Abstract
Background
Complementary medicines (CM) are frequently used by patients with cancer. Controversy exists over the effectiveness and risk that CM may add to conventional cancer therapy. The incidence of CM use among patients enrolled in phase III clinical trials is unknown.
Methods
Medication lists from 6 international phase III clinical trials were retrospectively reviewed to identify patients using CM. Patients had metastatic breast, colorectal, or lung cancers. Quality of life, adverse events, overall survival, and progression-free survival were compared between CM users and non-users. Baseline differences between groups were adjusted with propensity score matching groups.
Results
Seven hundred and six of 3446 patients (20.5%) used at least one CM. CM use was highest among patients with breast cancer (35.6%). CM users had more favorable baseline prognostic factors (ECOG 0-1, non-smoking status, younger age, and fewer metastases). CM use was associated with lower rates of adverse events (50% vs. 62%, P = .002) and quality of life was similar between both groups. After adjustment with propensity score matching, CM use was also associated with longer overall survival in patients with lung cancer (adjusted hazard ratio 0.80, 95%CI, 0.68-0.94, P =.0054). However, several key control variables like EGFR status were not available.
Conclusion
One in 5 patients in phase III clinical trials report using CM. CM was not associated with worse cancer-specific outcomes. However, CM users had more favorable baseline prognostic factors, and likely other confounders that may have contributed to improved outcomes observed in the lung cohort. Physicians should monitor for CM use and potential interactions with clinical trial drugs.
Keywords: complementary medicine, cancer, clinical trial
There has been increasing interest in the combination of capecitabine and temozolomide (CAPTEM) for the treatment of gastroenteropancreatic neuroendocrine neoplasm (GEPNEN). This review reports a large retrospective analysis to report the efficacy and tolerability of temozolomide (TEM)-containing regimens, particularly CAPTEM, in patients with grade 3 GEPNENs.
Implications for Practice.
This retrospective study reviewed complementary medicine use among patients with metastatic breast, lung, and colorectal cancers who were enrolled in phase III clinical trials. We found that at least 20% of patients enrolled in phase III cancer clinical trials were also using complementary medicines. Complementary medicines were not associated with worse cancer specific-outcomes; however, this may be influenced by having more favorable baseline characteristics and other uncaptured variables. Physicians should continue to monitor for potential interactions between complementary medicines and clinical trial drugs.
Background
Complementary medicine (CM) includes a broad range of substances used concurrently with conventional medical therapies, such as herbal and dietary supplements, homeopathy, and traditional medicines.1,2 CM use is popular amongst the general public and cancer patients, with typical CM usage rates varying from% 34 to 88%.3-6 A previous assessment of phase I clinical trials suggested that up to 88% of patients were using CM.4 No study has yet tried to assess CM use in large randomized clinical trials.
Although CM is typically viewed as “natural” and therefore less toxic, there are concerns that CM may have drug interactions, especially among patients enrolled in clinical trials with experimental agents.7-10 Among women with newly diagnosed early-stage breast cancer who receive standard therapies, the new use of alternative medicine was a marker of greater psychosocial distress and worse quality of life.11 Studies by Johnson et al suggested that the complementary and alternative medicine use were associated with worse overall survival (OS) among patients with non-metastatic cancers.12,13 These data suggest that there may be detriment associated with CM use in terms of toxicity, quality of life, and survival.
The goal of this study was to determine the incidence of CM use among patients with metastatic cancers enrolled in phase III clinical trials and to evaluate for negative associations between the CM use and cancer-specific outcomes. To this end, we retrospectively reviewed the CM use in 6 large randomized control trials conducted by the Canadian Clinical Trials Group (CCTG).
Methods
Clinical Trials and Patient Population
Six prospective randomized controlled clinical trials conducted by the CCTG were used in this analysis. Trials were conducted between 2003 and 2014. Three trials were performed in patients with metastatic colorectal cancer (NCT00079066, NCT00640471, and NCT01830621), 2 in metastatic non–small cell lung cancer (NCT00036647 and NCT01000025), and one in metastatic HER2 positive breast cancer (NCT00667251).14-19 See Supplementary Table S1 summarizing the trial details. All patients had progressive disease despite previous anti-neoplastic therapy with the exception of the breast cancer trial, which was performed in a first-line setting. NCT00667251 (MA.31; lapatinib versus trastuzumab with taxane therapy in metastatic breast cancer) was the only trial that explicitly prohibited medications that may interact with CYP3A4, of which, St John’s wort, star fruit, pawpaw, ginkgo biloba, kava, grape seed, valerian, ginseng, and echinacea were mentioned specifically.
