis). Patients were followed up from the index date through the end of data follow-up (date of the last record) or death, whichever occurred first.
Balancing Approach on Observable Characteristics
A two-step matching procedure was used to reduce confounding because of systematic differences in observed baseline characteristics between ICI-treated and non-ICI-treated cohorts when estimating the treatment effect. First, matching was performed based on propensity score, with nearest neighbor matching with a ≤0.05 caliper width. Propensity scores were estimated from 17 variables including age, gender, region, race, smoking status, tumor type, presence of metastatic disease, month of COVID-19+ diagnosis, Charlson comorbidity index [CCI], and 9 comorbid conditions (cardiovascular disease, chronic obstructive pulmonary disease [COPD], chronic respiratory disease, diabetes mellitus, hypertension, liver disease, obesity, HIV, and kidney disease). Comorbidities were assessed using the relevant ICD-10 diagnosis codes in the year before the index date; the CCI is a summary metric describing comorbidity burden.21 The calendar month of COVID-19+ diagnosis was included in the model to account for the fast-evolving nature of the pandemic and treatments for COVID-19 as the pandemic progressed. In addition to matching by propensity score, patients in the ICI-treated cohort were exactly matched in a 1:2 ratio to non-ICI-treated patients who shared the same tumor type (lung cancer vs. others), gender, region, and month of COVID-19+ diagnosis.
Assessment of Treatment Effect
Descriptive statistics and outcomes were compared to assess the differences between the matched ICI-treated versus non-ICI-treated patient cohorts. The Wilcoxon 2-sample test (2-sided) was used to compare means for continuous variables, and the chi-square test was used for categorical variables. Kaplan-Meier survival analysis was conducted to compare survival between the matched study cohorts, with patients censored by their last activity date. Differences between groups were examined using P values from log-rank tests.
Subgroup and Sensitivity Analyses
For patients in the ICI-treated cohort, a descriptive subgroup analysis was conducted to further compare the outcomes between patients who received ICI-based regimens with chemotherapy versus those whose cancer treatments only included ICI. Patients were stratified based on evidence of chemotherapy use within 90 days before the index date. We compared stratum-specific outcomes between the 2 subgroups of patients within the ICI-treated cohort.
A sensitivity analysis using the Cox proportional hazards model was conducted on the entire patient cohort with COVID-19 to further investigate the association between patient survival and the receipt of ICI treatment (Supplementary Table S2). All variables used in the propensity score model and exact matching were included in the Cox proportional hazards model as relevant covariates.
All analyses were performed using SAS Studio Release: 3.7 (Enterprise Edition), 2012-2017, SAS Institute Inc., Cary, NC, USA.