CM and Patient Classification
Per trial protocols, medications at the time of enrollment were recorded for each patient. Medication lists were reviewed by 2 independent investigators (JCW and AS) who assessed for complementary medicines based on the following criteria:
-
a. Products found on natural medicine databases including:
i. The Natural Medicines Comprehensive Database20
ii. The Memorial Sloan Kettering Cancer Centre Integrative Medicine Database21
iii. The National Center for Complementary and Integrative Health22
iv. The National Cancer Institute list of complementary medicines23
v. The Cochrane Complementary Medicine list of Operational definition of complementary medicine24
b. Products not located in a natural medicine database but collectively thought to be consistent with a CM (present on herbal websites/absence in drug databases)
Interrater reliability was assessed with Cohen’s kappa. All discrepancies were reviewed by additional authors (DYCH, JGM, and MS) and final coding was decided through consensus. The final list was then reviewed and approved by all authors prior to statistical analysis. CM were not assessed for intention (ie, used as anti-cancer therapy) as this information was not available. Vitamins and minerals were not included as CM in this analysis.
Outcomes and Statistical Analysis
Baseline Factors Associated with CM Use
Patients were considered users of CM if their medication list included one or more CMs. Patients using CM were compared to those not using CM. Baseline demographic data were obtained from variables reported in the original clinical trials. These variables (listed in Tables 1-3) were also used to develop a logistic regression model for each disease site, stratified by study treatment arms, to identify factors that predicted CM use, using backward variable elimination and a .05 significance level.
Table 1.
Baseline characteristics for CM and non-CM users in colorectal cancer patients from CO.20, 21, and 23.
| Variable | CM yes (%) N = 251 | CM no (%) N = 1353 | Total (%) | Univariate P value |
|---|---|---|---|---|
| Gender | ||||
| Female | 90 (35.9) | 481 (35.6) | 571 (35.6) | .926 |
| Male | 161 (64.1) | 872 (64.4) | 1033 (64.4) | |
| Age ∗∗ | ||||
| ≤65 | 167 (66.5) | 745 (55.1) | 912 (56.9) | .001 |
| >65 | 84 (33.5) | 608 (44.9) | 692 (43.1) | |
| ECOG Performance Status ∗∗ | ||||
| ECOG PS 0 or 1 | 236 (94.0) | 1159 (85.7) | 1395 (87.0) | <.001 |
| ECOG PS 2 or 3 | 15 (6.0) | 194 (14.3) | 209 (13.0) | |
| Presence of liver metastases | ||||
| No | 62 (24.7) | 338 (25.0) | 400 (24.9) | .925 |
| Yes | 189 (75.3) | 1015 (75.0) | 1204 (75.1) | |
| Number of previous chemo drug classes | ||||
| >2 | 240 (95.6) | 1306 (96.5) | 1546 (96.4) | .479 |
| ≤2 | 11 (4.4) | 47 (3.5) | 58 (3.6) | |
| Number of disease sites ∗∗ | ||||
| >2 | 65 (25.9) | 509 (37.6) | 574 (35.8) | < .001 |
| ≤2 | 186 (74.1) | 844 (62.4) | 1030 (64.2) | |
| Hemoglobin ∗∗ | ||||
| Grade ≥1 | 132 (52.6) | 913 (67.5) | 1045 (65.1) | < .001 |
| Grade 0 | 119 (47.4) | 440 (32.5) | 559 (34.9) | |
| LDH | ||||
| Missing | 4 (1.6) | 57 (4.2) | 61 (3.8) | .089 |
| >UNL | 154 (61.4) | 880 (65.0) | 1034 (64.5) | |
| ≤UNL | 93 (37.1) | 416 (30.7) | 509 (31.7) | |
| Alkaline phosphatase | ||||
| Missing | 2 (0.8) | 12 (0.9) | 14 (0.9) | .440 |
| >UNL | 155 (61.8) | 869 (64.2) | 1024 (63.8) | |
| ≤UNL | 94 (37.5) | 472 (34.9) | 566 (35.3) | |
| Study treatment in each trial | ||||
| Napabucasin + BSC (CO23) | 21 (8.4) | 117 (8.6) | 138 (8.6) | .031 |
| Placebo + BSC (CO23) | 23 (9.2) | 121 (8.9) | 144 (9.0) | |
| BSC (CO17) | 34 (13.5) | 251 (18.6) | 285 (17.8) | |
| Cetuximab + BSC (CO17) | 51 (20.3) | 236 (17.4) | 287 (17.9) | |
| Cetuximab + Brivanib (CO20) | 47 (18.7) | 329 (24.3) | 376 (23.4) | |
| Cetuximab + Placebo (CO20) | 75 (29.9) | 299 (22.1) | 374 (23.3) | |
∗∗Significance in univariate and multivariate analysis (P < .05)
Table 2.