Results
Patient Selection and Clinical Characteristics
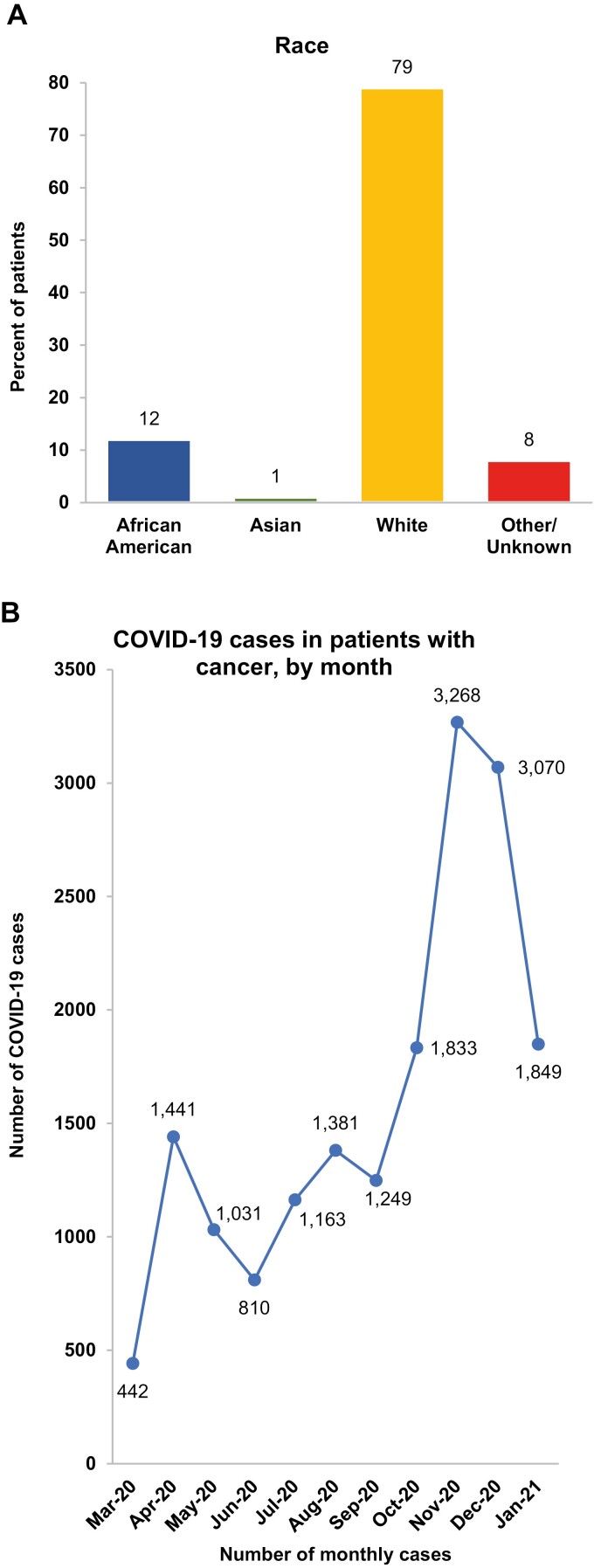
Among all patients included in the COVID-19-enriched Optum EHR dataset, 591 198 were confirmed as having a diagnosis of COVID-19 between February 20, 2020, and January 28, 2021. Among these, a total of 17 545 adults were identified as being diagnosed with cancer and having valid follow-up information. The overall cohort of COVID-19+ cancer patients was composed primarily of Whites (79%), followed by African Americans (12%), as shown in Fig. 1A. The mean age of patients was 66 years, with nearly equal gender distribution (51% female vs. 49% male). Geographically, the cohort is represented by patients from across the US, with 50.2% residing in the Midwest, 25.6% in the Northeast, 16.6% in the South, and 5.6% in the West.
Figure 1.
Characteristics of the COVID-19-enriched Optum electronic health record (EHR) database. (A) Proportion of patients by race/ethnicity in the COVID-19 enriched Optum EHR database. (B) Number of monthly cases of COVID-19 in patients with cancer in the COVID-19 enriched Optum EHR database.
The trend of COVID-19+ diagnoses in cancer patients initially peaked in April 2020, and after decreasing in May and June 2020, began gradually rising throughout the summer of 2020; diagnoses rose sharply in the late fall, peaking in November 2020 at more than twice the level in April 2020, as shown in Fig. 1B. Overall, nearly half the total COVID-19+ cancer patients were diagnosed during the holiday season from November 2020 to January 2021.
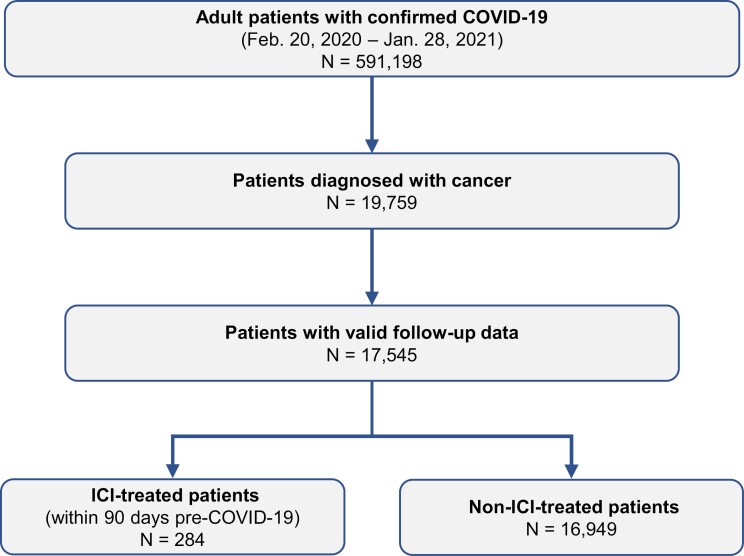
The final study population of COVID-19+ adult cancer patients consisted of 284 patients who were treated with ICIs and 16 949 non-ICI-treated patients (Fig. 2). Before matching, patients with and without recent exposure to ICIs had significantly different clinical characteristics, as shown in Table 1A. Compared with non-ICI-treated patients, metastatic disease was significantly more common in the ICI-treated group (78.9% vs. 14.5%), as was the presence of lung cancer (53.2% vs. 7.6%) and other solid tumors (96.8% vs. 83.6%), all P < .001. More ICI-treated patients had smoking experience than those in the non-ICI-treated group (11.6% vs. 7.2% current smoker; 54.6% vs. 33.6% former smoker, all P < .001), although the smoking status of nearly 20% of patients in the non-ICI-treated group was unknown, which could have influenced the data on smoking status. Patients with recent exposure to ICIs also had higher comorbidity burden (mean CCI: 6.4 vs. 2.2, P < .001). In particular, pulmonary conditions were significantly more common among patients in the ICI-treated group including COPD (34.5% vs. 14.7%, P < .001) and chronic respiratory disease (45.8% vs. 24.6%, P < .001). In contrast, obesity was significantly more common in the non-ICI-treated group than in the ICI-treated group (26.8% vs. 36.2%, P = .001), and patients in the non-ICI-treated group had a higher mean body mass index (27.0 vs. 29.7, P < .001).