Baseline patient characteristics for CM and non-CM users in lung cancer patients from BR.21 and BR.26.
| Variables | CM yes (%) N = 269 | CM no (%) N = 1182 | Total (%) | Univariate P value |
|---|---|---|---|---|
| Gender | ||||
| Female | 118 (43.9) | 494 (41.8) | 612 (42.2) | .534 |
| Male | 151 (56.1) | 688 (58.2) | 839 (57.8) | |
| Age | ||||
| ≤65 | 164 (61.0) | 686 (58.0) | 850 (58.6) | .379 |
| > 65 | 105 (39.0) | 496 (42.0) | 601 (41.4) | |
| ECOG Performance Status ∗∗ | ||||
| ECOG PS 0 or 1 | 214 (79.6) | 815 (69.0) | 1029 (70.9) | .001 |
| ECOG PS 2 or 3 | 55 (20.4) | 367 (31.0) | 422 (29.1) | |
| Weight Loss in previous 6 months ∗ | ||||
| Missing | 5 (1.9) | 27 (2.3) | 32 (2.2) | .034 |
| ≥5% | 51 (19.0) | 295 (25.0) | 346 (23.8) | |
| < 5% | 213 (79.2) | 860 (72.8) | 1073 (73.9) | |
| Smoking Status ∗∗ | ||||
| Never-smoker | 94 (34.9) | 311 (26.3) | 405 (27.9) | .006 |
| Unknown | 5 (1.9) | 35 (3.0) | 40 (2.8) | |
| Ever smoked | 170 (63.2) | 836 (70.7) | 1006 (69.3) | |
| Race ∗ | ||||
| Others | 196 (72.9) | 953 (80.6) | 1149 (79.2) | .005 |
| Eastern Asian | 73 (27.1) | 229 (19.4) | 302 (20.8) | |
| Study treatment in each trial | ||||
| BR21 Placebo (BR21) | 44 (16.4) | 199 (16.8) | 243 (16.7) | .702 |
| BR26 Placebo (BR26) | 51 (19.0) | 189 (16.0) | 240 (16.5) | |
| Erlotinib (BR21) | 87 (32.3) | 401 (33.9) | 488 (33.6) | |
| Dacomotinib (BR26) | 87 (32.3) | 393 (33.2) | 480 (33.1) | |
∗Significance in univariate analysis (P < .05).
∗∗Significance in both univariate and multivariate analysis (P < .05).
Table 3.
Baseline characteristics for CM and non-CM users in patients with breast cancer from MA.31.
| Variables | CM yes (%) N = 232 | CM no (%) N = 420 | Total | Univariate P value |
|---|---|---|---|---|
| Age ∗∗ | ||||
| <50 | 104 (44.8) | 118 (28.1) | 222 (34.0) | <.001 |
| ≥50 | 128 (55.2) | 302 (71.9) | 430 (66.0) | |
| ECOG Performance Status | ||||
| ECOG PS 0 or 1 | 227 (97.8) | 403 (96.0) | 630 (96.6) | .200 |
| ECOG PS 2 or 3 | 5 (2.2) | 17 (4.0) | 22 (3.4) | |
| Presence of liver metastases | ||||
| No | 120 (51.7) | 233 (55.5) | 353 (54.1) | .357 |
| Yes | 112 (48.3) | 187 (44.5) | 299 (45.9) | |
| ER Status | ||||
| Unknown/missing | 10 (4.3) | 18 (4.3) | 28 (4.3) | .889 |
| Negative | 73 (31.5) | 130 (31.0) | 203 (31.1) | |
| Positive | 149 (64.2) | 272 (64.8) | 421 (64.6) | |
| PR Status | ||||
| Unknown/missing | 16 (6.9) | 22 (5.2) | 38 (5.8) | .417 |
| Negative | 134 (57.8) | 260 (61.9) | 394 (60.4) | |
| Positive | 82 (35.3) | 138 (32.9) | 220 (33.7) | |
| Prior adjuvant anti-HER2/neu targeted therapy | ||||
| No | 193 (83.2) | 341 (81.2) | 534 (81.9) | .526 |
| Yes | 39 (16.8) | 79 (18.8) | 118 (18.1) | |
| Prior other adjuvant chemotherapy | ||||
| No | 119 (51.3) | 226 (53.8) | 345 (52.9) | .538 |
| Yes | 113 (48.7) | 194 (46.2) | 307 (47.1) | |
| Study treatment in each trial | ||||
| Lapatinib plus taxane | 121 (52.2) | 205 (48.8) | 326 (50.0) | .413 |
| Trastuzumab plus taxane | 111 (47.8) | 215 (51.2) | 326 (50.0) | |
∗∗Significance in both univariate and multivariate analysis (P < .05).
Patient Outcomes/Statistical Analysis
Outcomes between CM and non-CM users were analyzed collectively and by disease site. The baseline factors listed in Tables 1-3 were used to generate a logistic regression model; a propensity score analysis model was then applied to further optimize patient matching. Propensity score analysis was performed by each disease site, using a linear propensity score to generate the linear propensity scores by CM users and non-users. Patients whose propensity score was below the larger of the 2 minimums (for CM users and non-users) were excluded, as were patients whose score was above the smaller of the 2 maximums. The propensity scores were then used to stratify patients into 5 approximately sized subgroups. Stratified analysis was performed on the classified strata. Final groups were compared with Cochran-Mantel-Haenszel chi-square testing to ensure the balance of baseline characteristics.