Figure 2.
Selection of study cohorts: ICI-treated and non-ICI-treated. Feb, February; ICI, immune checkpoint inhibitor; Jan, January.
Table 1.
Patient demographics and clinical characteristics.
| A: All patients before matching | B: Matched cohorts | |||||
|---|---|---|---|---|---|---|
| ICI-treated (N = 284) | Non-ICI-treated (N = 16 949) | Difference P value | ICI-treated (N = 228) | Non-ICI-treated (N = 456) | Difference P value | |
| Demographics | ||||||
| Age, years, mean (SD) | 66.4 (12.9) | 66.4 (14.0) | .914 | 66.5 (13.7) | 68.6 (12.7) | .978 |
| Gender, % female | 43.3 | 51.3 | .025 | 43.4 | 43.4 | 1.000 |
| Race, % | .250 | .394 | ||||
| African American | 13.4 | 11.7 | 11.8 | 14.7 | ||
| Asian | 1.4 | 1.4 | 0.9 | 2.0 | ||
| White | 74.3 | 78.8 | 75.9 | 74.3 | ||
| Other/unknown | 10.9 | 8.1 | 11.4 | 9.0 | ||
| Ethnicity, % | .792 | .345 | ||||
| Hispanic | 7.0 | 6.1 | 8.3 | 5.7 | ||
| Not Hispanic | 85.9 | 87.1 | 83.8 | 84.7 | ||
| Unknown | 7.0 | 6.7 | 7.9 | 9.7 | ||
| Region, % | <.001 | 1.000 | ||||
| Midwest | 50.0 | 50.3 | 47.8 | 47.8 | ||
| Northeast | 34.9 | 25.4 | 37.3 | 37.3 | ||
| Other/unknown | 1.8 | 2.1 | 0.9 | 0.9 | ||
| South | 7.4 | 16.7 | 8.3 | 8.3 | ||
| West | 6.0 | 5.6 | 5.7 | 5.7 | ||
| Clinical characteristics | ||||||
| Type of cancer, % | ||||||
| Solid tumor | 96.8 | 83.6 | <.001 | 97.4 | 92.5 | .011 |
| Lung cancer | 53.2 | 7.6 | <.001 | 41.7 | 41.7 | 1.000 |
| Hematologic | 3.2 | 16.4 | <.001 | 2.6 | 7.5 | .011 |
| Metastatic disease, % | 78.9 | 14.5 | <.001 | 74.6 | 74.6 | 1.000 |
| Smoking status, % | <.001 | .063 | ||||
| Current smoker | 11.6 | 7.2 | 9.7 | 12.9 | ||
| Previously smoked | 54.6 | 33.6 | 50.4 | 41.9 | ||
| Never smoked | 26.4 | 41.1 | 32.0 | 32.2 | ||
| Unknown | 7.4 | 18.1 | 7.9 | 12.9 | ||
| Comorbidities | ||||||
| CCI, mean (SD) | 6.4 (3.1) | 2.2 (2.9) | <.001 | 6.1 (3.3) | 6.0 (3.3) | .639 |
| Pulmonary | ||||||
| COPD, % | 34.5 | 14.7 | <.001 | 29.4 | 27.0 | .507 |
| Chronic respiratory disease, % | 45.8 | 24.6 | <.001 | 41.7 | 35.8 | .132 |
| Cardiovascular, % | 65.9 | 60.4 | .060 | 65.8 | 68.4 | .488 |
| Hypertension, % | 57.0 | 57.5 | .881 | 56.6 | 66.0 | .016 |
| Obesitya, % | 26.8 | 36.2 | .001 | 28.5 | 30.7 | .555 |
| BMI, mean (SD) | 27.0 (6.6) | 29.7 (7.3) | <.001 | 27.3 (6.7) | 28.4 (6.8) | .051 |
Note: P values are shown for Wilcoxon 2-sample test (2-sided) to compare means for continuous variables and chi square test for categorical variables.
Obesity was defined by BMI > 30 or having ICD code for obesity.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitor; SD, standard deviation.
After implementing the 2-step matching procedure, 228 patients from the ICI-treated cohort were matched to 456 patients in the non-ICI-treated cohort. Characteristics of ICI-treated patients and their matched counterparts are presented in Table 1B. After matching, patients in both cohorts had similar demographic and clinical characteristics, except for the proportion of patients with hematological malignancies and hypertension. Because of the exact-matching requirements, patients in both groups had the same gender and geographic distributions. The percentages of patients with metastatic disease and lung cancer were also the same in both cohorts. Smoking status for patients who had and had not received ICIs was not statistically different. Most patients in both cohorts had a history of smoking (50.4% for ICI-treated vs. 41.9% for non-ICI-treated), and percentages of active smokers were also similar between cohorts (9.7% vs. 12.9%, respectively). Patients in both cohorts had similar comorbidity burden including pulmonary comorbidities and obesity.
COVID-19 Outcomes
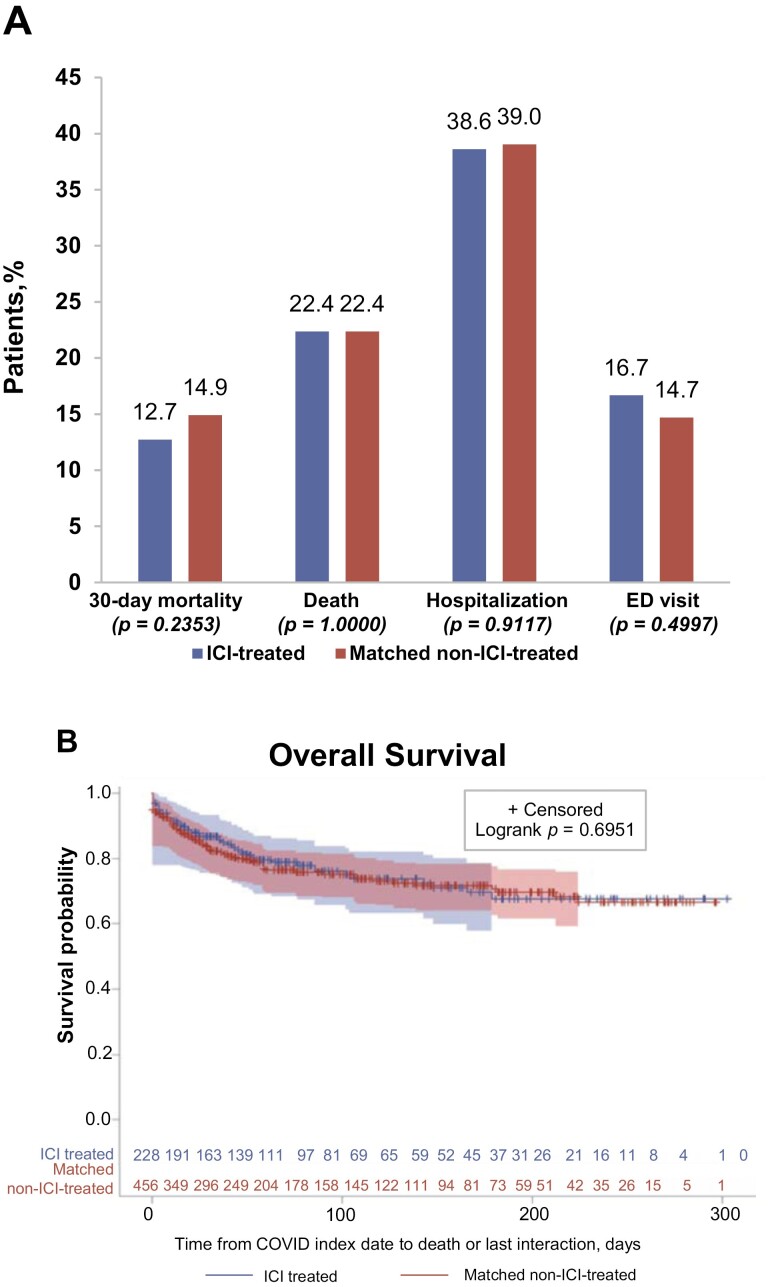
As of January 28, 2021, patients in both cohorts had a similar mean number of follow-up days: 87.5 days (SD: 79.5) for the ICI-treated cohort versus 81.7 days (SD: 81.3) for the non-ICI-treated cohort (P = .097). There were no significant differences in COVID-19 severity or mortality outcomes between the ICI-treated and the non-ICI-treated matched cohorts (Fig. 3A). Similar overall and 30-day mortality rates were observed among ICI-treated versus non-ICI-treated patients (overall mortality of 22.4% for each cohort, P = 1.000; 30-day mortality of 12.7% vs. 14.9%, P = .235). The percentages of patients who had at least one hospitalization were 38.6% for the ICI-treated group versus 39.0% for the non-ICI-treated group (P = .912), and the percentages of patients who had at least one ED visit were 16.7% versus 14.7%, respectively (P = .500). A Kaplan-Meier survival analysis indicates that estimated trends of overall survival were similar between the 2 cohorts, as shown in Fig. 3B.