OS was defined as the time from randomization to the date of death or censored at the last follow-up. Progression-free survival (PFS) was defined as the time from randomization until the date of progressive disease, death, change of therapy, or censored at the date of change of therapy or last disease assessment. Kaplan-Meier curves were generated and data were compared with a log-rank test stratified by propensity score group and treatment arms. Adjusted hazard ratios (aHR) and 95%CI were generated from the Cox regression model stratified by propensity score group and treatment arms.
CM use effect on the quality of life (QoL) was assessed in each trial using the EORTC QLQ-C30, a self-administered cancer-specific questionnaire assessing 5 functional domains (physical, role, emotional, cognitive, and social), 3 symptom domains (fatigue, nausea, and pain), and 6 single symptom items (dyspnea, sleep, appetite, bowels, and global assessment). For each domain, a linear transformation was applied to standardize the raw score from 0 to 100.
QoL data was also used to assess time to deterioration in physical function and global QoL, defined as the time from randomization to the time that the patient’s first evaluation as deteriorated; this condition was met if a 10-point decrease in function’s score from baseline was recorded. Patients without deterioration were censored at the time of last assessment in QoL. Kaplan-Meier curves were used with log-rank testing stratified by propensity score group and treatment arms to assess median time to deterioration.
QoL was also assessed through changes in the domains outlined above; a change in score of 10 points from baseline was considered clinically relevant. Patients were considered improved if a score was 10-points or higher than baseline at any time in QoL assessment whereas, patients were considered worsened if a score 10 or more points lower than baseline occurred at any time without improvement. Patients with scores between 10 points of baseline were considered stable. Domains were compared for stable, improved, and worsened between CM users and non-CM users with Chi-square test stratified by propensity score group and treatment arms; this was followed by Mantel-Haenszel chi-square test for trend.
Adverse events (AE) were assessed using the Common Toxicity Criteria of the National Cancer Institute (versions 2.0 and 3.0). Incidence of grade 3+ AEs was compared between CM users and non-users using the Cochran-Mantel-Haenzel chi-square test, stratified by propensity score group and treatment group.
For all comparisons, a P-value of <.05 was considered significant. P-values were unadjusted for multiple comparisons.
Results
Medication and Population Characteristics
A total of 24, 908 medications were reviewed for all 3707 patients. Agreement between medication reviewers was substantial (Kappa value = 0.65). After the final review, a total of 651 medications were identified as CMs. The most frequent CMs included herbal/natural products (46.6%), dietary supplements (22.1%), fish oils (18.0%), glucosamine (11.0%), and homeopathy (2.3%). Overall, 20.3% (752/3707) of patients used at least one type of CM. CM use was more common among patients with breast cancer [232/652 (35.6%)], and less so among patients with colorectal cancer [251/1604 (15.6%)] and lung cancer [269/1451 (18.5%)].
Baseline characteristics by disease site are listed in Tables 1-3. Multivariate analysis demonstrated that CM use in patients with lung cancer was associated with the favorable ECOG status (0 or 1) and non-smoker status; weight loss <5% and Eastern Asian ethnicity were significantly associated with CM use in univariate but not multivariate analysis. CM used in CRC patients was associated with age ≤65 years old, ECOG 0 or 1, ≤2 sites of metastases, and normal hemoglobin after multivariate analysis. CM use in patients with breast cancer was associated with age less than 50 after multivariate analysis.
Propensity Score Matching
All preselected baseline factors were included in the logistic regression model to generate the linear propensity score as the means to balance all factors between the CM use groups. After propensity matching analysis, all baseline characteristics were well balanced following the propensity match (data not shown). This resulted in the inclusion of 706 patients using the CM and 2740 patients using non-CM for the final analysis. See Supplementary Fig. S1 for a consort-flow depiction of the study design.