Figure 3.
Comparison of outcomes between the ICI-treated and matched non-ICI-treated study cohorts. (A) Mortality and COVID-19 severity outcomes in ICI-treated and matched non-ICI-treated cohorts, expressed as percentages of patients affected in each cohort. (B) Kaplan-Meier plot of overall survival after the COVID-19 diagnosis in the 2 study cohorts. ED, emergency department; ICI, immune checkpoint inhibitor.
Subgroup Analysis: Patients Treated With ICI Only Versus ICI Plus Chemotherapy
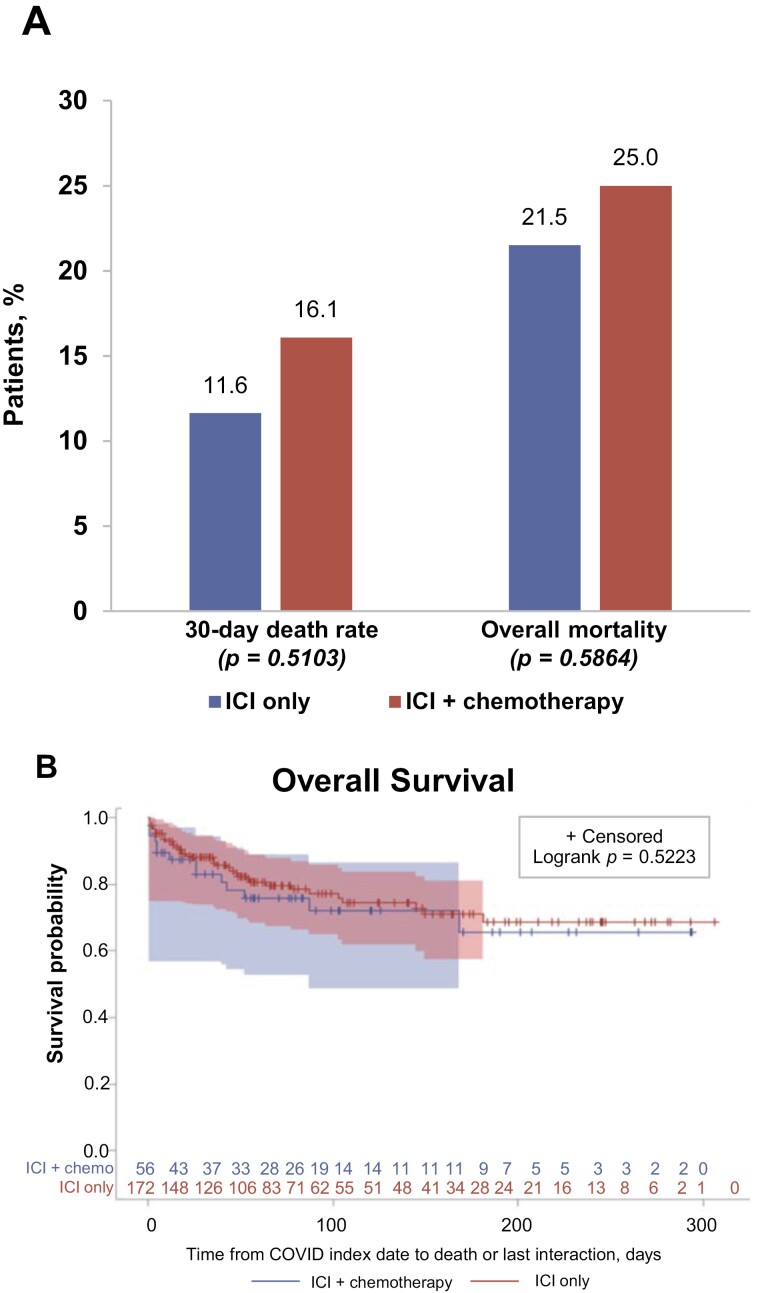
In the ICI-treated cohort, 56 (25% of the 228) patients were treated with ICI combined with chemotherapy; the remaining 172 patients received ICI without chemotherapy. Stratification of the ICI-treated cohort by receipt of chemotherapy revealed interesting similarities and differences, although the sample sizes in this subgroup analysis are small. Demographic characteristics between the 2 subgroups remained similar except for race (Supplementary Table S3). Many clinical characteristics also remained similar, particularly the proportion of patients with comorbidities. However, the distribution of cancer types differed significantly between the 2 subgroups such that higher percentages of patients treated with ICI plus chemotherapy had lung cancer compared with patients treated with ICI only (62.5% vs. 34.9%, P < .001).