Overall Survival and Progression-Free Survival
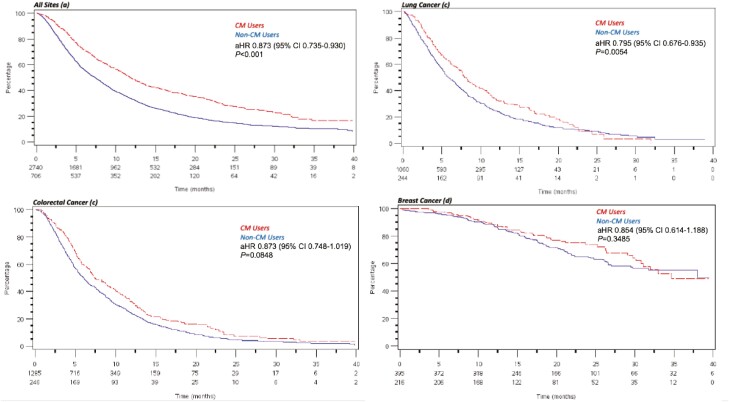
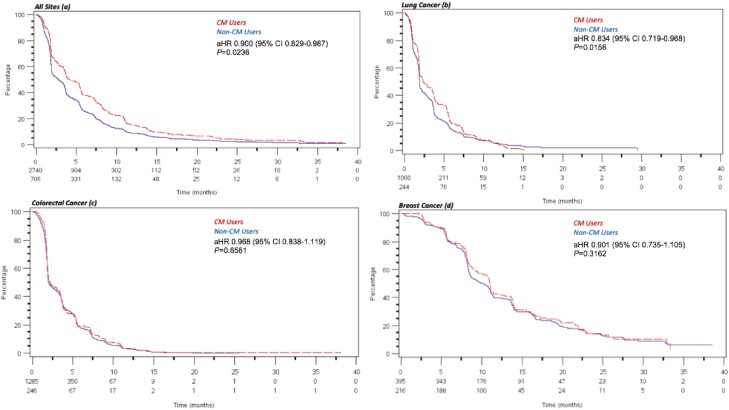
Collectively, CM use was associated with longer OS (aHR 0.84, 95%CI, 0.75-0.93, P =.001). By site, CM use was associated with longer OS in lung cancer (aHR 0.80, 95%CI, 0.68-0.94, P = .0054) but not in CRC (aHR 0.87, 95%CI, 0.75-1.02) or in patients with breast cancer (aHR 0.85, 95%CI, 0.61-1.19) (see Fig. 1a-d). Similarly, the collective risk of progression was also significantly lower in patients using CM (aHR 0.90, 95% CI, 0.82-0.987, P = .024) and in patients with lung cancer but not in CRC or patients with breast cancer (Fig. 2a-d).
Figure 1.
a-d: Kaplan-Meier curves depicting overall survival between complementary medicine (CM) users (n = 706) and non–CM users (n = 2740). Risk of death was lower in CM users (aHR 0.873, 95% CI, 0.735-0.930, P < .001) (a) and among CM users with lung cancer (aHR 0.795, 95% CI, 0.676-0.935, P = .005) (b). No significant differences in OS are noted between CM users and non-users with colorectal cancer (c) or breast cancer (d).
Figure 2.
a-d: Kaplan-Meier curves depicting progression-free survival between complementary medicine (CM) users (n = 706) and non–CM users (n = 2740). Risk of progression was lower in CM users (aHR 0.900, 95% CI, 0.829-0.987, P = .024) (a) and among CM users with lung cancer (aHR 0.834, 95% CI, 0.719-0.968, P = .016) (b). No significant differences in progression-free survival are noted between CM users and non-users with colorectal cancer (c) or breast cancer (d).
Quality of Life and Adverse Events
CM was not associated with improved QoL between CM and non-CM users. Time to deterioration of global quality of life was similar between CM users and non-users (aHR 1.07, P = .94, Supplementary Fig. S2). Time to deterioration of physical function QoL was also similar between CM users and non-users (aHR 0.97, 95% CI, 0.85-1.11, P = .67). CM use was associated with less improvement in pain, constipation, and role identity in the QoL response analysis. (Table 4) Grade 3+ adverse events were collectively lower in patients using CM (50.0% vs. 61.6%, P = .002).
Table 4.
Quality of life response analysis pooled for all trials.
| Domain | CM use (N, %) | No CM use (N, %) | P CMH | P Mantel Haenszel | ||||
|---|---|---|---|---|---|---|---|---|
| Improved | Stable | Worsen | Improved | Stable | Worsened | |||
| Physical | 123 (21) | 202 (34) | 273 (46) | 540 (25) | 610 (28) | 1014 (47) | .02 | .13 |
| Emotional | 191 (32) | 217 (36) | 189 (32) | 715 (33) | 733 (34) | 709 (33) | .10 | .09 |
| Role | 166 (28) | 147 (25) | 279 (47) | 752 (35) | 397 (18) | 1007 (47) | .00 | .05 |
| Cognitive | 165 (28) | 215 (36) | 215 (26) | 633 (29) | 680 (32) | 833 (39) | .28 | .92 |
| Social | 200 (34) | 143 (24) | 250 (42) | 797 (37) | 459 (21) | 885 (41) | .24 | .20 |
| Fatigue | 241 (40) | 63 (11) | 292 (49) | 936 (43) | 197 (9) | 1030 (48) | .28 | .14 |
| Nausea/vomiting | 94 (16) | 271 (45) | 231 (39) | 444 (21) | 848 (39) | 859 (40) | .05 | .15 |
| Pain | 226 (38) | 125 (21) | 238 (40) | 964 (45) | 392 (18) | 779 (36) | .00 | .00 |
| Dyspnea | 149 (25) | 220 (37) | 225 (38) | 582 (27) | 780 (36) | 795 (37) | .74 | .44 |
| Sleep | 180 (30) | 200 (34) | 214 (36) | 751 (35) | 616 (29) | 784 (36) | .01 | .07 |
| Appetite loss | 142 (24) | 201 (34) | 253 (42) | 565 (26) | 615 (29) | 974 (45) | .42 | .31 |
| Constipation | 116 (19) | 311 (52) | 168 (28) | 572 (27) | 972 (45) | 606 (28) | .00 | .03 |
| Diarrhea | 81 (14) | 240 (40) | 274 (46) | 358 (17) | 846 (39) | 952 (44) | .41 | .42 |
| Global health status | 185 (31) | 134 (22) | 277 (46) | 721 (34) | 456 (21) | 966 (45) | .15 | .05 |
Abbreviation: CMH, Cochran-Mantel-Haenszel chi-square.