Although the number of patients in each subgroup is small, we observed similar COVID-19 outcomes between the subgroups, suggesting that the addition of chemotherapy to an ICI-based regimen does not appear to be associated with different outcomes (Fig. 4A and B). The mean follow-up time for both subgroups was similar: 82.7 days for the ICI plus chemotherapy subgroup versus 89.1 days for the ICI only subgroup (P = .542).
Figure 4.
Subgroup analysis: COVID-19 outcomes in the ICI-treated cohort, stratified by receipt of chemotherapy. (A) COVID-19 mortality outcomes in subgroups treated with ICI only or ICI plus chemotherapy, expressed as percentages of patients affected in each subgroup. (B) Kaplan-Meier plot comparing survival after COVID-19 diagnosis in subgroups treated with ICI only and ICI plus chemotherapy.
Sensitivity Analysis
A sensitivity analysis conducted on the full sample population, after adjusting for demographics, clinical characteristics including tumor types and comorbidities, and key risk factors of COVID-19 mortality, demonstrated that recent exposure to ICI treatment does not predict higher mortality risk in patients (hazard ratio: 0.82, 95% CI [0.63-1.07], P = .1452).
Discussion
The findings of this large real-world study support the safe use of ICIs in cancer patients diagnosed with COVID-19. Before matching, the ICI cohort consisted of patients with compromised lung function, as characterized by high rates of lung cancer, metastatic disease, smoking, COPD, and chronic respiratory disease, rendering patients susceptible to poor COVID-19 outcomes. We used matching methods that accounted for differences in observable characteristics. Among matched ICI-treated and non-ICI-treated cohorts, no significant differences in COVID-19 outcomes emerged, including mortality (overall and 30-day) and severity (hospitalization and ED visits), suggesting that use of ICIs before COVID-19+ diagnosis did not negatively impact severity of COVID-19 or survival outcomes.
The findings of this study in the US are consistent with real-world studies conducted within and outside of the US.4,5,9-12,14,22 An analysis examining data collected from CCC19, a large registry of patients with cancer and COVID-19 in the US, found that immunotherapy did not affect mortality risk.12 A study conducted using the United Kingdom Coronavirus Cancer Monitoring Project (UKCCMP) COVID-19 database showed that mortality in cancer patients who contracted COVID-19 was not affected by immunotherapy.9 Multivariate analyses of data collected from the TERAVOLT registry, a global database that exclusively contains COVID-19+ patients with thoracic neoplasms, showed that immunotherapy was not associated with an increased risk of death, although these results may not be generalizable given the relatively small sample size.4 Our findings also corroborate with a recent meta-analysis of 16 studies showing that recent immunotherapy or chemoimmunotherapy was not associated with an increased risk of death in COVID-19+ cancer patients.23
Our study builds upon and adds to the existing literature on ICIs and COVID-19 in several ways. First, to our knowledge, our study cohort of cancer patients with COVID-19 in the US is the largest reported to date in the published literature. In contrast with prior studies that focus exclusively on PD-1 or patients diagnosed with a specific type of cancer, our analyses include all ICI targets and patients with a range of tumor types.4,10,11
Second, our data span 11 months of the pandemic, from February 2020 to January 2021, with nearly half of the patients diagnosed during the recent surge between November 2020 and January 2021. In contrast, all prior studies focused on earlier periods of the pandemic, none accruing after November 2020.4-14 In the context of the COVID-19 pandemic, which is fast-evolving and changing the practice of oncology in real time, the recency of our data may also ensure that the study’s findings bear the greatest relevance to real-world clinical practice.
Third, the existing literature is limited to data from either single institution7,10,11,13 or prospective registries.4,6,8,9,12,14 While findings from single-center analyses add to the evidence base, these data were typically collected during the epicenter of the outbreak when levels of resource scarcity were highest; the single institution experience also may not be generalizable to the rest of the population. Other studies that used registries (eg, CCC19) gathered data via a survey-based collection process in which data sharing is voluntary by design. This may result in selection bias and reporting errors. In contrast, leveraging the richness and broad coverage of automated data collection from EHRs representing multiple institutions across the US, our study included a cohort that better represents real-world clinical practice.
Lastly, the robustness of our findings was strengthened by utilizing 2 different assessment approaches. The primary approach, propensity score matching, was implemented to estimate treatment effects. We were able to demonstrate the balance between treatment groups in a transparent way. Our findings were further supported by the sensitivity analysis, which was conducted using a multivariate regression model to control for confounding effects.
A notable finding from our study is that there was no significant difference in outcomes between cohorts treated with ICI alone and those treated with ICI plus chemotherapy. Past studies have found that receipt of cytotoxic chemotherapy was associated with greater COVID-19 severity and 30-day mortality.4,6,12 However, data on patients treated with chemoimmunotherapy remain limited. The subgroup analysis in this study is purely descriptive. One caveat is that the sample sizes of subgroups after stratification were not sufficiently powered to distinguish the difference between each ICI-treated and non-ICI-treated subgroup. Although we found that many clinical characteristics are similar between patients who received ICI plus chemotherapy and those who received ICI without chemotherapy, it is possible that patients who received ICI plus chemotherapy had confounding factors that were not fully evaluable in our population. The finding presented in our study is encouraging for clinicians who treat patients with multimodality regimens that include both chemotherapy and ICIs. However, until further evidence is available, it is prudent to interpret these findings with caution.
Limitations
Despite the distinct strengths of this study, certain limitations should be considered. First, in the absence of a randomized, controlled trial, it should be acknowledged that, notwithstanding our best attempts, the observational nature of this study does not allow us to determine causality. We can only account for measurable confounders, while ruling out other unmeasured and residual factors that may impact the interpretation of our findings. Second, the issue of missing data is an important consideration and impacts a number of key study outcomes. For example, some of the clinical characteristics and prognostic factors that may impact outcomes (eg, tumor burden, site of metastasis) remain unknown. Other outcomes of interest such as intensive care unit (ICU) admission were likely underreported in the data. Third, the case definition used in this study relied on either a diagnosis code or positive diagnostic test. This definition excludes a subset of asymptomatic patients and patients who did not get tested or received tests outside of the relevant window. This may result in potential selection bias whereby our cohort reflects a COVID-19+ population with more-severe illness; therefore, our hospitalization and mortality rates in the overall COVID-19+ cohort should be interpreted with caution. Fourth, our cohort had a higher representation of Whites than the US COVID-19+ population (78.7% vs. 50%).24 The discrepant racial distribution was likely due to the fact that most patients in our study resided in the Northeast and Midwest where data were more heavily sourced. During the pandemic, public health response, availability of testing, social distancing policies, hospital and ICU capacity, use of COVID-19 treatment, and cancer management differ by geographic region. This may limit our ability to generalize findings to the overall US population.
Our final ICI-treated study cohort was reduced from 284 to 228 patients because 56 of the 284 ICI-treated cancer patients had baseline and clinical characteristics that were markedly different from those of patients who had not been treated with ICIs, and thus these 56 cancer patients could not be exactly matched. While the incorporation of exact matching, in addition to propensity-score matching, enhances the robustness of the ICI-treated versus non-ICI-treated comparison, the generalizability of the result is limited to the subset of patients for whom matches could be found, which excludes, in particular, many patients with more aggressive forms of lung cancer. We adopted an alternative methodology in the sensitivity analysis using the entire patient cohort, and the results confirmed the main study findings from the matched analysis.
Finally, whereas several other studies were able to attribute the cause of death to COVID-19 or cancer (or another cause), our database did not contain the cause of death, so all mortality was reported as all-cause mortality. It would have been informative to know whether patients died from COVID-19 or from their cancer.
Conclusion
Our large study of patients with cancer who contracted COVID-19 meaningfully adds to the evidence base that use of ICIs (any class, with or without chemotherapy) before SARS-CoV-2 infection does not affect COVID-19 severity or survival outcomes. These findings are consistent with the results from several registries of patients with COVID-19 and cancer and large single-institution studies. Together this expanded knowledge base supports the safety of the continued use of ICIs in cancer patients during the pandemic. Future research can address the long-term safety and generalizability of our findings with expanded sample sizes and further examine if there is a temporal association between ICI exposure and COVID-19 outcomes as well as how different ICI dosing frequencies might impact COVID-19 outcomes.
Supplementary Material
Acknowledgments
Fern Alexander, PhD, and Esther Tazartes, MS, of Global Outcomes Group, provided editorial assistance.
Contributor Information
Ruoding Tan, U.S. Medical Affairs, Genentech, Inc., South San Francisco, CA, USA.
Cindy Yun, U.S. Medical Affairs, Genentech, Inc., South San Francisco, CA, USA.