Discussion
This large, retrospective analysis of CM use in patients enrolled in phase III clinical trials has several important findings. First, we demonstrate that CM use remains high among patients with advanced cancers who are receiving experimental therapies and was highest amongst women with breast cancer. Previous reports on the incidence of CM use among patients with cancer have varied tremendously, from as low as 6% in the 2018 ASCO National Cancer Opinion Survey, to as high as 88% in a 2004 survey of patients enrolled in phase I clinical trials at the Mayo Center in the US.4,25 Such variation is expected based on study design (ie, medication list chart review versus survey-based studies) and definition of CM, as other studies have included non-pharmacological agents such as meditation and acupuncture.4,26 Our study included only pharmacological CMs identified by reputable sources and explicitly excluded vitamins and minerals, which would contribute to a lower incidence of CM compared to other studies.27,28 Collectively, the high incidence of CM use in this patient population supports the role of screening for CM at the time of trial enrollment, especially in women with breast cancer.
Second, we did not observe any negative association between CM use and cancer-specific outcomes. Our results did find that CM use was associated with longer OS, specifically in patients with lung cancer, however, this must be interpreted with caution given the retrospective and ad hoc nature of the study. Patients with Lung cancer using CM in this study had more favorable prognostic factors (better ECOG status, non-smokers) and while these were adjusted for, there are likely further unadjusted variables confounding the results. For example, EGFR mutation status was not assessed in many patients and therefore not controlled for in this study—CM patients were more likely to be Eastern Asian and non-smokers, both of which are associated with EGFR mutation status and consequently better response to the EGFR-targeted therapies evaluated in the lung cancer trials.29-31 We were also unable to control for variables such as education and economic status—variables known to be associated with both CM use and more favorable cancer outcomes.32,33 Our results are also limited by using trial enrollment medication lists, from which patients may not have reported or been asked about CM use. Based on the above, we cannot conclude that CM use improves cancer outcomes.
A recent study by Johnson et al (2018) found that in patients with non-metastatic cancers, users of CM had a worse 5-year OS compared to no CM use (82.2% vs. 86.6%).12 CM users were more likely to have delayed treatment or refuse additional treatment all together, suggesting that lack of additional treatment contributed to the greater mortality risk.12 These populations are inherently different than the patients included in our study, who had advanced incurable cancer, were typically previously treated with cancer treatments, and were accepting of medical treatment via experimental clinical trials. Moreover, the data collected from our study was obtained from the lists of concomitant medications from the CCTG clinical trials database, while in the Johnson and other studies CM use was obtained from a non-trial database. These differences in study design may explain the different outcomes observed between studies and highlight the importance of designing studies to accurately collect this data.
Beyond attempting to improve OS, patients with cancer use CM to improve QoL and to decrease cancer- and treatment-associated symptoms.3,8,26 Our results show no difference in time to deterioration of QoL between CM users and non-users and overall lower incidences of AEs, however, similar limitations apply including better baseline ECOG status in the CM users. The evidence for CM improving QoL and symptomology are conflicting.34-37 Non-pharmacological CMs including acupuncture, mindfulness, and relaxation therapies may have a short-term benefit toward the quality of life and symptom improvements such as nausea and pain, however, limited high-level data is available for pharmacological CMs.38-41 Conversely, a study by Burstein and colleagues, demonstrated greater psychosocial distress and worse quality of life with CM use in women who had received standard therapy for early-stage breast cancer.11
While we did not observe any associations between CM use and worse patient outcomes, we recognize that the CMs used in this study were heterogeneous and included a variety of herbal products, probiotics, dietary supplements, and fish oils. As such, to generalize all CM as being safe would be untrue—indeed, there are many known CMs that can interact with cancer therapies.42 As such, it remains pertinent to screen trial patients for CM use to identify and prohibit the use of CMs that have shared metabolism pathways, as done in the MA.31 breast cancer trial. Given the clear public interest in CMs and scarcity of data CM-drug interactions, further studies are warranted in the interest of patient safety.43-45
Conclusion
Our study demonstrates that CM use remains popular among patients with cancer, with at least 20% use among those enrolled in phase III trials. We did not observe worse cancer-specific outcomes in CM users. CM use was associated with numerous favorable prognostic baseline factors. This, in conjunction with other unmeasured confounders, may have contributed to the more favorable outcomes in the lung cohort. Given the absence of conclusive clinical trial data demonstrating the efficacy of CMs, the use of these agents remains controversial. Our findings do not replace the need for an open patient-physician relationship to encourage disclosure of CM use and for careful pharmacological review of CMs to identify potential drug interactions.
Supplementary Material
Contributor Information
J Connor Wells, Queen’s University, Kingston, ON, Canada.
Aven Sidhu, Fraser Health and Veralife Health Centre, Surrey, BC, Canada.
Keyue Ding, Canadian Cancer Trials Group, Kingston, ON, Canada.
Martin Smoragiewicz, Canadian Cancer Trials Group, Kingston, ON, Canada.
Daniel Y C Heng, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada.
Frances A Shepherd, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Peter M Ellis, Juravinski Hospital and Cancer Centre, McMaster University, Hamilton, ON, Canada.
Penelope A Bradbury, The Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada.
Derek J Jonker, BC Cancer Agency, Vancouver, BC, Canada.
Lillian L Siu, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Karen A Gelmon, BC Cancer Agency, Vancouver, BC, Canada.
Christos Karapetis, Flinders Medical Centre and Flinders University, Adelaide, Australia.
Jeremy Shapiro, Cabrini Health, Melbourne, Australia.
Louise Nott, Royal Hobart Hospital, Tasmania, Australia.
Christopher J O’Callaghan, Canadian Cancer Trials Group, Kingston, ON, Canada.
Wendy R Parulekar, Canadian Cancer Trials Group, Kingston, ON, Canada.
Lesley Seymour, Canadian Cancer Trials Group, Kingston, ON, Canada.
Jose G Monzon, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada.
Funding
None declared.
Conflict of Interest
J. Connor Wells: Pfizer (Other—travel support); Daniel Y.C. Heng: Pfizer, Novartis, BMS (C/A, RF); Penelope Bradbury: Abbvie, Lilly, Merck, Pfizer (H), Boehringer Ingelheim, Abbvie (SAB); Lillian Siu: Merck, Pfizer, Celgene, AstraZeneca/Medimmune, Morphosys, Roche, GeneSeeq, Loxo, Oncorus, Symphogen, Seattle Genetics, GSK, Voronoi, Treadwell Therapeutics (C/A), Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Inhelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca/Medimmune, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics, Mirati, Shattucks, Avid (RF), Agios (OI--spouse); Peter Ellis: AsztraZeneca, Pfizer, Abbvie, Takeda (H); Jose Monzon: Amgen, BMS, Merck, Novartis, Taiho (SAB), Merck (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Author Contributions
Conception/design: J.C.W., A., K.D., M.S., D.Y.C., L.S., J.G.M. Provision of study material/patients: K.D., F.A.S., P.M.E., P.A.B., D.J.J., L.L.S., K.A.G., C.K., J.S., L.N., C.J.O., W.R.P., L.S., J.G.M. Collection and/or assembly of data: All authors. Data analysis and interpretation: J.C.W., A.S., K.D., M.S., L.S., J.G.M. Manuscript writing: J.C.W., A.S., K.D., M.S., J.G.M. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. NCCIH. Complementary, Alternative, or Integrative Health: What’s In a Name?. https://nccih.nih.gov/health/integrative-health. Accessed October 23, 2019.
- 2. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Rep. 2015;(79):1-16. [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18(13):2505-2514. [DOI] [PubMed] [Google Scholar]
- 4. Dy GK, Bekele L, Hanson LJ, et al. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J Clin Oncol. 2004;22(23):4810-4815. [DOI] [PubMed] [Google Scholar]
- 5. Naing A, Stephen SK, Frenkel M, et al. Prevalence of complementary medicine use in a phase 1 clinical trials program: the MD Anderson Cancer Center Experience. Cancer. 2011;117(22):5142-5150. [DOI] [PubMed] [Google Scholar]
- 6. Hlubocky FJ, Ratain MJ, Wen M, Daugherty CK. Complementary and alternative medicine among advanced cancer patients enrolled on phase I trials: a study of prognosis, quality of life, and preferences for decision making. J Clin Oncol. 2007;25(5):548-554. [DOI] [PubMed] [Google Scholar]
- 7. Markman M. Safety issues in using complementary and alternative medicine. J Clin Oncol. 2002;20(18 Suppl):39S-41S. http://www.ncbi.nlm.nih.gov/pubmed/12235223. Accessed July 7, 2019. [PubMed] [Google Scholar]
- 8. Berretta M, Della Pepa C, Tralongo P, et al. Use of complementary and alternative medicine (CAM) in cancer patients: an Italian multicenter survey. Oncotarget. 2017;8(15):24401-24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai HH, Lin HW, Simon Pickard A, Tsai HY, Mahady GB. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: a systematic literature review. Int J Clin Pract. 2012;66(11):1056-1078. [DOI] [PubMed] [Google Scholar]
- 10. Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22(12):2489-2503. [DOI] [PubMed] [Google Scholar]
- 11. Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340(22):1733-1739. [DOI] [PubMed] [Google Scholar]
- 12. Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4(10):1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson SB, Park HS, Gross CP, et al. Use of alternative medicine for cancer and its impact on survival. J Natl Cancer Inst. 2018;110(1):121-124. [DOI] [PubMed] [Google Scholar]
- 14. Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040-2048. [DOI] [PubMed] [Google Scholar]