Arpamas Seetasith, U.S. Medical Affairs, Genentech, Inc., South San Francisco, CA, USA.
Daniel Sheinson, U.S. Medical Affairs, Genentech, Inc., South San Francisco, CA, USA.
Robert Walls, Safety and Risk Management, Product Development, F. Hoffmann-La Roche AG, Basel, Switzerland.
Innocent Ngwa, Safety and Risk Management, Product Development, F. Hoffmann-La Roche AG, Basel, Switzerland.
Josina C Reddy, Safety and Risk Management, Product Development, F. Hoffmann-La Roche AG, Basel, Switzerland.
Qing Zhang, Personalized Healthcare Data Science, Global Product Development, Genentech, South San Francisco, CA, USA.
Matthew H Secrest, Personalized Healthcare Data Science, Global Product Development, Genentech, South San Francisco, CA, USA.
Peter Lambert, Personalized Healthcare Data Science, Global Product Development, Genentech, South San Francisco, CA, USA.
Khaled Sarsour, Personalized Healthcare Data Science, Global Product Development, Genentech, South San Francisco, CA, USA.
Funding
Research reported in this publication was supported by Genentech, Inc. The sponsor was involved in all aspects of the study.
Conflict of Interest
Ruoding Tan, Cindy Yun, Arpamas Seetasith, Daniel Sheinson, Matthew H. Secrest, Peter Lambert, Khaled Sarsour: Genentech, Inc. (E, OI), F. Hoffmann-La Roche (OI); Robert Walls, Innocent Ngwa, Josina C. Reddy: F. Hoffmann-La Roche Ltd (E, OI); Qing Zhang: Genentech, Inc. (E), F. Hoffmann-La Roche, Regeneron, BMS, Abbvie, AC Immune (OI).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/Design: R.T., C.Y., A.S., D.S., R.W., I.N., J.C.R., Q.Z., M.H.S., P.L., and K.S. Provision of study material/patients: R.T., C.Y., A.S., D.S., R.W., I.N., J.C.R., Q.Z., M.H.S., and K.S. Collection and/or assembly of data: R.T., C.Y., A.S., D.S., R.W., I.N., J.C.R., Q.Z., M.H.S., and P.L. Data analysis and interpretation: R.T., C.Y., A.S., D.S., R.W., I.N., J.C.R., Q.Z., P.L., and K.S. Manuscript writing: R.T., C.Y., A.S., D.S., R.W., I.N., J.C.R., Q.Z., M.H.S., P.L., and K.S. Final approval of manuscript: All authors
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Addeo A, Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treat Rev. 2020;88:102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakouny Z, Hawley JE, Choueiri TK, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38(5):629-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nature Med. 2020;26(5):665-671. [DOI] [PubMed] [Google Scholar]
- 4. Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7) :914-922. 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuderer NM, Choueiri TK, Shah DP, et al. ; COVID-19 and Cancer Consortium . Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crolley VE, Hanna D, Joharatnam-Hogan N, et al. COVID-19 in cancer patients on systemic anti-cancer therapies: outcomes from the CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London) cohort study. Ther Adv Med Oncol. 2020;12:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee LY, Cazier JB, Angelis V, et al. ; UK Coronavirus Monitoring Project Team . COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31(10):1386-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wise-Draper T. Systemic cancer treatment-related outcomes in patients with SARS-CoV-2 infection: a CCC19 registry analysis. Ann Oncol. 2020;30:S1142-S1215. 10.1016/annonc/annonc325 [DOI] [Google Scholar]
- 13. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogiers A, Pires da Silva I, Tentori C, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9:e001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12(5):269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frebel H, Nindl V, Schuepbach RA, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209(13):2485-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chawla D, Rizzo S, Zalocusky K, et al. Descriptive epidemiology of 16,780 hospitalized COVID-19 patients in the United States. medRxiv Preprint. https://doi.org/10/1101/2020.07.17.20156265 [Google Scholar]
- 21. Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12(4):188-197. [PMC free article] [PubMed] [Google Scholar]
- 22. Horn L, Whisenant JG, Torri V, et al. TERAVOLT: Thoracic Cancers International COVID-19 Collaboration: impact of cancer therapy and COVID therapy on survival. Presented at the 2020 Annual Meeting of the American Society of Clinical Oncology. www.teravolt-consortium.org. [DOI] [PMC free article] [PubMed]
- 23. Park R, Lee SA, Kim SY, de Melo AC, Kasi A. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: a systematic review and meta-analysis of patient data. Acta Oncol. 2021;60(1):13-19. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. COVID-19 Racial and Ethnic Disparities; 2020. Downloaded April 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/increased-risk-illness.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.