- 15. Siu LL, Shapiro JD, Jonker DJ, et al. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 trial. J Clin Oncol. 2013;31(19):2477-2484. [DOI] [PubMed] [Google Scholar]
- 16. Jonker DJ, Nott L, Yoshino T, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(4):263-270. [DOI] [PubMed] [Google Scholar]
- 17. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. ; National Cancer Institute of Canada Clinical Trials Group . Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132. [DOI] [PubMed] [Google Scholar]
- 18. Ellis PM, Shepherd FA, Millward M, et al. ; NCIC CTG; Australasian Lung Cancer Trials Group; NCI Naples Clinical Trials Unit . Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15(12):1379-1388. [DOI] [PubMed] [Google Scholar]
- 19. Gelmon KA, Boyle FM, Kaufman B, et al. Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer: final results of NCIC CTG MA.31. J Clin Oncol. 2015;33(14):1574-1583. [DOI] [PubMed] [Google Scholar]
- 20. Welcome to the Natural Medicines Research Collaboration. https://naturalmedicines.therapeuticresearch.com/. Accessed November 25, 2019.
- 21. Integrative Medicine. Memorial Sloan Kettering Cancer Center. https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine. Accessed November 25, 2019.
- 22. NCCIH. National Center for Complementary and Integrative Health. https://nccih.nih.gov/. Accessed November 25, 2019.
- 23. National Cancer Institute. Complementary and alternative medicine (CAM). https://www.cancer.gov/about-cancer/treatment/cam. Accessed November 25, 2019.
- 24. Cochrane Complementary Medicine. Operational definition of complementary medicine. https://cam.cochrane.org/operational-definition-complementary-medicine. Accessed November 25, 2019.
- 25. American Society of Clinical Oncology. ASCO 2018 Cancer Opinions Survey. 2018. https://www.asco.org/sites/new-www.asco.org/files/content-files/research-and-progress/documents/2018-NCOS-Results.pdf. Accessed October 10, 2019.
- 26. Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM, Want DA. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr Oncol. 2018;25(4):e275-e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggiore RJ, Gross CP, Togawa K, et al. ; Cancer and Aging Research Group . Use of complementary medications among older adults with cancer. Cancer. 2012;118(19):4815-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Posadzki P, Watson LK, Alotaibi A, Ernst E. Prevalence of use of complementary and alternative medicine (CAM) by patients/consumers in the UK: systematic review of surveys. Clin Med (Lond). 2013;13(2):126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28(30):4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci. 2004;101(36):13306-13311. doi: 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339-346. [DOI] [PubMed] [Google Scholar]
- 32. Paltiel O, Avitzour M, Peretz T, et al. Determinants of the use of complementary therapies by patients with cancer. J Clin Oncol. 2001;19(9):2439-2448. [DOI] [PubMed] [Google Scholar]
- 33. Boon H, Stewart M, Kennard MA, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18(13):2515-2521. [DOI] [PubMed] [Google Scholar]
- 34. Lesser GJ, Case D, Stark N, et al. ; Wake Forest University Community Clinical Oncology Program Research Base . A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013;11(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology. 2002;60(4):640-644. [DOI] [PubMed] [Google Scholar]
- 36. Jin X, Ruiz Beguerie J, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2016;4:CD007731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ostermann T, Raak C, Büssing A. Survival of cancer patients treated with mistletoe extract (Iscador): a systematic literature review. BMC Cancer. 2009;9:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lotfi-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J Clin Oncol. 2008;26(34):5618-5629. [DOI] [PubMed] [Google Scholar]
- 40. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yap KY, See CS, Chan A. Clinically-relevant chemotherapy interactions with complementary and alternative medicines in patients with cancer. Recent Pat Food Nutr Agric. 2010;2(1):12-55. [DOI] [PubMed] [Google Scholar]
- 43. Jermini M, Dubois J, Rodondi PY, et al. Complementary medicine use during cancer treatment and potential herb–drug interactions from a cross-sectional study in an academic centre. Sci Rep. 2019;9(1):5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen M, Zhou SY, Fabriaga E, Zhang PH, Zhou Q. Food–drug interactions precipitated by fruit juices other than grapefruit juice: an update review. J Food Drug Anal. 2018;26(2S):S61-S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mouly S, Lloret-Linares C, Sellier PO, Sene D, Bergmann JF. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacol Res. 2017;118:82-92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.